Abstract
Vacuolar H+-ATPases (V-ATPases) are large electrogenic proton pumps composed of numerous subunits that play vital housekeeping roles in the acidification of compartments of the endocytic pathway. Additionally, V-ATPase play specialized roles in certain cell types, a capacity that is linked to cell type selective expression of isoforms of some of the subunits. We detected low levels of the a3 isoform of the a-subunit in mouse brain extracts. Examination of various brain-derived cell types by immunoblotting showed a3 was expressed in the N9 microglia cell line and in primary microglia, but not in other cell types. The expression of a3 in osteoclasts requires stimulation by Receptor Activator of Nuclear Factor κ B -ligand (RANKL). We found that Receptor Activator of Nuclear Factor κ B (RANK) was expressed by microglia. Stimulation of microglia with RANKL triggered increased expression of a3. V-ATPases in microglia were shown to bind microfilaments, and stimulation with RANKL increased the proportion of V-ATPase associated with the detergent-insoluble cytoskeletal fraction and with actin. In summary, microglia express the a3-subunit of V-ATPase. The expression of a3 and the interaction between V-ATPases and microfilaments was modulated by RANKL. These data suggest a novel molecular pathway for regulating microglia.
Keywords: V-ATPase, actin, microfilaments, central nervous system, Receptor Activator of Nuclear Factor κ B-ligand
Introduction
Vacuolar H+-ATPases (V-ATPases1) are multisubunit enzymes (11–13 subunits in mammals) that are required for vital housekeeping acidification in eukaryotic cells [1]. In addition, V-ATPases play vital roles in certain differentiated cell types [2–4]. In the brain V-ATPases are known to be required to generate membrane potential for loading neurotransmitters into presynaptic vesicles [5–7]. Kidney epithelial cells express high levels of V-ATPases in plasma membrane where they are involved in urinary acid/base regulation [2]. Likewise, osteoclasts target high levels of V-ATPases to the plasma membrane in order support the acidification necessary for bone resorption [8; 9]. Although each V-ATPase is thought to have the same basic subunit composition, a number of subunits have multiple isoforms that, when expressed, appear to be linked to the utilization of V-ATPases for specialized functions [2]. There are four isoforms of subunit a (a1-a4), a large transmembrane protein [10; 11]. Subunit a1 and a2 are expressed ubiquitously, a4 is expressed in specific cells in the kidney, and a3 has been described in osteoclasts and pancreatic beta cells [12; 13]. The a-subunit is thought to have a vital role in the enzymatic function of V-ATPase. In addition, evidence suggests that it carries vital targeting information, although the nature of that information has not been defined. Subunit a3 is required for osteoclast function, and children lacking functional a3 have a fatal form of osteopetrosis (autosomal malignant osteopetrosis) [14; 15]. Bone marrow transplants are beneficial, but long term survival remains problematic even with marrow transplant [16]. There have been reports of central nervous system problems associated with this syndrome, but it is not clear whether these are the result of primary defects of the lack of a3 in the brain, or whether these result from factors (calcemic imbalance for example) secondary to the osteopetrosis [17].
Recently it was reported that a3 is expression in rodent brains was confined to a crude glial cell isolate [18]. The precise identity of the cells expressing a3 was not reported. The goal of this study was to identify the specific cell-types expressing the a3-subunit in mice brains and to determine if its expression is regulated by Receptor Activator of Nuclear Factor κ B-ligand (RANKL) as it is in osteoclasts.
Materials and Methods
Reagents
Unless otherwise noted, reagents were obtained from Sigma-Aldrich Chemicals (St. Louis MO). Polyclonal antibodies against V-ATPase subunits E, a1, and a3 were described previously [19; 20].
Mouse Brain Extracts
Mice were sacrificed by cervical dislocation, brains were dissected free and homogenized by 20 strokes with a loose fitting Dounce homogenizer in an equal volume of 20 mM Tris-HCl pH 7.4, 100 mM NaCl, 5 mM MgCl2, 0.2 mM dithiothreitol plus a protease inhibitor cocktail. All protocols were approved by the University of Florida Institutional Animal Care and Usage Committee. The brains lysates were subjected to centrifugation at 100 KXg for 30 minutes and the upper flocculent layer was collected and washed by resuspending in homogenization buffer and repelleting the membranes. The samples were then subjected to SDS-PAGE, blotted to nitrocellulose and probed with antibodies as described in Figure Legends.
