Abstract
Objective
We examined the protein anabolic effects of Pro-Stat 64, a high nitrogen-containing, enzyme-hydrolyzed, tryptophan-fortified, collagen protein supplement administrated during hemodialysis, at two different dosing regimens.
Design
This was a randomized, controlled, prospective study with 3 different groups: control, single dose of supplementation, and double dose of supplementation.
Setting
This study was performed at a clinical research center.
Patients
Six prevalent chronic hemodialysis (HD) patients were enrolled: 5 males, 1 female, 4 African Americans, and 2 Caucasians. Their mean age was 45 ± 11 years. Two patients were diabetic.
Methods
Protein turnover studies were performed using amino-acid (AA) balance and primed constant infusion of L-(1-13C) leucine.
Main Outcome Measure
Whole-body protein balance was determined according to substrate kinetics.
Results
There were no statistically significant difference at any time point between protocols for blood chemistries and hormonal markers, except for minor variations in plasma glucose. All plasma AA groups displayed decreases during control. Compared with the control group, plasma nonessential AA and total AA concentrations were statistically significantly higher during HD after both single and double doses of supplementation. The forearm arteriovenous AA balance was statistically significantly better for essential, nonessential, and total AA uptake after both single-dose and double-dose supplementation compared with the control group, except for nonessential AA, which was significantly better only after a double dose. Whole-body protein breakdown and net protein balance were statistically significantly better during HD with a double-dose administration in a dose-dependent manner, compared with the control and single-dose groups.
Conclusions
Oral AA supplementation alone improves whole-body and skeletal muscle protein anabolism in a dose-dependent manner in chronic HD patients. These data should be taken into account during clinical decision-making or when designing clinical trials of nutritional supplementation.
NUTRITIONAL SUPPLEMENTATION has been an effective strategy to overcome overt uremic protein-energy wasting, a state of metabolic and nutritional derangements known to be strongly associated with major adverse clinical outcomes in chronic hemodialysis (CHD) patients.1,2 Several studies showed that parenteral and oral nutritional supplementation administered during hemodialysis led to a protein anabolic response in CHD patients.3–5 In all these studies, the nutritional supplementation, oral or parenteral, contained variable amounts of protein, carbohydrate, and fat. A very limited number of studies examined the efficacy and feasibility of more selective protein supplementation in CHD patients.
Amino acids are considered the most physiologically active substrate for promoting net protein anabolism.6 Amino-acid administration enhances protein synthesis primarily via increased delivery to the muscle tissue. On the other hand, concurrent carbohydrate intake may be necessary to promote net anabolism, insofar as this would lead to increased insulin levels, which are also known to exhibit protein anabolic properties. The role of lipids in nutritional supplementation is less well-defined. Overall, there is a need for detailed studies examining the metabolic effects of more specialized regimens, such as protein supplementation alone in patient populations at high risk for protein energy wasting, similar to CHD patients.
The aim of this study was to examine the protein anabolic effects of a high nitrogen-containing, enzyme-hydrolyzed, tryptophan-fortified, collagen protein supplement, Pro-Stat 64 (Medical Nutrition USA, Inc.), administrated during hemodialysis at two different doses. We hypothesized that there would be a dose-response relationship between the quantity of this selective protein supplement and the corresponding protein anabolic response. In order to test our hypothesis, we studied 6 CHD patients in 3 different groups: one set of controls, and two different amino-acid supplementation doses during a metabolic substrate protocol.
Methods
Patients
Patients were recruited from the Vanderbilt University Outpatient Dialysis Unit. Inclusion criteria for the study involved patients who had received CHD for more than 6 months, with less than 100 mL/min of urine output, who used a bio-compatible hemodialysis membrane (Fresenius Optiflux 180, Fresenius USA, Lexington, MA), and who received an adequate dose of dialysis (double-pool Kt/V ≥ 1.4) in a thrice-weekly program. Patients with active infectious or inflammatory disease (e.g., vascular access infections or overt periodontal disease), patients hospitalized within the 3 months before the study, patients with recirculation in the vascular access and/or vascular access blood flow of less than 500 mL/min as detected on an arteriovenous (AV) shunt, patients receiving steroids and/or immunosuppressive agents, and those with a history of intolerance to the nutritional supplements were excluded from the study. The Institutional Review Board of Vanderbilt University approved the study protocol, and written, informed consent was obtained from all study patients.
