Abstract
The role of CTLA-4 in Regulatory T cell (Treg) function is not well understood. We have examined the role of CTLA-4 and its relationship with the transcription factor FoxP3 using a model of Treg induction in human peripheral blood. Activation of human CD4+CD25- T cells resulted in the appearance of a de novo population of FoxP3-expressing cells within 48h. These cells expressed high levels of CTLA-4 and cell sorting on expression of CTLA-4 strongly enriched for FoxP3+ expressing cells with suppressive function. Culture in IL-2 alone also generated cells with suppressive capacity which also correlated with the appearance of CTLA-4. To directly test the role of CTLA-4, we transfected resting human T cells with CTLA-4, and found that this conferred suppression, similar to that of natural Treg, even though these did not express FoxP3. Furthermore, transfection of FoxP3 did not induce CTLA-4 and these cells were not suppressive. By separating the expression of CTLA-4 and FoxP3, our data show that FoxP3 expression alone is insufficient to upregulate CTLA-4, however, activation of CD4+ CD25- T cells can induce both FoxP3 and CTLA-4 in a sub-population of T cells which are capable of suppression. These data suggest that the acquisition of suppressive behaviour by activated CD4+ CD25- T cells requires the expression of CTLA-4, a feature which is appears to be facilitated by, but is not dependent on, expression of FoxP3.
Keywords: CTLA-4, FoxP3, T cells, differentiation, suppression
Introduction
Over the last decade, CD4+ CD25+ regulatory T cells (Treg) have emerged as major regulators of immunity(1, 2). Accordingly, in situations where Treg are depleted or lacking, autoimmune disease is observed. Recently, the transcription factor FoxP3 has been identified as a master regulator of Treg(3, 4) and accordingly, scurfy mice which lack FoxP3 expression, suffer from lethal autoimmunity due to the absence of Treg(5). Similarly humans lacking FoxP3 suffer from a severe autoimmune syndrome known as IPEX(6). Thus FoxP3 expression has become widely recognised as the hallmark of regulatory T cells. Whilst FoxP3 expression was initially thought to decisively identify Treg, recent evidence particularly in humans, suggests that FoxP3 can be induced upon T cell activation (7, 8).
Activated T cells and Treg share expression of a number of proteins, including CD25, GITR and CTLA-4. In particular, the role of CTLA-4 in Treg function has been an area of some debate. It is clear that CTLA-4-deficient mice, which suffer profound autoimmunity(9), possess Foxp3+ Treg(10), however, it is not certain that these Treg function normally(11, 12). Indeed, studies have suggested that suppression mediated by CTLA-4-deficient Treg may involve cytokines such as TGFβ or IL-10. In contrast, in wild-type Treg a role for CTLA-4 in suppressive function is indicated(10, 12-15). Thus, the regulation of both FoxP3 and CTLA-4 expression is of considerable interest and recently, it has been proposed that FoxP3 may be a direct regulator of the CTLA-4 promoter via an interaction with NF-AT(16). However, since CTLA-4 is normally expressed by all activated T cells, the majority of which do express Foxp3, it appears that FoxP3 is not an obligatory requirement for CTLA-4 expression.
The generation of “adaptive” or “inducible” Treg cells derived from mature CD25- T cells is an area of intense interest and therapeutic potential. In particular, such adaptive Treg have been shown to be induced via the upregulation of FoxP3 in the presence of cytokines, particularly TGFβ, (17-21) and these appear to be functional in mice. Interestingly, during human T cell activation, induction of FoxP3 is readily observed following stimulation of CD4+ CD25- T cells in vitro without the overt addition of cytokines. This has raised questions as to whether induced FoxP3+ human T cells are indeed competent Treg and whether FoxP3 expression is a reliable marker of Treg. Some studies have indicated that expression of FoxP3 is insufficient to generate Treg(8, 21, 22) whilst others support the opposite conclusion(7, 23). However, addressing this issue in humans is generally hampered by the fact that it is not possible to isolate FoxP3+ cells in order to test their function due to the lack of useful cell surface markers. It therefore remains unclear whether FoxP3 positive cells induced following T cell activation are functional or not.
In this study we have analysed the induction of FoxP3 and its functional relationship with CTLA-4 in human T cells. We observed that stimulation of CD4+ CD25- T cells in vitro resulted in the appearance of a discrete population of FoxP3+ T cells which occurred prior to T cell division. These cells also expressed CTLA-4 and there was a strong correlation between the level of CTLA-4 and FoxP3 expression. Interestingly, we also observed that treatment of CD25- T cells with interleukin-2 alone could upregulate FoxP3 in the absence of CTLA-4 but that these cells were not suppressive. In contrast, sorting of activated CTLA-4+ T cells strongly enriched for FoxP3 expression and these cells were suppressive in functional assays. To determine whether CTLA-4 was capable of suppression we transfected CTLA-4 into CD25- FoxP3- T cells. This did not induce FoxP3 but could confer suppressive activity. Conversely, expression of FoxP3 alone did not upregulate CTLA-4 and these cells were not suppressive. Taken together these data provide evidence that a bona fide population of CTLA-4+ FoxP3+ Treg is induced upon human T cell activation which are regulatory and that CTLA-4, but not FoxP3, expression is critical for suppressive function.