Brain-derived cell types
The murine microglial cell line N9 [21] was grown in Iscove’s Modified Dulbecco’s Medium with 25 mm HEPES and l-glutamine, supplemented with 5% fetal calf serum (Hyclone, Logan UT), 100 IU/mL penicillin, 100 μg/mL streptomycin and 50 nm β-mercaptoethanol. Cells were cultured in a humidified 5% CO2 atmosphere at 37 °C.
CG-4 cells are a rat cell line that can by stimulated to differentiate into oligodendrocytes and astrocytes depending on the culture conditions [22]. To differentiate CG-4 cells into oligodendrocytes, tissue culture plates were pre-coated sequentially with poly-ornithine solution, and fibronectin in DME-N2 biotin plus 30% B104-conditioned media (CM). (B104 cells are a neuronal cell line which produce soluble factors required for CG-4 growth). CG-4 cells were grown and expanded under these conditions in a serum free medium relying on mitogens produced by the B104 cells.
To differentiate CG-4 cells into oligodendrocytes, the conditioned medium was withdrawn. During a period of 48 hours the cells differentiated into oligodendrocyte-like cells. CG-4 cells were also induced to differentiate into astrocytes. The cells are grown and passaged as described above, and then induced to differentiate by withdrawing the B104 conditioned medium and replacing it with fetal bovine serum.
The rat cell line rtSc95.1was used as a model for Schwann cells. RtSc95.1 cells were grown for 3 days in Dulbecco’s Modified Eagle Medium (dMEM) plus 10% fetal bovine serum.
Glia were obtained from Swiss Webster mouse pups using the methods described previously[23]. Briefly, cortices were removed, cleaned of meninges and trypsinized, and dissociated by trituration. Cells were plated in culture flasks at 50,000 cells cm2, and grown in modified eagles medium (MEM), 10% fetal calf serum, penicillin and streptomycin, essential amino acids and nonessential amino acids. Microglia were harvested from astrocytes that become confluent prior to 3-weeks in culture. Passaging microglia was accomplished by shaking and slapping the flask on a table several times and vigorously swirling the flasks to dislodge the microglia that were attached to the monolayer of astrocytes. The growth medium containing the dislodged microglia cells was centrifuged at 800 rpm for 5 minutes, most of the supernatant was removed and the cells in the pellet were resuspended in the remaining 2–3 ml, yielding a density of ~85,000 cells ml−1. The density of cells was 20,000 cells cm2 after plating.
Stimulation of N9 microglia and primary mouse microglia was performed using recombinant GST-RANKL which contains amino acids 158–316 of the mouse RANKL gene [24]. Expression of GST-RANKL and isolation from bacterial extracts was performed by standard methods.
PCR
RT-PCR was performed on mRNA isolated from N9 microglia and RAW 264.7 osteoclast-like cells. Cells were scraped and lysed in TRIZOL reagent (Invitrogen), and total RNA was extracted according to the manufacturer’s instructions. RNA was quantified spectrophotometrically, and 1 μg was reversely transcribed. The standard PCR conditions were 95°C (10 min), and then 30 cycles of 94°C (1 min), 54°C (1 min), and 72°C (2 min). In pilot experiments, this number of cycles did not reach saturation of the PCR reaction. The primer sequences used were as follows: RANK forward 5′GGGTGGGGCGCAGACTTCAC 3′; RANK reverse 5′ATGCCAGCAGCCTGCACCAG 3′; GAPDH forward 5′AAATTCCATGGCACCGTCAA 3′; GAPDH reverse 5′AGGGATCTCGCTCCTGGAA 3′. Primers were obtained from Invitrogen. For each run, water was used as the negative control and RAW 264.7 osteoclast-like cells were used as a positive control.. The reaction product was quantified by measuring the densities of bands using a Spot-denso-program with Alpha Ease software (Alpha Innotech, San Leandro, CA), and values representing the average density per area unit were plotted. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene.
