Abstract
OBJECTIVES
Tropical enteropathy is characterized by an increased urinary lactulose-to-mannitol (L:M) ratio on a site-specific sugar absorption test and is associated with increased intestinal permeability and decreased nutrient absorptive capacity. The etiology of tropical enteropathy is postulated to be intestinal bacterial overgrowth. This study tested the hypothesis that treatment with a nonabsorbable, broad-spectrum antibiotic, rifaximin, reduces the L:M ratio in rural Malawian children, among whom tropical enteropathy is common.
METHODS
All children aged 3–5 years from one village were enrolled in a randomized, double-blind, placebo-controlled trial of treatment with rifaximin for 7 days. The L:M ratio was measured before and after treatment, and the change in the L:M ratio was the primary outcome. Secondary outcomes were changes in the urinary sucrose-to-lactulose (SUC:L) and sucralose-to-lactulose (SCL:L) ratios, as well as changes in the fractions of each test sugar recovered in the urine.
RESULTS
A total of 144 children participated in this study, of whom 76% had an elevated L:M ratio on enrollment (L:M≥0.10). Children who received rifaximin did not show an improvement in their L:M ratio compared with those who received placebo (−0.01±0.12 vs. 0.02±0.16, respectively, P = 0.51, mean±s.d.), nor were there significant differences between the two groups in excretion of lactulose, mannitol, sucralose, or sucrose, or in the SUC:L and SCL:L ratios.
CONCLUSIONS
Rifaximin had no effect on the tropical enteropathy of 3–5-year-old Malawian children, suggesting that small-bowel bacterial overgrowth is not an important etiological factor in this condition.
INTRODUCTION
Tropical enteropathy is characterized by a diffuse villous atrophy of the small bowel associated with inflammatory T-cell infiltration (1–4) and subclinical malabsorption. It is associated with stunting of otherwise healthy children who are < 5 years of age in developing countries (5). Although tropical enteropathy is widely attributed to some environmental exposure (6), the specific association is unknown. Given the high prevalence of tropical enteropathy in rural communities of developing countries (7), hygiene may well play a role (8,9). Microbial contamination of food and water can lead to changes in the populations of enteric microbiota, and may lead to small-bowel bacterial overgrowth (10) that manifests as tropical enteropathy.
Damage to the normal intestinal architecture compromises the intestine's capacity to absorb macronutrients (11–14). The small-intestinal sugar-permeability test is a noninvasive means of assessing tropical enteropathy and correlates well with the severity of histological changes (15), nutrient absorptive capacity (16,17), diarrhea (18), and growth (5). Traditionally, the small-intestinal sugar-permeability test uses the non-metabolized sugars, mannitol and lactulose, which are administered orally and measured in the urine as the ratio of lactulose to mannitol (L:M) (19). More recently, it has been shown that permeability can be measured in a site-specific manner using the additional sugars, sucrose and sucralose. Sucrose measures gastric permeability, the sucrose:lactulose ratio (SUC:L) measures proximal gastrointestinal permeability, sucralose measures whole-bowel permeability, and the sucralose:lactulose (SCL:L) ratio measures colonic permeability (20–22).
We explored the effect of rifaximin, a semi-synthetic nonabsorbable antibiotic with broad-spectrum activity against aerobic and anaerobic Gram-positive and Gram-negative bacteria (23,24), in a population at high risk for tropical enteropathy. Rifaximin has shown some efficacy for use in “traveler's diarrhea” (25,26), Clostridium difficile colitis (27), inflammatory bowel disease (28,29), and for the treatment of small-intestinal bacterial overgrowth (30,31). Given the experience with rifaximin in these diverse clinical problems provoked by alterations in the gut microbiota, and given the speculation that tropical enteropathy may be the result of small-intestinal bacterial overgrowth, we undertook a trial of 7 days of rifaximin in asymptomatic Malawian children, who were at high risk for tropical enteropathy, to test the hypothesis that rifaximin therapy would improve the L:M ratio compared with that with placebo. As gastric, proximal small-bowel and colonic permeability have not been evaluated in children with tropical enteropathy, we also explored the effect of rifaximin treatment on permeability in these sites as secondary outcomes.
METHODS
Subjects
This study was conducted in the village of Limela, located in the Machinga district of southern Malawi. The diet of the children there consists primarily of maize, supplemented with small amounts of fish. People live in mud huts with thatched roofs, and meals are prepared outdoors over open wood fires. Local health care is provided by traditional healers or by government health-surveillance assistants who have completed 2 months of instruction in first aid and disease prevention.
All children in Limela aged 3–5 years were recruited for the study. Exclusion criteria included any chronic debilitating illnesses, such as cerebral palsy, or obvious congenital abnormalities. If a child was in need of acute medical treatment, arrangements were made for a visit with the local health staff. If a child showed any evidence of severe acute malnutrition (edema suggestive of kwashiorkor or a weight-for-height Z score < –3), the child was excluded from the study and enrolled in a home-based therapeutic feeding program (32).
