Abstract
Elucidation of the intricate transcriptional pathways leading to neural differentiation and the establishment of neuronal identity is critical to the understanding and design of therapeutic approaches. Among the important players, the basic helix-loop-helix (bHLH) transcription factors have been found to be pivotal regulators of neurogenesis. In this study, we investigate the role of the bHLH differentiation factor Nex1/MATH-2 in conjunction with the nerve growth factor (NGF) signaling pathway using the rat phenochromocytoma PC12 cell line. We report that the expression of Nex1 protein is induced after 5 hr of NGF treatment and reaches maximal levels at 24 hr, when very few PC12 cells have begun extending neurites and ceased cell division. Furthermore, our study demonstrates that Nex1 has the ability to trigger neuronal differentiation of PC12 cells in the absence of neurotrophic factor. We show that Nex1 plays an important role in neurite outgrowth and has the capacity to regenerate neurite outgrowth in the absence of NGF. These results are corroborated by the fact that Nex1 targets a repertoire of distinct types of genes associated with neuronal differentiation, such as GAP-43, βIII-tubulin, and NeuroD. In addition, our findings show that Nex1 up-regulates the expression of the mitotic inhibitor p21WAF1, thus linking neuronal differentiation to cell cycle withdrawal. Finally, our studies show that overexpression of a Nex1 mutant has the ability to block the execution of NGF-induced differentiation program, suggesting that Nex1 may be an important effector of the NGF signaling pathway.
Keywords: neurite outgrowth, GAP-43, nerve growth factor, differential gene expression
Little is known about the transcriptional cascade of molecular events leading to neuronal differentiation and the establishment of neuronal identity. Recent studies have shown that neurogenic basic helix-loop-helix (bHLH) transcription factors are central players in distinct and interconnected subprograms that give rise to pan-neural and neuronal-specific properties (for review see Brunet and Ghysen, 1999; Goridis and Brunet, 1999). Compelling evidence suggests that each bHLH has a distinct and nonredundant function in the formation of sub-populations of neurons. Thus, the diverse number of the neurogenic bHLH factors is thought to be essential to ensure different developmental events in different lineages (for review see Lee, 1997; Guillemot, 1999). These transcription factors are characterized by two adjacent domains, a DNA-binding domain and a helix-loop-helix dimerization domain. They modulate gene expression by binding to the E-box cis-acting element (CANNTG) as dimers (for review see Massari and Murre, 2000).
The mammalian neurogenic bHLH genes have been classified into two groups based on sequence homology, temporal expression pattern, and gain and loss of functions (for review see Anderson and Jan, 1997; Lee, 1997; Guillemot, 1999). The first group, referred to as determination factors, is transiently expressed during early neurogenesis, in proliferative neural multipotent progenitor cells of the developing central nervous system (CNS). They are believed to be critical factors in determining various cell lineages. Key members include MASH-1, MATH-1, Neurogenin-1 (NeuroD3), and Neurogenin-2 (MATH4A). The second group, referred to as differentiation factors, is composed of several members, such as the NeuroD subfamily members NeuroD, NeuroD2 (NDRF), and Nex1 (MATH-2). Upon their expression, neural precursor cells undergo cell cycle arrest and acquire a mature neuronal phenotype (Lee, 1997; Farah et al., 2000). Embryonic expression of NeuroD subfamily members is restricted to the intermediate zone and cortical plate of the developing cortex and parallels overt neuronal differentiation and synaptogenesis (Schwab et al., 1998). Their persistent expression in an adult nervous system suggests a role in promoting and maintaining gene expression in mature neurons.
The objective of this study is to dissect the transcriptional network leading to terminal differentiation, by exploring the transcriptional functions of the NeuroD subfamily member Nex1. Beyond its pattern of expression, the functional knowledge of Nex1 remains largely unexplored. Nex1-null mice studies did not reveal any distinct phenotype, so no singular function could be attributed to Nex1 (Schwab et al., 1998). Thus, our initial approach is to ascertain Nex1 properties in the well-established rat pheochromocytoma PC12 cells, which exhibit neurite outgrowth and neuronal differentiation upon nerve growth factor (NGF) treatment (Greene and Tischler, 1976). More specifically, we focus on the functional contribution of Nex1 in conjunction with the NFG pathway, by generating two Nex1-overexpressing PC12 cell lines, using either wild-type Nex1 or a truncated Nex1 mutant.
In this report, we demonstrate that constitutive expression of Nex1 triggers neuronal differentiation of PC12 cells in the absence of neurotrophin factor. Our study reveals that Nex1 links neuronal differentiation to cell cycle arrest by activating the expression of neuronal-specific genes, such as GAP-43, βIII-tubulin, and NeuroD, as well as the cell cycle modulator gene p21WAF1. Our findings also show that constitutive expression of Nex1 accelerates the initial outgrowth induced by NGF treatment and promotes neurite regeneration of differentiated PC12 cells independently of a NGF boost. Finally, our results suggest that Nex1 may behave as a critical component in the execution of the NGF-induced differentiation program.
MATERIALS AND METHODS
Expression Vector Constructs
The cDNA for Nex1, kindly provided by Dr. Nave, has been described previously (Bartholoma and Nave, 1994). For bacterial expression of the 16 kDa recombinant truncated Nex1 (Nex1-Ag), the PstI-HindIII 300-bp-long carboxy-terminal fragment was subcloned in frame into the pRSET/A bacterial expression vector (Invitrogen, La Jolla, CA) to generate pRSET/A–Nex1-Ag. To generate the PC12/Nex1 stable cell line, the Nex1 cDNA sequence was cloned in frame by polymerase chain reaction (PCR) into the BamHI and EcoRI restriction sites of pcDNA6/HisC to generate pcDNA6/HisC-Nex1. To generate a control PC12 cell line, Nex1 cDNA was subcloned by PCR into the BamHI and EcoRI restriction sites of pcDNA6/HisB to create a stop codon (TAA) at the third N-terminal position of the Nex1 sequence. This plasmid is referred to as pcDNA6/HisB-Nex1-OOF (for out-of-frame). To generate the PC12/Nex1-mut1 stable cell line, the first 41 amino acids of Nex1 were deleted by PCR and the Nex1-mut1cDNA sequence was subcloned in frame into the BamHI and EcoRI restriction sites of pcDNA6/HisA, creating the pcDNA6/HisA–Nex1-mut1 plasmid.
Cell Culture and Neurite Analysis
The rat pheochromocytoma PC12 cells were grown on collagen I-coated plates (Becton Dickinson Labware, San Jose, CA) and grown under conditions as described by Chiaramello et al. (1996). P12 cells (ATCC, Rockville, MD) were differentiated in the presence of specific concentration of NGF (2.5s murine, Roche Molecular Biochemicals, Nutley, NJ) as indicated in the figure legends. Neurites were defined as a phase-dark process that was at least two cell diameters in length. To calculate the percentage of neurite-bearing cells, the experiment was carried out in triplicate and repeated four times. The error is the standard deviation of the mean, with n = 300. Neurite regeneration was determined after incubating cells with 50 ng/ml of NGF for 7 days. The culture medium was removed, and the cells were carefully washed at least three times with NGF-free medium. The neurites were then mechanically sheared by triturating cells in a pasteur pipette, and the cells were replated on collagen I-coated plates in the absence or presence of NGF (50 ng/ml). The regeneration process was examined 5 hr after replating, the percentage of neurite-bearing cells was scored on at least 300 cells, and the experiment was repeated three times.
Generation of Stable Cell Line
Stable cell lines were generated in PC12 cells using the eukaryotic expression pcDNA6 vector series (Invitrogen) that carries the blasticidin resistance gene (bsd). Therefore, transfectants were selected using blasticidin (Invitrogen) at a concentration of 5 μg/ml. Transfections were carried out using FuGENE™6 (Roche Molecular Biochemicals) according to the manufacturer’s conditions. The PC12-Nex1 stable cell line was created by using the pcDNA6/HisC-Nex1 plasmid, whereas the control stable cell line (PC12-Nex1-OOF) was engineered using the pcDNA6/HisB–Nex1-OOF plasmid. Finally, the pcDNA6/HisC–Nex1-mut1 plasmid was used to create the PC12–Nex1-mut1 stable cell line.