Triton-extracted cytoskeletons
N9 cultures were treated with GST-RANKL or vehicle control, then incubated in extraction buffer (20 mM Tris-HCl pH 7.4, 100 mM NaCl, 5 mM MgCl2, 0.2 mM CaCl2, 0.2 mM dithiothreitol, 0.5% Triton X-100 plus a protease inhibitor mixture) plus 10 μM phalloidin and 10 μM DNase1 to ensure that actin filaments neither polymerized nor depolymerized after cell disruption [19], and subjected to ultracentifugation at 100,000 Xg for 30 min. Supernatants and pellets were collected in equivalent volumes, subjected to SDS-PAGE, blotted to nitrocellulose and probed with antibodies as indicated.
Immunoprecipitations
Immunoprecipitations were performed as described previously [19]. N9 cells were cultured as described above, washed in PBS and solubilized in Triton X-100 buffer (1% Triton X-100, 20 mM Tris, pH 7.4, 1 mM EDTA, 1 mM dithiothreitol, 0.1% SDS, 10% glycerol, 5 mM sodium azide, and protease inhibitors). Following a centrifugation at 20,000 × g for 10 min to remove insoluble material, the extracts were incubated for 1 h at 4 °C with a 1:200 dilution of anti-a3 or anti-a1, 50 μl of protein A beads (Sigma) were added, and the mixture was incubated for 1 h at 4 °C with rocking. The protein A beads were collected by centrifugation at 10,000 × g for 15 s at 4 °C and washed three times with the Triton X-100 buffer. The wash buffers were removed by aspiration with a bent 23-gauge needle, and Laemmli sample buffer was added. The samples were heated at 85 °C for 10 min, cooled to room temperature, and centrifuged at 10,000 × g for 1 min, and the supernatants were applied to SDS-polyacrylamide gels, transferred to nitrocellulose, and probed with antibodies as described in the legends
Results
Expression of subunit a3 in mouse brains
From the literature it was known that subunit a3 is expressed in glial cells derived from rodent brains [18; 25; 26]. Whole brains were isolated from 20 week old mice, the brains were homogenized, and the microsomal fraction was collected and separated by SDS-PAGE and Western Blotting (Fig. 1). Consistent with the previous reports, a3 was present at low levels compared with subunit a1.
Fig. 1.
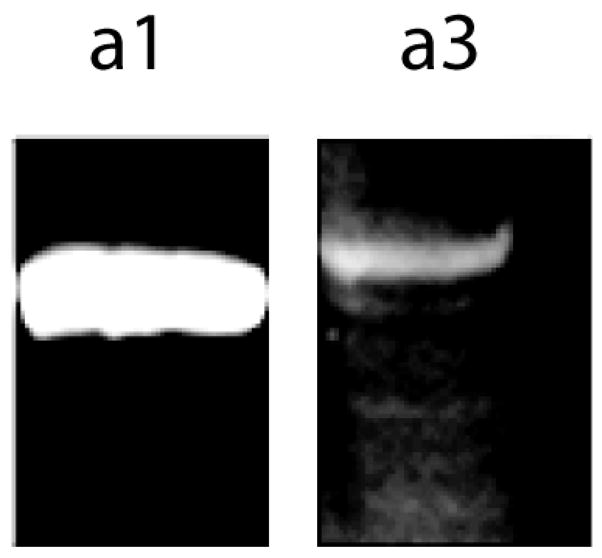
The a3-subunit of V-ATPase is expressed at low levels in the brains of mice. Mouse brains were homogenized and microsomal fractions were collected as described in Materials and methods, and 5 μg of microsomal protein was subjected to SDS-PAGE, blotted to nitrocellulose and probed with anti-a1 and anti-a3 antibodies as indicated. The a3-subunit was detected but was present at much lower levels than the a1-subunit, which is found in neurons.