Material and methods
Purification of T cells
Human CD4+CD25-T and CD4+CD25+T cells were purified by either by cell sorting or using specific anti-CD25-microbeads. PBMC were isolated from fresh buffy coats (provided by national blood transfusion services, Birmingham, U.K) using Ficoll-paque density centrifugation. CD4+T cells were isolated by incubating PBMC with human CD4+T cell-enrichment cocktail according to the manufacture’s instruction (Stemsep). To purify CD25+ T cells CD4+ cells were incubated with anti-CD25-microbeads (Miltenyi Biotech) at 4°C for 30 minutes. CD4+CD25- T cells which did not bind to the column, were collected from the flow-through and washed before use. CD4+CD25+T cells were subsequently retrieved from the column. For cell sorting, CD4+T cells were double stained using FITC conjugated anti-CD4 and PE-Cy5 conjugated anti-CD25 and CD4+CD25-T and CD4+CD25+T cells sorted on a Mo-Flo cytometer (DakoCytomation).Where cells were sorted for CTLA-4 expression, stimulated T cells were labelled for 1h at 37°C with anti-CTLA-4 -PE, placed on ice and stained for 30min with CD25 APC. Cells were sorted on a Mo-Flo cytometer (DakoCytomation) based on the expression of CTLA-4 and CD25.
Flow cytometry
CD25-PE-CY5, CD69-FITC, CTLA4-PE, and CTLA-4-APC were purchased from Pharmingen. Anti-Foxp3-PE (clone PCH101) was from eBioscience. The 206D-alexa 488 FoxP3 antibody was purchased from Biolegend. Cells were stained for total CTLA-4 and using anti-CTLA4 on fixed and permeabilised cells and recycling CTLA-4 in live cells at 37°C. Cells were acquired on a BD FACScalibur and analysed using FlowJo software
T cell stimulation
Purified CD25- T cells were stimulated with anti-CD3/anti-CD28 coupled dynabeads (Dynal) or mature DCs (10:1 or 100:1 T cells :DCs). DCs were grown from monocytes using GM-CSF and IL-4 as previously described (15). For PPD stimulation, autologous DC were generated as above and 5ug/ml of PPD added 6h prior to addition of CD4+CD25-T cells. Cells were cultured for 5 days and pulsed with 1uCi/well of 3[H] thymidine during the last 16 hours of culture and incorporation measured by scintillation counting. In some experiments CD25- T cells were stimulated using CD80 or CD86 transfectants plus anti-CD3 (1μg/ml) as previously described (24) and analysed by FACS at the time points indicated. For IL-2 treatment, CD4+CD25-T cells were cultured in RPMI 1640 medium containing IL-2 (10ng/ml)for the times shown. Fresh IL-2 was added to the culture every 2 days.
Suppression assays
Functional suppression assays were performed using an MLR where induced Treg (1× 105) were co-cultured with responder T cells at the ratio of 1:1in the presence of monocyte derived dendritic cells. Blocking anti-CTLA-4 antibody(BNI3-Pharmingen) was used at 10μg/ml where indicated. For time course experiments, IL-2-treated CD4+CD25-T cells were harvested at each time point and frozen in 40% FCS 10% DMSO 50%RPMI and stored in liquid nitrogen. At the end of the IL-2 culture all samples were thawed on the same day and added to the 96-well containing responder T cells and DCs to measure inhibition. Assays were incubated for a further 5 days, and during the last 16 hours 3H thymidine was added at 1uCi/well. 3H thymidine incorporation was measured by scintillation counting, and proliferative responses were expressed as the mean of triplicate wells ± SD. Results shown are representative of a minimum of three experiments performed.
PCR analysis of gene expression
Foxp3 expression was measured by RT-PCR and real-time RT-PCR. Total RNA extracted from purified CD4+CD25-T cells treated with IL2 with Trizol reagent (Invitrogen) and first-strand cDNAs were synthesized by reverse transcription (superscript ™ first-strand synthesis system for RT-PCR kit, Invitrogen). Quantitative mRNA expression was measured by real-time PCR with ABI/PRISM 7700 sequence detection system (PE Applied Biosystem) and the TaqMan mastermix kit with β -actin mRNA was used as internal control. The primers and the internal fluorescence TaqMan probes were designed as follows:
| Foxp3 F | 5′-GAGAAGCTGAGTGCCATGCA-3′ and |
| FoxP3rev | 5′-AGGAGCCCTTGTCGGATGAT-3′ |
| probe: 5′-FAM-ATGGCACTGACCAAGGCTTCATCTGTG-eclipse dark-3′; | |
| β-actin primers, | 5′-CCTGGCACCCAGCACAAT-3′ |
| and | 5′-GCCGATCCACACGGAGTACT-3′ and the |
| β-actin probe, 5′-VIC-ATCAAGATCATTGCTCCTCCTGAGCGC-Yahima yellow-3′. | |
The program used for amplification was 45 cycles of 15 sec at 95°C and 1 min at 60°C.
CFSE labelling
To determine cell division, T cells were washed twice with PBS and incubated with 2.5μM of CFSE for 10 min at 37°C, agitating gently. The labelling reaction was quenched by the addition of RPMI 1640 containing 10% FCS. The cells were washed three times with medium and resuspended in medium at a concentration of 2×106 cell/ml.
T cell transfection
CD4+CD25-T cells freshly isolated from buffy coat were transfected with plasmids containing full length CTLA4 and Foxp3 cDNA using Amaxa human T cell nucleofector kit according to manufacturers’ protocol. Briefly, 4× 106 CD4+CD25-T cells were resuspended in the 100μl of nucleofector solution. 3μg of plasmid DNA was added and transfected using program U014 or V024. 24 hours post nucleofection, cells were analyzed for expression by flow cytometry and used for functional assays by co-cultured with responder T cells and dendritic cells as described above.
