Abstract
INTRODUCTION
The aim of this study was to review the management and outcome of patients with Boerhaave's syndrome in a specialist centre between 2000–2007.
PATIENTS AND METHODS
Patients were grouped according to time from symptoms to referral (early, < 24 h; late, > 24 h). The effects of referral time and management on outcomes (oesophageal leak, reoperation and mortality) were evaluated.
RESULTS
Of 21 patients (early 10; late 11), three were unfit for surgery. Of the remaining 18, immediate surgery was performed in 8/8 referred early and 6/10 referred late. Four patients referred late were treated conservatively. Oesophageal leak (78% versus 12.5%; P < 0.05) and mortality (40% versus 0%; P < 0.05) rates were higher in patients referred late. For patients referred late, mortality was higher in patients managed conservatively (75% versus 17%; not significant).
CONCLUSIONS
The best outcomes in Boerhaave's syndrome are associated with early referral and surgical management in a specialist centre. Surgery appears to be superior to conservative treatment for patients referred late.
Keywords: Oesophagus, Oesophageal perforation, Boerhaave's syndrome
Spontaneous oesophageal perforation (Boerhaave's syndrome) is rare and carries a high mortality even in specialist centres.1–6 The best outcomes are achieved if surgical repair is performed within 24 h of perforation.7 Diagnosis and referral to specialist centres is often delayed due to absence of physical signs, non-specific findings on chest X-ray and low index of suspicion.8–11 Management of patients who are diagnosed late remains unclear, in particular the choice between conservative treatment and surgery.1,12 The aim of this study was to assess the outcome of patients with Boerhaave's syndrome following either early or late referral to a tertiary unit for management.
Patients and Methods
Clinical assessment and diagnosis
A retrospective review was performed of 21 consecutive patients with Boerhaave's syndrome treated at a tertiary oesophagogastric specialist centre between 2000 and 2007. The following data were collected concerning initial presentation to the referring hospital: presenting symptoms; time from symptom onset to initial presentation (h); initial chest X-ray findings; method of diagnosis; and time from symptom onset to diagnosis and transfer to the specialist centre (days). Baseline clinical and laboratory data were also recorded at the time of arrival at the specialist centre, including premorbid status, vital signs and laboratory indices (full blood count, renal function, arterial blood gas analysis).
Principles of treatment
All patients were assessed in the specialist centre by an experienced oesophagogastric surgeon. The principles of treatment were to control persistent oesophageal leakage and to drain mediastinal and/or pleural sepsis aggressively.3 Patients who were unfit for definitive surgery (n = 3) were managed non-operatively: (i) nil-by-mouth; (ii) wide-bore intercostal chest drain; (iii) intravenous antibiotics; (iv) high dependency/intensive care support; (v) gastric decompression (nasogastric tube); and (vi) nutritional support (parenteral). Patients who were fit for surgery (n = 18) were managed according to timing of referral to the specialist centre and clinical condition on arrival. Patients referred within 24 h of symptom onset (early group, n = 8) underwent immediate surgical repair if technically possible. Patients referred later than 24 h after symptom onset (late group, n = 10) were considered for either active conservative management (n = 4) or definitive surgery (n = 6). Patients in the late group who were unstable on arrival at the specialist centre underwent immediate operation. Since 2004, a more aggressive surgical policy has been adopted for stable patients in the late group, who were considered for immediate surgery rather than conservative management, according to clinical condition and surgeon preference.