Western Blot Analysis
Bacterial whole-cell extracts were prepared as described by Uittenbogaard et al. (1999). PC12 cells and stably transfected PC12 cells were lysed in M-Per mammalian protein extraction buffer (Pierce, Rockford, IL). Cell extracts were spun at 13,000 rpm for 10 min, and protein concentration was determined by Bradford assay (Bio-Rad, Hercules, CA). Proteins were resolved on either a 15% or 10% SDS-PAGE gels and transferred onto nitrocellulose membrane. Nex1 levels were determined using our polyclonal antibody (1:10,000). Transfected recombinant Nex1 protein was detected by the anti-Xpress-horseradish peroxidase (HRP) monoclonal antibody (Invitrogen) at a 1:5,000 dilution. NeuroD levels were determined using our polyclonal antibody (1:4,000). A mouse monoclonal anti-GAP-43 antibody (Zymed, South San Francisco, CA) was used at a 1:1,000 dilution, a mouse monoclonal anti-βIII-tubulin (Covance, Richmond, CA) at a 1:1,000 dilution, and a mouse monoclonal anti-p21WAF1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used at a 1:400 dilution. An HRP-conjugated secondary antibody (Pierce) was used at a 1:100,000 dilution. The antigen–antibody complex was detected using the SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce).
Protein Purification and Antibody Production
The bacterial expression vector pRSETA/Nex1-Ag was transformed into the Escherichia coli strain BL21 DE3pLysS (Invitrogen). The polyhistidine-containing recombinant Nex1-Ag was purified to 99% homogeneity by Ni2+-NTA chromatography (Qiagen, Chatsworth, CA) as described by Chiaramello et al. (1995). The purified Nex1-Ag recombinant protein was suspended in a buffer containing 10% glycerol, 20 mM HEPES (pH 7.2), 1 mM EDTA, 0.1 M NaCl, 1 mM dithiothreitol (DTT), and 1 mM phenylmethylsulfonyl fluoride (PMSF), and injected in New Zealand white rabbits as described by Chiaramello et al. (1995). The IgG fraction was purified by affinity chromatography using protein G Sepharose Fast Flow (Pharmacia, Piscataway, NJ), and the titer (1:10,000) was determined by an ELISA assay and the specificity tested by western blot using whole-cell extracts.
RESULTS
Nex1 Expression Is Induced Upon NGF Treatment in PC12 Cells
To investigate the transcriptional events controlled by Nex1 during neuronal differentiation, we used the PC12 cell line, which is a well-established system with which to study neurite outgrowth and terminal differentiation (Greene and Tischler, 1976). Initially, we examined the temporal expression pattern of Nex1 protein during NGF exposure by immunoblot analysis. For this purpose, we generated a polyclonal antibody against the 16 kDa recombinant carboxy-terminal portion of Nex1 protein (Nex1-Ag). To avoid cross reactivity with other bHLH proteins, Nex1-Ag did not carry the common bHLH motif; however, it contained a stretch of six histidines for purification purposes (Fig. 1). We used the pLysS E. coli strain and the T7 RNA polymerase system to express the recombinant Nex1-Ag protein. Nex1-Ag protein was purified to homogeneity (99%) by Ni2+-NTA chromatography and visualized on a silver-stained SDS gel. We first tested the specificity of our antibody by western assay, using bacterial whole-cell extracts overexpressing Nex1-Ag before and after 3 hr of IPTG induction. Figure 1A,B shows that Nex1 antigen is expressed at low levels prior to the IPTG induction (t = 0). This leakiness has often been observed with the pLysS strain, which is known to express low levels of T7 RNA polymerase in the absence of IPTG (Studier and Moffat, 1986; Studier et al., 1990). The generated Nex1 antibody detected a single band migrating at the expected MW of Nex1-Ag (16 kDa) in uninduced and induced bacterial cell extracts and did not react with other bacterial proteins, establishing the specificity of the generated antibody (Fig. 1B). Its specificity was further validated by the observation that it failed to detect any mammalian proteins in undifferentiated PC12 cells (Fig. 1C). It was previously reported that naïve PC12 cells do not express Nex1 (Bartholoma and Nave, 1994). Finally, the specificity of the Nex1 antibody was corroborated by the fact that it detected only a 36 kDa protein upon NGF induction of PC12 cells. Thus, the anti-Nex1 polyclonal antibody failed to cross-react with other NGF-induced bHLH proteins, such as NeuroD, as expected (Fig. 1C).
Fig. 1.
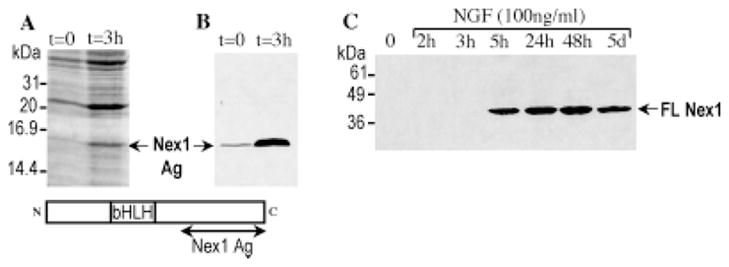
Bacterial expression of the truncated Nex1-Ag protein and NGF-induced expression of full-length Nex1 expression in PC12 cells. A: Coomassie blue staining of SDS-PAGE-separated E. coli whole-cell extracts expressing the truncated recombinant Nex1 protein Nex1-Ag. Bacterial whole-cell extracts were prepared before and after induction with IPTG as indicated at the top. Molecular weights (kDa) are indicated on the left, and the overexpressed truncated Nex1-Ag protein is indicated by an arrow. A schematic representation of the full-length Nex1 protein is shown at the bottom, to illustrate the C-terminal portion of Nex1 (Nex1-Ag) used to raise the anti-Nex1 rabbit polyclonal antibody. B: Specificity of the anti-Nex1 polyclonal antibody. Overexpressing truncated Nex1-Ag bacterial whole-cell extracts before and after IPTG induction were analyzed by western blot using the generated anti-Nex1 polyclonal antibody. The antigen–antibody complexes were detected by chemiluminescence. Truncated Nex1 protein is detected prior to IPTG induction because of leaky expression of the T7 RNA polymerase in the pLysS strain, resulting in a basal level of Nex1 expression. Among the many thousands proteins present in the uninduced and induced whole-cell extracts, only a single band at the expected MW is detected by the anti-Nex1 antibody. C: Timing of full-length Nex1 expression in untreated and NGF-treated PC12 cells. PC12 cells were grown in the absence or presence of NGF (100 ng/ml) for specific periods of time as indicated at the top. Nex1 protein was detected by Western assay using the anti-Nex1 polyclonal antibody. Nex1 protein is detected after 5 hr of NGF treatment and remains highly expressed after 5 days of NGF exposure.
To determine whether Nex1 is expressed during NGF-induced differentiation of PC12 cells, we performed a time-course analysis using PC12 whole-cell extracts before and during NGF treatment (100 ng/ml). Figure 1C shows that Nex1 expression is triggered upon NGF exposure in PC12 cells. We found that induction of Nex1 expression was detected only after 5 hr of NGF treatment and reached maximal levels at 24 hr (Fig. 1C). Nex1 protein remained expressed at significant levels after 5 days of NGF treatment (Fig. 1C). These results suggest that Nex1 may be an inducible component of the NGF signaling pathway and that the Nex1 gene may behave as a “delayed-response” gene rather than an immediate early gene. This pattern of expression is also in agreement with the known in vivo expression pattern of Nex1 during neurogenesis (Bartholoma and Nave, 1994; Schwab et al., 1998). Finally, these observations indicate that the PC12 cell line is an appropriate model with which to investigate the molecular mechanisms of action of Nex1 during the neuronal differentiation process.