Results
CTLA4 expression induced after activation of CD4+ CD25- T cells is strongly correlated with Foxp3 expression
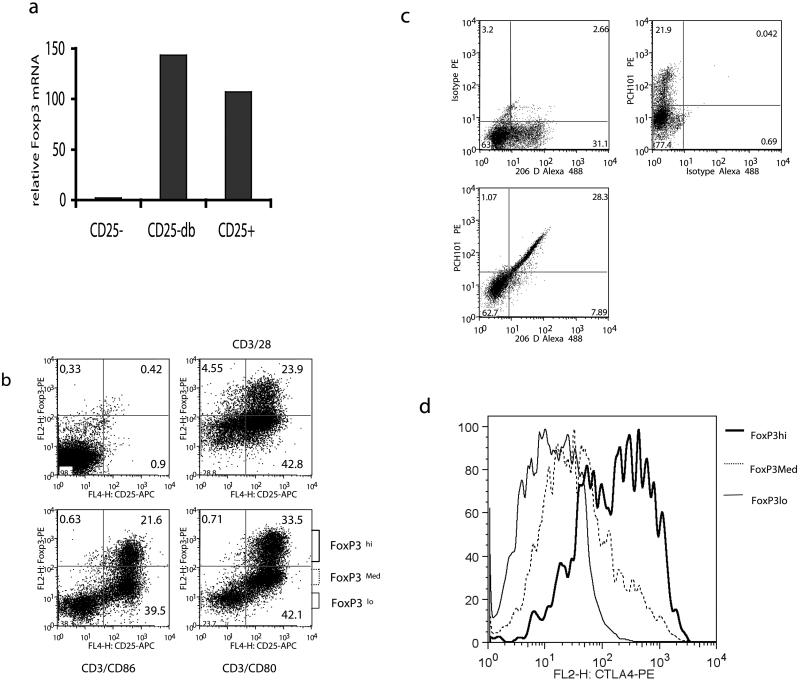
During T cell activation experiments, we observed that human CD25- T cells showed a significant increase in FoxP3 mRNA, as measured by RT-PCR (Figure 1a). To confirm this observation and to analyse the expression of FoxP3 protein, we carried flow cytometric analysis to determine FoxP3 expression under different activation conditions. Purified CD4+ CD25- T cells depleted of endogenous CD25+ regulatory cells were stimulated with anti-CD3/CD28 beads or anti-CD3 plus CD80 or CD86 transfectants and examined for FoxP3 induction. This revealed (Figure 1b) that activation of CD4+ CD25- cells resulted in the appearance of a distinct population (between approximately 10-30%) of FoxP3hi expressing cells. This increase was seen with both anti-CD3/CD28 antibody stimulation and importantly with co-stimulation using transfectants expressing the natural CD28/CTLA-4 ligands. It was also clear that some cells expressed lower levels of FoxP3 staining, however, whether this reflects genuine differences in FoxP3 protein levels is the subject of debate (21)(25). To further validate our FoxP3 staining we therefore activated T cells and compared FoxP3 expression using a second FoxP3 antibody 206D (Figure 1c). Staining with both 206D and PCH101 revealed induction of Foxp3 in a similar fraction of the cells. Notably, the presence of a significant population of non-activated cells provided an internal negative population. Dual staining with both antibodies clearly showed that these antibodies recognised the same cell populations expressing FoxP3, providing no evidence that PCH101 erroneously identifies FoxP3+ cells. Finally, we also analysed how the level of FoxP3 affected the expression of CTLA-4, since CTLA-4 expression is a notable feature of FoxP3+ Treg. Activated CD25- T cells were therefore gated on low medium or high expression of FoxP3 as shown in figure 1b and analysed for CTLA-4 expression. This revealed a strong correlation between FoxP3 and CTLA-4 expression, (Figure 1d) with cells gated for high levels of FoxP3 expressing much higher levels of CTLA-4. Taken together the above data were consistent with the concept that FoxP3 enhances CTLA-4 expression and that activation of human CD4+ CD25- T cells induced a population of cells expressing FoxP3 and high levels of CTLA-4.
Figure 1. Activation of CD4+ CD25- T cells induces FoxP3 and CTLA-4.
a). Purified CD25-T cells were left unstimulated (CD25-) or stimulated with anti-CD3 /CD28 coated beads (CD25-db) and compared to natural Treg (CD25+) for FoxP3 mRNA assessed by quantitative RT-PCR at 48h. (b) CD4+CD25- T cells stimulated with either CD3/CD28 beads or with anti-CD3 and transfectants expressing CD80 or CD86. After 72h, cells were stained for intracellular FoxP3 in conjunction with surface CD25 and analysed by flow cytometry. (c) Purified CD25-T cells were stimulated with anti-CD3 /CD28 coated beads and stained 36h later for Foxp3 with the antibodies shown. (d)CD4+CD25- T cells stimulated with CD3/CD28 beads were stained for intracellular CTLA-4 and FoxP3. CTLA-4 expression is shown for cells gated on different levels of FoxP3. In all FACS plots numbers indicate the percentage of total cells within the quadrant shown. Data shown are representative of a minimum of 3 experiments performed
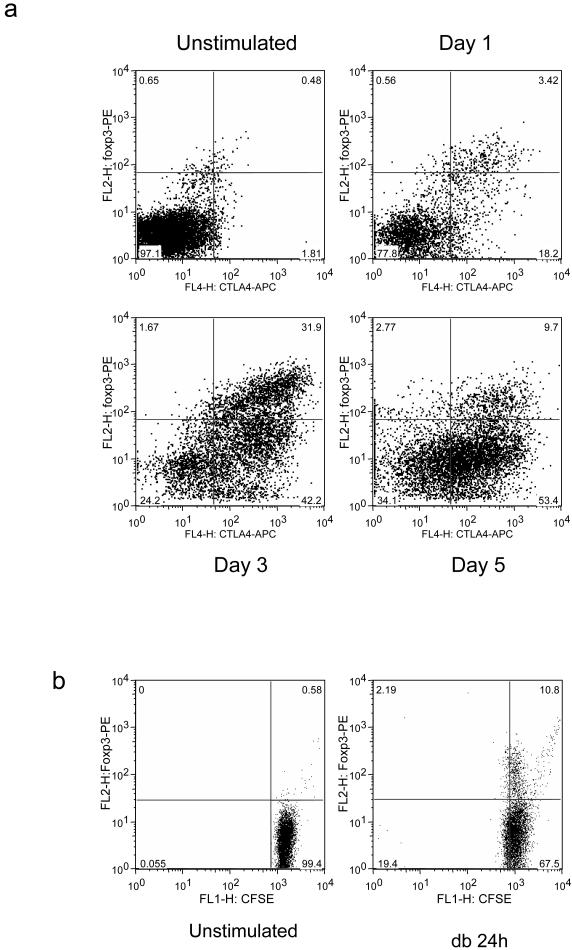