Design
This was a randomized, prospective, crossover study. Patients were assigned a random computer-generated sequence of study protocols, including control, Pro-Stat single dose, and Pro-Stat double dose. All patients participated in all protocols, with at least 4 weeks between each study, to allow for total clearance of stable isotopes. Within a week before each part of the study, dual-energy x-ray absorptiometry was performed to estimate lean and fat body masses.
Patients were admitted to the General Clinical Research Center the day before the study at approximately 7 pm. They received a meal (18% protein and 30% lipids) at least 10 hours before initiation of the study, and then fasted. Energy in-take was kept at maintenance levels, as based on the Harris-Benedict equation and each subject's gender, height, weight, and activity levels.
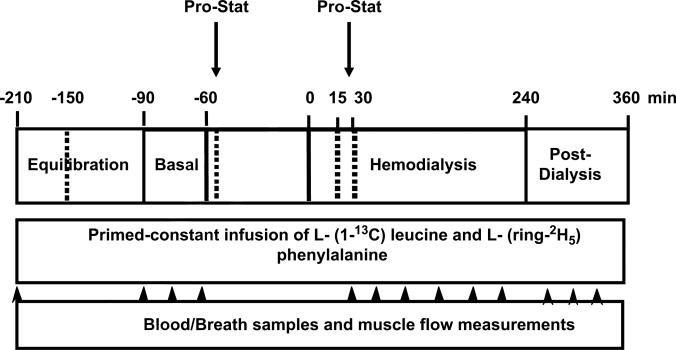
A schematic diagram of the metabolic study day protocol is given in Figure 1. Each metabolic study consisted of a pre-hemodialysis (HD) phase (a 2-hour equilibration phase followed by a 0.5-hour basal sampling phase), a 4-hour HD phase, and a 2-hour post-HD phase. A dialysis catheter was placed at the venous site of the AV shunt of the forearm at 6 am, to collect a baseline blood sample for biochemical nutritional markers and isotopic backgrounds, and then to initiate isotope infusion. Arterial vascular access obtained through the arterial side of the AV shunt was used to perform HD and to sample arterial blood. Another catheter was placed in a deep vein (with a retrograde insertion) of the contralateral forearm to sample the blood draining the forearm muscle bed. At the start of the infusion, subjects received a bolus injection of NaH13CO3 (0.12 mg/kg) and L-(1-13C) leucine (7.2 μmol/kg) to prime the carbon dioxide (CO2) and leucine pools, respectively. A continuous infusion of leucine (0.12 μmol/kg/min) was then started, and continued throughout the remainder of the study. Constant infusion of isotopes continued throughout the study. Blood samples were collected once before the start of the study, 3 times during the basal sampling phase, 6 times during HD, and 3 times during the post-HD phase. Simultaneously with each blood sample, breath samples were collected from the subjects via a Douglas bag, with duplicate 20-mL samples placed in nonsiliconized glass vacutainer tubes for measurement of breath 13CO2 enrichment. In addition, forearm blood flow was estimated using capacitance plethysmography (Model 2560 with URI/CP software, version 3.0, Moro Bay, CA).
Figure 1.
Schematic diagram of metabolic study day protocol, denoting time-points for blood draws, breath-sample collections, and muscle plasma flow measurements. A primed-constant infusion of L-(1-13C) leucine and L-(ring-2H5) phenylalanine was maintained throughout entire study (360 minutes). Pro-Stat was administered twice at 2 different doses.
During the control protocol, no nutritional supplementation was given. In the protocols using Pro-Stat, an initial dose was given 30 minutes before initiation of dialysis, and the second dose was given 30 minutes after initiation of dialysis. Each administration consisted of either 30 mL (single dose) or 60 mL (double dose) of Pro-Stat. The total amount of amino-acid supplementation in the double dose consisted of 9 essential amino acids (lysine, 2.7 g; leucine, 1.86 g; phenylalanine, 1.32 g; valine, 1.56 g; histidine, 0.44 g; isoleucine, 1.02 g; methionine, 0.48 g; threonine, 1.32 g; and tryptophan, 0.264 g) and 8 nonessential amino acids (alanine, 5.58 g; arginine, 5.13 g; aspartate, 3.96 g; cystine, 0.042 g; glutamate, 6.66 g; glycine, 16.14 g; proline, 8.88 g; serine, 1.92 g; and tyrosine, 0.12 g). The total supplementation provided 120 kcal and 240 kcal for each single and double dose, respectively.
