Abstract
Objectives
We sought to determine whether the technique of celloidin removal influences the results of immunostaining in celloidin-embedded cochleae.
Methods
We compared four protocols of celloidin removal, including those using clove oil, acetone, ether-alcohol, and methanol saturated with sodium hydroxide. By optimally fixing our tissue (perfused mice), and keeping constant the fixative type (formalin plus acetic acid), fixation time (25 hours), and decalcification time (ethylenediaminetetraacetic acid for 7 days), we determined whether the technique of celloidin removal influenced the immunostaining results. Six antibodies were used with each removal method: prostaglandin D synthase, sodium, potassium adenosine triphosphatase (Na+,K+-ATPase), aquaporin 1, connective tissue growth factor, tubulin, and 200 kd neurofilament.
Results
Clove oil, acetone, and ether-alcohol resulted in incomplete removal of the celloidin, thereby negatively affecting the results of immunostaining. The methanol–sodium hydroxide method was effective in completely removing the celloidin; it produced the cleanest and most reproducible immunostaining for all six antibodies.
Conclusions
Freshly prepared methanol saturated with sodium hydroxide and diluted 1:2 with methanol was the best solvent for removing celloidin from mouse temporal bone sections, resulting in consistent and reproducible immunostaining with the six antibodies tested.
Keywords: cochlea, immunostaining, sodium hydroxide–methanol
INTRODUCTION
Otologists must rely on postmortem analysis for knowledge about pathology, because biopsy of the labyrinth is not practical because of the risks to auditory and vestibular function. Standard light microscopy using hematoxylin and eosin staining can provide much information to the skilled observer.1 However, other techniques, such as immunostaining, can lead to additional insights by providing information about protein localization in the inner ear.
The easiest and most reliable immunostaining is obtained by using frozen sections or sections embedded in paraffin after fixation. Unfortunately, the human inner ear does not lend itself well to either of these methods. The inner ear is encased by the dense petrous bone, which necessitates decalcification. It also requires a rather large block size to keep all of the relevant structures of interest intact. Therefore, frozen sectioning is not a practical option. Paraffin embedding requires high heat (55°C), which tends to adversely affect morphology.2 The embedding medium of choice for the human temporal bone during the past 100 years has been celloidin, because it gives superior preservation of morphology.1 Celloidin, however, does not lend itself easily to immunostaining. It is difficult to remove celloidin from the sections — in contrast to paraffin, which is easily dissolved. It is imperative for appropriate antigen-antibody binding that the embedding medium be removed to allow penetration of the section by the antibody.
In his analysis of the feasibility of immunohistochemical investigation of the human cochlea, Arnold3 concluded that the sensitivity of the method was limited by both fixation and decalcification. In addition, postmortem autolysis, disease processes, and embedding media are additional variables complicating the interpretation of immunohistochemical staining of the human cochlea.
In the early 1990s, Shi et al4,5 had some success with immunostaining of formalin-fixed, acid-decalcified, celloidin-embedded human temporal bones using an aged sodium hydroxide (NaOH)–methanol protocol. Throughout the 1990s, several different removal techniques were described, including use of clove oil,6–8 acetone,9,10 ether-alcohol,11,12 and fresh NaOH-methanol.12 Some researchers initially worked out their assays in animal controls in order to determine what constitutes appropriate staining for any given antibody.9,13
The goal of the present study was to determine which technique(s) of removal of celloidin give(s) optimum results with conventional immunostaining. We utilized perfused mouse temporal bones that were processed by the same protocol for light microscopy that we use for human specimens. However, by using animal tissue, we were able to control the postmortem time, fixation time, and decalcification time, thus controlling these potential confounding variables. We used a panel of six antibodies (prostaglandin D synthase, sodium, potassium adenosine triphosphatase [Na+,K+-ATPase], aquaporin 1, connective tissue growth factor, tubulin, and 200 kd neurofilament) with which we have past experience in studying mouse cochleae and that have worked well in paraffin tissue. It is our long-term objective to eventually be able to translate our findings to more-effective immunostaining of human temporal bone sections.
MATERIALS AND METHODS
Five-week-old CBA/CAJ mice underwent intracardiac perfusion with 4% formaldehyde plus 1% acetic acid after deep anesthesia. After 25 hours of additional fixation, the temporal bones were dissected out and decalcified in 0.1 mol/L ethylenediaminetetraacetic acid. The cochleae were then processed for celloidin (parlodion strips; Mallinckrodt Chemicals, Phillipsburg, New Jersey) embedding.1 The celloidin blocks were serially sectioned at 20 µm, and a few sections were stained with hematoxylin and eosin. The unstained sections were used as described below.