Overexpression of Nex1 Leads to Neurite Outgrowth and Differentiation of PC12 Cells in the Absence of NGF
To dissect the potential functions of Nex1 during neuronal differentiation, we investigated whether Nex1 could trigger differentiation of PC12 cells in the absence of NGF. For this purpose, we engineered a PC12 stable cell line that constitutively expressed Nex1 under the control of the CMV promoter. To distinguish the recombinant Nex1 protein from the endogenous Nex1 protein, we subcloned the Nex1 cDNA sequence into the eukaryotic expression vector pcDNA6/HisC in frame downstream of an N-terminal peptide encoding a tag epitope (Xpress epitope) recognized by a monoclonal antibody (Invitrogen). As a negative control, we generated a PC12 stable cell line (PC12-OOF) that did not express Nex1 because of an out-of-frame insertion of the Nex1 cDNA into pcDNA6/HisB, creating a stop codon right after the first Nex1 amino acid in the pcDNA6/OOF plasmid (see Materials and Methods).
Upon constitutive expression of Nex1 in untreated PC12 cells, we obtained two types of phenotype, an extreme differentiation phenotype and a moderate differentiation phenotype. During the early stages of the selection process (first 2 weeks), we observed a dozen clusters of cells displaying an extreme differentiation phenotype. These cells, referred to as PC12–Nex1-E cells, progressively differentiated and established small clusters of neurite network on the transformed cell culture plate (Fig. 2). These cells failed to propagate and survive beyond 1 month, suggesting that they were terminally differentiated. The presence of these PC12–Nex1-E clusters was consistently observed in three independent transfection experiments, suggesting that the level of Nex1 expression may modulate the degree of differentiation phenotype. As expected, the selection of the negative control stable cells PC12-OOF failed to display similarly differentiated phenotype. They showed morphological characteristics identical to those of untreated PC12 cells (Fig. 2).
Fig. 2.
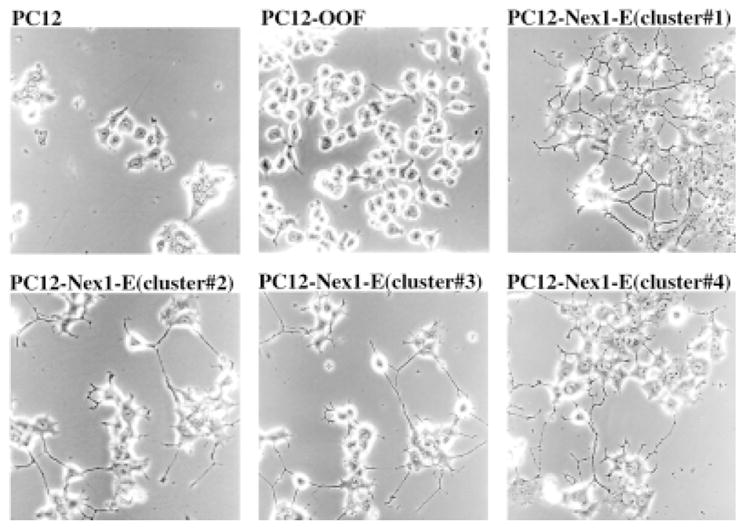
Constitutive expression of Nex1 in PC12 cells generates several clusters displaying an extreme differentiation phenotype. PC12 cells were stably transfected with either the eukaryotic expression vector pcDNA6/Nex or the control vector pcDNA/OOF, which contains a stop codon right after the Nex1 first amino acid. On the transformed plate, a dozen PC12–Nex1-E clusters established an extensive neurite network independently of NGF induction. In contrast, PC12 cells and control pC12-OOF cells failed to display such a differentiation phenotype. Four of these PC12–Nex1-E clusters are illustrated.
The selection process also gave rise to several PC12-Nex1 clones displaying a moderate differentiation phenotype (Fig. 3). Three representative clones, named PC12–Nex1-M(A), PC12–Nex1-M(B), and PC12–Nex1-M(C), were studied for each experimental procedure to reduce the effect of clonal variability on data interpretation. The constitutive overexpression of the recombinant Nex1 protein in these clones was confirmed by western blot analysis using either the anti-Nex1 polyclonal antibody or the anti-Xpress monoclonal antibody specific for recombinant protein. We found that Nex1 protein was expressed at similar levels in the three PC12–Nex1-M clones (Fig. 3A,B). As expected, PC12 cells did not express endogenous Nex1 protein or recombinant Nex1 protein (Fig. 3A,B). These PC12–Nex1-M clones exhibited a generation time of 7 days, whereas PC12 cells and PC12-OOF control cells were doubling every 3.5 days (data not shown). They also acquired some neuronal differentiation characteristics, such as flattened cell body and neurite extensions (Fig. 3D–F). Our statistical analysis revealed that the three PC12–Nex1-M clones exhibited a 25% increase in neurite outgrowth in the absence of NGF (Fig. 3C). Thus, taken together, these results suggest that Nex1 may execute key initial steps of neuronal differentiation similarly to the NGF-induced differentiation process.
Fig. 3.
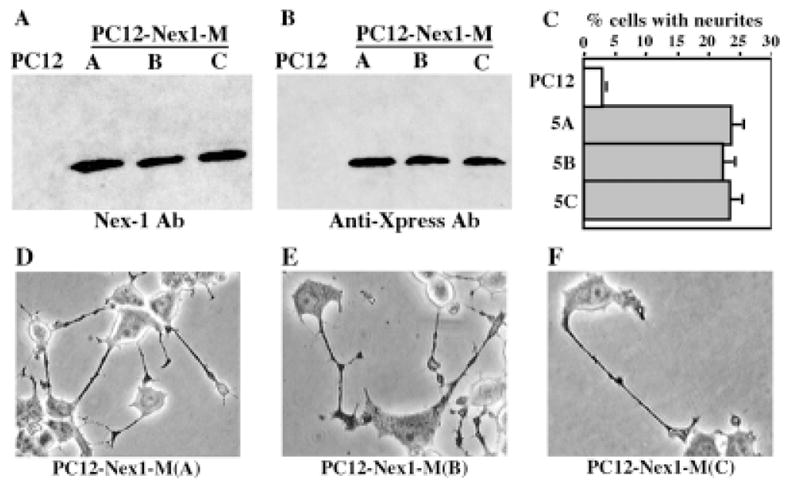
Overexpression of Nex1 induces a substantial spontaneous neurite outgrowth in untreated PC12–Nex1-M clones. The expression level of exogenous Nex1 protein was assessed by Western blot analysis using equal amounts of total protein from control PC12 cells and three stable PC12–Nex1-M(A), PC12–Nex1-M(B), and PC12–Nex1-M(C) clones. The three PC12–Nex1-M clones express similar amounts of the Nex1 protein, whereas control PC12 cells do not express Nex1 protein. Nex1 was detected with either our polyclonal antibody (A) or the anti-Xpress antibody raised against the N-terminal tag epitope (B). C: The clones PC12–Nex1-M(A), PC12–Nex1-M(B), and PC12–Nex1-M(C) show a significant increase in spontaneous neurite projections compared with PC12 cells. This is a representative graph from a triplicate experiment that was repeated four times. The error is the standard deviation of the mean. D–F: The phase-contrast micrograph of PC12–Nex1-M cells reveals their moderate differentiation phenotype. Cells from the three stably transfected PC12–Nex1-M clones are characterized by a flattened cell body and spontaneous neurite extensions.