Subunit a3 is expressed in microglia
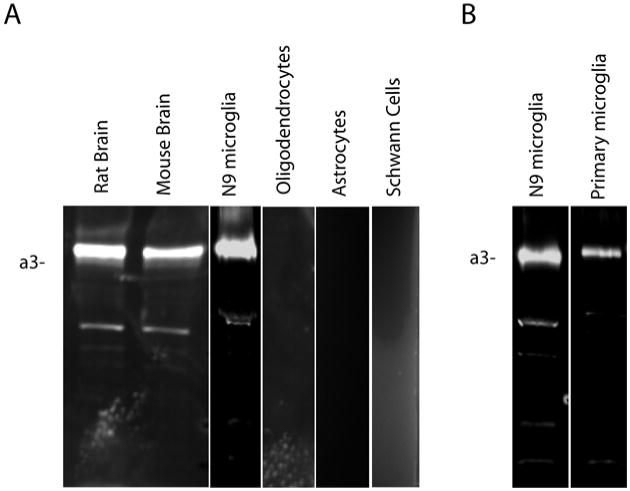
The expression of the a3-subunit is confined to the glial cells [18] To determine the specific glial cell types that express a3, we tested brain-derived glial cell lines that represent oligodendrocytes, Schwann cells, astrocytes, and microglia. Of these, only the microglia cell line N9, expressed detectable amounts of the a3-subunit (Fig. 2A). To confirm this result, primary microglia were analyzed As with the N9 cells, primary microglia grown by standard methods expressed subunit a3 (Fig. 2B).
Fig 2.
The a3-subunit is expressed in the N9 microglia cell line but not in other glial cells. A). 5 μg of total protein from rat and mice microsomes as well as the cell-types indicated was separated by SDS-PAGE, blotted to nitrocellulose, and probed with the anti-a3 polyclonal antibody. Note that while a3 was easily detected in the brain samples and in N9 microglia, it was not detected in other glial cell extracts. B.) Subunit a3 was present in both N9 microglia and primary microglia. 2.5 μg total protein from N9 microglia or primary microglia was separated by SDS-PAGE, blotted and probed with the anti-a3 antibody.
Expression of subunit a3 is stimulated by RANKL
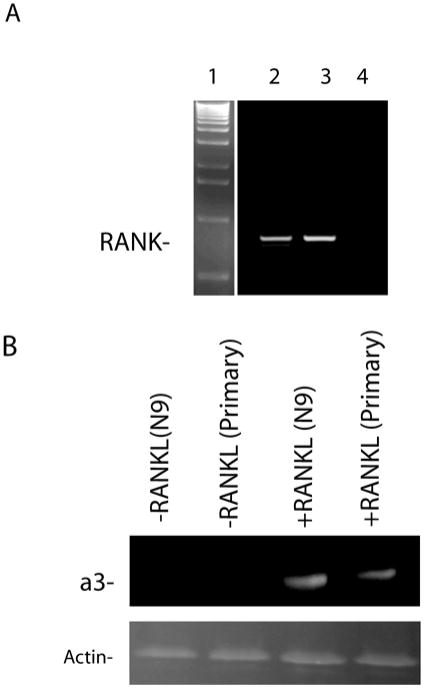
During osteoclastogenesis expression of subunit a3 is triggered by RANKL. We first examined by PCR whether N9 microglia and primary microglia express RANK, the receptor of RANKL, and confirmed the expression of the mRNA for RANK (Fig. 3A). We then treated N9 microglia and primary microglia with recombinant RANKL, and assayed the resulting cells by SDS-PAGE and immunoblot analysis. In both cases, RANKL stimulated increased the relative expression of subunit a3 (Fig. 3B).
Fig. 3.
RANK is expressed by microglia, and microglia respond to RANKL by increasing the level of the a3-subunit. A) RT-PCR was performed and the PCR products were separated on agarose gels.Lane 1, 1100 bp ladder; Lane 2, N9 microglia; Lane 3 RAW 264.7 cells (as a positive control); Lane 4, primers were omitted. A PCR product of 159 bp was detected as expected. B.) Treatment of microglia increased the relative levels of the a3-subunit. N9 cells or primary microglia were stimulated with 5 ng/ml recombinant GST-RANKL for 3 Days, the cells were harvested, and 2 μg total protein was separated by SDS-PAGE. Blotted to nitrocellulose, and probed with either anti-a3 antibody or anti-actin antibody. Both N9 microglia and primary microglia responded to RANKL with a large relative increase in the expression of the a3-subunit.