Before celloidin removal, the celloidin sections were mounted on gelatin subbed glass slides as follows. The celloidin sections were floated onto cigarette paper in a dish of 80% ethanol, and the paper was trimmed around each section. The sections were then placed face down onto a subbed slide smeared with albumin. A piece of bibulous paper was placed over each section, and a roller was used to flatten and smooth the section. After removal of the bibulous paper, a second piece of bibulous paper (soaked in 10% formalin) was placed over the cigarette paper, followed by a block of wood and a 500-g lead weight. The slides were left to dry in air for 1 hour. The wood block and lead weights were removed, and the bibulous and cigarette papers were removed gently.
Clove Oil
Sections were adhered to glass slides as described above. The air-dried slides were dehydrated in 80%, 95%, and 100% (2 changes) ethanol for 3 minutes each and then transferred into clove oil and left overnight.7,8 The following morning, the slides were immersed in 100%, 100%, 95%, 80%, and 50% ethanol for 3 minutes each, followed by distilled water for 3 minutes. Before immunostaining, the slides were transferred to 0.01 mol/L phosphate-buffered saline solution (PBS) and left for 3 minutes.
Ether-Alcohol
Removal was accomplished in one of two ways. After the sections were adhered to the slides, the slides were transferred directly to ethyl ether and 100% ethanol (1:1) for 30 minutes.11 The slides were repeatedly dipped in the solution to help remove the celloidin. The slides were transferred to a second change of ethyl ether and 100% ethanol (1:1) and left for 30 minutes and agitated once again. The slides were then hydrated in 100%, 100%, 95%, 80%, and 50% ethanol and distilled water (3 minutes each) and then transferred into PBS before immunostaining. The second method involved placing the slides in ethanols and dehydrating them before the ethyl ether and ethanol solution.
Acetone
Sections were adhered to glass slides as described above. The celloidin was dissolved in acetone for 30 minutes.9 Sections were hydrated in 100%, 100%, 95%, 80%, and 50% ethanol to distilled water (3 minutes each) and transferred to PBS before immunostaining.
Sodium Methoxide
Sections were adhered to glass slides as described above. Then 50 g of NaOH was mixed with 50 mL of methanol vigorously and allowed to settle for 30 minutes at room temperature. 12 The solution (cloudy solution on top of pellets) was diluted 1:2 with methanol and used immediately. Enough of the diluted sodium methoxide solution to cover each section was applied by drops to the dried celloidin sections for 5 minutes and then rinsed with 100% methanol.14 These two steps were repeated twice. Further rinsing with 100% and 70% methanol for 10 minutes each followed. The slides were transferred to distilled water and left for 10 minutes, followed by exposure to PBS for 10 minutes.
Immunostaining was accomplished with anti-bodies to prostaglandin D synthase (PGDS; Cay-men Chemical, Ann Arbor, Michigan) at a dilution of 1:5,000; Na+,K+-ATPase (provided by Dr George Siegel, University of Michigan, Ann Arbor) at a dilution of 1:10,000; aquaporin 1 (Aqp1, Chemicon International, Temecula, California) at a dilution of 1:1,000; connective tissue growth factor (CTGF; Cell Sciences, Canton, Massachusetts) at a dilution of 1:1,000; tubulin (Sigma, St Louis, Missouri) at a dilution of 1:15,000; and 200 kd neurofilament (NF, Boehringer Mannheim, Mannheim, Germany) at a dilution of 1:2,000. After 14-hour primary antibody incubations, sections were rinsed in 3 washes of PBS. Secondary antibodies appropriate for the host species of primaries, at a dilution of 1:200, were applied and incubated for 1 hour. After another 3 rinses in PBS, avidin-biotin-horseradish peroxidase (Standard ABC Kit, Vector Laboratories, Burlingame, California) was applied to the sections and left to incubate for 1 hour, followed again by 3 washes with PBS. Finally, the sections were colorized with 0.01% diaminobenzidine and 0.01% hydrogen peroxide for 5 to 10 minutes, rinsed, and dehydrated followed by application of coverslips. The immunostaining results were compared to those of controls using paraffin sections (considered the standard), in which the embedding medium is easily removed with xy-lenes. For each antibody, staining was at least duplicated, but most combinations of antibody and removal method were tested 3 to 12 times.
