Abstract
Background
Primary neuroendocrine carcinomas of the liver have rarely been reported in humans and domestic animals, but not in non-human primates.
Methods
We describe the morphologic and immunohistochemical features of a primary hepatic neuroendocrine carcinoma found in a 29-year-old female baboon.
Results and conclusions
The neoplasm was characterized by multiple solid nodules that were multifocally distributed in the liver. Metastases were not observed. Histologically, the neoplasm was composed of cords and nests of epithelial cells arranged in a neuroendocrine pattern, occasionally forming glandular and rosette-like structures. On immunohistochemical evaluation, the neoplastic cells were immunopositive for pancytokeratin, chromogranin A, neuron-specific endolase, and synaptophysin and were negative for vimentin, S100 protein, glucagon, and insulin.
Keywords: Endocrine, liver, non-human primate, tumor
Introduction
The neuroendocrine cell system is composed of the cell types that form endocrine glands and those diffusely distributed throughout the body, including skin, lungs, gastrointestinal tract, and biliary tract [4]. These were once called amine precursor uptake and decarboxylation (APUD) cells and are thought to originate from either the neural crest, migrating out of the ectodermal layer, or from the endodermal layer [9]. Neuroendocrine tumors arising from these cells vary from benign carcinoids to aggressive neuroendocrine carcinomas. In humans neuroendocrine tumors most commonly originate in the gastropancreatic and bronchopulmonary organs; only rarely is the liver involved.[3].
Only 44 primary hepatic neuroendocrine carcinomas have been reported in humans [3], and when liver metastases from other neoplastic sites are included, the reported cases do not exceed 125 [6]. Likewise, in domestic animals, this neoplasm has rarely been described [8].
Histologically, hepatic neuroendocrine carcinomas in humans are described as having cords, trabeculae, or glandular (rosette-like) patterns [8]. Immunohistochemically, they consistently express chromogranin A, neuron-specific enolase (NSE), and synaptophysin, and occasionally cytokeratin, somatostatin, serotonin, and other peptides [8].
In the recent and comprehensive reviews of spontaneous neoplasia in baboons by Cianciolo et al. [1, 2], neuroendocrine carcinomas were not reported in the liver or in other organs and systems. We describe the morphologic and immunohistochemical features of a primary hepatic neuroendocrine carcinoma in a 29-year-old female baboon (Papio sp.). To the best of our knowledge, this is the first description of a primary neuroendocrine carcinoma of the liver in a non-human primate.
Materials and methods
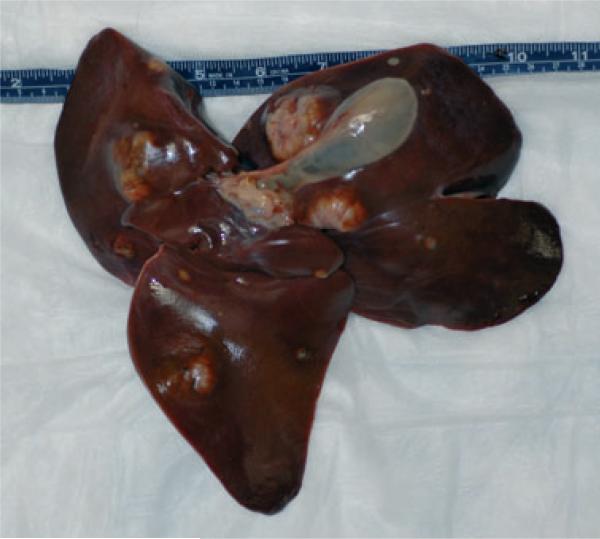
A 29-year-old, 16.27 kg, female baboon (Papio sp.) was selected for euthanasia (Euthasol, 3.5 ml i.v.; Virbac, Fort Worth, TX, USA) due to dark loose stools, and senescence and submitted for necropsy. On external examination, the animal was found to be in good body condition and adequately hydrated. Other findings included multiple pale-tan to white irregularly shaped firm nodules, up to 5 cm diameter, multifocally distributed in the hepatic parenchyma (Fig. 1) and a pale white, homogeneous nodule, 2 cm diameter, on the left body of the uterus, which blended with the uterine tissue on cut surface. Careful necropsy observation revealed no additional extrahepatic nodules.
Fig. 1.
Hepatic neuroendocrine carcinoma. Note the multifocal distribution of variably sized neoplastic nodules in the liver parenchyma.
Representative sections of the lung, tracheobronchial lymph node, spleen, heart, kidney, liver, gall bladder, thyroid glands, adrenal glands, axillary lymph node, stomach, duodenum, colon, mesenteric lymph node, pituitary gland, midbrain, ovary, urinary bladder, uterus, salivary gland, and thymus were fixed in 10% neutral buffered formalin, processed conventionally, embedded in paraffin, cut at 5 μm, and stained with hematoxylin and eosin.