RANKL triggers associations between V-ATPases and the actin cytoskeleton
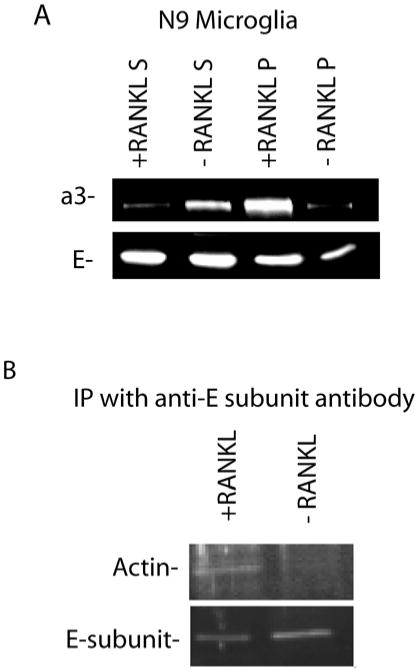
Osteoclastogenesis occurs when hematopoietic precursors are stimulated with RANKL, and is associated with increased amounts of V-ATPase bound to microfilaments [27]. We determined the amount of a3 associated with the detergent-insoluble cytoskeletal fraction after ultracentrifugation of microglia that had been extracted with Triton X-100. We found that the amount of a3 in the cytoskeletal fraction increased from 32% to 81% (by scanning densitometry) in cultures treated with RANKL (Fig. 4A). To confirm that the V-ATPases were bound to microfilaments, we performed immunoprecipitations using antibodies directed against the E-subunit to determine the relative amount of actin bound to V-ATPase complexes [19]. Under conditions in which the same amount of E-subunit was pulled down, the amount of actin was increased in immunoprecipiations from cells treated with RANKL (Fig. 4B).
Fig. 4.
Stimulation of N9 microglia with RANKL increases the association of V-ATPases with the detergent-insoluble cytoskeletal fraction and with actin. A.) N9 microglia were treated with 5 ng/ml RANKL (+RANKL) or with vehicle (-RANKL) for 3 days, then homogenized in 1% Triton X-100 and subjected to ultracentrifugation at 100,000 Xg for 30 min. Supernatants (S) and pellets (P) were collected in equal volumes, subjected to SDS-PAGE, blotted to nitrocellulose and probed with either anti-a3 or anti-E subunit antibodies. Less a3 was expressed in the absence of RANKL, and most of that was found in the supernatant fraction. When stimulated with RANKL, more a3 was expressed and more than 90% (by densitometry) was in the pellet which is the detergent-insoluble cytoskeletal fraction. A larger portion of the E-subunit (a peripheral subunit was also found in the pellet derived from RANKL-stimulated cells. B.) Immunoprecipitations from 1% Triton X-100 extracts with anti-E subunit antibody were performed. Immunoprecipitates were separated by SDS-PAGE, blotted and probed with anti-actin and anti-E subunit antibodies. Although the same amount of E-subunit was pulled down in each case, the amount of actin detected was higher after RANKL-stimulation.
Discussion
In this study we report for the first time that subunit a3 of V-ATPase is expressed in N9 microglia and in primary microglia grown in tissue culture. We found that microglia express RANK, and are stimulated by RANKL to produce increased levels of a3 and to bind a larger portion of their V-ATPases to the cytoskeletal fraction. These findings identify new players in microglial regulation and function.
We confirmed the results of a previous study that reported low levels of subunit a3 in mouse brains, and that a3 expression was confined to crude microglial fraction [18]. Because that fraction contained a number of cell types, we assayed representatives of each of these cell types, and found that only microglia expressed detectable levels of a3. Both N9 microglia and primary microglia isolated from mouse brains expressed the a3-subunit. The fact that a3 was detected in both the crude brain extracts and in primary microglia suggests that a3 expression is a component of normal brain physiology. Whether the expression of a3 in the brain was the result of RANKL stimulation of RANK is not clear. Although in osteoclasts, a3 expression is closely tied to the RANKL-RANK interaction other pathways might also stimulate a3 expression.
Osteoprotegerin, which competitively inhibits the interaction between RANKL and RANK [28], was found in cerebrospinal fluid [29]. In addition it has been reported that flt3 positive macrophage precursors differentiate sequentially into osteoclasts, dendritic cells and microglia [30]. RANKL was found in both embryonic and adult brains of mice [31]. In E15 mouse brains RANKL was expressed in neuroblasts. In adult mice, RANKL was expressed in neurons of the cerebral cortex. Another possible source of RANKL in the brain are T-cells, which cross the blood brain barrier in response to certain antigens [32–34]. T-cells express high levels of RANKL on their cell surface [35; 36]. When they migrate into the CNS, they would be expected to stimulate microglia through the RANKL/RANK interaction [37; 38]. These data suggest that it is likely microglia are regulated by the RANKL/RANK/osteoprotegerin system.
