Abstract
Artemisinins are derived from extracts of sweet wormwood (Artemisia annua) and are well established for the treatment of malaria, including highly drug-resistant strains. Their efficacy also extends to phylogenetically unrelated parasitic infections such as schistosomiasis. More recently, they have also shown potent and broad anticancer properties in cell lines and animal models. In this review, we discuss recent advances in defining the role of artemisinins in medicine, with particular focus on their controversial mechanisms of action. This safe and cheap drug class that saves lives at risk from malaria can also have important potential in oncology.
Introduction
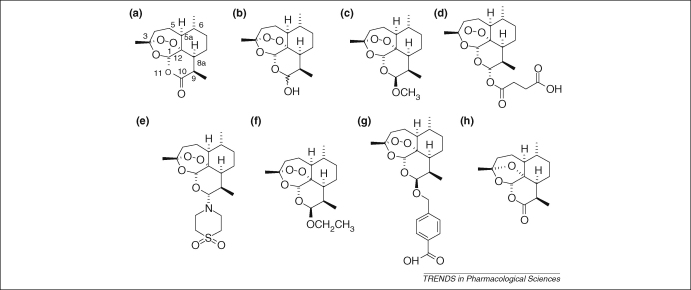
The remarkable story of the discovery of artemisinin (Figure 1a) and establishment of its antimalarial activity by Chinese scientists represents one of the great discoveries in medicine in the latter half of the 20th century [1]. Through a collaborative effort, collectively referred to as ‘Project 523’, the Chinese prepared dihydroartemisinin (DHA; Figure 1b), artemether (Figure 1c) and artesunate (Figure 1d) in the 1970s. It is these derivatives [with others, including artemisone (Figure 1e), arteether (Figure 1f) and artelinic acid (Figure 1g), generically known as ‘artemisinins’] that are now making a crucial contribution to the management of malaria, one of our most important infections. The magnitude of the malaria problem is represented in the annual burden of 500 million cases. This fascinating class of drug, with structures so different from the classical quinoline antimalarials, is particularly valuable when used in combination with other antimalarials [2,3].
Figure 1.
Chemical structures of artemisinins. Artemisinin (a) isolated in crystalline form in 1973 from Artemisia annua and derivatives dihydroartemisinin (DHA) (b), artemether (c), artesunate (d) and arteether (f) were first prepared by Chinese scientists in the 1970s [1]. Artemisone (e), representative of a new class of artemisinin known as amino-artemisinins, is curative in clinical trials at one-third the dose regimen of artesunate. It is characterized by low toxicity [56]. Artelinate (g) was prepared at the Walter Reed Army Institute of Research (http://wrair-www.army.mil), but was withdrawn because of toxicity concerns [112]. Deoxyartemisinin (h), lacking the peroxide bridge, is biologically inert.
Artemisinins have also been submitted to studies aimed at exploring other uses for this drug class. Artemisinins are active against other parasite species in vitro, including protozoa that are phylogenetically unrelated to apicomplexan parasites such as the Plasmodium species that cause malaria. Artemisinins also act against metazoan parasites such as Schistosoma spp. Their anti-disease properties include potent anticancer activity in in vitro studies and in an in vivo model of colorectal cancer. Taken together with case reports describing benefits in diverse cancers, a recently published clinical trial of short-term use in lung cancer, their established record of safety in children and adults with malaria, and their permissive cost, there are compelling reasons to study their contribution to management of tumours that require adjuvant and neo-adjuvant therapies. This selective review focuses on rapidly advancing areas of artemisinin science and usage and illustrates why artemisinins have the potential to rival acetylsalicylic acid in the breadth of their anti-disease properties.
There is considerable debate regarding the mechanisms of antimalarial action of artemisinins. An endoperoxide bridge (Figure 1) lies at the heart of antiparasitic activity of artemisinins, although the chemical nature of the interaction between artemisinins (particularly the essential endoperoxide) and parasite target(s) is not well understood. The role of ferrous species in the antimalarial actions of artemisinins is also debated [4] because these cations can catalyse in vitro reactions of some artemisinins, including their decomposition in aqueous solutions.
One issue focuses further discussions: is there a single important target for artemisinins in Plasmodium spp. or are there multiple targets? Fully synthetic trioxolanes that contain an endoperoxide bridge but lack other features of artemisinins have increased complexity of the debate on mechanisms of action of artemisinins [5]. Many groups, including our own, have reviewed recent developments [6–9]. Clarifying mechanisms of action of artemisinins is important for understanding both how structurally related drugs, such as the fully synthetic trioxolanes, might work and the basis for the development of resistance by parasites to this class of antimalarial. Clearly, a structural appreciation of the putative targets should contribute to the design of derivatives that are not crippled by mutations in target, as exemplified by approaches used in the development of new dihydrofolate reductase inhibitors [10,11].
Rodent malarias are also useful models for understanding possible mechanisms of resistance to different classes of antimalarials [12,13]. Genetic analyses permitted by Plasmodium chabaudi infection in mice identified a locus linked to artemisinin resistance that is stable after mosquito passage [14,15]. Linkages to artemisinin resistance have been narrowed down to a de-ubiquitination enzyme (among others) that might function in the endoplasmic reticulum of parasites and be involved in the stress response. Other groups have established stable artemisinin-resistant strains, confirming that artemisinin resistance can develop through standard selection procedures rather than (unfortunately) being an extremely rare event and can also arise by more than one mechanism [16–18].
Molecular targets of artemisinins
Plasmodium falciparum multiplies in red blood cells, and digestion of haemoglobin during its 48 h asexual life cycle is essential for parasite survival (Box 1). For many years, artemisinins have been proposed to act on parasite haemoglobin-digestion processes within the ‘food vacuole’ (Box 1, Figure Ib). Other studies have indicated that artemisinins could also target the parasite mitochondrion or the translationally controlled tumour protein (TCTP) and PfATP6, a parasite-encoded sarcoplasmic–endoplasmic reticulum calcium ATPase (SERCA). These hypotheses are discussed in more detail here.
Box 1. The intraerythrocytic parasite and proposed targets of artemisinins.