Unstained sections of the neoplasm were cut at 5 μm, deparaffinized, and immunostained for pancytokeratin, NSE, synaptophysin, chromogranin A (1:1000; DakoCytomation, Carpinteria, CA, USA), vimentin, S100 protein, glucagon, and insulin. All immunohistochemistry except chromogranin A was performed by the Armed Forces Institute of Pathology (AFIP) immunohistochemistry laboratory personnel using the standard AFIP materials and methods.
Results
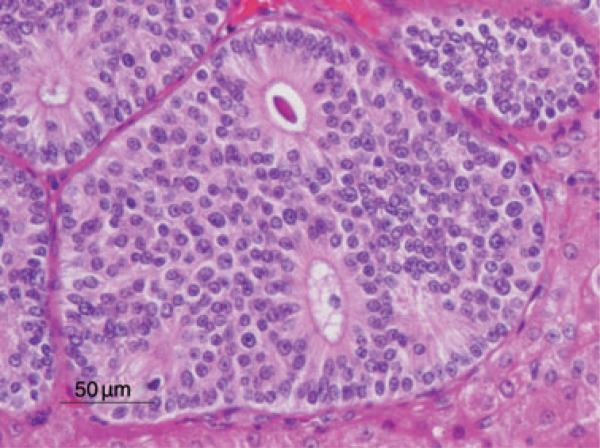
Examination of the liver architecture by light microscopy revealed multifocal effacement and replacement by a well-demarcated, unencapsulated, infiltrative, highly cellular neoplasm composed of nests, cords, and occasionally sheets of epithelial cells, often forming rosettes and pseudorosettes, supported by a fine to moderate fibrovascular stroma. The neoplastic cells were cuboidal to columnar with variably distinct cell borders and a moderate amount of eosinophilic, finely granular cytoplasm. The nuclei were central to basilar, round to oval, averaging 10–15 μm in diameter, with coarsely stippled chromatin. There was mild anisokaryosis and rarely large nuclei (40 μm in diameter). Mitoses were rare (<1 per 10 high-powered field, 40× magnification). Additional features included scattered necrosis and hemorrhage, and variable amounts of homogeneous, eosinophilic material filling the central lacunae of the rosette formations (Fig. 2). The adjacent portions of the non-affected liver parenchyma showed mild to moderate atrophy from neoplastic compression.
Fig. 2.
Hepatic neuroendocrine carcinoma. Note the islands of neoplastic cells forming rosettes occasionally containing homogeneous, eosinophilic material. HE stain. Bar = 50 μm.
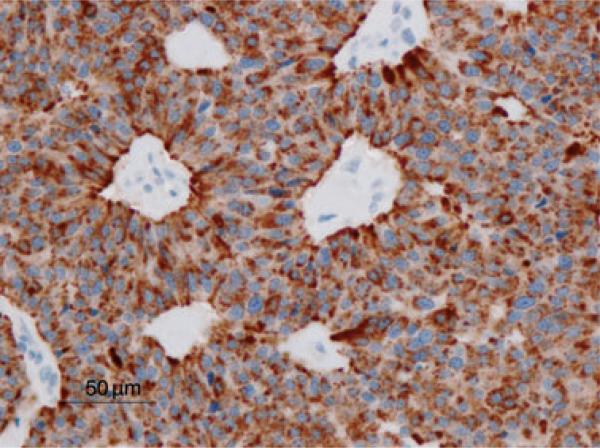
The neoplastic cells were reactive for pancytokeratin, chromogranin A (Fig. 3), NSE, and synaptophysin and were not reactive for vimentin, S100 protein, glucagon, or insulin. The nodule found in the uterus showed histologic characteristics consistent with adenomyosis. Adenomyosis is commonly encountered in baboons and was considered an unrelated finding.
Fig. 3.
Hepatic neuroendocrine carcinoma. Neoplastic cells are strongly immunoreactive for chromogranin A. Immunohistochemical staining for chromogranin A by the avidin-bioin-horseradish peroxidase (ABC) method. Bar = 50 μm.
Discussion
The morphologic features and the positive staining for the neuroendocrine markers led to the classification of this neoplasm as a neuroendocrine carcinoma. As hepatic neuroendocrine carcinomas in other species often present with multifocal to diffuse hepatic involvement [7, 8], and this neoplasm was not observed in any other tissues, the liver was considered to be the primary site of origin. In humans, this tumor has a slightly higher predilecton for females (1.4:1) [6]. The same trend has been reported in dogs but is reversed in the few cases reported in cats [7, 8]. Although this isolated case does not allow any epidemiologic consideration, the baboon was female. As in this case, neuroendocrine carcinomas in humans are consistently immunopositive for chromogranin A, NSE, and synaptophysin [8]. This is contrary to what has been observed in dogs and cats, where the expression of chromogranin A is inconsistent, and NSE and synaptophysin are considered better indicators [8].