RESULTS
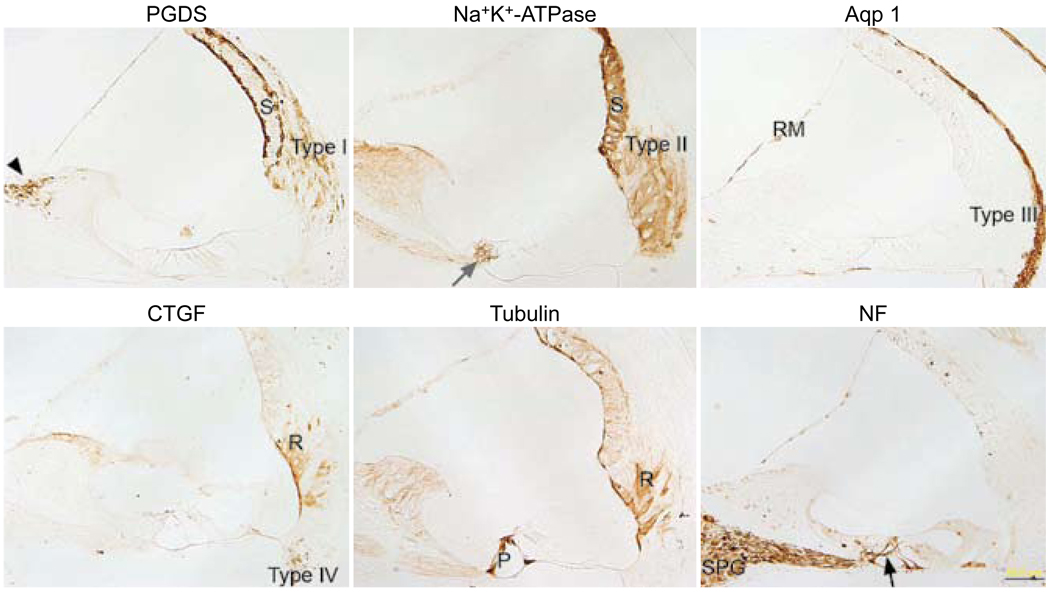
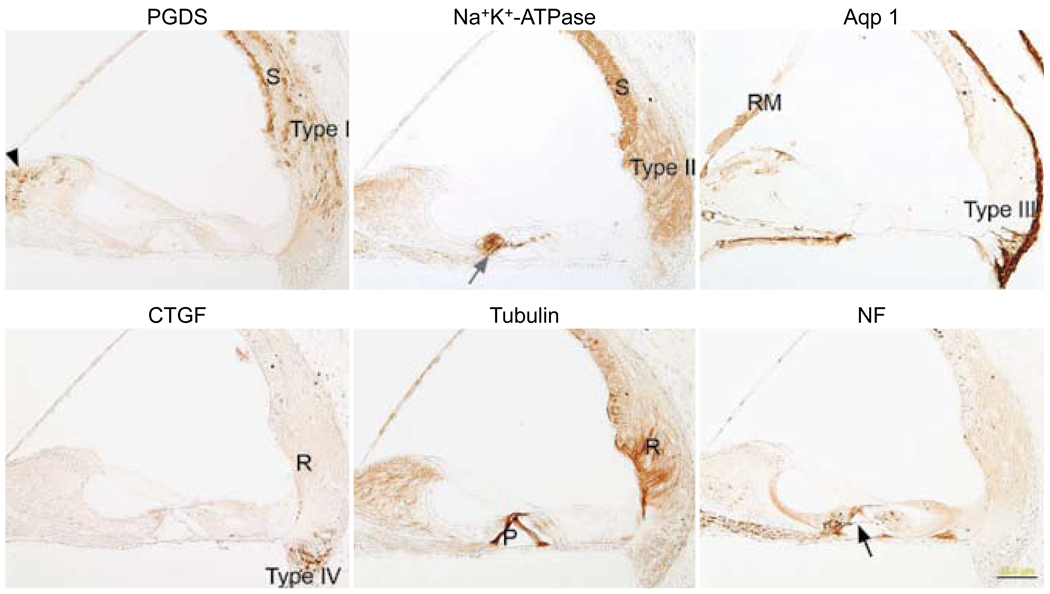
Immunostaining for the six antibodies was accomplished in paraffin sections to serve as controls (Fig 1). The PGDS antibody stained the marginal and basal cells of the stria vascularis, the type I fibrocytes of the spiral ligament, and the fibrocytes of the spiral limbus. The Na+,K+-ATPase antibody stained the stria vascularis, the type II fibrocytes of the spiral ligament, and the nerve endings below the inner and outer hair cells. The aquaporin 1 antibody stained the type III fibrocytes of the spiral ligament, some type IV fibrocytes of the spiral ligament, the medial portion of Reissner’s membrane, and the mesenchymal cells lining the bone of the inner osseous lamina. The CTGF antibody stained type IV fibrocytes of the spiral ligament and the root cells. Staining for tubulin was evident in the pillar cells and in the root cells. The NF antibody stained spiral ganglion cells, nerve fibers in Rosenthal’s canal, nerve fibers crossing the tunnel of Corti, and nerve endings below the inner and outer hair cells.