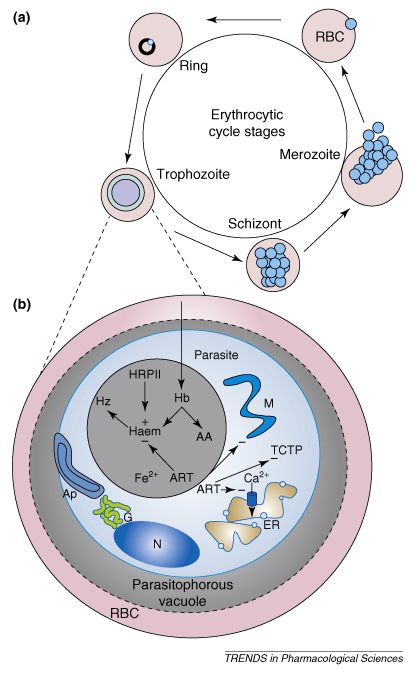
Human malaria-causing parasites have complex life cycles requiring both mosquito vectors and human hosts with three cycles of asexual and one cycle of sexual reproduction. One of the asexual phases takes place within the red blood cells of its host (Figure Ia). Invasive forms, termed merozoites, enter the red blood cell and remain relatively metabolically inactive (compared with the later asexual stages of development) for 10–15 h (the ring stage). The parasite then undergoes a rapid phase of growth over the next 25 h (forming the trophozoite stage), during which time the parasite digests the majority of the haemoglobin of the host cell and grows to fill >50% of the volume of the host cell. Haemoglobin is digested within a food vacuole (Figure Ib), which results in the formation of haem. As the haem is formed, it associates via one of the peripheral carboxyl groups with the Fe3+ of an adjacent haem to form insoluble haemozoin. It has been proposed, although not proven, that this process is aided by a protein termed the histidine-rich protein II. At the end of the trophozoite stage the parasite divides several times (the schizont stage) before the host cell lyses (some 48 h after invasion) to release the newly formed meroziotes that continue the cycle.
Artemisinins, which might not require activation by Fe2+, have been proposed over several years to target several different pathways (Figure Ib), including the heam detoxification pathway, the mitochondrion, the TCTP and a Ca2+ pump localized to the endoplasmic reticulum (termed PfATP6).
Figure I.
Diagram showing the complex life cycle of Plasmodium falciparum. Abbreviations: AA, amino acids; Ap, apicoplast; ART, artemisinins; DV, digestive vacuole; ER, endoplasmic reticulum; G, Golgi apparatus; Hb, haemoglobin; Hz, haemozoin; M, mitochondrion; N, nucleus; RBC, red blood cell; TCTP, translationally controlled tumour protein.
Haem pathway
Haemozoin is parasite pigment deposited within a food vacuole (Box 1) after digestion of haemoglobin. It has long been proposed as a target of artemisinins, although the plasmodial stages most susceptible to the activity of artemisinins are too young to manifest visible pigment (reviewed in Refs [19,20]). The endoperoxide bridge of artemisinins is proposed to be activated by ferrous iron to generate free radicals (of the oxy or C-centred variety) in in vitro experiments and, subsequently, to alkylate haem. As iron is the principal element deposited in haemozoin, digestion of haemoglobin by parasites is suggested to render them susceptible to killing by locally activated artemisinins.
However, several localization studies indicate that most artemisinin taken up into parasites is outside of their food vacuoles [21,22]. Some studies with fluorescent artemisinin derivatives show food vacuolar localization [23], perhaps representing trafficking of the fluorophore itself. This trafficking of a fully synthetic fluorescent antimalarial trioxolane might also explain differential localization results (one parasite with signal in the cytosol and the other in the food vacuole) observed for two parasites sharing the same erythrocyte [24]. Synthetic trioxolanes, such as OZ277, are more fragile than the semi-synthetic artemisinin derivatives when assayed in aqueous solutions [4,25,26], and they also seem to degrade easily within parasitized erythrocytes [27]. These properties might influence estimates of potency.
Further evidence for the irrelevance of parasite pigment in the action of artemisinins comes from their potent activity against non-pigment-producing apicomplexan parasites (see later). There is also divergence between some in vitro assays of haem alkylation by trioxolanes and natural and semi-synthetic artemisinins [25]. The correlation observed between antimalarial potencies of trioxolanes and their propensity to alkylate haem [25] is not observed for artemisinins, implying either that these classes of antimalarial might have different modes of action or that, indeed, the haem pathway might be irrelevant. The trioxolane OZ277 inhibits PfATP6 calcium ATPase activity when expressed in oocytes [24] at low (μM) concentrations. This might be owing to decomposition of the compound under the assay conditions or other aspects of the in vitro assay system. Study of more stable trioxolanes might resolve some of these issues. There is also correlation (r2 = 0.5, n = 38; p = 0.002) between parasiticidal activities of artesunate and OZ277 tested against field isolates, with no correlation between OZ277 and other classes of antimalarial such as quinolines [28]. This correlation might represent a general (non-target-specific) propensity of parasites to be susceptible to endoperoxides, but it is also consistent with the shared-target hypothesis for mechanisms of action, with PfATP6 being an example of such a target.
As a variant of the haem hypothesis, reaction with a histidine-rich protein of parasites (HRPII; Box 1) might also be involved in antimalarial activity [29] because HRPII aids digestion of haemoglobin. However, very little HRPII is secreted in early ring stages (Box 1), which are most susceptible to artemisinins [30,31].
Understanding interactions between haemoglobins and artemisinins is complicated by alterations in iron status associated with haemoglobinopathies. Higher concentrations of free iron in haemoglobin-E-containing and thalassaemic erythrocytes reduces parasiticidal potencies of artemisinins when assayed in vitro [32]. However, in vivo kinetic studies using bioassays of artesunate and its active metabolite, DHA, show approximately tenfold higher plasma concentrations in α-thalassaemic subjects when areas under the time–concentration curves were assessed [33], and the haemoglobin E trait might increase parasite clearance by artemisinins [34]. Despite these differences between in vitro activities of artemisinins related to the haemoglobin status of host erythrocytes, thalassaemia is not an influential co-variate in population pharmacokinetic analysis of rectal artesunate used to treat Plasmodium vivax or P. falciparum infections. Additionally, antimalarial activities of artemisinin against P. falciparum parasites cultured in the presence of carboxy-haemoglobin are significantly higher than in the presence of oxy-haemoglobin. This increase in artemisinin activity is unexpected if Fe2+ is important in activating artemisinins because carboxy-haemoglobin inhibits haem-Fe2+ reactivity, indicating that haemoglobin iron plays no part in activating artemisinin for antimalarial activity and that competitive degradation of the artemisinin by haemoglobin actually attenuates its antimalarial activity [35,36].