Written and oral informed consent was obtained from each child's parent or caretaker before enrollment in the study by a native-speaking research nurse. Caretakers were free-to-end participation in the study at any time. The study was approved by the institutional review boards of both the University of Malawi and Washington University in St Louis (ClinicalTrials.gov number NCT00858988).
Study design
This study was a randomized, double-blinded, placebo-controlled trial of the efficacy of rifaximin to reduce tropical enteropathy. All participants were enrolled on the same day in September 2007. Initial demographic information was collected about the child's household, including whether the child's mother and father were alive and what type of water source was available to the household. Initial anthropometric information collected included an evaluation for edema and measurements of weight, height, and mid-upper-arm circumference. Information was also collected regarding the number of days of diarrhea, visible blood in stool, abdominal pain, vomiting, cough, and rash during the earlier week. After the collection of this information, each child completed the pre-intervention site-specific sugar-absorption test.
Starting from the morning after the site-specific sugar-absorption test, each child received for 7 consecutive days either 100 mg rifaximin (Salix Pharmaceuticals Inc., Morrisville, NC) or a starch placebo,twice daily, under the direct supervision of the field research staff. The medication was given as a flavored solution with water in a 30 ml cup, and the child was observed to consume the entire amount. The randomization to rifaximin or placebo was carried out prospectively with the use of a coded list compiled from a random number generator; the code was fully blinded to the field staff and trial participants. A total of 150 small plastic sacks with either rifaximin or placebo were then prepared and numbered ahead of time in accordance with the list generated by a pharmacy student who had no contact with the children or the researchers in the field. The rifaximin and placebo were indistinguishable in appearance and taste so that the children and field staff had no knowledge of each child's allocation status to intervention or placebo. The code list was unblinded only after all the clinical and laboratory analyses were completed and data analysis was undertaken. Each child was visited at home 14 and 21 days after the start of the study to check on the child's clinical status, assess for side effects, and collect symptom information from the preceding week. Finally, the children returned to the research site in the village 28 days after starting treatment for a second site-specific sugar-absorption test and for anthropometric assessment.
The primary outcome was the difference in the urinary L:M ratio before and after the intervention. Secondary outcomes were the amounts of lactulose, mannitol, sucralose, and sucrose excreted in the urine as a percentage of the amount ingested before and after the intervention and changes in SUC:L and SCL:L. Adverse drug effects were monitored and included the incidence of diarrhea, bloody stool, abdominal pain, vomiting, cough, and rash.
A sample size of 128 was calculated as being necessary to detect a decrease of 0.06 units in the L:M ratio, with 95% specificity and 80% power. This decrease was chosen because such a change would correlate well with a clinically significant improvement in gut function. The sample size calculations assumed that urinary mannitol and lactulose measurements would be distributed in a manner similar to our earlier study, which showed that >70% of apparently healthy rural Malawian children had tropical enteropathy (33).
Site-specific sugar-absorption test
Caretakers were instructed to not give their children any food after 10 p.m. on the day before the site-specific sugar-absorption tests and to assemble at the village research site at 6 a.m. on the day of the tests. After arrival at the research site, each child voided and this urine was collected and discarded.
To initiate the test, each child drank a 100 ml sugar solution containing 5 g lactulose, 1 g mannitol, 1 g sucralose, and 10 g sucrose. This sugar solution tastes quite sweet and all the children drank the solution eagerly. The children remained at the village research site for 4 h after ingestion of the sugar solution, during which time all of the child's urine was collected in a sterile cup with 10 mg merthiolate added to limit the bacterial degradation of excreted sugars. To facilitate the collection of urine, the children were given additional water to drink 3.5 h after ingestion of the sugar solution. During the 4 h collection time, the children were supervised at the research site by their mothers or by other guardians and the field research staff, so that no urine is lost. They were kept entertained with games and songs to help facilitate their cooperation in having to remain in a relatively small area for so long a time. When the children voided at least 4 h after the consumption of the sugar solution, they completed the urine collection and were dismissed. At the end of the collection period, the total urine volume was measured and a 4 ml aliquot was transferred into a plastic cryovial. Urine specimens were flash frozen, transported in a liquid nitrogen dry shipper, and stored in –70°C freezers.