In osteoclasts, both a3 expression and the association between V-ATPase and actin filaments are linked with transport of V-ATPases to the osteoclast plasma membrane, an event that is necessary for bone resorption [13; 39; 40]. Although it is possible that V-ATPases might be transported to the plasma membrane of microglia, we think that it is more likely that upregulation of a3, and changes in the association between V-ATPase and the cytoskeleton, are indicators that a subset of V-ATPases are being utilized by the cell in a manner different from the normal housekeeping functions of V-ATPase.
In summary, we report for the first time that microglia express subunit a3 and RANK, and respond to RANKL. These molecules may have an unexpected role in microglial physiology and pathophysiology.
Acknowledgments
We thank Dr. Beth S. Lee, The Ohio State University, for providing us with the anti-a3, anti-a1 and anti-E subunit antibodies. We thank Dr. David Muir, University of Florida College of Medicine for providing cell lines for the project, and Dr. Wolfgang J. Streit, University of Florida College of Medicine for teaching us how to isolate primary microglia. We acknowledge support by the University of Florida, University Scholars Program, in which EMS participated.
Footnotes
vacuolar H+-ATPase, V-ATPase; Receptor Activator of Nuclear Factor κ B-ligand, RANKL; Receptor Activator of Nuclear Factor κ B, RANK; modified eagles medium, MEM; Dulbecco’s Modified Eagle Medium, dMEM; conditioned media, CM; Glyceraldehyde-3-phosphate dehydrogenase, GADH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cipriano DJ, Wang Y, Bond S, Hinton A, Jefferies KC, Qi J, Forgac M. Structure and regulation of the vacuolar ATPases. Biochim Biophys Acta. 2008;1777:599–604. doi: 10.1016/j.bbabio.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar H+-ATPase. Physiol Rev. 2004;84:1263–1314. doi: 10.1152/physrev.00045.2003. [DOI] [PubMed] [Google Scholar]
- 3.Breton S, Brown D. New insights into the regulation of V-ATPase-dependent proton secretion. Am J Physiol Renal Physiol. 2007;292:F1–10. doi: 10.1152/ajprenal.00340.2006. [DOI] [PubMed] [Google Scholar]
- 4.Gluck SL, Underhill DM, Iyori M, Holliday LS, Kostrominova TY, Lee BS. Physiology and biochemistry of the kidney vacuolar H+-ATPase. Annu Rev Physiol. 1996;58:427–445. doi: 10.1146/annurev.ph.58.030196.002235. [DOI] [PubMed] [Google Scholar]
- 5.Nelson N, Harvey WR. Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol Rev. 1999;79:361–385. doi: 10.1152/physrev.1999.79.2.361. [DOI] [PubMed] [Google Scholar]
- 6.Hiesinger PR, Fayyazuddin A, Mehta SQ, Rosenmund T, Schulze KL, Zhai RG, Verstreken P, Cao Y, Zhou Y, Kunz J, Bellen HJ. The v-ATPase V-0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell. 2005;121:607–620. doi: 10.1016/j.cell.2005.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moriyama Y, Maeda M, Futai M. The role of V-ATPase in neuronal and endocrine systems. J Exp Biol. 1992;172:171–178. doi: 10.1242/jeb.172.1.171. [DOI] [PubMed] [Google Scholar]
- 8.Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;245:855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- 9.Holliday LS, Bubb MR, Jiang J, Hurst IR, Zuo J. Interactions between vacuolar H+-ATPases and microfilaments in osteoclasts. Journal of Bioenergetics and Biomembranes. 2005;37:419–423. doi: 10.1007/s10863-005-9483-y. [DOI] [PubMed] [Google Scholar]
- 10.Oka T, Murata Y, Namba M, Yoshimizu T, Toyomura T, Yamamoto A, Sun-Wada GH, Hamasaki N, Wada Y, Futai M. a4, a unique kidney-specific isoform of mouse vacuolar H+-ATPase subunit a. J Biol Chem. 2001;276:40050–40054. doi: 10.1074/jbc.M106488200. [DOI] [PubMed] [Google Scholar]