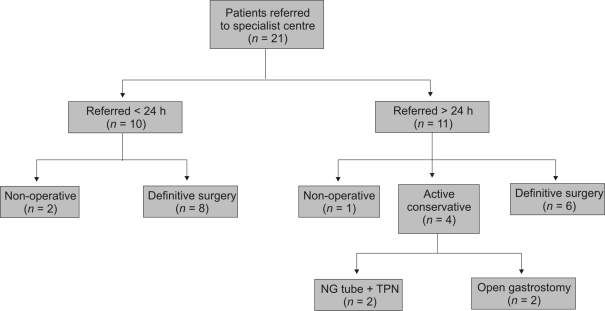
The principles of active conservative management were identical to those of non-operative management. However, patients who were actively managed conservatively were considered for delayed surgical intervention in the event of clinical deterioration, whereas those in the non-operative group were not. In addition, gastric decompression was achieved by open surgical gastrostomy rather than by nasogastric tube in two patients who were managed conservatively. A jejunostomy tube was also inserted in these patients at the time of laparotomy to allow enteral nutrition. Patients in the conservative group underwent delayed surgery if they exhibited signs of systemic sepsis, general deterioration or persistent oesophageal leakage. Patient subgroups are summarised in Figure 1.
Figure 1.
Overview of patient subgroups. NG, nasogastric; TPN, total parenteral nutrition.
Surgical techniques
The principal objectives in patients undergoing primary surgical management were: (i) pleural and mediastinal decontamination; (ii) debridement or resection of devitalised oesophageal tissue; (iii) primary repair of perforation (if possible); (iv) drainage of pleural and mediastinal spaces; (v) gastric decompression; and (vi) distal enteral feeding access. The operative approach was determined by the location of oesophageal perforation, patient fitness and surgeon preference. Transthoracic approaches were either via posterolateral thoracotomy (left or right) or a left thoraco-abdominal incision. In addition, all patients underwent laparotomy for insertion of decompressing gastrostomy and feeding jejunostomy. Since 2004, the senior author (AB) has adopted a transhiatal technique to achieve the surgical objectives in five patients,13 in an attempt to avoid the potential morbidity associated with thoracotomy.
The operative procedure was influenced by the patient's general condition, suitability of the oesophagus for primary repair and degree of intrathoracic contamination, and included: (i) primary repair; (ii) repair over T-tube; (iii) debridement and drainage; and (iv) oesophageal exclusion (cervical oesophagostomy, distal oesophageal transection ± oesophagectomy). Large-bore apical and basal intercostal chest drains were inserted in all patients at the initial operation. A transhiatal drain was inserted in patients undergoing a pure transhiatal approach without thoracotomy.
Outcomes and statistical analysis
The effects of (i) time of referral (early versus late) and (ii) management strategy (conservative versus surgery) on outcomes (oesophageal leak, re-operation and survival) were evaluated. Oesophageal leaks were confirmed by water-soluble swallow in all patients undergoing primary repair. In patients who did not undergo primary repair, a leak was considered to be persistent if a second intervention was necessary (percutaneous drainage or surgery).
Results of continuous data are expressed as mean ± SD unless otherwise stated. Proportions were compared by means of the Fisher exact test or chi-squared test with Yates' correction, and continuous variables were compared by means of unpaired t-tests. A two-tailed P-value of < 0.05 was considered statistically significant.
Results
Clinical presentation
Clinical features at presentation were vomiting (100%), chest pain (90%) and subcutaneous emphysema (38%). The median time from onset of symptoms to presentation was 5 h (range, 0–336 h). Seven patients (33%) presented later than 12 h of onset of symptoms. Chest X-ray revealed pneumomediastinum in nine patients (43%), unilateral pleural effusion in 15 (71%) and bilateral pleural effusions in three (14%). Confirmation of diagnosis and side of perforation was obtained by water-soluble contrast swallow in 12 patients (57%), and by CT scan with oral contrast in nine patients (43%). Of 18 patients who underwent surgery, 17 perforations were in the lower oesophagus.
Ten patients (48%) were transferred to the specialist centre within 24 h of symptom onset (early group), and 11 patients were transferred after 24 h (late group; median, 3 days; range, 2–60 days). Delay in transfer of patients in the late group was due to either delayed presentation in six (55%) or delayed diagnosis in five (45%). Patients in the early group were more likely to have subcutaneous emphysema than patients in the late group (60% versus 18%; P = 0.08, Fisher exact test). Baseline clinical and laboratory values were similar between the two groups (Table 1).