PfATP6
The supportive arguments for PfATP6, the P. falciparum SERCA orthologue, as a target for artemisinins have been reviewed recently [9]. Evidence from transfection into parasites of DNA encoding PfATP6 that have altered sensitivity to some artemisinins will provide suitable genetic tests for the PfATP6 hypothesis (studies in progress), which has gained support from data from field isolates. An interesting study from French Guiana showed a clear association between mutation(s) in PfATP6 and decreased susceptibility to artemether, particularly with position 769 (Ser769Asn substitution) [37]. Parasites with Ser769Asn had a median IC50 value >20-times higher for artemether (indicating artemether resistance) compared with parasites without this mutation [9].
Detailed methodology for in vitro assays used in the earlier publication [37] is provided in a follow-up paper [38]. The lack of a laboratory-adapted line carrying the Ser769Asn mutation has been criticized, despite there being well-recognized ‘fitness-costs’ (i.e the ability of resistant parasites to persist in the absence of drug pressure) of some resistance mutations for cultured parasites, as shown for mutations in the P. falciparum multidrug resistance gene 1 (pfmdr1) [38–40]. Laboratory-derived transfectants carrying the Ser769Asn mutation will clarify its role in artemisinin resistance, especially when combined with ex vivo assays of susceptibility to artemisinins with the Xenopus oocyte model. An African isolate carrying the Ser769Asn mutation was still susceptible to DHA, and data for susceptibility to artemether were not reported (Table 1). These observations indicate that different artemisinin derivatives give rise to different inhibitory profiles when they encounter PfATP6 with a particular single-site polymorphism [41], as discussed elsewhere [42]. Structural modelling of the Ser769Asn mutation has proved difficult because the region containing this mutation has relatively low similarity to a mammalian SERCA (compared with other functionally conserved regions), a crystal structure of which is available [43]. This region is not related to the thapsigargin-binding site of mammalian SERCAs, which, in PfATP6, has also been hypothesized to accommodate artemisinins on the basis of mutational studies after expression in oocytes [44].
Table 1.
Polymorphism in the PfATPase6 gene and in vitro susceptibility to artemisinins of Plasmodium falciparum
| Region | Non-synonymous nucleotide substitution | Amino acid substitution | Artemether IC50 median [range] (nM) | DHA IC50 median [range] (nM) | Artesunate IC50 median [range] (nM) | Refs |
|---|---|---|---|---|---|---|
| Wild type | – | 5.6 [1.3–55.8] | 0.68 [0.1–31.8] | 0.25 [0.17–18.4] | [37,41,45] | |
| 5.46 [0.68–61.1] | ||||||
| Thailand | T266C | Ile89Thr | Not determined | Not determined | 3.38 [0.81–29.9] | [45] |
| Africa | C727T | His243Tyr | Not determined | 4.2; 6.4 | Not determined | [41] |
| G2306A | Ser769Asn | Not determined | 0.83 | Not determined | ||
| Senegal | G1291A | Glu431Lys | Not determined | Not determined | 20.8 | [37] |
| G1291A and C1868A | Glu431Lys and Ala623Glu | Not determined | Not determined | 44.7 | ||
| French Guiana | G2306A | Ser769Asn | 58.8 [38.2–100] | Not determined | Not determined | [37] |
| A1721C and G2306A | Gln574Pro and Ser769Asn | 116.8 | Not determined | Not determined |
Mutation elsewhere in field isolates (position 243 in PfATP6) decreases susceptibility to DHA, although data are only available from two isolates [41]. Monitoring of polymorphisms in PfATP6 (and indeed other transporter sequences) and relating the findings to phenotypes by assessing susceptibility to artemisinins is likely to be highly relevant to the objective of detecting early signs of artemisinin resistance (Table 1). For example, increased copy number of the multidrug resistance gene pfmdr1 modulates susceptibility of parasites to artemisinins in vitro, although the clinical relevance of this observation is not established [45].
Other targets
Recent studies with Baker's yeast indicate that mitochondrial membrane potential can be disrupted by artemisinin when grown in nonfermentable conditions (i.e. when carbon sources such as glycerol or ethanol are not metabolized by glycolysis) [46]. However, the relevance of these observations to antimalarial activity of artemisinins is unclear because other experiments indicate that higher concentrations (mM) of artemisinins are necessary to trigger resistance responses to artemisinins in yeast [47]. Additionally, the new clinically tested artemisinin derivative artemisone has no effect on mitochondrial membrane potential, reactive oxygen species levels or inhibition of the respiratory chain in neuronal cell lines [48].
The TCTP orthologue of P. falciparum was identified some years ago as a protein alkylated by radiolabelled artemisinin. There is no new evidence that supports the idea of TCTP as a target for artemisinins. Field isolates that have variable sensitivities to artemether are not associated with sequence polymorphisms in TCTP [37]. Neither do studies with animal models of artemisinin-resistant parasites support involvement of TCTP as a target [15].
Properties of artemisinins
Antimalarial activity of artemisinins – clinical applications
Using artesunate to treat severe malaria in adults has been emphasized in recent publications [49]. Parenteral artesunate (including intramuscular artesunate [50]) is easier to administer and is associated with fewer adverse effects (e.g. hypoglycaemia) when compared with quinine [51], the only other drug used in severe malaria. Mortality in adults is also lower with artesunate than with quinine. Intrarectal treatment with artesunate of children or adults who cannot take medicines by mouth and suffer from symptoms of malaria away from healthcare facilities has also been studied in large scale (Phase IV) studies that will be reported soon. Both safety and efficacy have been established in smaller studies [52,53]. However, a child treated with very high rectal doses of artesunate (88 mg kg−1 in total compared with a recommended 10–20 mg kg−1) recently died because of probable toxicity [54].
Curiously, oral artemether and DHA are more commonly used in fixed-dose formulations rather than artesunate. Artesunate might have more favourable properties, both in terms of stability and ease of co-formulation when compared with DHA, and in terms of adverse effects in animal models when compared with artemether [55]. Newer semi-synthetic artemisinin derivatives such as artemisone (Figure 1e) preserve safety but enhance efficacy and should be studied for performance against models of artemisinin resistance [56].