- 11.Smith AN, Finberg KE, Wagner CA, Lifton RP, Devonald MA, Su Y, Karet FE. Molecular cloning and characterization of Atp6n1b: a novel fourth murine vacuolar H+-ATPase a-subunit gene. J Biol Chem. 2001;276:42382–42388. doi: 10.1074/jbc.M107267200. [DOI] [PubMed] [Google Scholar]
- 12.Sun-Wada GH, Toyomura T, Murata Y, Yamamoto A, Futai M, Wada Y. The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J Cell Sci. 2006;119:4531–4540. doi: 10.1242/jcs.03234. [DOI] [PubMed] [Google Scholar]
- 13.Li YP, Chen W, Liang Y, Li E, Stashenko P. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat Genet. 1999;23:447–451. doi: 10.1038/70563. [DOI] [PubMed] [Google Scholar]
- 14.Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK, Wallbrandt P, Zecca L, Notarangelo LD, Vezzoni P, Villa A. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet. 2000;25:343–346. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- 15.Kornak U, Schulz A, Friedrich W, Uhlhaas S, Kremens B, Voit T, Hasan C, Bode U, Jentsch TJ, Kubisch C. Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet. 2000;9:2059–2063. doi: 10.1093/hmg/9.13.2059. [DOI] [PubMed] [Google Scholar]
- 16.Stark Z, Savarirayan R. Osteopetrosis Orphanet. J Rare Dis. 2009;4:5. doi: 10.1186/1750-1172-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogbureke KU, Zhao Q, Li YP. Human osteopetroses and the osteoclast V-H+-ATPase enzyme system. Front Biosci. 2005;10:2940–2954. doi: 10.2741/1750. [DOI] [PubMed] [Google Scholar]
- 18.Poea-Guyon S, Amar M, Fossier P, Morel N. Alternative splicing controls neuronal expression of v-ATPase subunit a1 and sorting to nerve terminals. J Biol Chem. 2006;281:17164–17172. doi: 10.1074/jbc.M600927200. [DOI] [PubMed] [Google Scholar]
- 19.Chen SH, Bubb MR, Yarmola EG, Zuo J, Jiang J, Lee BS, Lu M, Gluck SL, Hurst IR, Holliday LS. Vacuolar H+-ATPase binding to microfilaments: regulation in response to phosphatidylinositol 3-kinase activity and detailed characterization of the actin-binding site in subunit B. J Biol Chem. 2004;279:7988–7998. doi: 10.1074/jbc.M305351200. [DOI] [PubMed] [Google Scholar]
- 20.Ochotny N, Van VA, Chan N, Yao Y, Morel M, Kartner N, von Schroeder HP, Heersche JN, Manolson MF. Effects of human a3 and a4 mutations that result in osteopetrosis and distal renal tubular acidosis on yeast V-ATPase expression and activity. J Biol Chem. 2006;281:26102–26111. doi: 10.1074/jbc.M601118200. [DOI] [PubMed] [Google Scholar]
- 21.Righi M, Mori L, De LG, Sironi M, Biondi A, Mantovani A, Donini SD, Ricciardi-Castagnoli P. Monokine production by microglial cell clones. Eur J Immunol. 1989;19:1443–1448. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- 22.Louis JC, Magal E, Muir D, Manthorpe M, Varon S. Cg-4, A New Bipotential Glial-Cell Line from Rat-Brain, Is Capable of Differentiating Invitro Into Either Mature Oligodendrocytes Or Type-2 Astrocytes. Journal of Neuroscience Research. 1992;31:193–204. doi: 10.1002/jnr.490310125. [DOI] [PubMed] [Google Scholar]
- 23.Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krits I, Wysolmerski RB, Holliday LS, Lee BS. Differential Localization of Myosin II Isoforms in Resting and Activated Osteoclasts. Calcif Tissue Int. 2002;71:530–538. doi: 10.1007/s00223-001-1112-0. [DOI] [PubMed] [Google Scholar]
- 25.Mattsson JP, Li X, Peng SB, Nilsson F, Andersen P, Lundberg LG, Stone DK, Keeling DJ. Properties of three isoforms of the 116-kDa subunit of vacuolar H+-ATPase from a single vertebrate species. Cloning, gene expression and protein characterization of functionally distinct isoforms in Gallus gallus. Eur J Biochem. 2000;267:4115–4126. doi: 10.1046/j.1432-1327.2000.01445.x. [DOI] [PubMed] [Google Scholar]