Table 1.
Demographics and baseline clinical data
| Early group (< 24 h) (n = 10) | Late group (> 24 h) (n = 11) | |
|---|---|---|
| Mean age (years) | 68 ± 10 | 64 ± 11 |
| Sex (F:M) | 5:5 | 3:8 |
| Time from symptoms to hospital admission (h) (median, range) | 4 (1–14) | 6 (2–336) |
| Time from symptoms to transfer to specialist centre (days) (median, range) | 1 (0–1) | 3 (2–60) |
| Subcutaneous emphysema | 60% | 18% |
| Pneumomediastinum on chest X-ray | 50% | 36% |
| Systolic blood pressure (mmHg) | 115 ± 27 | 119 ± 19 |
| Heart rate (bpm) | 93 ± 17 | 95 ± 14 |
| Respiratory rate (per min) | 29 ± 8 | 24 ± 6 |
| Temperature (°C) | 37.0 ± 0.9 | 36.9 ± 0.8 |
| Haemoglobin (g/dl) | 14.0 ± 1.6 | 13 ± 2.9 |
| Leukocyte count (×109/dl) | 9.5 ± 4.1 | 14.5 ± 10.1 |
| Urea (mmol/l) | 7.7 ± 1.9 | 9.3 ± 4.7 |
| Creatinine (μmol/l) | 103 ± 24 | 125 ± 73 |
| Arterial pH | 7.34 ± 0.03 | 7.36 ± 0.1 |
| Base deficit | 3.8 ± 2.2 | 4.8 ± 4.2 |
Management and outcome
Three patients were deemed unfit for surgery and were managed non-operatively. Of these, two patients were moribund on arrival at the specialist centre and died within 24 h of perforation. The other patient, a 57-year-old woman with significant co-morbidity, presented later than 24 h and died from overwhelming sepsis after 3 weeks.
Of the remaining 18 patients who were fit for surgery, all eight patients referred within 24 h of perforation (early group) underwent immediate surgery (thoracotomy 6; transhiatal 2) after being stabilised on HDU or ITU, irrespective of time of arrival. Primary repair of the perforation was performed in all cases, including repair over a T-tube in one patient. Postoperatively, one patient developed a persistent oesophageal leak, which was successfully managed by percutaneous drainage on day 19. Three patients in the early group underwent further surgery for sepsis (2) and bleeding (1) as summarised in Table 2. The median length of hospital stay was 52 days (range, 10–120 days). There were no in-hospital deaths in this group.
Table 2.
Management and outcome of patients with Boerhaave's syndrome
| Case | Initial therapy | Year | Operative approach & technique | Leaka | Re-operation | Cause of death (days postoperatively) | |
|---|---|---|---|---|---|---|---|
| Patients unfit for surgery (n = 3) | |||||||
| N1 | Non-op | 2001 | – | – | + | Sepsis (1) | |
| N2 | Non-op | 2001 | – | – | + | Sepsis (22) | |
| N3 | Non-op | 2002 | – | – | + | Sepsis (1) | |
| Patients fit for surgery (n = 18) | |||||||
| Early group (< 24 h) | |||||||
| E1 | Surgery | 2001 | Thoracotomy | Repair | + | ||
| E2 | Surgery | 2001 | Thoracotomy | Repair | Laparotomy (bleeding) | ||
| E3 | Surgery | 2001 | Thoracotomy | Repair | bVATS → rib resection | ||
| E4 | Surgery | 2003 | Thoracotomy | Repair | |||
| E5 | Surgery | 2005 | Transhiatal | Repair | Transthoracic drainage | ||
| E6 | Surgery | 2006 | Transhiatal | Repair | |||
| E7 | Surgery | 2007 | Thoracotomy | Repair | |||
| E8 | Surgery | 2007 | Thoracotomy | T-tube | |||
| Late group (> 24 h) | |||||||
| L1 | Surgery | 2002 | Thoracotomy | T-tube | Pneumonia (19) | ||
| L2 | Surgery | 2005 | Thoracotomy | Resection | N/A | ||
| L3 | Surgery | 2005 | Transhiatal | Repair | |||
| L4 | Surgery | 2006 | Thoracotomy | T-tube | + | ||
| L5 | Surgery | 2007 | Thoracotomy | Repair | + | Exclusion | |
| L6 | Surgery | 2007 | Transhiatal | Repair | + | bVATS + debridement | |
| L7 | Conservativec | 2003 | Thoracotomy | Rib resection | + | Exclusion | Sepsis (99) |
| L8 | Conservativec | 2003 | – | + | dCVA (131) | ||
| L9 | Conservative | 2005 | Transhiatal | Drainage | + | Exclusion | |
| L10 | Conservative | 2007 | Thoracotomy | T-tube | + | Exclusion | Sepsis (72) |
In patients who underwent primary repair (including T-tube repair), diagnosis of leak was made by water-soluble contrast swallow. In patients who underwent a drainage procedure only, a leak was considered to be persistent if a second intervention (percutaneous drainage or surgery) was necessary.