Fig. 1.
Paraffin sections immunostained with each of six antibodies. Prostaglandin D synthase (PGDS) antibody stains basal and marginal cells of stria vascularis (S), type I fibrocytes of spiral ligament (type I), and fibrocytes of spiral limbus (black arrowhead). Sodium, potassium adenosine triphosphatase (Na+,K+-ATPase) antibody stains stria vascularis (S), type II fibrocytes of spiral ligament (type II), and nerve fibers below inner hair cells (gray arrow). Antibody against aquaporin 1 (Aqp 1) stains type III fibrocytes of spiral ligament (type III) and medial portion of Reissner’s membrane (RM). Connective tissue growth factor (CTGF) antibody stains type IV fibrocytes of spiral ligament (type IV) and root cells (R). Antibody against tubulin stains pillar cells (P) and root cells (R). Neurofilament (NF) antibody stains some of spiral ganglion cells (SPG) and nerve fibers in Rosenthal’s canal, beneath inner and outer hair cells and crossing tunnel of Corti (black arrow). Calibration bar — 50 µm.
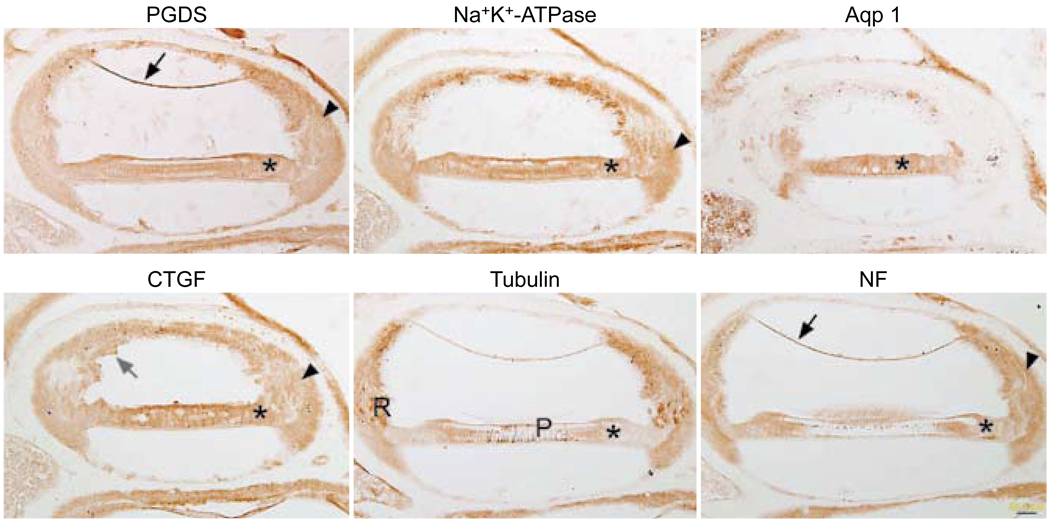
Immunostaining for all six antibodies had a similar pattern with the clove oil removal method (Fig 2), characterized by excessive nonspecific background staining. Most of the spiral ligament, Reissner’s membrane, the stria vascularis, and the supporting cells of the organ of Corti were stained. Pieces of residual, torn celloidin were still evident in the CTGF-stained section. The tubulin antibody started to stain the root cells and pillar cells appropriately, but there was also a great deal of extraneous background staining.
Fig. 2.