Constitutive Expression of Nex1 Increases NGF Effect on Neurite Outgrowth
The effect of Nex1 on promoting neurite outgrowth prompted us to examine whether Nex1 could accelerate the NGF response in PC12–Nex1-M cells. Previous studies have shown that neurite outgrowth in NGF-treated PC12 cells is initiated only after a lag period of 24 hr (Burstein and Greene, 1978; Greene et al., 1980; Greene and Tischler, 1982), so we focussed our analysis on that initial phase. Both PC12–Nex1-M and control PC12 cells were treated with NGF (50 ng/ml) for 6, 24, or 48 hr, and neurite processes were determined as described in Materials and Methods. After 6 hr of NGF treatment, 50% of PC12–Nex1-M cells already displayed substantial neurite outgrowth (Fig. 4A), compared to 25% with PC12–Nex1-M cells at t = 0 hr (Fig. 3D). Furthermore, the neurite elongation of PC12–Nex1-M cells was comparable to that observed in PC12 cells following 48 hr of NGF treatment (Fig. 4B). In contrast, at t = 6 hr, PC12 cells did not yet elaborate any significant neurite extension (Fig. 4A,B). After 24 hr of NGF exposure, PC12–Nex1-M cells started establishing a neurite network (Fig. 4B). By 48 hr of NGF treatment, nearly all the PC12–Nex1-M cells bore numerous and elongated neurite extensions, thus generating an extensive neurite network, whereas only 50% of PC12 cells displayed neurite extension (Fig. 4A,B). The control PC12-OOF cells behaved in a manner similar to PC12 cells in terms of neurite outgrowth during the time-course analysis (data not shown). Although Figure 4 shows the results obtained only from the PC12–Nex1-M(B) clones, similar results were observed with the other two clones, PC12–Nex1-M(A) and PC12–Nex1-M(C). Thus, these results indicate that constitutive expression of Nex1 in PC12–Nex1-M cells is sufficient to enhance NGF response and to induce accelerated initial neurite outgrowth. These observations suggest that the PC12–Nex1-M cells may be transcriptionally conditioned in a manner that parallels the NGF signaling-mediated transcription, thus resulting in a faster NGF response.
Fig. 4.
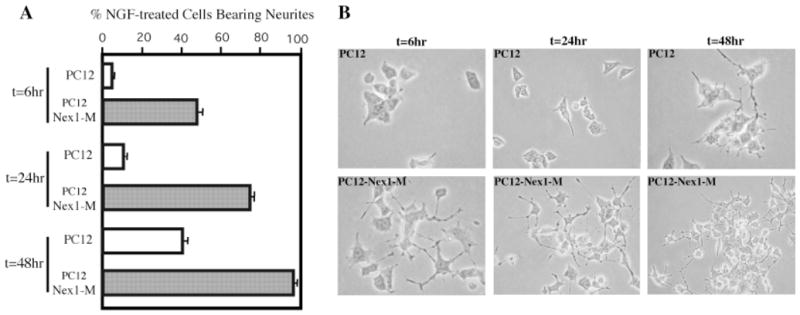
Constitutive expression of Nex1 accelerates the initial neurite outgrowth in NGF-treated PC12–Nex1-M cells. PC12 and PC12–Nex1-M(B) cells were grown on collagen I-coated plates and treated with NGF (50 ng/ml) for 6, 24, or 48 hr as indicated and neurite outgrowth was determined as described in Materials and Methods. A: Quantitation of the degree of neurite outgrowth observed in the NGF-treated PC12 and PC12–Nex1-M cells. The percentage of cells bearing neurites is consistently higher upon constitutive expression of Nex1 during the NGF treatment. PC12 cells are represented by open bars, whereas PC12–Nex1-M(B) cells are indicated by shaded bars. This is a representative graph from a triplicate experiment that was repeated four times. The error is the standard deviation of the mean. B: PC12–Nex1-M(B) cells already extend neurites after 6 hr of exposure to NGF, whereas PC12 cells require at least 24 hr of NGF treatment to extend neurites. Similar results were obtained with the two other PC12–Nex1-M clones. Micrographs of NGF-treated PC12–Nex1-M cells at t = 24 and 48 hr were taken at low magnification to reveal the appearing neurite network.
Constitutive Expression of Nex1 Promotes Neurite Regeneration of NGF-Differentiated PC12-Nex1 Cells
In view of our observations that Nex1 promotes neurite outgrowth, we investigated whether constitutive expression of Nex1 could either promote or enhance neurite regeneration of differentiated PC12–Nex1-M cells. To test this possibility, we differentiated PC12 cells and PC12–Nex1-M(B) cells with NGF (50 ng/ml) for 7 days, and we then mechanically sheared their neurites by trituration after extensive washes to remove NGF. Neurite regeneration was analyzed in the absence and presence of NGF (50 ng/ml) for 5 hr. As expected, PC12 cells showed limited neurite regeneration (21%) without an NGF boost, whereas PC12–Nex1-M(B) cells displayed substantial neurite regeneration (62%; Fig. 5B). Furthermore, PC12–Nex1-M cells were able to form a new neurite network readily (Fig. 5A). Although Figure 5 illustrates neurite regeneration obtained from PC12–Nex1-M(B) clone, similar results were seen with PC12–Nex1-M(A) and PC12–Nex1-M(C) clones. In the presence of NGF, PC12–Nex1-M(B) cells still exhibited significantly greater neurite regeneration than PC12 cells, suggesting that constitutive expression of Nex1 may enhance the propensity to initiate neurite outgrowth (Fig. 5B). This experiment indicates that constitutive expression of Nex1 is capable of triggering the neurite regeneration process in the absence of an NGF boost. Thus, these results further support the idea that a Nex1 transcription-dependent pathway may be emulating critical steps of the NGF-mediated pathway.
Fig. 5.
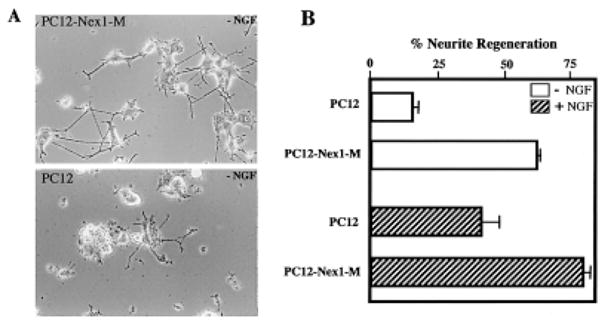
Ectopic expression of Nex1 promotes the neurite regeneration of NGF-differentiated cells in the absence of an NGF boost. PC12 cells and PC12–Nex1-M(B) cells were differentiated for 7 days in the presence of NGF (50 ng/ml), after which the cells were thoroughly washed with NGF-free medium. The neurites were mechanically sheared as described in Materials and Methods. The cells were replated on collagen I-coated plates in the absence or presence of NGF (50 ng/ml). The regeneration process was analyzed 5 hr after replating. A: PC12–Nex1-M(B) cells display substantial neurite regeneration in the absence of NGF compared to PC12 cells. B: Ectopic expression of Nex1 enhances the propensity to initiate neurite outgrowth even in the absence or presence of NGF. The percentage of neurite-bearing cells was scored on at least 300 cells from three distinct experiments. Cells cultured in the absence of NGF after trituration are indicated by open bars, whereas cells grown in the presence of NGF after trituration are represented by hatched bars. This is a representative graph from a triplicate experiment that was repeated four times. The error is the standard deviation of the mean.
Overexpression of Nex1 Induces Expression of Neuronal-Specific Genes and Cell Cycle Modulator in the Absence of NGF
To understand how constitutive expression of Nex1 modulates the genetic program of PC12 cells to promote neuronal differentiation in the absence of NGF, we assessed the expression of several markers associated with neuronal differentiation in the three PC12–Nex1-M clones. Considering our observations that constitutive expression of Nex1 promotes neurite outgrowth and regeneration, we focussed on the growth-associated protein GAP-43, because increased GAP-43 expression has been associated with neurite outgrowth in PC12 cells (Yankner et al., 1990; Mobarak et al., 2000). Immunoblot analysis using a monoclonal mouse antibody against GAP-43 (Zymed) revealed that overexpression of Nex1 increased the expression of GAP-43 protein in the absence of NGF (Fig. 6A). Thus, this result is consistent with the observed increased spontaneous neurite outgrowth of untreated PC12–Nex1-M cells (Figs. 2, 3).
Fig. 6.