Tumor cells are monoclonally derived from stem- or stem-like cells [10]; hence, the progenitor cell compartment of the liver has been addressed in the histogenesis of hepatic neuroendocrine and non-neuroendocrine carcinomas. The oval cell is considered to be the progeny of the hepatic stem cells and is bipotential in nature, giving raise to both hepatocytes and bile duct cells [5]. These cells are located in the terminal biliary ductules and canals of Hering, which represent the terminal branches of the biliary tree that connect the interhepatocytic bile canaliculi with the biliary ducts in the portal tracts. Oval cells express markers of both immature hepatocytes (α-fetoprotein) and bile duct cells (bile duct type cytokeratin) [9]. In addition, the hepatic progenitor cell (HPC) compartment has neuro/neuroendocrine features such as the expression of chromogranin A, neural cell adhesion molecule (NCAM), neurotrophin 4/5, neurotrophin receptor tyrosine kinase B, and parathyroid hormone-related peptide [5]. Roskams et al. [9] found that during the early stages of regeneration, bile duct epithelium displays neuroendocrine features including cytoplasmic dense core neuronsecretory granules and chromogranin A expression, while reactive bile ductules have been shown to express NSE [9]. Moreover, the presence of a peribiliary capillary plexus supports the hypothesis that biliary epithelium may exert an endocrine function [9]. The involvement of oval cells in liver carcinogenesis has been shown in a number of animal models in which the development of hepatocellular carcinomas is preceded by the activation of oval cells and the neoplastic cells express oval cell markers such as OV-6 and α-fetoprotein [5]. Thus, along with the evidence of neuroendocrine features expressed by the liver progenitor compartment, supports the hypothesis that neoplastic transformation in the progenitor cell compartment with further differentiation into the neuroendocrine lineage could explain the histogenic derivation of this neoplasm.
Acknowledgments
We thank Marie Silva, Michaelle Hohmann, Denise Trejo, Dr Andy Ambrus, and the personnel of the Armed Forces Institute of Pathology for pathology support. This research was funded in part by the base grant to the Southwest National Primate Research Center (National Institutes of Health/National Center for Research Resources grant P51 RR013986). The baboon was housed in facilities constructed with support from the Research Facilities Improvement Program grants C06 RR014578 and C06 RR015456.
References
- 1.Cianciolo RE, Butler SD, Eggers JS, Dick EJ, Jr, Leland M, De la Garza M, Brasky KM, Cummins LB, Hubbard GB. Spontaneous neoplasia in the baboon (Papio spp.). J Med Primatol. 2007;36:61–79. doi: 10.1111/j.1600-0684.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 2.Cianciolo RE, Hubbard GB. A review of spontaneous neoplasia in baboons (Papio spp.). J Med Primatol. 2005;34:51–66. doi: 10.1111/j.1600-0684.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaya G, Pasche C, Osterheld MC, Chaubert P, Fontolliet C. Primary neuroendocrine carcinoma of the liver: an autopsy case. Pathol Int. 2001;51:874–8. doi: 10.1046/j.1440-1827.2001.01295.x. [DOI] [PubMed] [Google Scholar]
- 4.Klöppel G. Tumor biology and histopathology of neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab. 2007;21:15–31. doi: 10.1016/j.beem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol. 2002;13:389–96. doi: 10.1016/s1084952102001258. [DOI] [PubMed] [Google Scholar]
- 6.Lingamfelter D, Hoffman L, Verma A, DePond A, Lankachandra K. Giant atypical carcinoid of the liver with vascular metastases and local sinusoidal invasion: a case report. J Med Case Reports. 2007;1:47. doi: 10.1186/1752-1947-1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patnaik AK, Lieberman PH, Erlandson RA, Antonescu C. Hepatobiliary neuroendocrine carcinoma in cats: a clinicopathologic, immunohistochemical, and ultrastructural study of 17 cases. Vet Pathol. 2005;42:331–7. doi: 10.1354/vp.42-3-331. [DOI] [PubMed] [Google Scholar]
- 8.Patnaik AK, Newman SJ, Scase T, Erlandson RA, Antonescu C, Craft D, Bergman PJ. Canine hepatic neuroendocrine carcinoma: an immunohistochemical and electron microscopic study. Vet Pathol. 2005;42:140–6. doi: 10.1354/vp.42-2-140. [DOI] [PubMed] [Google Scholar]
- 9.Roskams T, Cassiman D, De Vos R, Libbrecht L. Neuroregulation of the neuroendocrine compartment of the liver. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:910–23. doi: 10.1002/ar.a.20096. [DOI] [PubMed] [Google Scholar]
- 10.Trosko JE. The role of stem cells and gap junctional intercellular communication in carcinogenesis. J Biochem Mol Biol. 2003;36:43–8. doi: 10.5483/bmbrep.2003.36.1.043. [DOI] [PubMed] [Google Scholar]