VATS, video-assisted thoracoscopic surgery.
Two patients (L7 and L8) who were managed conservatively underwent laparotomy and insertion of decompressive gastrostomy and feeding jejunostomy as part of active conservative management.
Patient L8 developed an oesophago-pleuro-cutaneous fistula that was managed conservatively. However, she died due to hypoxic brain damage following a prolonged respiratory arrest.
Four of 10 patients in the late group were treated by active conservative management. Three out of four patients deteriorated and required delayed surgery after a median of 60 days (range, 4–60days; Table 2). Postoperatively, all three patients developed sepsis due to persistent leaks and underwent oesophageal exclusion. The remaining patient had a controlled oesophageal leak that developed into an oesophago-pleuro-cutaneous fistula. However, she died from hypoxic brain damage after a respiratory arrest.
Six of 10 patients in the late group underwent immediate surgery (Table 2). One patient (L2) had an extensive oesophageal injury, and underwent oesophagectomy with cervical oesophagostomy as a primary procedure. Five patients in this group had primary repair of the perforation either with or without a T-tube. Persistent leaks were treated by percutaneous drainage (1), thoracoscopic debridement (1) and oesophageal exclusion (1) after a median of 27 days (range, 17–28 days). The median length of hospital stay in this group was 40 days (range, 10–60 days). There was only one death in this group (patient L1), who died from pneumonia 19 days after thoracotomy. Table 3 compares the effects of time of referral, operative approach and time period on postoperative outcome in surgical candidates. Of patients referred later than 24 h, persistent oesophageal leak (100% versus 60%; not significant), re-operation (75% versus 33%; not significant) and mortality (75% versus 17%; not significant) rates were higher for patients initially managed conservatively compared to those managed surgically, but due to small numbers, this did not reach statistical significance.
Table 3.
Outcome of patients undergoing surgical intervention (n = 18)
| Persistent oesophageal leaka | Re-operation | Hospital mortality | |
|---|---|---|---|
| Timing of referral | |||
| < 24 h (early group) | 1/8 (12.5%)b | 2/8 (25%) | 0/8 (0%)b |
| > 24 h (late group) | 7/9 (78%) | 5/10 (50%) | 4/10 (40%) |
| Surgical approach | |||
| Transhiatal | 4/7 (57%) | 4/7 (57%) | 2/7 (29%) |
| Transthoracic | 4/10 (40%) | 3/11 (27%) | 2/11 (18%) |
| Time period | |||
| 2000–2003 | 3/7 (43%) | 2/7 (29%) | 3/7 (43%) |
| 2004–2007 | 5/10 (50%) | 5/11 (45%) | 1/11 (9%) |
An oesophageal leak was considered to be persistent if it failed to heal and required re-intervention (percutaneous drainage or surgery). Excludes patient L4 (see Table 2) who underwent primary oesophagectomy.
P < 0.05, Pearson chi-squared test with Yate's correction.