The concentrations of lactulose, mannitol, sucralose, and sucrose in the urine specimens were then analyzed as described earlier (22,33). For the analyses of sucrose, lactulose, and mannitol, a 20 μl aliquot of spun urine was injected into an Aminex HPX 87C 300 7.8 mm cation-exchange column and eluted with degassed pure water at a flow rate of 0.6 ml/min at 85°C. The column effluent was monitored with a differential refractometer (Dionex Corperation, Sunnyvale, CA), with the internal temperature set at 50°C. For the analysis of sucralose, the samples were processed through reverse solid-phase extraction using the “On Guard II RP 1 cc Cartridge” (Dionex Corporation, Sunnyvale, CA) to remove the majority of the background contaminants before being injected into the same analytical column but equipped with a “Carbo-C Cartridge” (BioRad Laboratories, Richmond, CA). The cartridge was washed with 5 ml of HPLC grade acetonitrile, and then with 5 ml of nanopure water, before applying 0.5 ml of the sample. The cartridge was then washed with 5 ml of nanopure water, followed by 0.3 ml of HPLC grade acetonitrile. The sucralose was eluted with 5 ml of HPLC grade acetonitrile. The effluent was evaporated to dryness and then reconstituted in 0.5 ml of nanopure water. A 20 μl aliquot was injected into the analytical column for analysis. The assays are sensitive to 1 μg/ml of sucrose, lactulose, and mannitol, and to 10 μg/ml of sucralose.
Statistical analysis
Data were tabulated on a spreadsheet, the randomization code was broken, and summary statistics were calculated for the groups that received rifaximin and placebo. The values for urinary sugar recovery were expressed as a percentage of the ingested dose. The reported values for normal L:M range from 0.03 to 0.12 (14,16). A value of ≥0.10 was chosen to be indicative of tropical enteropathy (14,33).
The differences in urinary sugar recovery and the sugar ratios before and after intervention were calculated and compared between the rifaximin and placebo groups by using the Wilcoxon rank-sum test. This nonparametric test was chosen because the ratios were not likely to be normally distributed, and the range of values includes only positive numbers. The changes in weight were compared using Student's t-test. The incidences of diarrhea, visible blood in stool, abdominal pain, vomiting, cough, and rash were compared using Fisher's exact test. For each comparison, P < 0.05 was considered statistically significant. Statistical analyses were carried out using the Excel (Microsoft Corp., Redmond, WA) and SPSS (SPSS, Inc., Chicago, IL) software packages.
RESULTS
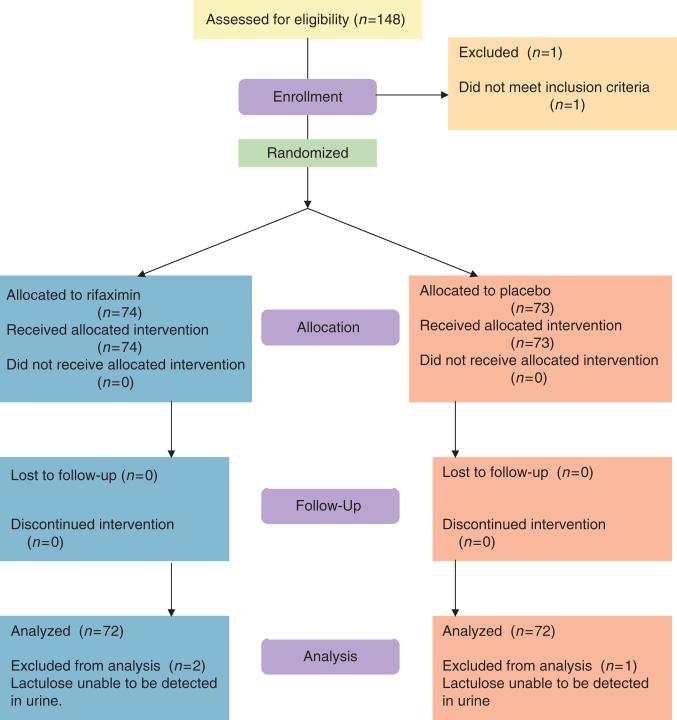
A total of 147 children met the inclusion criteria and completed the study; 74 were in the rifaximin group and 73 were in the placebo group. The urine specimens for two children in the rifaximin group and for one child in the placebo group could not be used for calculation of the L:M ratio because no lactulose was detected in the urine. These children were excluded from further analysis, leaving 72 children in each group (Figure 1). The baseline demographic, anthropometric, and clinical characteristics of the two groups did not differ significantly (Table 1). No children terminated the study early and all 144 children included in the analysis received their study intervention on all 7 days under the direct observation of study staff, except for one child who missed one dose of the intervention.
Figure 1.
Flowchart of study enrollment and follow-up.
Table 1.