Celloidin removal with clove oil followed by immunostaining. All six panels show similar nonspecific staining pattern, regardless of antibody used. Reissner’s membrane (black arrows) shows background staining with PGDS and NF. Supporting cells (asterisks) show similar staining patterns with all six antibodies. Staining in spiral ligament (arrowheads) is diffusely brown with most of antibodies. In CTGF-stained tissue, bits of celloidin (gray arrow) are still evident in section near stria vascularis. Tubulin antibody starts to stain expected structures such as root cells (R) and pillar cells (P), but there is also great deal of brown background staining in section. Calibration bar — 50 µm.
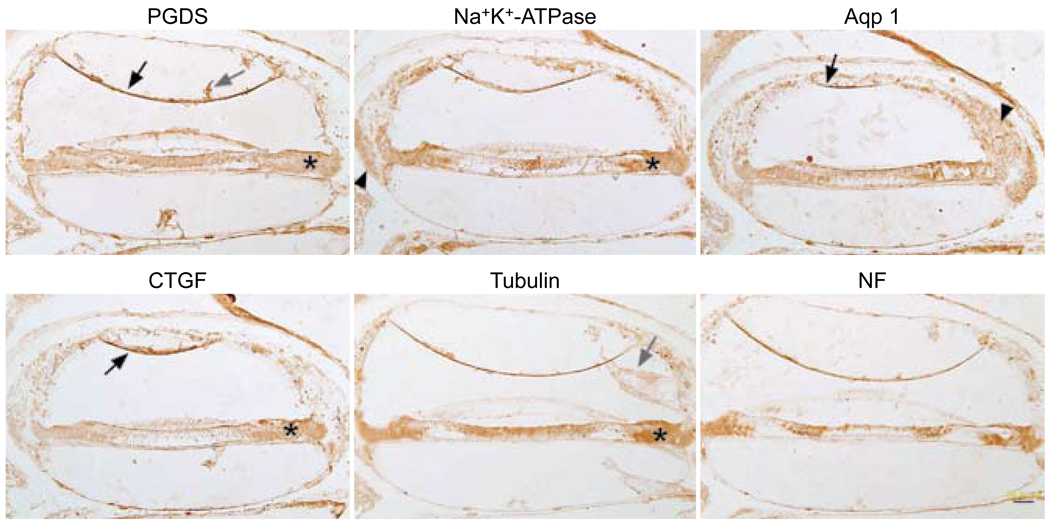
The ether-alcohol method was also not effective at removing celloidin. Figure 3 shows celloidin remaining in the sections attached to the tectorial membrane, attached to Reissner’s membrane, and within the scalae. The staining patterns for all six antibodies were similar. The supporting cells of the organ of Corti were typically a diffuse brown color, as were Reissner’s membrane and parts of the spiral ligament.
Fig. 3.
Celloidin removal with ether-alcohol followed by immunostaining. All six panels show similar nonspecific staining pattern, regardless of antibody used. Once again, Reissner’s membrane (black arrows) stains with most of antibodies. Supporting cells (asterisks) are all diffusely brown. There is spurious staining in spiral ligament (arrowheads). Celloidin is still evident in many sections (gray arrows). Calibration bar — 50 µm.