Discussion
Boerhaave's syndrome is characterised by oesophageal rupture due to severe vomiting against a closed glottis, which leads to mediastinitis, and is invariably fatal if left untreated.14 Three patients in our series were profoundly septic by the time of transfer to a specialist centre and were unsalvageable. In the majority of cases, however, surgical repair is possible and if undertaken early (within 24 h of symptom onset) can be associated with excellent survival (100% in our series). For patients referred later than 24 h, morbidity and mortality is significant. Management of late cases is less well defined and a conservative approach has been advocated for stable patients in this group.7,12 However, three out of four patients treated conservatively in this series deteriorated and required delayed surgery (Table 2). Subsequent re-operation and mortality rates in this subgroup were considerable. By contrast, only one patient (17%) died in the late group who underwent immediate surgery. Although not statistically significant due to small numbers, this difference is likely to be clinically relevant. Indeed, adoption of an aggressive surgical policy for late cases during the last 4 years has resulted in a reduction in mortality from 100% to 14% in this subgroup, and must be the preferred approach for the vast majority of cases irrespective of the patient's clinical condition on arrival. Conservative treatment should be reserved for a highly selected group of patients with minimal mediastinal or thoracic contamination, and must include large-bore drainage of the thoracic cavity. Patients should be closely monitored and undergo surgical intervention at the earliest sign of clinical deterioration.
Our data show that delayed referral to a specialist centre occurs in over 50% of cases and is due to delays in either clinical presentation or diagnosis.2 Since subcutaneous emphysema and pneumomediastinum were absent in over 50% of patients in this series, physical examination and plain radiography alone are insufficient to make the diagnosis.9,10 An urgent water-soluble contrast swallow and/or CT scan with oral contrast are essential in any patient presenting with severe vomiting followed by chest pain in whom a diagnosis of Boerhaave's syndrome is being considered. Oral contrast must be used routinely for CT scans in these patients to differentiate Boerhaave's syndrome from spontaneous pneumomediastinum, a rare condition with a benign course.15
Due to the relative rarity of Boerhaave's syndrome, patients should be transferred to a regional specialist centre with expertise in the management of complex oesophagogastric cases. An experienced surgeon should determine the timing of surgery, the operative approach and the most appropriate technique. Pre-operative, peri-operative and postoperative management of these patients requires input from experienced intensivists, anaesthetists and interventional radiologists in order to achieve zero mortality.
Based on our data, we feel that all patients presenting with Boerhaave's syndrome should be considered for early surgical intervention, ideally within 24 h of clinical presentation.1,16,17 In the absence of any prospective data, the operative approach should be determined by the patient's general condition and surgeon's preference. Although a transthoracic approach is considered to be the standard,15 acceptable results may be achieved with a transhiatal approach, which avoids the potential morbidity of a thoracotomy.18 In this series of unselected cases, respiratory complications and ITU stay were similar for both approaches (Table 4). Re-operation was more common in patients in the transhiatal group, but this did not translate into increased mortality compared to the transthoracic group. Irrespective of the approach used, debridement and lavage of the mediastinal and pleural cavities is an essential component of the surgical procedure; if this cannot be satisfactorily completed by a transhiatal approach, the surgeon should proceed to a thoracotomy without hesitation. Video-assisted thoracoscopy may be an alternative method to allow inspection and decontamination of the thoracic cavity without the morbidity of a thoracotomy incision, but experience with this technique in the management of oesophageal perforation is limited.
Table 4.