Baseline characteristics of study participants
| Rifaximin group (n=72) | Placebo group (n=72) | |
|---|---|---|
| Female (n (%)) | 32 (44) | 29 (40) |
| Age (mo) | 47.6±6.5a | 46.8±7.6 |
| Mother alive (n (%)) | 72 (100) | 71 (99) |
| Father alive (n (%)) | 69 (96) | 69 (96) |
| Water source | ||
| Well (n (%)) | 61 (85) | 70 (97) |
| Borehole (n (%)) | 7 (10) | 1 (1) |
| Public tap (n (%)) | 1 (1) | 0 (0) |
| Weight-for-height Z-score | 0.13±0.72 | 0.16±0.82 |
| Weight-for-age Z-score | –0.96±0.93 | –0.86±0.95 |
| Height-for-age Z-score | –1.72±1.40 | –1.63±1.43 |
| Diarrhea in the week before study (n (%)) | 3 (4) | 1 (1) |
| Visible blood in stool in the week before study (n (%)) | 0 (0) | 0 (0) |
| Vomiting in the week before study (n (%)) | 2 (3) | 5 (7) |
| Abdominal pain in the week before study (n (%)) | 10 (14) | 11 (15) |
| Cough in the week before study (n (%)) | 11 (15) | 13 (18) |
| Rash in the week before study (n (%)) | 3 (4) | 1 (1) |
Mean±s.d. (all such values).
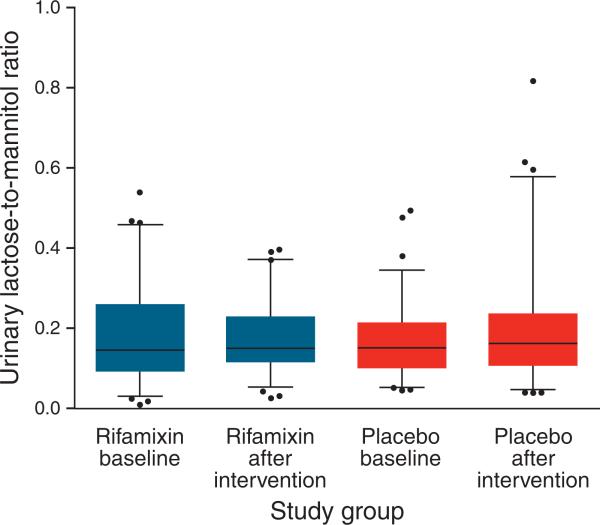
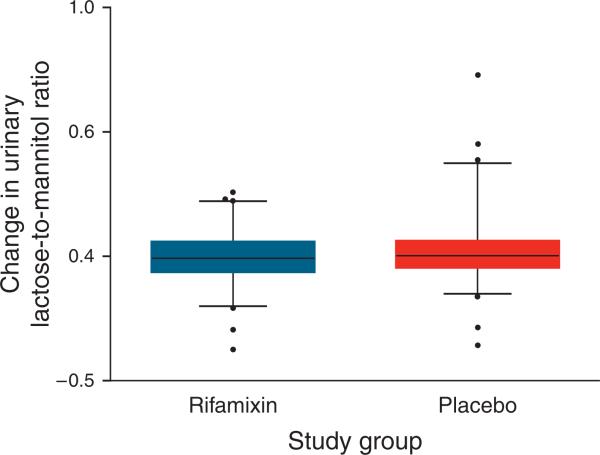
Upon enrollment, 108 (76%) of the children had an L:M ratio ≥0.10 beforethe intervention and 48 (34%) had an L:M ratio ≥0.20. The distribution of the L:M ratio (Figure 2) and the change in the L:M ratio after intervention (Figure 3) did not differ significantly after the intervention in either group. The administration of rifaximin for 7 days did not affect lactulose, mannitol, sucralose, or sucrose excretion, or the SUC:L or SCL:L ratios, in either the rifaximin or the placebo groups (Table 2).
Figure 2.
Distribution of urinary lactulose-to-mannitol ratios before and after intervention. Each box plot depicts the 25th percentile, mean, and 75th percentile values. Whiskers depict 5th–95th percentile values.
Figure 3.
Distribution of changes in the urinary lactulose-to-mannitol ratios before and after intervention. Each box plot depicts the 25th percentile, mean, and 75th percentile values. Whiskers depict 5th–95th percentile values.
Table 2.