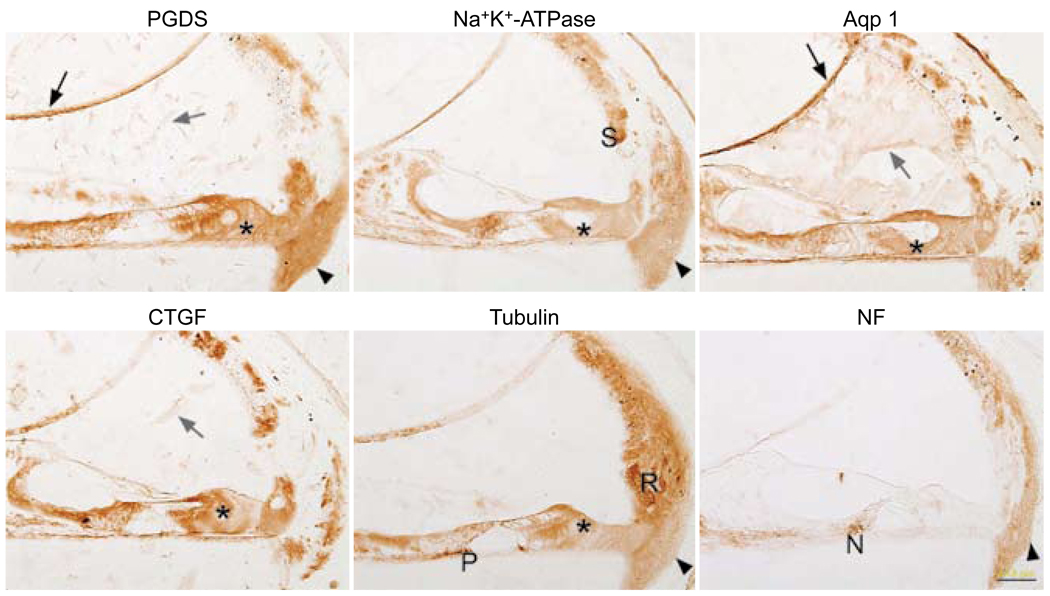
Removal of celloidin with acetone was less than optimal (Fig 4). There was diffuse brown staining in the spiral ligament and in supporting cells of the organ of Corti. The Na+,K+-ATPase antibody started to stain the stria and the nerve fibers below the inner hair cells, as well as tunnel crossing fibers, but there was also a diffuse brown background in the majority of other cells. The tubulin antibody started to stain the root cells and the pillar cells in a manner similar to the clove oil method, but there was a diffuse brown background.
Fig. 4.
Celloidin removal with acetone followed by immunostaining. Reissner’s membrane (black arrows) stains in many panels (nonspecific staining). Supporting cells (asterisks) are diffusely brown in many panels. With Na+,K+-ATPase antibody, stria (S) starts to stain appropriately, but there is a great deal of background staining. Celloidin (gray arrows) can still be seen in some panels. Spiral ligament (arrowheads) shows background staining. Tubulin panel shows appropriate staining in root cells (R) and in a portion of pillar cells (P), but background staining is also seen. Neurofilament antibody stains nerve fibers (N) below inner hair cell area, but it also spuriously stains spiral ligament (arrowhead). Calibration bar — 50 µm.
Methanol saturated with NaOH was the best method for removing celloidin and achieving successful immunostaining with all six antibodies used. Selective and appropriate staining was evident in all of the sections. Repeated comparisons showed that using fresh NaOH mixed with methanol was much more effective than using an aged solution. Figure 5 demonstrates the expected staining for the six antibodies. The PGDS antibody stained the type I fibrocytes of the spiral ligament, the marginal and basal cells of the stria vascularis, and the fibrocytes of the spiral limbus. The Na+,K+-ATPase antibody stained the type II fibrocytes of the spiral ligament, the stria vascularis, and the nerve endings below the inner and outer hair cells. The aquaporin 1 antibody stained the type III fibrocytes of the spiral ligament, some type IV fibrocytes of the spiral ligament, the medial portion of Reissner’s membrane, and the mesenchymal cells lining the bone of the inner osseous lamina. The CTGF antibody stained type IV fibrocytes of the spiral ligament and sometimes the root cells (not shown). Staining for tubulin was evident in the root cells and pillar cells. The NF antibody stained nerve fibers in Rosenthal’s canal and below the inner and outer hair cells. The findings were very similar to those seen in the paraffin sections (Fig 1). The findings were also in keeping with previous reports in applicable cases.15–18
Fig. 5.
Celloidin removal with methanol saturated with sodium hydroxide followed by immunostaining. Successful immunostaining for all six antibodies is possible when methanol saturated with sodium hydroxide is used. Each antibody shows selectivity for appropriate cells (compare to Fig 1), and there is very little background. PGDS staining is evident in marginal and basal cells of stria vascularis (S), type I fibrocytes of spiral ligament (type I), and fibrocytes of spiral limbus (black arrowhead). Staining for Na+,K+-ATPase is evident in stria vascularis (S), type II fibrocytes of spiral ligament (type II), and nerve fibers below inner and outer hair cells (gray arrow). Aquaporin 1 antibody stains type III fibrocytes of spiral ligament (type III), medial portion of Reissner’s membrane (RM), cells lining bone of scala tympani, and some cells in spiral limbus. CTGF antibody stains type IV fibrocytes of spiral ligament (type IV). Antibody against tubulin stains pillar cells (P), root cells (R), and spiral limbus. Neurofilament antibody stains nerve fibers in osseous spiral lamina, nerve fibers below inner hair cell, tunnel crossing fibers (black arrow), and nerve fibers below outer hair cells. Calibration bar — 50 µm.