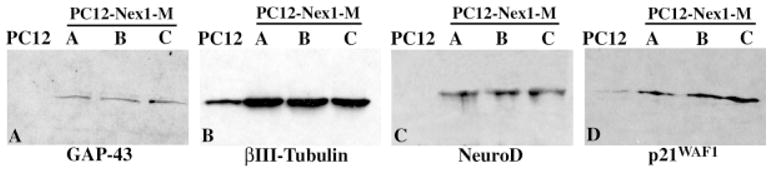
Overexpression of Nex1 induces expression of several genes associated with neuronal differentiation in the absence of NGF. Western blot analyses comparing the expression of neuronal marker protein using equal amounts of total protein from PC12 cells and the three PC12–Nex1-M(A), PC12–Nex1-M(B), and PC12–Nex1-M(C) clones. The specific antibodies used for this study are described in Materials and Methods. The antigen–antibody complexes were detected by chemiluminescence. Overexpression of Nex1 in untreated cells stimulates the expression of the GAP-43 protein (A), the neuronal specific βIII-tubulin protein (B), the bHLH differentiation factor NeuroD (C), and the mitotic inhibitor p21WAF1 protein (D). As expected, PC12 cells do not express GAP-43 and NeuroD proteins. The p21WAF1 protein and the neuronal-specific βIII-tubulin protein are expressed at much lower levels in PC12 cells.
Because PC12 differentiation is accompanied by cytoskeletal changes particularly involving microtubules (Tischler and Greene, 1978; Luckenbill-Edds et al., 1979), we investigated whether overexpression of Nex1 could modulate the expression level of the neuronal-specific βIII-tubulin. By immunoblot analysis using the mouse monoclonal antibody TuJ1, we observed a threefold increase in βIII-tubulin expression upon Nex1 overexpression in the three PC12–Nex1-M clones (Fig. 6B). This finding is consistent with the notion that Nex1 modulates a specific program of gene expression promoting neurite outgrowth and neuronal differentiation.
We investigated whether overexpression of Nex1 could activate the expression of the bHLH differentiation factor NeuroD, because they are coexpressed during neurogenesis (Schwab et al., 2000). We observed increased levels of the NeuroD protein in all three PC12–Nex1-M clones, whereas PC12 cells did not express any detectable levels of NeuroD (Fig. 6C). These data are consistent with the in vivo cross-regulatory network among neural bHLH genes occurring during neurogenesis (Lee et al., 1995; Ma et al., 1996; Cau et al., 1997).
The fact that neuronal differentiation is preceded by withdrawal from the cell cycle and that PC12–Nex1 cells display a doubling time twice as long as that of PC12 cells, we addressed the question of whether overexpression of Nex1 may induce the expression of cell cycle modulators. We focused on the mitotic inhibitor p21WAF1, because its expression increases during NGF-induced differentiation of PC12 cells (Dobashi et al., 1995). Immunoblot analysis using a mouse monoclonal antibody against p21 (Santa Cruz Biotechnology) revealed that all three PC12–Nex1-M clones expressed higher levels of p21 protein compared to PC12 cells (Fig. 6D). Taken together, these results show that Nex1 activates the expression of neuronal-specific genes and cell cycle regulators, linking neuronal differentiation and cell cycle arrest. Finally, these results imply that the spectrum of genes targeted by Nex1 may overlap with the NGF signaling-mediated transcriptional pathway.
Constitutive Expression of Mutated Nex1 Blocks NGF-Induced Differentiation of PC12 Cells
To challenge further the notion that Nex1 may be a critical effector of the NGF pathway, we generated a stable PC12 cell line constitutively expressing a dominant mutant-like form of Nex1, Nex1-mut1, which does not contain the critical N-terminal transcriptional domain TAD1. Our Nex1 transcriptional analysis revealed that TAD1 is specific and essential for the Nex1-mediated activation of GAP-43 promoter (Uittenbogaard and Chiaramello, manuscript in preparation). The Nex1-mut1 protein was expressed from the high-expression-level CMV promoter (see Material and Methods). We obtained several stable PC12–Nex1-mut1 clones, which exhibited round cell morphology accompanied by a lack of spontaneous neurite process compared to PC12 cells and control PC12-OOF cells. We found that four PC12–Nex1-mut1 clones did not responded to NGF treatment and that these mutant clones failed to differentiate for up to 6 days of NGF exposure (Fig. 7). However, with two other PC12–Nex1-mut1 clones, we observed a delayed response to NGF characterized by the presence of a few short neurite extensions at 6 days of NGF treatment (data not shown). As expected, control PC12-OOF cells behaved in a manner similar to PC12 cells after 6 days of NGF exposure (Fig. 7). Thus, it appears that there is a critical equilibrium between the levels of the recombinant Nex1-mut1 protein, the NGF-induced endogenous Nex1 protein, and the time of NGF exposure to determine the outcome of the NGF program. Altogether, these results imply that the Nex1-mut1 protein most likely acts in a dominant-negative fashion and may consequently interfere with NGF-induced gene expression, thus blocking NGF-induced neuronal differentiation.
Fig. 7.
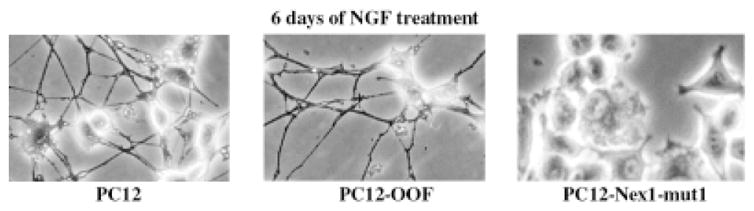
Constitutive expression of mutated Nex1-mut1 blocks NGF-induced differentiation in PC12 cells. PC12 cells were stably transfected with either the eukaryotic expression vector pcDNA6/Nex1-mut1 or the control expression vector pcDNA6/OOF. PC12, control PC12-OOF, and PC12–Nex1-mut1 cells were grown on collagen I-coated plates and differentiated with NGF (25 ng/ml) for 6 days.
DISCUSSION
The major goal of this study is to elucidate the dynamic interplay between the intrinsic functions of Nex1 and specific extrinsic signals responsible for generating transcriptional programs leading to neuronal differentiation. In this analysis, we provide compelling evidence that Nex1 is an important player in the NGF signaling pathway. Furthermore, we demonstrate that Nex1 is able to carry out independently key steps of neuronal differentiation as well as modulate molecular events leading to neurite regeneration.
The original motivation for examining Nex1 expression during NGF-induced differentiation of PC12 cells was that most studies of bHLH proteins have been concentrated on their roles during the different steps of neurogenesis. However, their contribution to the transcription-dependent pathways in association with neurotrophin-induced differentiation remains poorly explored. A time-course analysis of Nex1 expression upon NGF induction suggests that the Nex1 gene may behave as a delayed response gene rather than an immediate early gene. The fact that Nex1 expression peaks when very few PC12 cells have begun extending neurites and ceased cell division implies that Nex1 expression is triggered at the onset of neuronal differentiation and precedes cell cycle withdrawal. This is consistent with the cell cycle arrest-inducing properties of the other two members of the NeuroD subfamily, NeuroD and NeuroD2 (Farah et al., 2000; Olson et al., 2001). This raises the issue of the transcriptional mechanisms responsible for triggering Nex1 expression at the onset of NGF-induced differentiation. Based on the presence of a perfect CRE regulatory element in the proximal Nex1 promoter, it is conceivable that Nex1 expression is under the control of CREB, a critical mediator of NGF-dependent gene transcription. Alternatively, Nex1 expression may also be under the control of an upstream bHLH cross-regulatory network, insofar as functional E-boxes are present in its 5′-regulatory region (Bartholomae and Nave, 1994). Potential bHLH candidates are the SHARP-1 and SHARP-2 transcription factors, which behave as immediate early genes upon NGF induction (Rossner et al., 1997).