During each protocol, patients were dialyzed for 4 hours with a blood flow of 400 mL/min and a dialysate flow of 500 mL/min. Ultrafiltration rates were determined by the patients’ needs and estimated dry weight. The composition of the dialysate used during the study was identical for all treatments, and consisted of 139 mEq/L sodium, 2 mEq/L potassium, 2.5 mEq/L calcium, 200 mg/dL glucose, and 39 mEq/L bicarbonate. After completion of HD, dialysis lines were disconnected, and a 2-hour post-HD phase ensued. After the post-HD phase, all catheters were removed, the patients were given a meal and observed until they were stable, at which time they were discharged.
Analytical Procedures
Blood samples were collected into Venoject tubes containing 15 mg Na2EDTA (Terumo Medical Corp., Elkton, MD). All analytical procedures, including nutritional biochemical markers, were performed as described previously.7,8 Individual amino acids were grouped for purposes of analysis. They included essential amino acids (EAAs), the sum of arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine; total amino acids (TAAs), the sum of all individual amino acids; and nonessential amino acids (NEAAs), the difference between TAAs and EAAs. Plasma enrichments of (13C) leucine and (13C) ketoisocaproate were determined using gas chromatography/mass spectrometry (GC/MS-Hewlett-Packard 5890a GC and 5970 MS, Hewlett-Packard, San Fernando, CA), as previously described.7,8
Calculations
Estimation of net skeletal muscle protein balance (synthesis-breakdown) was determined by the A – V difference of amino acids in the forearm, as previously reported.7,8 The steady-state rates of total whole-body leucine appearance were calculated by dividing the (13C) leucine infusion rate by the plasma (13C) ketoisocaproate enrichment,9 as previously described.7,8
Statistical Analysis
Demographics, nutritional characteristics, and biochemical parameters of the subjects of the three protocols were analyzed. For each protocol, at each time-point, nutritional and biochemical parameters were measured 3 times for accuracy, and an average value per protocol per each time-point (pre-HD, during HD, and post-HD) for each patient was used as the unit of the analysis. Patients’ baseline characteristics are presented using means and standard deviations (SDs) for continuous variables, and frequency and proportion for categorical variables. Comparisons of baseline nutritional and biochemical parameters among study protocols were performed using repeated measurements of analysis of variance. Changes in plasma glucose concentration, insulin concentration, amino-acid concentration, forearm amino-acid concentration, and whole-body protein turnover over time (during HD vs. pre-HD, and post-HD vs. pre-HD) were compared among protocols using general linear models, with bootstrap covariance accounting for correlated measures within a patient. The normality of residuals of the general linear models was assessed, and transformation on the dependent variable was performed to achieve normality if needed. A two-sided P < .05 was required to reject the null hypothesis. All analyses were performed with R-software, version 2.6.1 (www.r-project.org), with consideration of within-subject correlations.
Results
Blood Chemistries
Table 1 depicts patient characteristics and baseline biochemical nutritional markers for the 3 study protocols. All measures were similar, without any statistically significant differences. Measurement of pre-HD and post-HD blood chemistries, including BUN, showed expected changes after HD treatment, without significant differences between HD sessions within patients (data not shown). Table 2 shows the results for glucose and insulin for the 2 study protocols compared to the control protocol during the 3 study periods. Plasma glucose concentrations increased less during HD during the double-dose Pro-Stat protocol, compared with the control group (P = .006). No other statistically significant differences were found at any time between protocols for plasma glucose or insulin concentrations (Fig. 2).
Table 1.