Activity against Toxoplasma gondii and other pathogenic apicomplexan parasites
Studying the susceptibility of non-plasmodial apicomplexans to artemisinins affords new therapeutic opportunities and provides new mechanistic insights. If organisms within the crown eukaryotic group are susceptible to artemisinins, then the simplest mechanistic interpretation is that they function in a similar way against these phylogenetically related organisms. For example, Toxoplasma gondii is a somewhat more tractable parasite than Plasmodium spp., particularly for studies using genetic manipulations or imaging technologies. Early work showed toxoplasma to be susceptible to artemisinins, albeit requiring concentrations within the micromolar range to kill parasites (online supplementary Table S1). Now studies show that T. gondii can be killed by nanomolar concentrations of artemisone in in vitro models and that TgSERCA (the PfATP6 orthologue) is susceptible to inhibition by artemisinin when expressed in yeast [57]. Furthermore, artemisinins trigger disturbances of calcium metabolism in parasites that have functional consequences on invasion machinery, and these might differ if parasites are cultured within host cells or as free living organisms [58]. These findings independently support the hypothesis that parasite SERCAs are targets for artemisinins (both in vivo and after heterologous expression). They also indicate that a glutamic acid residue predicted in transmembrane segment 3 of TgSERCA is permissive for artemisinin susceptibility [44], consistent with the suggestion made here that other key residues in TgSERCA might modulate artemisinin susceptibility.
Babesia species are tick-borne intraerythrocytic parasites that can infect humans in addition to a variety of domestic animals, depending on the species of parasite. Unlike plasmodial infections, babesia do not generate a parasitophorous vacuole and do not digest haemoglobin to make haemozoin [59]. Yet, some species are also susceptible to killing by artemisinins (online supplementary Table S1), once again making the haemoglobin digestion pathway a less compelling one for their mechanisms of action. Other related parasites have variable susceptibilities to artemisinins (online supplementary Table S1). These studies also establish that neither haemozoin nor haemoglobin is crucial to antiparasitic activity of artemisinins. It will be of interest to test the SERCA hypothesis for the mechanism of action of artemisinins in these related pathogenic parasites.
Activity against other protozoan and metazoan parasites
Artemisinins are also active against phylogenetically unrelated parasites, such as the single-celled kinetoplastids and metazoan helminths (online supplementary Table S2; efficacy against Schistosoma spp. is reviewed elsewhere [60]). Both salivarian (African) and stercorarian (American) trypanosomes can be killed by micromolar concentrations of artemisinins, indicating that artemisinins can be used as leads on which to optimize more potent derivatives [61]. Leishmania spp. are also killed by micromolar concentrations of artemisinins (online supplementary Table S2). As these infections are usually neglected in drug development portfolios, it would be regrettable if promising in vitro activities are not examined more thoroughly in relevant in vivo models perhaps used in combination with current therapies.
For metazoan infections, particularly Schistosoma spp., artemether and artesunate have shown useful activities in human studies and in models of infection [60,62]. First identified in Chinese studies [63], these observations have been extended to African infections. The limited portfolio of active trematocidal compounds reinforces the potential for artemisinins in the treatment of Schistosoma mansoni and Schistosoma haematobium.
Antitumour properties of artemisinins
Since the late 1980s, anticancer properties of artemisinins have been assayed in vitro (online supplementary Table S3). After more detailed studies, artemisinins such as artesunate were found to be active against a variety of unrelated tumour cells lines, from the most common types such as colon, breast and lung cancers to leukaemias and pancreatic cancer [64,65]. Studies have also identified potential general mechanisms such as normalization of the upregulated Wnt/β-catenin pathway in colorectal cancer [66]. Other pathways for anticancer activity include inhibition of enhanced angiogenesis associated with tumours [67–77]. Artemisinins inhibit proliferation, migration and tube formation of human umbilical vein endothelial cells (HUVEC), inhibit vascular endothelial growth factor (VEGF) binding to surface receptors on HUVEC and reduce expression of VEGF receptors Flt-1 and KDR/flk-1 on HUVECs [74,75,77]. In cancer cells, artemisinins reduce expression of the VEGF receptor KDR/flk-1 in tumour and endothelial cells and slow growth of human ovarian cancer HO-8910 xenografts in nude mice [67–69,75,77]. HUVEC apoptosis by artesunate is associated with downregulation of Bcl-2 (B-cell leukemia/lymphoma 2) and upregulation of BAX (Bcl-2-associated X protein) [78].
mRNA expression of 30 out of 90 angiogenesis-related genes correlated significantly with the cellular response to artemisinins [70]. In this microarray panel, there were many fundamental angiogenic regulators encoded by genes such as VEGFC, fibroblast growth factor-2 (FGF2), matrix metalloproteinase-9 (MMP9), thrombospondin-1 (THBS1) and hypoxia-inducing factor α (HIF1A). The fact that sensitivity and resistance of tumour cells can be predicted by mRNA expression levels of angiogenesis-related genes indicates that artemisinins reveal their antitumour effects, at least in part, by inhibition of tumour angiogenesis. Overexpression of enzymes associated with modulation of oxidative stress such as glutamylcysteine synthetase, glutathione S-transferases and the endothelial growth factor receptor reduce susceptibility of tumour cells to artemisinins [79,80]. Importantly, overexpression of genes encoding transporters that mediate drug resistance (e.g. multidrug resistance gene 1, multidrug resistance associated protein 1 and breast cancer resistance protein), dihydrofolate reductase and ribonucleotide reductase, which also confer resistance to established antitumour drugs, do not affect susceptibility, indicating that artemisinins function in different ways to classical cancer chemotherapeutic agents. These in vitro studies have also shown that for some cancer lines, delivery of iron, for example by the use of holotransferrin, enhances the anticancer properties of artemisinins [65,81–87].
Should artemisinins remain relegated to the large category of compounds that have interesting in vitro properties against cancers but have not been studied sufficiently to warrant more extensive clinical studies? Probably not, for many reasons. First, artesunate is a cheap, safe, easily administered and orally bioavailable compound that acts at targets different to those of many current cancer chemotherapeutic agents and is unlikely to interact adversely with existing anticancer interventions (P. Folb, personal communication). Second, study of an animal model carrying a human colorectal cancer cell line confirms that artesunate has independent antitumour activity and can shrink primary tumours and reduce the risk of hepatic metastases developing [66]. Additionally, human studies of individual cases [88,89], in addition to a recently published Phase II study of lung cancer [90], support rapid implementation of studies of artesunate as a primary or adjunct antitumour intervention, particularly for colorectal cancers and for leukaemia (as supported by results in online supplementary Table S3).