Comparison between transthoracic and transabdominal approaches
| Transthoracic (n = 12) | Transhiatal (n = 5) | |
|---|---|---|
| Operation within 24 h of perforation | 6 (50%) | 2 (40%) |
| Repair of perforation | 10 (83%) | 4 (80%) |
| Respiratory complications | 3 (25%) | 1 (20%) |
| Re-operation | 4 (33%) | 3 (60%) |
| Median length of hospital stay (days) (range) | 56 (10–99) | 49 (31–90) |
| Median length of ITU stay (days) (range) | 7 (1–58) | 4 (0–90) |
| Hospital mortality | 3 (25%) | 0 (0%) |
A variety of techniques were employed in our centre to deal with the oesophageal perforation. However, primary repair was the preferred technique whenever possible. Based on our own experience, it is usually necessary to open the muscle layer to define the proximal extent of the mucosal tear. If the margins of the perforation are viable and will hold sutures, an accurate mucosal repair should be performed with 3/0 absorbable sutures (Vicryl, Ethicon, UK). Repair of the muscle layer is optional. Repair over a T-tube may be useful for late cases to allow a controlled oesophago-pleuro-cutaneous fistula to develop.19 In late cases, oesophageal tissue may be non-viable and should be debrided. Primary suture repair in these cases may be attempted, accepting that the postoperative leak rate will be high.11 In all cases, whether or not repair is achieved, insertion of at least two wide-bore drains into the pleural and mediastinal spaces is essential. A gastrostomy provides excellent decompression of the stomach and obviates the requirement for long-term nasogastric tube placement.7 A feeding jejunostomy allows early enteral nutrition, and avoids the potential complications of parenteral nutrition.
Postoperative management consists of broad-spectrum antibiotics, nil orally and enteral nutrition via the jejunostomy tube. The gastrostomy is left on free drainage to minimise reflux of gastric contents into the oesophagus. A water-soluble contrast swallow is performed after 5–7 days to exclude a leak prior to recommencing oral intake. Postoperatively, all patients are monitored closely for signs of sepsis, and undergo ultrasonography and/or CT to guide drainage of any new or persistent collections. Intrathoracic collections are drained percutaneously, if possible.
Inadequate radiological drainage and/or systemic deterioration are indications for surgery, particularly in the presence of a persistent leak. Surgical options include thoracoscopic drainage, thoracotomy ± rib resection, or oesophageal exclusion. Oesophageal exclusion (cervical oesophagostomy, distal oesophageal transection, decompressive gastrostomy and feeding jejunstomy) is a last resort in patients with significant leaks associated with systemic sepsis, and prevents on-going leakage of salivary and gastric secretions into the thoracic cavity. Two patients in our series died despite oesophageal exclusion, and it could be argued that this technique should be employed earlier in the clinical course before the effects of mediastinitis become irreversible. Delayed oesophageal reconstruction in these patients using either colonic or small bowel conduits is associated with an acceptable quality of life in the long term.20
Conclusions
The best outcome in Boerhaave's syndrome is associated with early referral and surgical repair in a specialist centre. In patients referred later, conservative management appears to have a very limited role, and this group should also undergo surgical drainage and attempted repair as early as possible. Postoperatively, thoracic sepsis and persistent oesophageal leaks require aggressive multimodal treatment, including percutaneous drainage and re-operation, if necessary.