Demographic, Nutritional Characteristics, and Biochemical Parameters of Study Population (n = 6) as Assessed for Each Study Protocol
| Demographics | Value (n [%]; mean [SD]) |
|---|---|
| Age (y) | 45 (11.4) |
| Gender (M/F) | 5 (83%)/1 (17%) |
| Black race | 4 (67%) |
| Diabetes | 2 (33%) |
| Cause of endstage renal disease | |
| Hypertension | 5 (83%) |
| Diabetes | 1 (17%) |
| Control | Single-Dose | Double-Dose | P Value | |
|---|---|---|---|---|
| Body weight (kg) | 92.4 (20) | 91.0 (20) | 91.4 (22) | .58 |
| Body mass index (kg/m2) | 31.0 (5.4) | 30.5 (5.5) | 30.6 (5.7) | .64 |
| Fat mass: dual-energy x-ray absorptiometry (%) | 33.9 (3.8) | 33.9 (3.9) | 34.1 (4.5) | .97 |
| Albumin (g/dL) | 3.95 (0.17) | 3.85 (0.23) | 3.95 (0.19) | .38 |
| Prealbumin (mg/dL) | 32.95 (7.9) | 33.1 (5.9) | 33.2 (8.4) | .99 |
| Transferrin (mg/dL) | 175.2 (21) | 160.7 (27.0) | 179.2 (35) | .06 |
| Cholesterol (mg/dL) | 146.5 (33) | 143.2 (43) | 139.0 (50.0) | .81 |
| Total CO2 (mg/dL) | 26.9 (3.4) | 25.8 (1.2) | 24.7 (1.0) | .18 |
| CRP (mg/L) | 7.05 (4.8) | 7.5 (4.2) | 17.8 (28) | .53 |
values are absolute numbers and percentages or means (sds).
Table 2.
Plasma Insulin and Glucose Concentrations of Study Populations
| Parameter | Pre-HD, Mean ± SD | During HD, Mean ± SD | Post-HD, Mean ± SD |
|---|---|---|---|
| Glucose (mg/dL) | |||
| Control | 90 ± 14 | 112 ± 15 | 85 ± 13 |
| Δ | 22 ± 7.2 | –5 ± 7.1 | |
| Pro-Stat (single) | 95 ± 20 | 112 ± 21 | 89 ± 16 |
| Δ | 16 ± 7.1 | –5.9 ± 8.8 | |
| Pro-Stat (double) | 98 ± 19 | 113 ± 15 | 84 ± 12 |
| Δ | 16 ± 5.5 (P = .006)* | –14 ± 14 | |
| Insulin (μU/mL) | |||
| Control | 12.8 ± 5 | 17.0 ± 7.2 | 11.9 ± 7.1 |
| Δ | 4.2 ± 4.7 | –0.9 ± 3.3 | |
| Pro-Stat (single) | 11.5 ± 3.3 | 15.7 ± 6.1 | 11.4 ± 3.4 |
| Δ | 4.1 ± 4.6 | –0.2 ± 3.4 | |
| Pro-Stat (double) | 13.3 ± 4.8 | 17.5 ± 6.3 | 10.7 ± 3.2 |
| Δ | 4.2 ± 5 | –2.6 ± 2 |
Δ = [during-HD – pre-HD] or [post-HD – pre-HD].
P value compares difference from baseline [during HD – pre-HD] between single-dose and control protocols.
Figure 2.
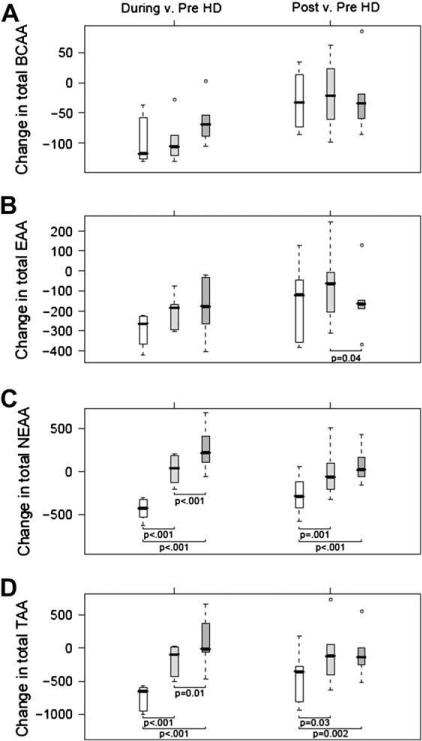
Box-and-whisker plot of changes from baseline (during-HD – pre-HD, and post-HD – pre-HD) are depicted for plasma BCAAs (A), EAAs (B), NEAAs (C), and TAAs (D). Open boxes represent control group, light gray boxes represent Pro-Stat single-dose group, and dark gray boxes represent Pro-Stat double-dose group. Units are μmol/L. P values were obtained from general linear models with bootstrap covariance to account for correlated measures within a patient.