Other potentially useful properties of artemisinin compounds
In in vitro studies, several groups have reported that artemisinins have antiviral properties. Artemisinins reduce replication rates of hepatitis B and C viruses [91,92], a range of human herpes viruses [93–95], HIV-1 [96], influenza virus A [93,97] and a bovine viral diarrhoea virus [98] in the low micromolar range. Artesunate was also effective at reducing CMV (human herpes virus 5) copy number in an immunosuppressed 12-year-old child [99] and was used (100 mg per day, orally) for 30 days without attributable toxicity. Artemisinins also have some antifungal properties against Pneumocystis carinii in vitro [100,101], although artemether was not curative in two in vivo studies in immunosuppressed rats [102,103]. There are several other disease models, such as those for rheumatoid arthritis [104–106], nephritic syndrome [107], pancreatitis [108] and lupus nephritis [109,110], in which artemisinins have produced promising results. In the case of lupus nephritis, artemisinin has been used for three years in a human study, with positive effects on the disease state [111].
Concluding remarks
Artemisinins are firmly established in combination therapies [2,3] to treat drug-resistant malaria. They are becoming established as anti-schistosomal agents. Their true potential now lies in broader anti-disease applications, particularly in addressing the difficult challenge posed by advanced cancers for which expensive treatments are providing, at best, incremental gains in outcome. Questions about dosing regimens, safety of long-term use and possible interactions (either positive or negative) with existing therapies and toxicities that might be related to the treatment of tumours should be answered by appropriate clinical studies as part of an urgent need to investigate drugs such as artesunate for oncological indications.
Acknowledgements
We thank Qinxue Hu for help translating numerous articles in Chinese and Elvira Derbyshire for graphics. SK thanks P. Folb, P. Kremsner, D. Kumar, Ajit Lalvani, Sir David Weatherall, Herwig Jensen and Tim Planche for exceptional advice. RKH acknowledges support from the Government of the Hong Kong Special Administrative Region University Grants Committee Areas of Excellence Fund, Project No. AoE P/10–01–2-II and the University Grants Council, Grants No. HKUST 6493/06M and 600507. HMS is a Wellcome Trust Career Development Fellow (Grant No. 077441), sponsored by SK. SK is funded by the Commission of the European Communities ANTIMAL Grant: 018834.
Footnotes
Comprehensive tables of the effects of artemisinins against apicomplexan species, other parasites and cancers are provided as supplementary data, which can be found at doi:10.1016/j.tips.2008.07.004.
Supplementary data
References
- 1.Zhang J.-F. Yang Cheng Evening News Publishing Company; 2005. A Detailed Chronological Record of Project 523 and the Discovery and Development of Qinghaosu (Artemisinin) [Google Scholar]
- 2.Kremsner P.G., Krishna S. Antimalarial combinations. Lancet. 2004;364:285–294. doi: 10.1016/S0140-6736(04)16680-4. [DOI] [PubMed] [Google Scholar]
- 3.White N.J. Antimalarial drug resistance. J. Clin. Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes R.K. The Fe2+-mediated decomposition, PfATP6 binding, and antimalarial activities of artemisone and other artemisinins: the unlikelihood of C-centered radicals as bioactive intermediates. Chem. Med. Chem. 2007;2:1480–1497. doi: 10.1002/cmdc.200700108. [DOI] [PubMed] [Google Scholar]
- 5.Vennerstrom J.L. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430:900–904. doi: 10.1038/nature02779. [DOI] [PubMed] [Google Scholar]
- 6.Gelb M.H. Drug discovery for malaria: a very challenging and timely endeavor. Curr. Opin. Chem. Biol. 2007;11:440–445. doi: 10.1016/j.cbpa.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golenser J. Current perspectives on the mechanism of action of artemisinins. Int. J. Parasitol. 2006;36:1427–1441. doi: 10.1016/j.ijpara.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Jefford C.W. New developments in synthetic peroxidic drugs as artemisinin mimics. Drug Discov. Today. 2007;12:487–495. doi: 10.1016/j.drudis.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Krishna S. Re-evaluation of how artemisinins work in light of emerging evidence of in vitro resistance. Trends Mol. Med. 2006;12:200–205. doi: 10.1016/j.molmed.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuthavong Y. Malarial (Plasmodium falciparum) dihydrofolate reductase-thymidylate synthase: structural basis for antifolate resistance and development of effective inhibitors. Parasitology. 2005;130:249–259. doi: 10.1017/s003118200400664x. [DOI] [PubMed] [Google Scholar]
- 11.Yuvaniyama J. Insights into antifolate resistance from malarial DHFR-TS structures. Nat. Struct. Biol. 2003;10:357–365. doi: 10.1038/nsb921. [DOI] [PubMed] [Google Scholar]
- 12.Peters W., Richards W.H.G. 2nd edn. Vol. 1. Springer-Verlag; 1984. (Handbook of Experimental Therapeutics Antimalarial Drugs). [Google Scholar]
- 13.Peters W., Richards W.H.G. 2nd edn. Vol. 2. Springer-Verlag; 1984. (Handbook of Experimental Therapeutics. Antimalarial Drugs). [Google Scholar]
- 14.Hunt P. Gene encoding a deubiquitinating enzyme is mutated in artesunate- and chloroquine-resistant rodent malaria parasites. Mol. Microbiol. 2007;65:27–40. doi: 10.1111/j.1365-2958.2007.05753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afonso A. Malaria parasites can develop stable resistance to artemisinin but lack mutations in candidate genes atp6 (encoding the sarcoplasmic and endoplasmic reticulum Ca2+ ATPase), tctp, mdr1, and cg10. Antimicrob. Agents Chemother. 2006;50:480–489. doi: 10.1128/AAC.50.2.480-489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrer-Rodriguez I. Plasmodium yoelii: identification and partial characterization of an MDR1 gene in an artemisinin-resistant line. J. Parasitol. 2004;90:152–160. doi: 10.1645/GE-3225. [DOI] [PubMed] [Google Scholar]