DISCUSSION
Researchers have tried to utilize human archival celloidin-embedded temporal bone sections for immunohistochemical stains with various levels of success. In order for antibodies to penetrate celloidin-embedded tissue, it is first necessary to remove the celloidin. Various removal protocols have been used; some methods have been more successful than others. With human postmortem tissue, autolysis, length of fixation, and disease processes complicate the interpretation of immunohistochemical staining. In order to better assess the various celloidin removal techniques without these potential confounding variables, optimally fixed normal mouse cochleae were used in this study. The fixative chosen for this study was 4% formaldehyde plus 1% acetic acid. Our previous results showed that this fixative gave uniform immunostaining results in both paraffin-and celloidin-embedded tissues.14
Four methods of celloidin removal were evaluated. The clove oil, acetone, and ether-alcohol methods did not remove enough celloidin from the sections to obtain successful immunostaining. Undis-solved celloidin could be seen in both the clove oil–treated and the ether-alcohol–treated slides. With acetone, the results were a little better, but still suboptimal. The antibodies appeared to only partially penetrate the tissue. Staining for tubulin was only partially successful for root cells and pillar cells. At the same time, there was extraneous staining in the spiral ligament. The same occurred with the staining for the Na+,K+-ATPase antibody. Some Na+,K+-ATPase staining was evident in the nerve fibers below the inner hair cells, but the staining in the inner sulcus, outer sulcus, and Hensen cells was all spurious. All three methods produced diffuse brown background staining in many cell types, not just the expected ones. It was only with the methanol-NaOH method that credible immunostaining was possible with all six antibodies.
It is not technically feasible to remove celloidin from floating sections. It is imperative to adhere the celloidin section to a glass slide before celloidin removal for tissue morphology to be well preserved. We believe that with the methanol-NaOH method, the removal of celloidin was complete. This method accomplished more than just etching the surface of the section; the celloidin lifted off the slide during its removal. In addition, the extreme pH provided by the methanol-NaOH is known to assist in immunostaining of formalin-fixed tissue, possibly by opening up antigenic sites.14
Although the parameters for the clove oil, ether alcohol, and acetone removal methods were not exhausted (we could have adjusted the times, numbers of rinses, etc), we believe that methanol-NaOH is the method of choice for removing celloidin from histologic sections and for achieving credible immunostaining with the six antibodies used in this study. Our experience also showed that freshly prepared sodium methoxide was much more effective than the aged solutions advocated in some protocols.
Embedding temporal bones in celloidin does not limit our ability to later successfully immunostain them (at least with the six antibodies used here). As stated previously, our ultimate goal is to be able to immunostain some of the thousands of celloidin-embedded human temporal bone sections presently stored in otopathology laboratories. Being able to reliably and repeatedly localize proteins with well-characterized antibodies will provide us with a trust-worthy and instructive tool for investigating pathology in the human inner ear.
CONCLUSIONS
Freshly prepared methanol saturated with NaOH was the best solution for removing celloidin from temporal bone sections. This removal technique produced the cleanest, most selective, and reproducible results with the six antibodies used in the present study. Because celloidin gives superb preservation of morphology, it may be the embedding medium of choice for both morphological and pathological studies, including immunostaining.
Acknowledgments
This work was supported by the National Institutes of Health (grant U24 DC 008559 Human Temporal Bone Consortium for Research Resource Enhancement of the National Institute on Deafness and Other Communication Disorders) and by private grants from Mr Lakshmi Mittal and Mr Axel Eliasen. This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Massachusetts Eye and Ear Infirmary.