To unravel the transcriptional functions of Nex1 during neuronal differentiation, we generated a Nex1-overexpressing PC12 cell line. Unlike the case for NeuroD and NeuroD2, no gain of function studies have been performed for Nex1 (Lee et al., 1995; McCormick et al., 1996). We found that constitutive overexpression of Nex1 promotes neuronal differentiation and mimics the effects of NGF in terms of neurite outgrowth and gene expression. The facts that Nex1 expression is regulated by NGF and that ectopic expression of Nex1 is capable of promoting a differentiation phenotype suggest that Nex1 may be an important effector of the NGF-mediated differentiation program. However, the degree of neuronal differentiation induced by ectopic expression of Nex1 appears to be modulated by Nex1 levels, as illustrated by the two types of phenotypes obtained during the selection of the PC12-Nex1 cell line. The extreme differentiated phenotype was observed in several clusters of cells during the early steps of the selection process and consisted of an extensive neurite network and cessation of proliferation. In contrast, the PC12–Nex1-M clones displaying a moderate phenotype could be propagated, albeit with a longer generation time than control PC12 cells. These cells were characterized by a flat and elongated cell body, increased neurite outgrowth, and increased adherence. Morphological characteristics similar to those of PC12–Nex1-M clones have been previously reported upon overexpression of NeuroD in PC12 cells, which also appears to be correlated with the levels of ectopic NeuroD expression (Noma et al., 1999). Furthermore, these studies show that NeuroD expression is triggered only upon NGF treatment. These prior results complement our findings that Nex1 up-regulates the expression of NeuroD protein in the absence of NGF. The fact that NeuroD is expressed during the progressive transition from proliferative to differentiating postmitotic PC12 cells is in agreement with recent in vivo results showing NeuroD expression in proliferating neural precursor cells (Miyata et al., 1999; Morrow et al., 1999; Liu et al., 2000). The expression of NeuroD protein in PC12–Nex1-M cells is most likely a direct regulatory effect of Nex1, because the human and mouse NeuroD promoter sequences contain functional conserved E-boxes involved in autoactivation as well as transactivation (Miyachi et al., 1999). This cross regulation is in accordance with the overlapping spatiotemporal patterns of expression of Nex1 and NeuroD during neurogenesis (Schwab et al., 2000). However, their precise timing of expression relative to each other and their degree of overlapping expression during NGF-induced differentiation remain to be defined.
One of the critical steps during initiation of the neuronal differentiation process is permanent withdrawal from the cell cycle. Our results suggest that a critical level of Nex1 may be necessary to trigger permanent cell cycle withdrawal, implying that Nex1 may modulate the expression of cell cycle regulators. Prior studies have reported that NGF induces neuronal differentiation of PC12 cells through the p21 pathway, resulting in an increased p21WAF1 protein expression (Yan and Ziff, 1995; van Grunsven et al., 1996; Erhardt and Pittman, 1998a). The fact that Nex1 up-regulates the expression of p21WAF1 protein in the slowly growing PC12–Nex1-M cells is consistent with these published results. However, unlike the case for Nex1, overexpression of p21WAF1 protein is not sufficient to trigger neuronal differentiation of PC12 cells (Erhardt and Pittman, 1998b). It is only in the presence of EGF that overexpression of p21WAF1 protein leads to neurite outgrowth (Erhardt and Pittman, 1998a). It is reasonable to speculate that the increased p21WAF1 protein expression may be the result of a direct Nex1 transcriptional modulation of the p21 promoter activity, because the mouse, rat, and human p21 promoter sequences contain multiple functional conserved E-boxes (Halevy et al., 1995; Prabhu et al., 1997). There are several paradigms of direct bHLH-mediated activation of the p21 promoter. MyoD up-regulates the level of p21WAF1 protein in muscle cells, and ectopic expression of NeuroD in HeLa cells increases the expression of p21WAF1 protein, resulting in cell cycle withdrawal (Halevy et al., 1995; Mutoh et al., 1998).
Another critical step of neuronal differentiation is the onset of neurite outgrowth. A wealth of evidence indicates that GAP-43 expression is critical to the establishment of axonal outgrowth during the initiation and remodeling of neural connections (Zuber et al., 1989; Benowitz and Perrone-Bizzozero, 1991; Aigner et al., 1995; Strittmatter et al., 1995; Benowitz and Routtenberg, 1997; Mani et al., 2000). Our study implies that there is a direct correlation between Nex1 expression and the increased expression of GAP-43 protein, as well as neurite outgrowth. This is most likely the result of a direct Nex1-mediated transcriptional regulation of the GAP-43 gene, as Nex1 binds to the conserved proximal E1–E2 cluster and up-regulates the GAP-43 promoter activity (Chiaramello et al., 1996; Uittenbogaard and Chiaramello, manuscript in preparation). Our observations appear to differ from previous studies reporting a poor GAP-43 protein expression in untreated PC12 cells as a result of a rapid decay of GAP-43 mRNA (Federoff et al., 1988; Perrone-Bizzozero et al., 1991, 1993). The GAP-43 mRNA is stabilized only upon NGF treatment, through a posttranscriptional regulation involving neuronal-specific RNA-binding proteins (Kohn et al., 1996; Irwin et al., 1997). In fact, overexpression of the RNA-binding protein HuD in untreated PC12 cells results in an increase of GAP-43 mRNA stability, accompanied by an increased spontaneous neurite outgrowth (Tsai et al., 1997; Mobarak et al., 2000; Anderson et al., 2000). These apparently contradictory findings could be explained by the ectopic expression of Nex1, which may regulate mRNA-binding protein-encoding genes, in a manner similar to NGF. Thus, the observed increased expression of GAP-43 protein in untreated PC12–Nex1-M cells might be the result of a combined transcriptional and posttranscriptional regulation of the GAP-43 gene. This dual regulation may be extended to the p21WAF1 gene, which is known to be regulated at both the transcriptional level by distinct transcription factors and the posttranscriptional level through the mediation of the mRNA-binding protein HuD (Halevy et al., 1995; Prabhu et al., 1997; Joseph et al., 1998). However, the fact that the differentiation-promoting genes, GAP-43, NeuroD, and p21WAF1, have E-boxes in their respective promoters, combined with the notion that overexpression of Nex1 may kinetically be the driving force in this situation, strongly implies that it is a direct effect, especially in the case of GAP-43, which was previously shown not to be regulated by NeuroD (McCormick et al., 1996). Nevertheless, this does not rule out that NeuroD is also regulating the other genes examined. The bHLH proteins are known to participate in a complex auto-regulatory and cross-regulatory network with each other.
It is known that neurite outgrowth in NGF-treated PC12 cells is initiated only after a 24 hr lag, which correlates with the full induction of GAP-43 expression (Costello et al., 1990). Thus, the increased GAP-43 expression in PC12–Nex1-M cells may in part be responsible for the accelerated NGF responsiveness and initial neurite outgrowth observed in NGF-treated PC12–Nex1-M cells. Our findings are consistent with a previous report showing that overexpression of GAP-43 in PC12 cells leads to increased NGF responsiveness and accelerated neurite outgrowth (Yankner et al., 1990). Thus, PC12–Nex1-M cells are transcriptionally “preprogrammed” to execute the NGF-induced neuronal differentiation program. Hence, it is reasonable to speculate that the accelerated NGF response may be due to a “priming” effect. In this case, the priming effect would appear to depend on the presence of a critical pool of differentiation-promoting transcripts generated by recombinant Nex1. However, beyond the first 5 hr, the possibility cannot be excluded that the increased NGF responsiveness is also partly due to the additional induction of the endogenous Nex1, which would result in higher levels of total Nex1 protein.