Urinary recovery of lactulose, mannitol, sucrose and sucralose in healthy 3- to 5-year-old Malawian children before and after 7 days administration of rifaximin or placebo
| Rifaximin (n=72) | Placebo (n=72) | |
|---|---|---|
| Lactulose excretion (% of dose administered) | ||
| Baseline | 0.30±0.18a | 0.34±0.25 |
| After intervention | 0.28±0.29 | 0.29±0.24 |
| Change in lactulose excretion | –0.02±0.33 | –0.05±0.36 |
| Mannitol excretion (% of dose administered) | ||
| Baseline | 9.57±5.24 | 10.29±6.62 |
| After intervention | 7.90±6.34 | 9.18±7.80 |
| Change in mannitol excretion | –1.67±8.71 | –1.11±10.8 |
| Sucralose excretion (% of dose administered) | ||
| Baseline | 0.51±0.29 | 0.58±0.53 |
| After intervention | 0.48±0.37 | 0.51±0.38 |
| Change in sucralose excretion | –0.028±0.51 | –0.072±0.70 |
| Sucrose excretion (% of dose administered) | ||
| Baseline | 0.062±0.040 | 0.074±0.058 |
| After intervention | 0.054±0.061 | 0.058±0.054 |
| Change in sucrose excretion | –0.008±0.072 | –0.016±0.078 |
| Ratio of sucrose-to-lactulose (SUC:L) excreted | ||
| Baseline | 0.50±0.34 | 0.64±0.90 |
| After intervention | 0.46±0.36 | 0.48±0.35 |
| Change in SUC:L | –0.057±0.41 | –0.16±0.84 |
| Ratio of lactulose-to-mannitol (L:M) excreted | ||
| Baseline | 0.18±0.12 | 0.17±0.09 |
| After intervention | 0.18±0.09 | 0.20±0.14 |
| Change in L:M | –0.01±0.12 | 0.02±0.16 |
| Ratio of sucralose-to-lactulose (SCL:L) excreted | ||
| Baseline | 0.42±0.32 | 0.39±0.23 |
| After intervention | 0.53±0.56 | 0.45±0.34 |
| Change in SCL:L | 0.12±0.67 | 0.06±0.42 |
Mean±s.d. (all such values). No statistically significant differences were found between all before–after and rifaximin–placebo comparisons.
Comparing only the children with an elevated L:M ratio on enrollment, the changes in the L:M ratio before and after treatment with rifaximin (n=53) or placebo (n=55) were −0.03±0.12 and −0.02±0.14, respectively (P=0.43). Likewise, the changes in SUC:L were −0.026±0.38 and −0.031±0.39, respectively, (P=0.94) and those in SCL:L were −0.48±3.46 and −0.87±2.99, respectively, (P=0.53).
The incidence of diarrhea during the study was higher in the rifaximin group compared with that in the placebo group (13 vs. 1%, respectively) (Table 3). Of the nine children in the rifaximin group who reported to have diarrhea during the study period, only one child had diarrhea during the first week while they were receiving rifaximin. Two additional children had diarrhea during the third week, one during both the third and fourth weeks, and five during the final week. The child with diarrhea in the placebo group reported symptoms during the third week. None of the episodes of diarrhea were clinically dehydrating and did not lead any of the caregivers to seek additional health care for their children. There was no clinical evidence of any other adverse effects in any of the children in the study by the time of final evaluation on the last day of the study.
Table 3.
Clinical signs and symptoms during 28-day study perioda
| Rifaximin (n=74) | Placebo (n=73) | |
|---|---|---|
| Diarrhea (n (%)) | 9 (13)b | 1 (1) |
| Visible blood in stool (n (%)) | 0 (0) | 1 (1) |
| Vomiting (n (%)) | 7 (10) | 4 (5) |
| Abdominal pain (n (%)) | 19 (26) | 16 (22) |
| Cough (n (%)) | 18 (25) | 14 (19) |
| Rash (n (%)) | 2 (3) | 4 (6) |
| Change in weight from day 0 to day 28 (g) | 72±886c | 4.0±762 |
Assessed daily during a 7-day intervention period and weekly during a 3-week follow-up period.
Statistically significant, P=0.02.
Mean±s.d.
Among the 34 children with a L:M ratio <0.10, which was considered normal, the SCL:L ratio was 0.60±0.33 on enrollment.
DISCUSSION
In an impoverished rural African population of 3–5-year-old children, of whom 76% had evidence of tropical enteropathy, a 7-day course of rifaximin, given in the context of a rigorous, double-blind, placebo-controlled trial, had no effect on intestinal absorptive dysfunction, as measured by the site-specific sugar-absorption test. This result suggests that intestinal bacterial overgrowth does not play a causal role in tropical enteropathy.
A limitation of the trial is that it was conducted in a rural sub-Saharan population in which corn is the staple food. In other populations with different habitual diets, environmental conditions, or the prevalence of intestinal conditions such as celiac disease, the findings may differ. The baseline measurements of the L:M ratio in the present population are very similar to that found earlier in a different Malawian village, but with similar environmental and dietary conditions (33), showing the reproducibility of the sugar-absorption method.
The use of sucralose in the site-specific absorption test has not been performed earlier in a developing world population. Sucralose serves as a useful marker of colonic permeability in individuals in whom the the L:M ratio is normal (21,22,34). Earlier work in healthy American children has shown that 0.4% of sucralose is recovered in the urine after 3 h and that a normal SCL:L value is approximately 0.8 (20). In comparison, 0.5% of sucralose was recovered in the urine in this study and the SCL:L ratio was approximately half of that seen in healthy American children. Some of these differences may be attributed to differences in methodology. In this study, urinary sugar recovery was determined over 4 h, whereas earlier data were collected in a 3 h urine collection, and sucralose can be expected to enter the body through the largest absorptive surface, the small bowel, which had increased permeability in our population. The similar fraction of sucralose recovered in the urine, however, and the lower SCL:L ratio indicate that colonic permeability was not increased in rural Malawian children. To fully evaluate the colonic permeability of sucralose, a longer time period for urine collection may have been helpful (21), but this would add significant complexity to the collection of urine from these young children in the field. We thus chose a 4 h time frame for urine collection as a compromise between these conflicting demands.