- 17.Puri S.K., Chandra R. Plasmodium vinckei: selection of a strain exhibiting stable resistance to arteether. Exp. Parasitol. 2006;114:129–132. doi: 10.1016/j.exppara.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Walker D.J. Mechanisms of artemisinin resistance in the rodent malaria pathogen Plasmodium yoelii. Antimicrob. Agents Chemother. 2000;44:344–347. doi: 10.1128/aac.44.2.344-347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bray P.G. Quinolines and artemisinin: chemistry, biology and history. Curr. Top. Microbiol. Immunol. 2005;295:3–38. doi: 10.1007/3-540-29088-5_1. [DOI] [PubMed] [Google Scholar]
- 20.Haynes R.K. Artemisinin antimalarials do not inhibit hemozoin formation. Antimicrob. Agents Chemother. 2003;47:1175. doi: 10.1128/AAC.47.3.1175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckstein-Ludwig U. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 22.Ellis D.S. The chemotherapy of rodent malaria, XXXIX. Ultrastructural changes following treatment with artemisinine of Plasmodium berghei infection in mice, with observations of the localization of [3H]-dihydroartemisinine in P. falciparum in vitro. Ann. Trop. Med. Parasitol. 1985;79:367–374. [PubMed] [Google Scholar]
- 23.Stocks P.A. Evidence for a common non-heme chelatable-iron-dependent activation mechanism for semisynthetic and synthetic endoperoxide antimalarial drugs. Angew. Chem. Int. Ed. Engl. 2007;46:6278–6283. doi: 10.1002/anie.200604697. [DOI] [PubMed] [Google Scholar]
- 24.Uhlemann A.C. Mechanism of antimalarial action of the synthetic trioxolane RBX11160 (OZ277) Antimicrob. Agents Chemother. 2007;51:667–672. doi: 10.1128/AAC.01064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creek D.J. Relationship between antimalarial activity and heme alkylation for spiro- and dispiro-1,2,4-trioxolane antimalarials. Antimicrob. Agents Chemother. 2008;52:1291–1296. doi: 10.1128/AAC.01033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Creek D.J. Kinetics of iron-mediated artemisinin degradation: effect of solvent composition and iron salt. J. Pharm. Sci. 2005;94:1820–1829. doi: 10.1002/jps.20400. [DOI] [PubMed] [Google Scholar]
- 27.Charman S.A. Second generation synthetic peroxide antimalarials. Am. J. Trop. Med. Hyg. 2007;77(Suppl. 5):95. [Google Scholar]
- 28.Kreidenweiss A. Antimalarial activity of a synthetic endoperoxide (RBx-11160/OZ277) against Plasmodium falciparum isolates from Gabon. Antimicrob. Agents Chemother. 2006;50:1535–1537. doi: 10.1128/AAC.50.4.1535-1537.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kannan R. Reaction of artemisinin with haemoglobin: implications for antimalarial activity. Biochem. J. 2005;385:409–418. doi: 10.1042/BJ20041170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ter Kuile F. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp. Parasitol. 1993;76:85–95. doi: 10.1006/expr.1993.1010. [DOI] [PubMed] [Google Scholar]
- 31.Desakorn V. Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 2005;99:517–524. doi: 10.1016/j.trstmh.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Charoenteeraboon J. Inactivation of artemisinin by thalassemic erythrocytes. Biochem. Pharmacol. 2000;59:1337–1344. doi: 10.1016/s0006-2952(00)00271-9. [DOI] [PubMed] [Google Scholar]
- 33.Ittarat W. Effects of α-thalassemia on pharmacokinetics of the antimalarial agent artesunate. Antimicrob. Agents Chemother. 1998;42:2332–2335. doi: 10.1128/aac.42.9.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutagalung R. Influence of hemoglobin E trait on the antimalarial effect of artemisinin derivatives. J. Infect. Dis. 2000;181:1513–1516. doi: 10.1086/315373. [DOI] [PubMed] [Google Scholar]
- 35.Monti D. Does chloroquine really act through oxidative stress? FEBS Lett. 2002;522:3–5. doi: 10.1016/s0014-5793(02)02881-8. [DOI] [PubMed] [Google Scholar]
- 36.Parapini S. Evidence that haem iron in the malaria parasite is not needed for the antimalarial effects of artemisinin. FEBS Lett. 2004;575:91–94. doi: 10.1016/j.febslet.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 37.Jambou R. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet. 2005;366:1960–1963. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- 38.Legrand E. In vitro monitoring of Plasmodium falciparum drug resistance in French Guiana: a synopsis of continuous assessment from 1994 to 2005. Antimicrob. Agents Chemother. 2008;52:288–298. doi: 10.1128/AAC.00263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayward R. pfmdr1 mutations associated with chloroquine resistance incur a fitness cost in Plasmodium falciparum. Mol. Microbiol. 2005;55:1285–1295. doi: 10.1111/j.1365-2958.2004.04470.x. [DOI] [PubMed] [Google Scholar]
- 40.Walliker D. Fitness of drug-resistant malaria parasites. Acta Trop. 2005;94:251–259. doi: 10.1016/j.actatropica.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Cojean S. Resistance to dihydroartemisinin. Emerg. Infect. Dis. 2006;12:1798–1799. doi: 10.3201/eid1211.060903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Legrand E. Resistance to dihydroartemisinin. Emerg. Infect. Dis. 2007;13:808–809. doi: 10.3201/eid1305.061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toyoshima C. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature. 2000;405:647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- 44.Uhlemann A.C. A single amino acid residue can determine the sensitivity of SERCAs to artemisinins. Nat. Struct. Mol. Biol. 2005;12:628–629. doi: 10.1038/nsmb947. [DOI] [PubMed] [Google Scholar]
- 45.Price R.N. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W. Yeast model uncovers dual roles of mitochondria in action of artemisinin. PLoS Genet. 2005;1:e36. doi: 10.1371/journal.pgen.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alenquer M. Adaptive response to the antimalarial drug artesunate in yeast involves Pdr1p/Pdr3p-mediated transcriptional activation of the resistance determinants TPO1 and PDR5. FEMS Yeast Res. 2006;6:1130–1139. doi: 10.1111/j.1567-1364.2006.00095.x. [DOI] [PubMed] [Google Scholar]
- 48.Schmuck G. Neurotoxic mode of action of artemisinin. Antimicrob. Agents Chemother. 2002;46:821–827. doi: 10.1128/AAC.46.3.821-827.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dondorp A. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 50.Nealon C. Intramuscular bioavailability and clinical efficacy of artesunate in gabonese children with severe malaria. Antimicrob. Agents Chemother. 2002;46:3933–3939. doi: 10.1128/AAC.46.12.3933-3939.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodrow C.J. Artesunate versus quinine for severe falciparum malaria: a randomised trial. Lancet. 2006;367:110–111. doi: 10.1016/S0140-6736(06)67957-9. [DOI] [PubMed] [Google Scholar]