References
- 1.Jougon J, McBride T, Delcambre F, Minniti A, Velly JF. Primary oesophageal repair for Boerhaave's syndrome whatever the free interval between perforation and treatment. Eur J Cardiothorac Surg. 2004;25:475–9. doi: 10.1016/j.ejcts.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence DR, Ohri SK, Moxon RE, Townsend ER, Fountain SW. Primary oesophageal repair for Boerhaave's syndrome. Ann Thorac Surg. 1999;67:818–20. doi: 10.1016/s0003-4975(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 3.Hafer G, Haunhorst WH, Stallkamp B. [Atraumatic rupture of the oesophagus (Boerhaave syndrome)] Zentralbl Chir. 1990;115:729–35. [PubMed] [Google Scholar]
- 4.Pate JW, Walker WA, Cole FH, Jr, Owen EW, Johnson WH. Spontaneous rupture of the oesophagus: a 30-year experience. Ann Thorac Surg. 1989;47:689–92. doi: 10.1016/0003-4975(89)90119-7. [DOI] [PubMed] [Google Scholar]
- 5.Nesbitt JC, Sawyers JL. Surgical management of oesophageal perforation. Am Surg. 1987;53:183–91. [PubMed] [Google Scholar]
- 6.Janjua KJ. Boerhaave's syndrome. Postgrad Med J. 1997;73:265–70. doi: 10.1136/pgmj.73.859.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan AZ, Strauss D, Mason RC. Boerhaave's syndrome: diagnosis and surgical management. Surgeon. 2007;5:39–44. doi: 10.1016/s1479-666x(07)80110-7. [DOI] [PubMed] [Google Scholar]
- 8.Haynes DE, Haynes BE, Yong YV. Oesophageal rupture complicating Heimlich maneuver. Am J Emerg Med. 1984;2:507–9. doi: 10.1016/0735-6757(84)90075-5. [DOI] [PubMed] [Google Scholar]
- 9.Hegenbarth R, Birkenfeld P, Beyer R. [Roentgen findings in spontaneous oesophageal perforation (Boerhaave syndrome)] Aktuelle Radiol. 1994;4:337–8. [PubMed] [Google Scholar]
- 10.Bladergroen MR, Lowe JE, Postlethwait RW. Diagnosis and recommended management of oesophageal perforation and rupture. Ann Thorac Surg. 1986;42:235–9. doi: 10.1016/s0003-4975(10)62725-7. [DOI] [PubMed] [Google Scholar]
- 11.Skinner DB, Little AG, DeMeester TR. Management of oesophageal perforation. Am J Surg. 1980;139:760–4. doi: 10.1016/0002-9610(80)90379-7. [DOI] [PubMed] [Google Scholar]
- 12.Vogel SB, Rout WR, Martin TD, Abbitt PL. Oesophageal perforation in adults: aggressive, conservative treatment lowers morbidity and mortality. Ann Surg. 2005;241:1016–21. doi: 10.1097/01.sla.0000164183.91898.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan AZ, Forshaw MJ, Davies AR, Youngstein T, Mason RC, Botha AJ. Transabdominal approach for management of Boerhaave's syndrome. Am Surg. 2007;73:511–3. [PubMed] [Google Scholar]
- 14.Vial CM, Whyte RI. Boerhaave's syndrome: diagnosis and treatment. Surg Clin North Am. 2005;85:515–24. doi: 10.1016/j.suc.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Langwieler TE, Steffani KD, Bogoevski DP, Mann O, Izbicki JR. Spontaneous pneumomediastinum. Ann Thorac Surg. 2004;78:711–3. doi: 10.1016/j.athoracsur.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Attar S, Hankins JR, Suter CM, Coughlin TR, Sequeira A, McLaughlin JS. Oesophageal perforation: a therapeutic challenge. Ann Thorac Surg. 1990;50:45–9. doi: 10.1016/0003-4975(90)90082-h. [DOI] [PubMed] [Google Scholar]
- 17.Richardson JD. Management of oesophageal perforations: the value of aggressive surgical treatment. Am J Surg. 2005;190:161–5. doi: 10.1016/j.amjsurg.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Whyte RI, Iannettoni MD, Orringer MB. Intrathoracic oesophageal perforation. The merit of primary repair. J Thorac Cardiovasc Surg. 1995;109:140–4. doi: 10.1016/S0022-5223(95)70429-9. [DOI] [PubMed] [Google Scholar]
- 19.Shenfine J, Dresner SM, Vishwanath Y, Hayes N, Griffin SM. Management of spontaneous rupture of the oesophagus. Br J Surg. 2000;87:362–73. doi: 10.1046/j.1365-2168.2000.01383-6.x. [DOI] [PubMed] [Google Scholar]
- 20.Varghese D, Patel H, Waters R, Dickson GH. Quality-of-life study on four patients who underwent oesophageal resection and delayed reconstruction for Boerhaave's syndrome. Dis Oesophagus. 2000;13:314–6. doi: 10.1046/j.1442-2050.2000.00138.x. [DOI] [PubMed] [Google Scholar]