There has been little experience with regard to the use of sucrose in a site-specific absorption test from the developing world; one other similar population in Malawi studied by our research group found a value for sucrose excretion to be 0.06%, very similar to the value found in this study. In healthy control populations of older children from the developed world, 0.02–0.03% of administered sucrose has been recovered in the urine. We speculate that this finding may represent modest, subclinical gastritis in this population, which could be the consequence of a Helicobacter pylori infection. The finding that urinary sucrose excretion is increased in Malawian children requires further investigation to understand its significance.
Although it has been suggested that tropical enteropathy is the result of bacterial overgrowth, the use of antibiotics in a clinical trial in children with tropical enteropathy has not been reported earlier. We chose to use rifaximin because of its safety, lack of systemic absorption, and broad-spectrum of antibacterial activity (23,35). A single-dosing regimen of rifaximin for a fixed duration was used, and thus a dose–response effect could not be specifically studied. The rifaximin dose used in adults was 400–800 mg/day for 3–5 days (23–25,28,35); it can be reasonably extrapolated that the dose of 200 mg/day for 7 days used with young children in this study was sufficient to achieve a comparable drug exposure in the intestinal tract. Allowing for a 21-day period before the reassessment of tropical enteropathy after the completion of intervention provided time for the regeneration of healthy enterocytes and for the restoration of the intestinal absorptive area. It seems unlikely that trials using different antibiotics or with the L:M ratio measured at different intervals would have a fundamentally different result.
The greater number of children who received rifamixin and who reported diarrhea was not significantly different from the fraction of children reported to have diarrhea in the week before intervention, nor was the diarrhea clinically serious. Administration of antibiotics often results in subclinical changes in stool patterns, so this finding is not unexpected. In addition, this finding may simply be one of chance, given that it was observed 2 weeks after medication had ceased. Further monitoring of diarrhea among children receiving rifaximin is warranted, given the limited experience with this drug in children.
This study should lead to a reconsideration of the hypothesis that overgrowth or disruption of the normal bacterial microbiota in the small bowel leads to tropical enteropathy in an environment in which the consumption of coliform-contaminated foods is common. It is apparent that the perturbations causing tropical enteropathy occur in the small bowel; the site-specific test for colonic and stomach permeability did not show marked derangements, and earlier work shows that the probiotic Lactobacillus GG offers no benefit in alleviating tropical enteropathy (33). Rifaximin has shown effectiveness in reducing small-bowel bacterial overgrowth; if a similar pathophysiological process were at work in tropical enteropathy, rifaximin should have been effective in this clinical setting as well. Our data suggest that efforts to improve hygiene aimed at reducing coliform contamination of the diet may not affect tropical enteropathy.
Given that an acute enteric infection because of viral and parasitic pathogens can also compromise intestinal mucosal integrity (18), perhaps chronic or repeated infections with nonbacterial organisms is linked to tropical enteropathy. Clinical studies to test this hypothesis may thus be warranted. For example, infection because of intestinal parasites could be correlated with intestinal integrity, or a trial carried out of empirical treatment with an antiparasitic agent (6). A specific study to detail the populations of bacteria present among the enteric microbiota (36) of children with and without tropical enteropathy may be helpful in determining the role that bacterial communities play in this process.
Recent evidence indicates that intestinal permeability is modulated by the human immune system, and that intestinal permeability plays an important role in allergic and autoimmune disease (37). The role of cell-mediated immunity, particularly specific T-cell subsets and cytokines, in the pathogenesis of tropical enteropathy may well be an important additional area for research.
The failure of empirical antibacterial therapy to improve tropical enteropathy suggests that nutritional etiologies must also be considered in the pathogenesis of tropical enteropathy. A supplementation trial of the micronutrients, vitamin A and zinc, in Brazilian children was effective in improving the intestinal barrier function and also correlated with an improvement in linear growth (17). An inadequate intake of macronutrients may play a role in the pathogenesis of tropical enteropathy, although the observation that enteropathy is prevalent in residents of the developing world who are of every socio-economic class and ethnic background makes this seem less plausible. Longitudinal studies evaluating the role of nutritional status and nonbacterial microbes are needed to elucidate the patho-physiology of this disease process.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Tropical enteropathy is associated with stunting the growth of otherwise healthy children in developing countries.
The etiology of tropical enteropathy is not understood.