- 52.Gomes M. Rectal artemisinins for malaria: a review of efficacy and safety from individual patient data in clinical studies. BMC Infect. Dis. 2008;8:39. doi: 10.1186/1471-2334-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krishna S. Bioavailability and preliminary clinical efficacy of intrarectal artesunate in Ghanaian children with moderate malaria. Antimicrob. Agents Chemother. 2001;45:509–516. doi: 10.1128/AAC.45.2.509-516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campos, M.S. et al. Fatal artesunate toxicity in a child. J. Pediatr. Infect. Dis. (in press)
- 55.Woodrow C.J. Artemisinins. Postgrad. Med. J. 2005;81:71–78. doi: 10.1136/pgmj.2004.028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haynes R.K. Artemisone – a highly active antimalarial drug of the artemisinin class. Angew. Chem. Int. Ed. Engl. 2006;45:2082–2088. doi: 10.1002/anie.200503071. [DOI] [PubMed] [Google Scholar]
- 57.Nagamune K. Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryot. Cell. 2007;6:2147–2156. doi: 10.1128/EC.00262-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagamune K. Artemisinin-resistant mutants of Toxoplasma gondii have altered calcium homeostasis. Antimicrob. Agents Chemother. 2007;51:3816–3823. doi: 10.1128/AAC.00582-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vial H.J., Gorenflot A. Chemotherapy against babesiosis. Vet. Parasitol. 2006;138:147–160. doi: 10.1016/j.vetpar.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 60.Utzinger J. Artemisinins for schistosomiasis and beyond. Curr. Opin. Investig. Drugs. 2007;8:105–116. [PubMed] [Google Scholar]
- 61.Mishina Y.V. Artemisinins inhibit Trypanosoma cruzi and Trypanosoma brucei rhodesiense in vitro growth. Antimicrob. Agents Chemother. 2007;51:1852–1854. doi: 10.1128/AAC.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao S.H. Development of antischistosomal drugs in China, with particular consideration to praziquantel and the artemisinins. Acta Trop. 2005;96:153–167. doi: 10.1016/j.actatropica.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Chen D.J. Experimental studies on antischistosomal activity of qinghaosu. Zhong Hui Yi Xue Zha Zhi. 1980;60:422–425. [Google Scholar]
- 64.Efferth T. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001;18:767–773. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- 65.Kelter G. Role of transferrin receptor and the ABC transporters ABCB6 and ABCB7 for resistance and differentiation of tumor cells towards artesunate. PLoS ONE. 2007;2:e798. doi: 10.1371/journal.pone.0000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li L.N. Artesunate attenuates the growth of human colorectal carcinoma and inhibits hyperactive Wnt/β-catenin pathway. Int. J. Cancer. 2007;121:1360–1365. doi: 10.1002/ijc.22804. [DOI] [PubMed] [Google Scholar]
- 67.Zhou H.J. Artesunate inhibits angiogenesis and downregulates vascular endothelial growth factor expression in chronic myeloid leukemia K562 cells. Vascul. Pharmacol. 2007;47:131–138. doi: 10.1016/j.vph.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Wu X.H. Dihydroartemisinin inhibits angiogenesis induced by multiple myeloma RPMI8226 cells under hypoxic conditions via downregulation of vascular endothelial growth factor expression and suppression of vascular endothelial growth factor secretion. Anticancer Drugs. 2006;17:839–848. doi: 10.1097/01.cad.0000224443.85834.32. [DOI] [PubMed] [Google Scholar]
- 69.Li J., Zhou H.J. Dihydroartemisinin inhibits the expression of vascular endothelial growth factor in K562 cells. Yao Xue Xue Bao. 2005;40:1041–1045. [PubMed] [Google Scholar]
- 70.Anfosso L. Microarray expression profiles of angiogenesis-related genes predict tumor cell response to artemisinins. Pharmacogenomics J. 2006;6:269–278. doi: 10.1038/sj.tpj.6500371. [DOI] [PubMed] [Google Scholar]
- 71.Longo M. Effects of the antimalarial drug dihydroartemisinin (DHA) on rat embryos in vitro. Reprod. Toxicol. 2006;21:83–93. doi: 10.1016/j.reprotox.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Dell’Eva R. Inhibition of angiogenesis in vivo and growth of Kaposi's sarcoma xenograft tumors by the anti-malarial artesunate. Biochem. Pharmacol. 2004;68:2359–2366. doi: 10.1016/j.bcp.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 73.Huan-huan C. Artesunate reduces chicken chorioallantoic membrane neovascularisation and exhibits antiangiogenic and apoptotic activity on human microvascular dermal endothelial cell. Cancer Lett. 2004;211:163–173. doi: 10.1016/j.canlet.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 74.Chen H.H. Antimalarial dihydroartemisinin also inhibits angiogenesis. Cancer Chemother. Pharmacol. 2004;53:423–432. doi: 10.1007/s00280-003-0751-4. [DOI] [PubMed] [Google Scholar]
- 75.Chen H.H. Inhibitory effects of artesunate on angiogenesis and on expressions of vascular endothelial growth factor and VEGF receptor KDR/flk-1. Pharmacology. 2004;71:1–9. doi: 10.1159/000076256. [DOI] [PubMed] [Google Scholar]
- 76.Wartenberg M. The antimalaria agent artemisinin exerts antiangiogenic effects in mouse embryonic stem cell-derived embryoid bodies. Lab. Invest. 2003;83:1647–1655. doi: 10.1097/01.lab.0000098424.38003.ff. [DOI] [PubMed] [Google Scholar]
- 77.Chen H.H. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol. Res. 2003;48:231–236. doi: 10.1016/s1043-6618(03)00107-5. [DOI] [PubMed] [Google Scholar]
- 78.Wu G.D. Apoptosis of human umbilical vein endothelial cells induced by artesunate. Vascul. Pharmacol. 2004;41:205–212. doi: 10.1016/j.vph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Efferth T., Oesch F. Oxidative stress response of tumor cells: microarray-based comparison between artemisinins and anthracyclines. Biochem. Pharmacol. 2004;68:3–10. doi: 10.1016/j.bcp.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 80.Efferth T. Role of antioxidant genes for the activity of artesunate against tumor cells. Int. J. Oncol. 2003;23:1231–1235. [PubMed] [Google Scholar]