Since, purified CD25-T cells contain a small population of FoxP3-expressing cells which cannot be depleted by removing CD25+ cells we sought to establish whether the increase in CTLA-4-Hi FoxP3+ cells resulted from the expansion of pre-existing Treg cells or whether it was due to de novo expression. We therefore performed kinetic experiments to determine the expression of CTLA-4 and FoxP3. Purified CD4+ CD25- T cells were stimulated using CD3/CD28 beads and then stained for FoxP3 and CTLA-4 at various time points. This revealed (Fig. 2a) that significant FoxP3 expression was observed by 24h which is before T cell division takes place in this system. Interestingly, we also observed that whilst there is initially a strong co-expression of CTLA-4+ and FoxP3, by 120h, the majority of CTLA-4+ cells clearly lacked FoxP3. This suggested that prior to cell division there is a strong correlation between CTLA-4 and FoxP3 expression, but that over time FoxP3 expression diminishes whilst CTLA-4 is maintained. To support these kinetic experiments we also performed CFSE labelling studies. Again this confirmed (Fig. 2b) that an increase in FoxP3 could be observed by 24h in the absence of cell division demonstrating that these cells are indeed induced FoxP3 expressing cells. Overall these data demonstrated that activation of human CD4+ CD25- T cells, induced a distinct population of FoxP3 expressing cells which, initially, strongly co-express CTLA-4 but where expression of FoxP3 gradually diminishes.
Figure 2. CTLA-4 and FoxP3 are co-expressed early after T cell activation.
(a) CD4+CD25- T cells were stimulated with CD3/CD28 beads for the times shown and analysed for FoxP3 and CTLA-4 induction by flow cytometry. (b) CD4+CD25- T cells were CFSE labelled and stimulated with CD3/CD28 beads (db) for the times shown. CFSE dilution and FoxP3 expression were analysed by flow cytometry. Data shown are representative of a minimum of 3 experiments performed.
Effect of Interleukin-2 FoxP3 and CTLA-4 expression
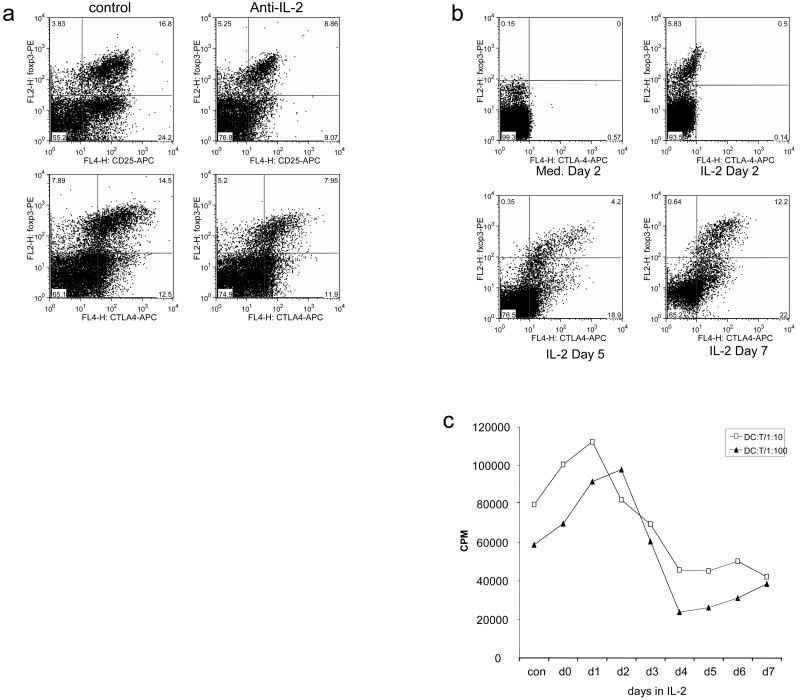
Since interleukin-2 is established as an important factor in Treg homeostasis, and has been proposed to influence CTLA-4 expression, we investigated the effect of IL-2 on the expression of FoxP3 and CTLA-4 in our in vitro system. The addition of blocking antibodies to IL-2 resulted in a significant decrease in the percentage of cells expressing FoxP3 (Figure 3a) as well as a decrease in the level of CD25, indicating that IL-2 is an important factor for maintaining inducible FoxP3+ cells. Interestingly, the inhibitory effect of blocking IL-2 on CD25 expression was more apparent in the FoxP3 negative cells, suggesting that FoxP3 may be able to sustain CD25 expression in the absence of IL-2. In addition, we observed that, FoxP3+ cells maintained CTLA-4 expression in the absence of IL-2. To compliment these studies, we examined the effect on FoxP3 and CTLA-4 expression of supplementing CD4+ CD25- T cell cultures with IL-2 (Figure 3b). This revealed, that CD4+ CD25- cells cultured in IL-2 could acquire expression of FoxP3 by 48h. However, in this system, where there was no overt T cell stimulation, FoxP3 expression could be induced without CTLA-4. Accordingly FoxP3 was evident by 48h whereas CTLA-4 expression was not observed until later timepoints. To examine the functional effects of treatment with IL-2, we tested whether these cells could influence T cell alloresponses stimulated by cultured dendritic cells. CD25- T cells were harvested each day from IL-2 culture and frozen. At the end of the experiment T cells were thawed and tested simultaneously in a suppression assay. The results of this analysis (figure 3c) revealed that cells cultured in IL-2 for 24-48h which lacked CTLA-4 expression did not possess suppressive capacity, however, from day 3 onwards there was evidence of substantial suppression of T cell responses which correlated with upregulation of CTLA-4.