One hypothesis is that small-bowel bacterial overgrowth leads to tropical enteropathy.
The site-specific sugar-absorption test serves to study tropical enteropathy in a noninvasive manner.
WHAT IS NEW HERE
The use of the broad-spectrum nonabsorbable antibiotic, rifaximin, did not improve tropical enteropathy in this randomized, double-blind, placebo-controlled trial.
The hypothesis that small-bowel bacterial overgrowth is the cause of tropical enteropathy should be reconsidered.
Financial support
Supported by Cooperative Agreement 58-6250-6001 from the United States Department of Agriculture Agricultural Research Service. Salix Pharmaceuticals donated the rifaximin. Indi Trehan is partly supported by NIH training grant T32 HD049338.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Mark J. Manary, MD.
Potential competing interests: None.
REFERENCES
- 1.Menzies IS, Zuckerman MJ, Nukajam WS, et al. Geography of intestinal permeability and absorption. Gut. 1999;44:483–9. doi: 10.1136/gut.44.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell DI, Murch SH, Elia M, et al. Chronic T cell-mediated enteropathy in rural west African children: relationship with nutritional status and small bowel function. Pediatr Res. 2003;54:306–11. doi: 10.1203/01.PDR.0000076666.16021.5E. [DOI] [PubMed] [Google Scholar]
- 3.Kelly P, Menzies I, Crane R, et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg. 2004;70:412–9. [PubMed] [Google Scholar]
- 4.Ramakrishna BS, Venkataraman S, Mukhopadhya A. Tropical malabsorption. Postgrad Med J. 2006;82:779–87. doi: 10.1136/pgmj.2006.048579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet. 1991;338:907–10. doi: 10.1016/0140-6736(91)91772-m. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons T, Fuchs GJ. Chronic enteropathy: clinical aspects. Nestle Nutr Workshop Ser Pediatr Program. 2007;59:89–101. doi: 10.1159/000098529. discussion 102–4. [DOI] [PubMed] [Google Scholar]
- 7.Fagundes-Neto U, Viaro T, Wehba J, et al. Tropicalent eropathy (environmental enteropathy) in early childhood: a syndrome caused by contaminated environment. J Trop Pediatr. 1984;30:204–9. doi: 10.1093/tropej/30.4.204. [DOI] [PubMed] [Google Scholar]
- 8.Fagundes Neto U, Martins MC, Lima FL, et al. Asymptomatic environmental enteropathy among slum-dwelling infants. J Am Coll Nutr. 1994;13:51–6. doi: 10.1080/07315724.1994.10718371. [DOI] [PubMed] [Google Scholar]
- 9.Omokhodion FO, Oyemade A, Sridhar MK, et al. Diarrhoea in children of Nigerian market women: prevalence, knowledge of causes, and management. J Diarrhoeal Dis Res. 1998;16:194–200. [PubMed] [Google Scholar]
- 10.Guarner F. Enteric flora in health and disease. Digestion. 2006;73(Suppl 1):5–12. doi: 10.1159/000089775. [DOI] [PubMed] [Google Scholar]
- 11.Brown KH, Khatun M, Ahmed G. Relationship of the xylose absorption status of children in Bangladesh to their absorption of macronutrients from local diets. Am J Clin Nutr. 1981;34:1540–7. doi: 10.1093/ajcn/34.8.1540. [DOI] [PubMed] [Google Scholar]
- 12.Carneiro Chaves FJ, Tavarela Veloso F, Cruz I, et al. Subclinical tropical enteropathy in Angola: peroral jejunal biopsies and absorption studies in asymptomatic healthy men. Mt Sinai J Med. 1981;48:47–52. [PubMed] [Google Scholar]
- 13.Chacko A, Begum A, Mathan VI. Absorption of nutrient energy in southern Indian control subjects and patients with tropical sprue. Am J Clin Nutr. 1984;40:771–5. doi: 10.1093/ajcn/40.4.771. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DI, Lunn PG, Elia M. Age-related association of small intestinal mucosal enteropathy with nutritional status in rural Gambian children. Br J Nutr. 2002;88:499–505. doi: 10.1079/BJN2002697. [DOI] [PubMed] [Google Scholar]
- 15.Ukabam SO, Cooper BT. Small intestinal permeability as an indicator of jejunal mucosal recovery in patients with celiac sprue on a gluten-free diet. J Clin Gastroenterol. 1985;7:232–6. doi: 10.1097/00004836-198506000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Goto K, Chew F, Torun B, et al. Epidemiology of altered intestinal permeability to lactulose and mannitol in Guatemalan infants. J Pediatr Gastroenterol Nutr. 1999;28:282–90. doi: 10.1097/00005176-199903000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Chen P, Soares AM, Lima AA, et al. Association of vitamin A and zinc status with altered intestinal permeability: analyses of cohort data from northeastern Brazil. J Health Popul Nutr. 2003;21:309–15. [PubMed] [Google Scholar]