The notion that Nex1 plays a regulatory role in neurite outgrowth is further substantiated by Nex1’s ability to regenerate neurite outgrowth in the absence of NGF. Our results suggest that constitutive expression of Nex1 may promote a more favorable pool of regeneration-dependent transcripts present at the time of the neural network disruption, such as GAP-43 and βIII-tubulin. To support this notion further, it has been shown that constitutive expression of GAP-43 results not only in an accelerated NGF response but also in an enhanced regenerative response in NGF-treated PC12 cells (Yankner et al., 1990). A complementary study has shown that neurite regeneration of NGF-treated PC12 cells is the result of the accumulation of RNA transcripts during the NGF priming period (Twiss and Shooter, 1995). This raises the question of whether the spectrum of genes targeted by Nex1 during initial neurite outgrowth overlaps with the Nex1-mediated program of gene expression during neurite regeneration. The fact that the GAP-43 and βIII-tubulin genes participate in both processes lends credence to this notion (Skeene, 1989; Yankner et al., 1990; Moskowitz et al., 1993; Moskowitz and Oblinger, 1995; Bomze et al., 2001; Woolf, 2001). Finally, the critical regulatory role of Nex1 during neurite outgrowth is further corroborated by the phenotype of the NGF-treated PC12–Nex1-mut1 cells. Constitutive expression of the truncated Nex1 mutant mut1 prevents NGF-induced differentiation, confirming that Nex1 modulates key NGF transcription-dependent pathways.
In conclusion, our results support the idea that Nex1 is a critical effector of the NGF pathway. Whether Nex1 participates in other NGF-dependent pathways is a key differentiation effector in other extrinsic signaling pathways is a critical question that remains to be addressed. More specifically, it is critical to explore the question of whether Nex1 also indirectly modulates the expression of certain target genes through a posttranscriptional regulation involving the expression of mRNA-stabilizing proteins. Finally, it will be illuminating to carry out a comprehensive analysis of Nex1-mediated gene regulation at different steps of neuronal differentiation and neurite regeneration.
Acknowledgments
This work was supported by NSF grant IBN 9727443 and NIH grant R01-NS41391 to A.C. We thank Dr. Nave for providing the Nex1 cDNA clone. We also thank Dr. Robyn Rufner for her assistance and technical help at the Center for Microscopic Image Analysis.
References
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Jan YN. The determination of neuronal phenotype. In: Cowan WM, Jessel TM, Zipursky SL, editors. Molecular and cellular approaches to neural development. New York: Oxford University Press; 1997. pp. p26–63. [Google Scholar]
- Anderson KD, Morin MA, Beckel-Mitchener A, Mobarak CD, Neve RL, Furneaux HM, Burry R, Perrone-Bizzozero NI. Overexpression of HuD, but not of its truncated form HuD I+II, promotes GAP-43 gene expression and neurite outgrowth in PC12 cells in the absence of nerve growth factor. J Neurochem. 2000;75:1103–1114. doi: 10.1046/j.1471-4159.2000.0751103.x. [DOI] [PubMed] [Google Scholar]
- Bartholoma A, Nave KA. NEX-1: a novel brain-specific helix-loop-helix protein with autoregulation and sustained expression in mature cortical neurons. Mech Dev. 1994;48:217–228. doi: 10.1016/0925-4773(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Perrone-Bizzozero NI. The expression of GAP-43 in relation to neuronal growth and plasticity: when, where, how, and why? Progr Brain Res. 1991;89:69–87. doi: 10.1016/s0079-6123(08)61716-1. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Pate Skene JH. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci. 2001;4:38–43. doi: 10.1038/82881. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Ghysen A. Deconstructing cell determination: proneural genes and neuronal identity. Bioessays. 1999;21:313–318. doi: 10.1002/(SICI)1521-1878(199904)21:4<313::AID-BIES7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Burstein DE, Greene LA. Evidence for RNA synthesis-dependent and -independent pathways in stimulation of neurite outgrowth by nerve growth factor. Proc Natl Acad Sci USA. 1978;75:6059–6063. doi: 10.1073/pnas.75.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Fode C, Guillemot F. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. doi: 10.1242/dev.124.8.1611. [DOI] [PubMed] [Google Scholar]
- Chiaramello A, Neuman K, Palm K, Metsis M, Neuman T. Helix-loop-helix transcription factors mediate activation and repression of the p75LNGFR gene. Mol Cell Biol. 1995;15:6036–6044. doi: 10.1128/mcb.15.11.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaramello A, Neuman T, Peavy DR, Zuber MX. The GAP-43 gene is a direct downstream target of the basic helix-loop-helix transcription factors. J Biol Chem. 1996;271:22035–22043. doi: 10.1074/jbc.271.36.22035. [DOI] [PubMed] [Google Scholar]
- Costello B, Meymandi A, Freeman JA. Factors influencing GAP-43 gene expression in PC12 pheochromocytoma cells. J Neurosci. 1990;10:1398–1406. doi: 10.1523/JNEUROSCI.10-04-01398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobashi Y, Kudoh T, Matsumine A, Toyoshima K, Akiyama T. Constitutive overexpression of CDK2 inhibits neuronal differentiation of rat pheochromocytoma PC12 cells. J Biol Chem. 1995;270:23031–23037. doi: 10.1074/jbc.270.39.23031. [DOI] [PubMed] [Google Scholar]
- Erhardt JA, Pittman RN. Ectopic p21(WAF1) expression induces differentiation-specific cell cycle changes in PC12 cells characteristic of nerve growth factor treatment. J Biol Chem. 1998a;273:23517–23523. doi: 10.1074/jbc.273.36.23517. [DOI] [PubMed] [Google Scholar]
- Erhardt JA, Pittman RN. p21WAF1 induces permanent growth arrest and enhances differentiation, but does not alter apoptosis in PC12 cells. Oncogene. 1998b;16:443–451. doi: 10.1038/sj.onc.1201577. [DOI] [PubMed] [Google Scholar]
- Farah MH, Olson JM, Sucic HB, Hume RI, Tapscott SJ, Turner DL. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development. 2000;127:693–702. doi: 10.1242/dev.127.4.693. [DOI] [PubMed] [Google Scholar]
- Federoff HJ, Grabczyk E, Fishman MC. Dual regulation of GAP-43 gene expression by nerve growth factor and glucocorticoids. J Biol Chem. 1988;263:19290–19295. [PubMed] [Google Scholar]
- Goridis C, Brunet JF. Transcriptional control of neurotransmitter phenotype. Curr Opin Neurobiol. 1999;9:47–53. doi: 10.1016/s0959-4388(99)80006-3. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. PC12 pheachromocytoma cultures in neurobiological research. Adv Cell Neurobiol. 1982;3:373–414. [Google Scholar]
- Greene LA, Burstein DE, Black MM. The priming model for the mechanism of action of nerve growth factor: evidence derived from clonal PC12 pheochromocytoma cells. In: Giacobini E, Veradakis A, Shahar A, editors. Tissue culture in neurobiology. New York: Raven Press; 1980. pp. p313–319. [Google Scholar]
- Guillemot F. Vertebrate bHLH genes and the determination of neuronal fates. Exp Cell Res. 1999;253:357–364. doi: 10.1006/excr.1999.4717. [DOI] [PubMed] [Google Scholar]
- Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–10121. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- Irwin N, Baekelandt V, Goritchenko L, Benowitz LI. Identification of two proteins that bind to a pyrimidine-rich sequence in the 3′-untranslated region of GAP-43 mRNA. Nucleic Acids Res. 1997;25:1281–1288. doi: 10.1093/nar/25.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B, Orlian M, Furneaux H. p21(waf1) mRNA contains a conserved element in its 3′-untranslated region that is bound by the Elav-like mRNA-stabilizing proteins. J Biol Chem. 1998;273:20511–20516. doi: 10.1074/jbc.273.32.20511. [DOI] [PubMed] [Google Scholar]