Plasma Amino Acids
Table 3 shows the functional groups of amino acids, branched-chain amino acids (BCAAs), NEAAs, EAAs, and TAAs during each study protocol and at the three study time-points. There were no differences during the pre-HD period for any of the variables. As expected, during the control protocol, all plasma amino-acid groups displayed substantial decreases in the during-HD phase (P < .05 for all amino-acid groups). During both single-dose and double-dose Pro-Stat administrations, NEAA and TAA concentrations were significantly higher in the during-HD and post-HD groups compared with the control group. When examining changes in NEAA and TAA concentrations during HD compared with baseline, there was a statistically significant and dose-dependent benefit with Pro-Stat administration (Fig. 2), such that double-dose Pro-Stat maintained positive NEAA and TAA concentrations. The beneficial effect of single-dose and double-dose Pro-Stat on NEAAs and TAAs also carried over to the postdialysis phase.
Table 3.
Plasma Amino-Acid Concentrations During 3 Study Protocols
| Parameter (μmol/L) | Pre-HD, Mean ± SD | During HD, Mean ± SD (P Value) | Post-HD, Mean ± SD (P Value) |
|---|---|---|---|
| BCAA | |||
| Control | 407 ± 77 | 309 ± 96 | 377 ± 73 |
| Δ | –98 ± 40 | –29 ± 47 | |
| Pro-Stat (single) | 391 ± 101 | 295 ± 78 | 372 ± 92 |
| Δ | –96 ± 37 | –19 ± 62 | |
| Pro-Stat (double) | 403 ± 90 | 339 ± 70 | 379 ± 60 |
| Δ | –64 ± 37 | –24 ± 59 | |
| EAA | |||
| Control | 1120 ± 254 | 825 ± 219 | 969 ± 183 |
| Δ | –295 ± 83 | –151 ± 195 | |
| Pro-Stat (single) | 1040 ± 236 | 838 ± 157 | 972 ± 196 |
| Δ | –202 ± 85 | -68 ± 190 | |
| Pro-Stat (double) | 1135 ± 244 | 955 ± 154 | 984 ± 135 |
| Δ | –180 ± 147 | –151 ± 160 (.04)† | |
| NEAA | |||
| Control | 1424 ± 343 | 981 ± 253 | 1149 ± 242 |
| Δ | –443 ± 126 | –274 ± 225 | |
| Pro-Stat (single) | 1342 ± 312 | 1363 ± 245 | 1332 ± 295 |
| Δ | 21 ± 167 (<.001)* | 10 ± 304 (.001)* | |
| Pro-Stat (double) | 1298 ± 347 | 1560 ± 240 | 1370 ± 261 |
| Δ | 262 ± 256 (<.001)*,† | 71 ± 209 (<.001)* | |
| TAA | |||
| Control | 2543 ± 565 | 1805 ± 454 | 2118 ± 413 |
| Δ | –738 ± 191 | –425 ± 405 | |
| Pro-Stat (single) | 2382 ± 505 | 2201 ± 343 | 2304 ± 465 |
| Δ | –181 ± 236 (<.001)* | –78 ± 487 (.03)* | |
| Pro-Stat (double) | 2433 ± 587 | 2515 ± 373 | 2353 ± 390 |
| Δ | 82 ± 389 (<.001)*,† | -80 ± 362 (.002)* |
Δ = [during-HD – pre HD] or [post HD – pre HD].
P values compare difference from baseline [Δ] between single-dose or double-dose with control protocols.
P values compare difference from baseline [Δ] between single-dose and double-dose protocols.