Figure 3. Effect of IL-2 on CTLA-4 and FoxP3 induction.
(a) CD4+CD25- T cells stimulated with CD3/CD28 beads for 48h in the presence or absence (control) of anti-IL-2 antibody. Cells were stained for intracellular CTLA-4, FoxP3, surface CD25 and analysed by flow cytometry. (b) CD4+CD25- T cells were cultured in IL-2 alone and harvested at the times shown. Intracellular FoxP3 and CTLA-4 were detected by flow cytometry. Numbers shown indicate percentage of cells in each quadrant. (c) The suppressive capacity of T cells harvested from IL-2 cultures at different times (day 1-day 7) was tested for the ability to suppress alloresponses to DC. Fresh CD4+CD25- T cells were stimulated in response to allogeneic DC at the ratios shown(1:10 or 1:100 - DC:T cells). These cultures were supplemented with an equal number of T cells cultured in IL-2 for the time shown on the x-axis. Tritiated thymidine incorporation was measured by scintillation counting at day 5 of culture. Data shown are representative of a minimum of 3 experiments performed.
CTLA-4 expression identifies functional Treg
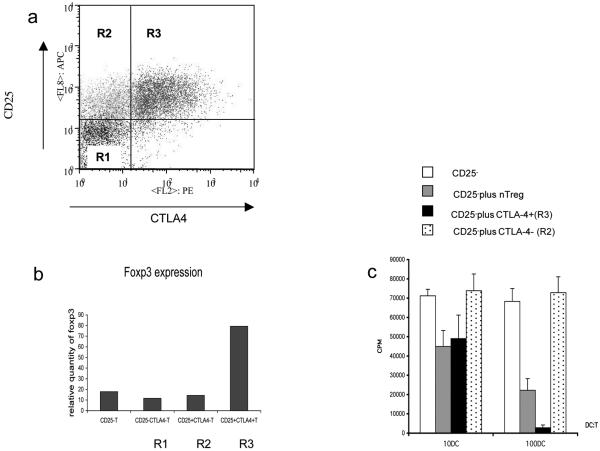
The above experiment suggested that CTLA-4 expression was associated with T cell suppression. We therefore carried out cell sorting experiments based on CTLA-4 expression. By staining cells for CTLA-4 at 37°C we could detect live cells which traffic CTLA-4 to the plasma membrane. This effectively labelled CTLA-4+ cells but did not require cell permeabilisation which precludes subsequent functional analysis. Since we had observed that up to 48h after stimulation with CD3/CD28 beads, CTLA-4 expression is largely confined to FoxP3+ cells, we stimulated CD25- T cells for 48h and sorted on the basis of CTLA-4 expression. Cells were sorted into tree populations CD25- / CTLA-4- (R1), CD25+ /CTLA-4- (R2), and CD25+ /CTLA-4+ (R3) populations as shown in figure 4a. Populations were then analysed for expression of FoxP3 by RT-PCR and for functional suppression. This analysis demonstrated that the CTLA-4+ cells labelled at 37°C contained the high levels of FoxP3 mRNA in comparison to CTLA-4- cells (figure 4b). Furthermore, CTLA-4+ cells were very effective at suppressing alloresponses whereas CTLA-4- cells were not (figure 4c). These data clearly demonstrate that selecting CTLA-4+ cells at 48h after stimulation (i.e. before cell division) enriches for a fraction of functionally competent Treg cells which have been induced following stimulation. We therefore concluded that induced CTLA-4+ FoxP3+ cells generated during in vitro stimulation were functional and possessed suppressive capacity similar to natural Treg.
Figure 4. Sorting for CTLA-4 expression at 48h identifies functional Treg.
(a) CD4+CD25- T cells stimulated with CD3/CD28 beads were stained for CTLA-4 (37°C) and CD25 and sorted into the regions shown (R1-R3). (b) Cells were sorted into the regions shown in (a) and recovered for mRNA analysis of FoxP3 expression by PCR. (c) CD4+CD25- T cells stimulated and sorted as shown in (a) were compared with natural Treg for their ability to suppress alloresponses. Fresh CD4+ CD25- T cells were stimulated by DC at different ratios (1:10 or 1:100 - DC:T cells) in the presence of sorted populations shown. Proliferative responses were determined by tritiated thymidine incorporation as measured by scintillation counting at day 5 of culture. Data shown are representative of 3 experiments.