- 26.Toyomura T, Oka T, Yamaguchi C, Wada Y, Futai M. Three subunit a isoforms of mouse vacuolar H(+)-ATPase. Preferential expression of the a3 isoform during osteoclast differentiation. J Biol Chem. 2000;275:8760–8765. doi: 10.1074/jbc.275.12.8760. [DOI] [PubMed] [Google Scholar]
- 27.Lee BS, Gluck SL, Holliday LS. Interaction between vacuolar H(+)-ATPase and microfilaments during osteoclast activation. J Biol Chem. 1999;274:29164–29171. doi: 10.1074/jbc.274.41.29164. [DOI] [PubMed] [Google Scholar]
- 28.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 29.Hofbauer LC, Cepok S, Hemmer B. Osteoprotegerin is highly expressed in the spinal cord and cerebrospinal fluid. Acta Neuropathol (Berl) 2004;107:575–577. doi: 10.1007/s00401-004-0854-y. [DOI] [PubMed] [Google Scholar]
- 30.Servet-Delprat C, Arnaud S, Jurdic P, Nataf S, Grasset MF, Soulas C, Domenget C, Destaing O, Rivollier A, Perret M, Dumontel C, Hanau D, Gilmore GL, Belin MF, Rabourdin-Combe C, Mouchiroud G. Flt3+ macrophage precursors commit sequentially to osteoclasts, dendritic cells and microglia. BMC Immunol. 2002;3:15. doi: 10.1186/1471-2172-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kartsogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK, Quinn JM, Niforas P, Ng KW, Martin TJ, Gillespie MT. Localization of RANKL (receptor activator of NF kappa B ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone. 1999;25:525–534. doi: 10.1016/s8756-3282(99)00214-8. [DOI] [PubMed] [Google Scholar]
- 32.Mokhtarian F, Huan CM, Roman C, Raine CS. Semliki Forest virus-induced demyelination and remyelination--involvement of B cells and anti-myelin antibodies. J Neuroimmunol. 2003;137:19–31. doi: 10.1016/s0165-5728(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 33.Compston A. Inflammation and the brain. Mol Chem Neuropathol. 1993;19:47–64. doi: 10.1007/BF03160168. [DOI] [PubMed] [Google Scholar]
- 34.Huang Z, Ha GK, Petitto JM. IL-15 and IL-15Ralpha gene deletion: Effects on T lymphocyte trafficking and the microglial and neuronal responses to facial nerve axotomy. Neurosci Lett. 2007;417:160–164. doi: 10.1016/j.neulet.2007.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alnaeeli M, Penninger JM, Teng YT. Immune interactions with CD4+ T cells promote the development of functional osteoclasts from murine CD11c+ dendritic cells. J Immunol. 2006;177:3314–3326. doi: 10.4049/jimmunol.177.5.3314. [DOI] [PubMed] [Google Scholar]
- 36.Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265:144–150. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]
- 37.Biernacki K, Prat A, Blain M, Antel JP. Regulation of Th1 and Th2 lymphocyte migration by human adult brain endothelial cells. J Neuropathol Exp Neurol. 2001;60:1127–1136. doi: 10.1093/jnen/60.12.1127. [DOI] [PubMed] [Google Scholar]
- 38.Archambault AS, Sim J, Gimenez MA, Russell JH. Defining antigen-dependent stages of T cell migration from the blood to the central nervous system parenchyma. Eur J Immunol. 2005;35:1076–1085. doi: 10.1002/eji.200425864. [DOI] [PubMed] [Google Scholar]
- 39.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuo J, Jiang J, Chen SH, Vergara S, Gong Y, Xue J, Huang H, Kaku M, Holliday LS. Actin Binding Activity of Subunit B of Vacuolar H(+)-ATPase Is Involved in Its Targeting to Ruffled Membranes of Osteoclasts. J Bone Miner Res. 2006;21:714–721. doi: 10.1359/jbmr.060201. [DOI] [PubMed] [Google Scholar]