- 18.Zhang Y, Lee B, Thompson M, et al. Lactulose-mannitol intestinal permeability test in children with diarrhea caused by rotavirus and cryptosporidium. Diarrhea working group, Peru. J Pediatr Gastroenterol Nutr. 2000;31:16–21. doi: 10.1097/00005176-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Bjarnason I, Macpherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–81. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 20.Meddings JB, Sutherland LR, Byles NI, et al. Sucrose: a novel permeability marker for gastroduodenal disease. Gastroenterology. 1993;104:1619–26. doi: 10.1016/0016-5085(93)90637-r. [DOI] [PubMed] [Google Scholar]
- 21.Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology. 1998;114:83–92. doi: 10.1016/s0016-5085(98)70636-5. [DOI] [PubMed] [Google Scholar]
- 22.Shulman RJ, Eakin MN, Czyzewski DI, et al. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr. 2008;153:646–50. doi: 10.1016/j.jpeds.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scarpignato C, Pelosini I. Experimental and clinical pharmacology of rifaximin, a gastrointestinal selective antibiotic. Digestion. 2006;73(Suppl 1):13–27. doi: 10.1159/000089776. [DOI] [PubMed] [Google Scholar]
- 24.Adachi JA, DuPont HL. Rifaximin: a novel nonabsorbed rifamycin for gastrointestinal disorders. Clin Infect Dis. 2006;42:541–7. doi: 10.1086/499950. [DOI] [PubMed] [Google Scholar]
- 25.Castelli F, Saleri N, Tomasoni LR, et al. Prevention and treatment of traveler's diarrhea. Focus on antimicrobial agents. Digestion. 2006;73(Suppl 1):109–18. doi: 10.1159/000089786. [DOI] [PubMed] [Google Scholar]
- 26.DuPont HL, Haake R, Taylor DN, et al. Rifaximin treatment of pathogen-negative travelers’ diarrhea. J Travel Med. 2007;14:16–9. doi: 10.1111/j.1708-8305.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 27.Gerard L, Garey KW, DuPont HL. Rifaximin: a nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections. Expert Rev Anti Infect Ther. 2005;3:201–11. doi: 10.1586/14787210.3.2.201. [DOI] [PubMed] [Google Scholar]
- 28.Prantera C, Lochs H, Campieri M, et al. Antibiotic treatment of Crohn's disease: results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment Pharmacol Ther. 2006;23:1117–25. doi: 10.1111/j.1365-2036.2006.02879.x. [DOI] [PubMed] [Google Scholar]
- 29.Gionchetti P, Rizzello F, Lammers KM, et al. Antimicrobials in the management of inflammatory bowel disease. Digestion. 2006;73(Suppl 1):77–85. doi: 10.1159/000089782. [DOI] [PubMed] [Google Scholar]
- 30.Majewski M, Reddymasu SC, Sostarich S, et al. Efficacy of rifaximin, a nonabsorbed oral antibiotic, in the treatment of small intestinal bacterial overgrowth. Am J Med Sci. 2007;333:266–70. doi: 10.1097/MAJ.0b013e3180536784. [DOI] [PubMed] [Google Scholar]
- 31.Scarpellini E, Gabrielli M, Lauritano CE, et al. High dosage rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2007;25:781–6. doi: 10.1111/j.1365-2036.2007.03259.x. [DOI] [PubMed] [Google Scholar]
- 32.Linneman Z, Matilsky D, Ndekha M, et al. Alarge-scale operational study of home-based therapy with ready-to-use therapeutic food in childhood malnutrition in Malawi. Matern Child Nutr. 2007;3:206–15. doi: 10.1111/j.1740-8709.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galpin L, Manary MJ, Fleming K, et al. Effect of Lactobacillus GG on intestinal integrity in Malawian children at risk of tropical enteropathy. Am J Clin Nutr. 2005;82:1040–5. doi: 10.1093/ajcn/82.5.1040. [DOI] [PubMed] [Google Scholar]
- 34.Anderson AD, Jain PK, Fleming S, et al. Evaluation of a triple sugar test of colonic permeability in humans. Acta Physiol Scand. 2004;182:171–7. doi: 10.1111/j.1365-201X.2004.01347.x. [DOI] [PubMed] [Google Scholar]
- 35.Huang DB, DuPont HL. Rifaximin—a novel antimicrobial for enteric infections. J Infect. 2005;50:97–106. doi: 10.1016/j.jinf.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 36.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–8. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 37.Teshima CW, Meddings JB. The measurement and clinical significance of intestinal permeability. Curr Gastroenterol Rep. 2008;10:443–9. doi: 10.1007/s11894-008-0083-y. [DOI] [PubMed] [Google Scholar]