- Kohn DT, Tsai KC, Cansino VV, Neve RL, Perrone-Bizzozero NI. Role of highly conserved pyrimidine-rich sequences in the 3′ untranslated region of the GAP-43 mRNA in mRNA stability and RNA–protein interactions. Brain Res Mol Brain Res. 1996;36:240–250. doi: 10.1016/0169-328x(95)00239-o. [DOI] [PubMed] [Google Scholar]
- Lee JE. Basic helix-loop-helix genes in neural development. Curr Opin Neurobiol. 1997;7:13–20. doi: 10.1016/s0959-4388(97)80115-8. [DOI] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Liu M, Pleasure SJ, Collins AE, Noebels JL, Naya FJ, Tsai MJ, Lowenstein DH. Loss of BETA2/NeuroD leads to malformation of the dentate gyrus and epilepsy. Proc Natl Acad Sci USA. 2000;97:865–870. doi: 10.1073/pnas.97.2.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckenbill-Edds L, Van Horn C, Greene LA. Fine structure of initial outgrowth of processes induced in a pheochromocytoma cell line (PC12) by nerve growth factor. J Neurocytol. 1979;8:493–511. doi: 10.1007/BF01214805. [DOI] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Mani S, Schaefer J, Meiri KF. Targeted disruption of GAP-43 in P19 embryonal carcinoma cells inhibits neuronal differentiation as well as acquisition of the morphological phenotype. Brain Res. 2000;853:384–395. doi: 10.1016/s0006-8993(99)02042-9. [DOI] [PubMed] [Google Scholar]
- Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eukaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick MB, Tamimi RM, Snider L, Asakura A, Bergstrom D, Tap-scott SJ. NeuroD2 and neuroD3: distinct expression patterns and transcriptional activation potentials within the NeuroD gene family. Mol Cell Biol. 1996;16:5792–5800. doi: 10.1128/mcb.16.10.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi T, Maruyama H, Kitamura T, Nakamura S, Kawakami H. Structure and regulation of the human NeuroD (BETA2/BHF1) gene. Brain Res Mol Brain Res. 1999;69:223–231. doi: 10.1016/s0169-328x(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Miyata T, Maeda T, Lee JE. NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev. 1999;13:1647–1652. doi: 10.1101/gad.13.13.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobarak CD, Anderson KD, Morin M, Beckel-Mitchener A, Rogers SL, Furneaux H, King P, Perrone-Bizzozero NI. The RNA-binding protein HuD is required for GAP-43 mRNA stability, GAP-43 gene expression, and PKC-dependent neurite outgrowth in PC12 cells. Mol Biol Cell. 2000;11:3191–3203. doi: 10.1091/mbc.11.9.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- Moskowitz PF, Oblinger MM. Sensory neurons selectively upregu-late synthesis and transport of the beta III-tubulin protein during axonal regeneration. J Neurosci. 1995;15:1545–1555. doi: 10.1523/JNEUROSCI.15-02-01545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz PF, Smith R, Pickett J, Frankfurter A, Oblinger MM. Expression of the class III beta-tubulin gene during axonal regeneration of rat dorsal root ganglion neurons. J Neurosci Res. 1993;34:129–134. doi: 10.1002/jnr.490340113. [DOI] [PubMed] [Google Scholar]
- Mutoh H, Naya FJ, Tsai MJ, Leiter AB. The basic helix-loop-helix protein BETA2 interacts with p300 to coordinate differentiation of secretin-expressing enteroendocrine cells. Genes Dev. 1998;12:820–830. doi: 10.1101/gad.12.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma T, Yoon YS, Nakazawa A. Overexpression of NeuroD in PC12 cells alters morphology and enhances expression of the adenylate kinase isozyme 1 gene. Brain Res Mol Brain Res. 1999;67:53–63. doi: 10.1016/s0169-328x(99)00038-8. [DOI] [PubMed] [Google Scholar]
- Olson JM, Asakura A, Snider L, Hawkes R, Strand A, Stoeck J, Hallahan A, Pritchard J, Tapscott SJ. NeuroD2 is necessary for development and survival of central nervous system neurons. Dev Biol. 2001;234:174–187. doi: 10.1006/dbio.2001.0245. [DOI] [PubMed] [Google Scholar]
- Perrone-Bizzozero NI, Neve RL, Irwin N, Lewis S, Fisher I, Benowitz LI. Post-transcriptional regulation of GAP-43 mRNA levels during neuronal differentiation and nerve regeneration. Mol Cell Neurosci. 1991;2:402–409. doi: 10.1016/1044-7431(91)90027-l. [DOI] [PubMed] [Google Scholar]
- Perrone-Bizzozero NI, Cansino VV, Kohn DT. Posttranscriptional regulation of GAP-43 gene expression in PC12 cells through protein kinase C-dependent stabilization of the mRNA. J Cell Biol. 1993;120:1263–1270. doi: 10.1083/jcb.120.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu S, Ignatova A, Park ST, Sun XH. Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol Cell Biol. 1997;17:5888–5896. doi: 10.1128/mcb.17.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossner MJ, Dorr J, Gass P, Schwab MH, Nave KA. SHARPs: mammalian enhancer-of-split- and hairy-related proteins coupled to neuronal stimulation. Mol Cell Neurosci. 1997;10:460–475. [PubMed] [Google Scholar]
- Schwab MH, Druffel-Augustin S, Gass P, Jung M, Klugmann M, Bartholomae A, Rossner MJ, Nave KA. Neuronal basic helix-loop-helix proteins (NEX, neuroD, NDRF): spatiotemporal expression and targeted disruption of the NEX gene in transgenic mice. J Neurosci. 1998;18:1408–1418. doi: 10.1523/JNEUROSCI.18-04-01408.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab MH, Bartholomae A, Heimrich B, Feldmeyer D, Druffel-Augustin S, Goebbels S, Naya FJ, Zhao S, Frotscher M, Tsai MJ, Nave KA. Neuronal basic helix-loop-helix proteins (NEX and BETA2/NeuroD) regulate terminal granule cell differentiation in the hippocampus. J Neurosci. 2000;20:3714–3724. doi: 10.1523/JNEUROSCI.20-10-03714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene JH. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Strittmatter SM, Fankhauser C, Huang PL, Mashimo H, Fishman MC. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell. 1995;80:445–452. doi: 10.1016/0092-8674(95)90495-6. [DOI] [PubMed] [Google Scholar]
- Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tischler AS, Greene LA. Morphologic and cytochemical properties of a clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Lab Invest. 1978;39:77–89. [PubMed] [Google Scholar]
- Tsai KC, Cansino VV, Kohn DT, Neve RL, Perrone-Bizzozero NI. Post-transcriptional regulation of the GAP-43 gene by specific sequences in the 3′ untranslated region of the mRNA. J Neurosci. 1997;17:1950–1958. doi: 10.1523/JNEUROSCI.17-06-01950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twiss JL, Shooter EM. Nerve growth factor promotes neurite regeneration in PC12 cells by translational control. J Neurochem. 1995;64:550–557. doi: 10.1046/j.1471-4159.1995.64020550.x. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard M, Peavy DR, Chiaramello A. Expression of the bHLH gene NSCL-1 suggests a role in regulating cerebellar granule cell growth and differentiation. J Neurosci Res. 1999;57:770–781. [PubMed] [Google Scholar]
- van Grunsven LA, Billon N, Savatier P, Thomas A, Urdiales JL, Rudkin BB. Effect of nerve growth factor on the expression of cell cycle regulatory proteins in PC12 cells: dissection of the neurotrophic response from the anti-mitogenic response. Oncogene. 1996;12:1347–1356. [PubMed] [Google Scholar]
- Woolf CJ. Turbocharging neurons for growth: accelerating regeneration in the adult CNS. Nat Neurosci. 2001;4:7–9. doi: 10.1038/82921. [DOI] [PubMed] [Google Scholar]
- Yan GZ, Ziff EB. NGF regulates the PC12 cell cycle machinery through specific inhibition of the Cdk kinases and induction of cyclin D1. J Neurosci. 1995;15:6200–6212. doi: 10.1523/JNEUROSCI.15-09-06200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner BA, Benowitz LI, Villa-Komaroff L, Neve RL. Transfection of PC12 cells with the human GAP-43 gene: effects on neurite outgrowth and regeneration. Brain Res Mol Brain Res. 1990;7:39–44. doi: 10.1016/0169-328x(90)90071-k. [DOI] [PubMed] [Google Scholar]
- Zuber MX, Goodman DW, Karns LR, Fishman MC. The neuronal growth-associated protein GAP-43 induces filopodia in non-neuronal cells. Science. 1989;244:1193–1195. doi: 10.1126/science.2658062. [DOI] [PubMed] [Google Scholar]