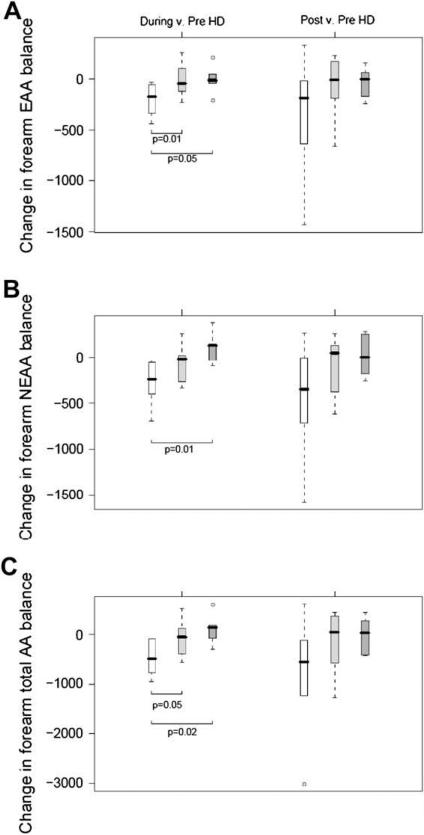
Forearm Amino-Acid Uptake
Table 4 shows the forearm uptake of amino acids by functional groups, and Figure 3 depicts changes in forearm EAA, NEAA, and TAA uptake relative to baseline values. There was a substantial release of amino acids from the forearm during the control protocol, indicating a high protein catabolic state. This adverse effect was greatly reduced by Pro-Stat administration, and was to some extent reversed to a positive state during the double-dose protocol. These beneficial effects reached statistical significance for EAAs during the single dose (P = .01) and double dose (P = .05) when each was compared with the control group, for NEAAs during the double dose compared with the control group (P = .01) and for TAAs during both the single dose (p = 0.05) and double dose (p = 0.02) versus the control group, all during HD. There was no statistically significant difference between single or double doses.
Table 4.
Forearm Amino-Acid Balance During 3 Study Protocols
| Parameter (nmol/100 mL per min) | Pre-HD, Mean ± SD (P Value) | During HD, Mean ± SD (P Value) | Post-HD, Mean ± SD (P Value) |
|---|---|---|---|
| EAA | |||
| Control | 81 ± 172 | –122 ± 132 | –275 ± 540 |
| Δ | –204 ± 165 | –356 ± 613 | |
| Pro-Stat (single) | –29 ± 168 | –43 ± 212 | –108 ± 183 |
| Δ | –14 ± 170 (.01) | –80 ± 319 | |
| Pro-Stat (double) | 86 ± 165 | 80 ± 127 | 51 ± 126 |
| Δ | –6 ± 135 (.05) | –35 ± 147 | |
| NEAA | |||
| Control | –6 ± 115 | –284 ± 286 | –462 ± 669 |
| Δ | –278 ± 251 | –456 ± 665 | |
| Pro-Stat (single) | –57 ± 219 | –117 ± 249 | –140 ± 164 |
| Δ | –60 ± 216 | –83 ± 342 | |
| Pro-Stat (double) | –5 ± 186 | 108 ± 84 | 15 ± 174 |
| Δ | 112 ± 166 (.01) | 19 ± 223 | |
| Total | |||
| Control | 75±273 | –406 ± 410 | –737 ± 1190 |
| Δ | –482 ± 348 | –812 ± 1251 | |
| Pro-Stat (single) | –86 ± 380 | –160 ± 459 | –248 ± 337 |
| Δ | –74 ± 381 (.05) | –163 ± 652 | |
| Pro-Stat (double) | 82 ± 341 | 188 ± 193 | 66 ± 272 |
| Δ | 106 ± 296 (0.02) | –16 ± 358 |
Δ = [during-HD – pre-HD] or [post-HD – pre-HD].
*P values compare difference from baseline [Δ] between single-dose or double-dose with control protocols.
Figure 3.
Box-and-whisker plots of changes from baseline (during-HD – pre-HD, and post-HD – pre-HD) are depicted for forearm EAA (A), NEAA (B), and TAA (C) uptakes. Open boxes represent control group, light gray boxes represent Pro-Stat single-dose group, and dark gray boxes represent Pro-Stat double-dose group. Units are nmol/100 mL per minute. P values were obtained from general linear models with bootstrap covariance to account for correlated measures within a patient.