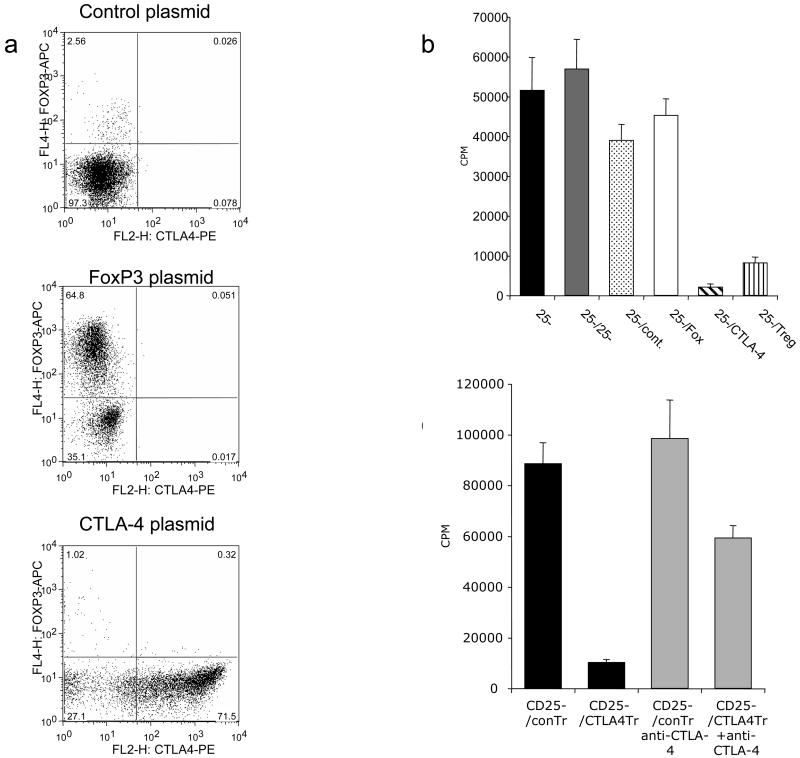
To determine if CTLA-4 expression was sufficient to confer suppression to CD4+ CD25- T cells, we transfected resting T cells with CTLA-4 cDNA using the Amaxa nucleofector and used them in a suppression assay. This revealed that cells transfected with CTLA-4 efficiently expressed CTLA-4 protein (Figure 5a) compared to mock transfected cells, furthermore these cells were themselves were unresponsive to stimulation (data not shown). Strikingly, CTLA-4-transfected T cells potently suppressed the activation of normal resting CD4+CD25- T cells (Figure 5b). Furthermore, CTLA-4 transfected cells did not upregulate FoxP3, indicating that FoxP3 expression was not required to for this regulatory function. Conversely, we also transfected FoxP3 into resting T cells and observed that FoxP3 expression alone did not induce CTLA-4 and nor did these cells have suppressive capacity. Taken together these data suggested that the acquisition of suppressive function by Treg requires CTLA-4 but not FoxP3 expression. Finally to confirm the role of CTLA-4 in suppression we carried out experiments in the presence of anti-CTLA-4 antibody. This showed that anti-CTLA-4 was able to reverse the suppressive capacity of CTLA-4 transfected T cells (Fig 5c).
Figure 5. (a) Transfection of CTLA-4 confers suppressive capacity.
CD4+CD25- T cells were transfected with CTLA-4 or FoxP3 cDNA and stained for CTLA-4 and FoxP3 expression at 24h. Expression was detected by flow cytometry using permeabilised cells. (b) Transfected cells were tested for their ability to suppress alloresponses of fresh CD4+ CD25- T cells stimulated by DC at different ratios (1:10 or 1:100 - DC:T cells). Responses were measured from CD25- cells alone (CD25-), CD25- cells plus additional CD25- cells as mock suppressors, or CD25- cells with added control transfected cells (CD25- /Cont.), FoxP3 expressing cells (CD25-/Fox), CTLA-4 transfected cells (CD25- /CTLA-4) or natural CD25+ Treg (CD25- / Treg). (c) CTLA-4 transfected (25-/CTLA-4Tr) cells or control transfected cells (25-/conTr) were tested for their ability to suppress alloresponses of fresh CD4+ CD25- T cells stimulated by DC in the presence or absence of blocking CTLA-4 antibody (10μg/ml). Proliferative responses were determined by tritiated thymidine incorporation as measured by scintillation counting at day 5 of culture. Data shown are representative of a minimum of 4 experiments performed.
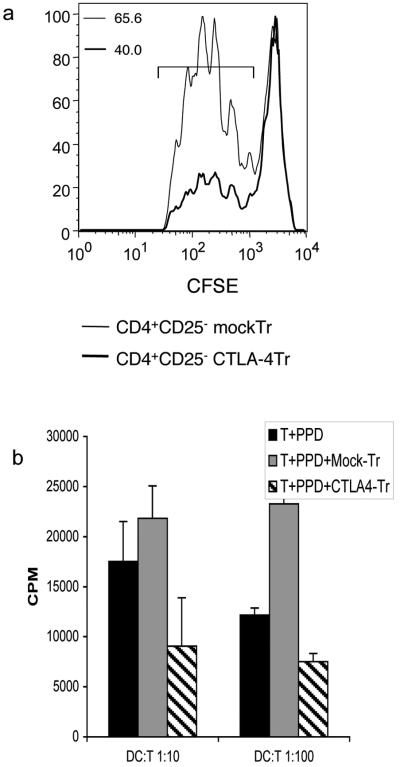
To confirm that suppression by CTLA-4-transfected cells reflected inhibition of responder T cells rather than differences in proliferation by the transfected cells, we labelled responder T cells with CFSE and monitored cell division. This result (Fig. 6a) clearly showed that the suppressive effect of CTLA-4 transfected cells seen in thymidine incorporation assays, reflects a decrease in responder T cell division as measured by CFSE. Finally, we also tested whether the CTLA-4 mediated suppression could affect antigen-specific responses other than alloantigen. We therefore stimulated T cells with autologous DCs plus the recall antigen PPD. Once again the addition of CTLA-4 transfected T cells compared to mock transfected T cells resulted in clear inhibition of PPD responses and similar data were also seen when stimulating with the superantigen SEB (data not shown). Overall we concluded that expression of CTLA-4 conferred suppressive activity in several different T cell stimulation settings.