- 81.Singh N.P., Lai H.C. Synergistic cytotoxicity of artemisinin and sodium butyrate on human cancer cells. Anticancer Res. 2005;25:4325–4331. [PubMed] [Google Scholar]
- 82.Kim S.J. Dihydroartemisinin enhances radiosensitivity of human glioma cells in vitro. J. Cancer Res. Clin. Oncol. 2006;132:129–135. doi: 10.1007/s00432-005-0052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Efferth T. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic. Biol. Med. 2004;37:998–1009. doi: 10.1016/j.freeradbiomed.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 84.Singh N.P., Lai H.C. Artemisinin induces apoptosis in human cancer cells. Anticancer Res. 2004;24:2277–2280. [PubMed] [Google Scholar]
- 85.Sadava D. Transferrin overcomes drug resistance to artemisinin in human small-cell lung carcinoma cells. Cancer Lett. 2002;179:151–156. doi: 10.1016/s0304-3835(02)00005-8. [DOI] [PubMed] [Google Scholar]
- 86.Singh N.P., Lai H. Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci. 2001;70:49–56. doi: 10.1016/s0024-3205(01)01372-8. [DOI] [PubMed] [Google Scholar]
- 87.Lai H., Singh N.P. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett. 1995;91:41–46. doi: 10.1016/0304-3835(94)03716-v. [DOI] [PubMed] [Google Scholar]
- 88.Berger T.G. Artesunate in the treatment of metastatic uveal melanoma – first experiences. Oncol. Rep. 2005;14:1599–1603. [PubMed] [Google Scholar]
- 89.Singh N.P., Panwar V.K. Case report of a pituitary macroadenoma treated with artemether. Integr. Cancer Ther. 2006;5:391–394. doi: 10.1177/1534735406295311. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Z.Y. Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2008;6:134–138. doi: 10.3736/jcim20080206. [DOI] [PubMed] [Google Scholar]
- 91.Paeshuyse J. Hemin potentiates the anti-hepatitis C virus activity of the antimalarial drug artemisinin. Biochem. Biophys. Res. Commun. 2006;348:139–144. doi: 10.1016/j.bbrc.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 92.Romero M.R. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antiviral Res. 2005;68:75–83. doi: 10.1016/j.antiviral.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 93.Efferth T. Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J. Mol. Med. 2002;80:233–242. doi: 10.1007/s00109-001-0300-8. [DOI] [PubMed] [Google Scholar]
- 94.Kaptein S.J. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antiviral Res. 2006;69:60–69. doi: 10.1016/j.antiviral.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 95.Naesens L. Antiviral activity of diverse classes of broad-acting agents and natural compounds in HHV-6-infected lymphoblasts. J. Clin. Virol. 2006;37(Suppl. 1):S69–S75. doi: 10.1016/S1386-6532(06)70015-4. [DOI] [PubMed] [Google Scholar]
- 96.Efferth T. Activity of drugs from traditional Chinese medicine toward sensitive and MDR1- or MRP1-overexpressing multidrug-resistant human CCRF-CEM leukemia cells. Blood Cells Mol. Dis. 2002;28:160–168. doi: 10.1006/bcmd.2002.0492. [DOI] [PubMed] [Google Scholar]
- 97.Qian R.S. The immunologic and antiviral effect of qinghaosu. J. Tradit. Chin. Med. 1982;2:271–276. [PubMed] [Google Scholar]
- 98.Romero M.R. Antiviral effect of artemisinin from Artemisia annua against a model member of the Flaviviridae family, the bovine viral diarrhoea virus (BVDV) Planta Med. 2006;72:1169–1174. doi: 10.1055/s-2006-947198. [DOI] [PubMed] [Google Scholar]
- 99.Shapira M.Y. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin. Infect. Dis. 2008;46:1455–1457. doi: 10.1086/587106. [DOI] [PubMed] [Google Scholar]
- 100.Merali S., Meshnick S.R. Susceptibility of Pneumocystis carinii to artemisinin in vitro. Antimicrob. Agents Chemother. 1991;35:1225–1227. doi: 10.1128/aac.35.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ni X., Chen Y. In vitro study of the anti-pneumocystis carinii effect of arteminsin derivatives. Zhonghua Jie He He Hu Xi Za Zhi. 2001;24:164–167. [PubMed] [Google Scholar]
- 102.Brun-Pascaud M. Lack of activity of artemether for prophylaxis and treatment of Toxoplasma gondii and Pneumocystis carinii infections in rat. Parasite. 1996;3:187–189. doi: 10.1051/parasite/1996032187. [DOI] [PubMed] [Google Scholar]
- 103.Chen Y.T. An experimental trial of artemether in treatment of Pneumocystis carinii in immunosuppressed rats. Chin. Med. J. (Engl.) 1994;107:673–677. [PubMed] [Google Scholar]
- 104.Xu H. Anti-malarial agent artesunate inhibits TNF-α-induced production of proinflammatory cytokines via inhibition of NF-κB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology (Oxford) 2007;46:920–926. doi: 10.1093/rheumatology/kem014. [DOI] [PubMed] [Google Scholar]
- 105.Mirshafiey A. Design of a new line in treatment of experimental rheumatoid arthritis by artesunate. Immunopharmacol. Immunotoxicol. 2006;28:397–410. doi: 10.1080/08923970600927447. [DOI] [PubMed] [Google Scholar]
- 106.Cuzzocrea S. Artemether: a new therapeutic strategy in experimental rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2005;27:615–630. doi: 10.1080/08923970500418786. [DOI] [PubMed] [Google Scholar]
- 107.Razavi A. Treatment of experimental nephrotic syndrome with artesunate. Int. J. Toxicol. 2007;26:373–380. doi: 10.1080/10915810701493293. [DOI] [PubMed] [Google Scholar]
- 108.Zhao M. Induction of apoptosis by artemisinin relieving the severity of inflammation in caerulein-induced acute pancreatitis. World J. Gastroenterol. 2007;13:5612–5617. doi: 10.3748/wjg.v13.i42.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li W.D. Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-α and blocking the signaling pathway NF-κB translocation. Int. Immunopharmacol. 2006;6:1243–1250. doi: 10.1016/j.intimp.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 110.Dong Y.J. Effect of dihydro-qinghaosu on auto-antibody production. TNFα secretion and pathologic change of lupus nephritis in BXSB mice. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23:676–679. [PubMed] [Google Scholar]
- 111.Lu L. Study on effect of Cordyceps sinensis and artemisinin in preventing recurrence of lupus nephritis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22:169–171. [PubMed] [Google Scholar]
- 112.Li Q. Toxicokinetics and hydrolysis of artelinate and artesunate in malaria-infected rats. Int. J. Toxicol. 2005;24:241–250. doi: 10.1080/10915810591007201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.