REFERENCES
- 1.Schuknecht HF. Pathology of the ear. 2nd ed. Philadelphia, Pa: Lea and Febiger; 1993. [Google Scholar]
- 2.Merchant SN, Burgess B, O’Malley J, Jones D, Adams JC. Polyester wax: a new embedding medium for the histopathologic study of human temporal bones. Laryngoscope. 2006;116:245–249. doi: 10.1097/01.mlg.0000192171.85406.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold W. Immunohistochemical investigation of the human inner ear. Limitations and prospects. Acta Otolaryngol. 1988;105:392–397. doi: 10.3109/00016488809119491. [DOI] [PubMed] [Google Scholar]
- 4.Shi S-R, Coté C, Kalra KL, Taylor CR, Tandon AK. A technique for retrieving antigens in formalin-fixed, routinely acid-decalcified, celloidin-embedded human temporal bone sections for immunohistochemistry. J Histochem Cytochem. 1992;40:787–792. doi: 10.1177/40.6.1588025. [DOI] [PubMed] [Google Scholar]
- 5.Shi S-R, Tandon AK, Haussmann RRM, Kalra KL, Taylor CR. Immunohistochemical study of intermediate filament proteins on routinely processed, celloidin-embedded human temporal bone sections by using a new technique for antigen retrieval. Acta Otolaryngol. 1993;113:48–54. doi: 10.3109/00016489309135766. [DOI] [PubMed] [Google Scholar]
- 6.Portmann D, Fayad J, Wackym PA, Shiroishi H, Linthicum FH, Jr, Rask-Andersen H. A technique for reembed-ding celloidin sections for electron microscopy. Laryngoscope. 1990;100:195–199. doi: 10.1288/00005537-199002000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Sone M, Paparella MM, Schachern PA, Morizono N, Le CT, Lin J. Expression of glycoconjugates in human eustachian tubes with otitis media. Laryngoscope. 1998;108:1474–1479. doi: 10.1097/00005537-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Sone M, Schachern PA, Paparella MM, Morizono N. Study of systemic lupus erythematosus in temporal bones. Ann Otol Rhinol Laryngol. 1999;108:338–344. doi: 10.1177/000348949910800404. [DOI] [PubMed] [Google Scholar]
- 9.Keithley EM, Horowitz S, Ruckenstein MJ. Na,K-ATPase in the cochlear lateral wall of human temporal bones with endolymphatic hydrops. Ann Otol Rhinol Laryngol. 1995;104:858–863. doi: 10.1177/000348949510401106. [DOI] [PubMed] [Google Scholar]
- 10.Tian Q, Linthicum FH, Jr, Keithley EM. Application of labeling techniques to archival temporal bone sections. Ann Otol Rhinol Laryngol. 1999;108:47–53. doi: 10.1177/000348949910800107. [DOI] [PubMed] [Google Scholar]
- 11.Pawlowski KS, Wright CG, Meyerhoff WL. Histologic demonstration of glycosaminoglycans in inner ear fluids. Acta Otolaryngol. 1998;118:505–510. doi: 10.1080/00016489850154630. [DOI] [PubMed] [Google Scholar]
- 12.Miguel-Hidalgo JJ, Rajkowska G. Immunohistochemistry of neural markers for the study of the laminar architecture in celloidin sections from the human cerebral cortex. J Neurosci Methods. 1999;93:69–79. doi: 10.1016/s0165-0270(99)00114-4. [DOI] [PubMed] [Google Scholar]
- 13.Keithley EM, Tian Q, Robins-Browne R. Fibronectin-like immunoreactivity of the basilar membrane of celloidin-embedded human temporal bone sections. Acta Otolaryngol. 1994;114:613–619. doi: 10.3109/00016489409126114. [DOI] [PubMed] [Google Scholar]
- 14.O’Malley JT, Merchant SN, Burgess BJ, Jones DD, Adams JC. Effects of fixative and embedding medium on morphology and immunostaining of the cochlea. Audiol Neurootol. 2009;14:78–87. doi: 10.1159/000158536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hafidi A, Despres G, Romand R. Cochlear innervation in the developing rat: an immunocytochemical study of neurofilament and spectrin proteins. J Comp Neurol. 1990;300:153–161. doi: 10.1002/cne.903000202. [DOI] [PubMed] [Google Scholar]
- 16.Schulte BA, Adams JC. Distribution of immunoreactive Na+,K+-ATPase in gerbil cochlea. J Histochem Cytochem. 1989;37:127–134. doi: 10.1177/37.2.2536055. [DOI] [PubMed] [Google Scholar]
- 17.Slepecky NB, Ulfendahl M. Actin-binding and microtubule-associated proteins in the organ of Corti. Hear Res. 1992;57:201–215. doi: 10.1016/0378-5955(92)90152-d. [DOI] [PubMed] [Google Scholar]
- 18.Stanković KM, Adams JC, Brown D. Immunolocalization of aquaporin CHIP in the guinea pig inner ear. Am J Physiol. 1995;269:C1450–C1456. doi: 10.1152/ajpcell.1995.269.6.C1450. [DOI] [PubMed] [Google Scholar]