Figure 6. CTLA-4 transfectants inhibit responder T cell division.
a) CFSE-labelled CD4+ CD25- T cells were stimulated by DC in an alloresponses for 5 days in the presence of either CTLA-4-transfected or mock-transfected CD25-T cells. DCs were added in the ratio of 1:10 or 1:100 T cells. Responder T cell division was monitored by CFSE dilution and analysed by flow cytometry. Numbers shown are percentage of cells dividing in the region indicated. b) T cell responses to PPD antigen were measured using autologous DCs pulsed with 5μg/ml of PPD in the presence of CTLA-4 or mock transfected T cells. Proliferative responses were determined by tritiated thymidine incorporation as measured by scintillation counting at day 5 of culture. Data shown are representative of 2 independent experiments.
Discussion
The role of CTLA-4 in Treg function has been the subject of considerable debate. Here we investigated the relationship between CTLA-4 and FoxP3 induced during human T cell activation. We provide evidence of an initial strong correlation between FoxP3 expression that of CTLA-4 in the early stages of activation and that isolating these cells demonstrates suppressive function, consistent with an induced Treg. Furthermore we provide evidence that suppressive function relates to CTLA-4 expression rather than to FoxP3.
It is now clear that human CD4+CD25- T cells can be induced to express FoxP3 by T cell stimulation without additional cytokines(7, 8, 23). Nonetheless the issue of whether these induced FoxP3+ cells reflect the induction of functional Treg remains controversial. We examined the regulation of both CTLA-4 and FoxP3 on activated human CD4+ CD25- T cells and observed a strong correlation between expression of CTLA-4 and FoxP3 at early timepoints (24-48h). This tight relationship is consistent with the view that CTLA-4 is a possible target of FoxP3 transcriptional regulation which may occur in co-operation with other transcription factors such as NF-AT(16). However, we also observed that normally activated T cell blasts can express CTLA-4 in the absence of FoxP3 at later time points. Thus, whilst FoxP3 is not absolutely required for CTLA-4 expression, our data suggest that FoxP3 may facilitate the expression of CTLA-4 prior to cell division and that following cell division FoxP3 is not required. This concept is in keeping with the fact that natural FoxP3+ Treg maintain CTLA-4 expression in the absence of overt stimulation. In contrast, T cell blasts appear to express higher levels of CTLA-4 with increasing cell divisions(24, 26). Therefore the regulation of CTLA-4 expression in Treg and non-regulatory T cell blasts appears to differ, in both kinetics and the dependence on FoxP3. We suggest that FoxP3 expression may facilitate the early expression of high levels of CTLA-4 which could then contribute to the “anergic” status typically ascribed to Treg in vitro(27). In keeping with this we observe that CTLA-4 transfected T cells are relatively unresponsive to normal stimulation.
By exploiting the relationship between CTLA-4 and FoxP3 early after T cell activation we have been able to purify induced FoxP3+ cells and assess their suppressive function. Strikingly, we observed that cell sorting on CTLA-4 expression selected for functionally suppressive T cells, expressing FoxP3. Thus our observations appear similar to those observed originally by Walker et.al (7). One possible reason for the differences between our conclusions and those who find that CD25- cells induced to express FoxP3 are not suppressive(8, 22, 28), is that it has not been possible previously to isolate these FoxP3+ cells effectively. Consequently, proliferation or cytokine production from contaminating non-Treg cells may mask any regulatory effects. In some studies, T cells with induced FoxP3 have been analysed for their intrinsic ability to produce cytokines such as IL-2 and interferon gamma as a measure of FoxP3 function. This has led conclusion that since these cytokines are still transcribed in FoxP3+ cells, that FoxP3 is therefore not functionally active (8, 22). However, interpretation of this analysis is complicated by the relative timings of cytokine gene expression compared to FoxP3. Thus, if cytokine genes are transcribed before FoxP3 is induced it might appear that FoxP3 expression does not inhibit cytokine gene expression and is therefore non functional. In contrast, we find clear evidence that sorting of CTLA-4+ FoxP3+ T cells identifies induced FoxP3+ cells that are capable of suppressing alloresponses.
Experiments directly expressing FoxP3 using retroviral vectors to infect CD25- T cells have also yielded conflicting results, regarding the role of FoxP3 in suppression. Some authors have concluded that FoxP3 expression confers suppression(29), whilst others found it insufficient(28). In our experiments transfection of FoxP3 into resting T cells did not confer Treg activity. We suggest that whether or not FoxP3 confers suppression may depend on its ability to induce CTLA-4. Our ability to transfect CTLA-4 and confer suppression strongly supports a role for CTLA-4. Importantly, we found clear evidence that FoxP3 expression alone is not sufficient for the induction of CTLA-4 in resting T cells. Thus, the level of CTLA-4 expression achieved during T cell stimulation may underlie the differences in suppression observed by several groups. It is noteworthy that in studies using retroviral FoxP3 transduction that the conditions used involve “pre-activation” of T cells prior transduction. However, these are quite variable and make use soluble anti-CD28 antibodies or CD3 antibodies and APC(28, 29). Nonetheless, these conditions are designed not induce FoxP3 themselves and it is therefore likely that CTLA-4 is also not effectively induced. Consequently, variable levels of CTLA-4 expression caused by pre-conditioning of T cells prior to transduction may affect the conclusions from these studies.
An additional possibility is that CTLA-4 expression and suppressive capacity may be influenced by the level of FoxP3 expression during activation. Transgenic mice generated by Wan et.al (30), expressed low levels of FoxP3 and demonstrated impaired suppression. Notably these cells also expressed much lower levels of CTLA-4 compared to normal FoxP3+ T cells. Indeed, in other studies, lack of suppressive function also correlates with low levels of CTLA-4 expression in Treg. For example in FoxP3-null T cells(31), it is clear that despite undergoing aspects of Treg differentiation, these cells were non-functional and again CTLA-4 expression was highly impaired. Likewise in FoxP3 retroviral transduction studies(28), very modest CTLA-4 expression was achieved, however, in experiments where some suppression was observed, this correlated with higher expression of CTLA-4(28).
We directly addressed the role of CTLA-4 expression in human Treg function by transfecting CTLA-4 directly into resting human T cells. This allowed CTLA-4 expression prior to T cell activation, thereby mimicking the expression pattern of CTLA-4 seen in Treg. Strikingly, we observed that T cells acquired potent suppressive capabilities demonstrating that CTLA-4 may be a direct mediator of Treg function. This finding is consistent with a number of murine studies which also indicate a functional role for CTLA-4 on Treg. Anti-CTLA-4 antibodies can clearly abrogate Treg function in settings where CTLA-4 expression was restricted only to Treg(12). Furthermore, bone marrow chimera experiments(32) demonstrated a suppressive role for CTLA-4 expressing cells consistent with that of Treg, a finding further supported by more recent chimera experiments(33). Whilst our data are supportive of a role for CTLA-4 in Treg function, the level of expression obtained by transfection is higher than that found in natural Treg. Therefore, experiments using variable levels of CTLA-4 expression would be useful to further validate our findings.
The question of how CTLA-4 may affect the function of regulatory cells is at present unresolved. However, several distinct mechanisms are possible see (34) for review. In the context of the present experiments perhaps the most plausible mechanisms include the activation of IDO pathway or direct competition for CD28 ligands on APC, however it is also possible that CTLA-4 ligation signals the generation of additional downstream effectors in the Treg. To date, we have been unable to demonstrate a role for IDO in our system and therefore prefer other models in which CTLA-4 affects the APC since we observe clear dose effects of APC numbers on the efficacy of CTLA-4 mediated suppression. Whether or not CTLA-4 expressed on activated T cells acts in a regulatory capacity is unknown. However, whilst it is not impossible that activated CTLA-4-positive T cells could act in a regulatory manner, it is notable that Treg have significantly higher levels of CTLA-4. Furthermore the ability of T cell blasts to make cytokines such as IL-2 could serve to override regulatory potential conferred by CTLA-4 expression.
Despite good evidence for CTLA-4 in Treg function, some data from CTLA-4 knockout mice have suggested that CTLA-4 expression is not absolutely required for suppression indicating that alternative suppressive mechanisms can operate in the absence of CTLA-4(10, 12). Overall, however, a significant body of data indicate that CTLA-4 is an important, component of Treg suppression and is likely to represent a significant therapeutic target for modulating Treg behaviour.
Acknowledgments
YZ was supported by the BBSRC and the MRC. CNM was supported by the Wellcome Trust, OQ was supported by the BBSRC. LW is an MRC Career Development Fellow
Abbreviations used in this paper
- Treg
regulatory T cells
REFERENCES
- 1.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 3.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 5.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 6.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 7.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 9.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 10.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 11.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzotti CN, Tipping H, Perry LCA, Mead KI, Blair PJ, Zheng Y, Sansom DM. Inhibition of human T cell proliferation by CTLA-4 utilizes CD80 and requires CD25+ regulatory T cells. Eur J Immunol. 2002;32:2888–2896. doi: 10.1002/1521-4141(2002010)32:10<2888::AID-IMMU2888>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 Differentially Modulate the Suppressive Function of Human Regulatory T Cells. J Immunol. 2004;172:2778–2784. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 20.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3- T cells by T cell receptor stimulation is TGF{beta}-dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25- cells. Proc Natl Acad Sci U S A. 2005;102:4103–4108. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manzotti CN, Liu MKP, Burke F, Dussably L, Zheng Y, Sansom DM. Integration of CD28 and CTLA-4 function results in differential responses of T cells to CD80 and CD86. Eur. J Immunol. 2006;36:1413–1422. doi: 10.1002/eji.200535170. [DOI] [PubMed] [Google Scholar]
- 25.Pillai V, Karandikar NJ. Attack on the clones? Human FOXP3 detection by PCH101, 236A/E7, 206D, and 259D reveals 259D as the outlier with lower sensitivity. Blood. 2008;111:463–464. doi: 10.1182/blood-2007-09-111823. author reply 464-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle AM, Mullen AC, Villarino AV, Hutchins AS, High FA, Lee HW, Thompson CB, Reiner SL. Induction of cytotoxic T lymphocyte antigen 4 (CTLA-4) restricts clonal expansion of helper T cells. J Exp Med. 2001;194:893–902. doi: 10.1084/jem.194.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 28.Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, Ziegler SF, Roncarolo MG, Levings MK. The role of 2 FOXP3 isoforms in the generation of human CD4 Tregs. J Clin Invest. 2005;115:3276–3284. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 30.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 31.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 32.Bachmann MF, Kohler G, Ecabert B, Mak TW, Kopf M. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol. 1999;163:1128–1131. [PubMed] [Google Scholar]
- 33.Chikuma S, Bluestone JA. Expression of CTLA-4 and FOXP3 in cis protects from lethal lymphoproliferative disease. Eur J Immunol. 2007;37:1285–1289. doi: 10.1002/eji.200737159. [DOI] [PubMed] [Google Scholar]
- 34.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]