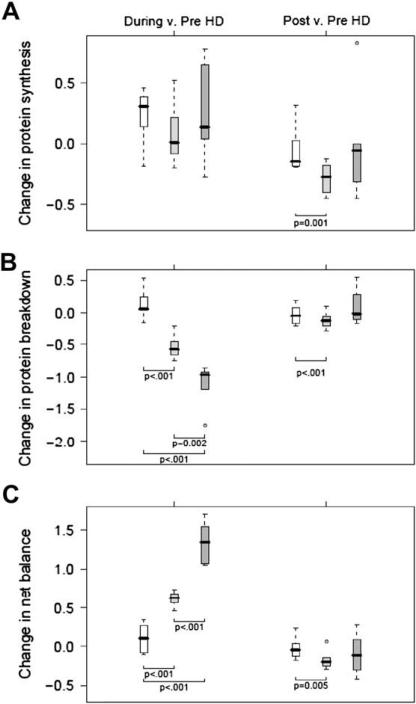
Whole-Body Protein Metabolism
Table 5 shows the dynamic components of whole-body protein homeostasis for the three study protocols pre-HD, during HD, and post-HD. In the pre-HD period, there were no statistically significant differences among protocols for whole-body protein synthesis, proteolysis, or net balance. As shown in Figure 4, Pro-Stat supplementation resulted in a dose-dependent benefit in whole-body protein breakdown (Fig. 4B) and in whole-body net protein balance (Fig. 4C). These anabolic effects were statistically significantly more pronounced for double-dose Pro-Stat administration. There was no effect of Pro-Stat administration in both single doses and double doses on whole-body protein synthesis (Fig. 4A). The beneficial effects of Pro-Stat post-HD compared with beneficial effects during HD were maintained only for the single dose, albeit not as strongly as observed during the HD period.
Table 5.
Whole-Body Protein Turnover During 3 Study Periods
| Parameter (mg/kg Fat-Free Mass per min) | Pre-HD, Mean ± SD | During HD, Mean ± SD | Post-HD, Mean ± SD |
|---|---|---|---|
| Protein synthesis | |||
| Control | 4.71 ± 0.84 | 4.95 ± 0.88 | 4.66 ± 0.97 |
| Δ | 0.23 ± 0.24 | –0.06 ± 0.2 | |
| Pro-Stat (single) | 5.02 ± 0.8 | 5.10 ± 0.67 | 4.73 ± 0.84 |
| Δ | 0.08 ± 0.26 | –0.29 ± 0.13 (.001)* | |
| Pro-Stat (double) | 4.38 ± 0.19 | 4.62 ± 0.38 | 4.37 ±0.35 |
| Δ | 0.24 ± 0.4 | –0.01 ± 0.45 | |
| Protein breakdown | |||
| Control | 4.11±0.4 | 4.23 ± 0.31 | 4.07 ± 0.38 |
| Δ | 0.13 ± 0.24 | –0.04 ± 0.16 | |
| Pro-Stat (single) | 4.11±0.59 | 3.57 ± 0.52 | 3.99 ± 0.55 |
| Δ | –0.54 ± 0.19 (<.001)* | –0.12 ± 0.14 (<.001)* | |
| Pro-Stat (double) | 3.84 ± 0.67 | 2.72 ± 0.74 | 3.92 ± 0.5 |
| Δ | –1.1 ± 0.33 (<.001)*,† | 0.08 ± 0.28 | |
| Net Balance | |||
| Control | 0.61 ± 0.77 | 0.71 ± 0.94 | 0.59 ± 0.88 |
| Δ | 0.11 ± 0.2 | –0.02 ± 0.15 | |
| Pro-Stat (single) | 0.91 ± 0.54 | 1.52 ± 0.55 | 0.74±0.53 |
| Δ | 0.61 ± 0.1 (<.001)* | –0.17 ± 0.13 (.005)* | |
| Pro-Stat (double) | 0.54 ± 0.55 | 1.89 ± 0.73 | 0.45 ± 0.63 |
| Δ | 1.3 ± 0.27 (<.001)*,† | –0.10 ± 0.26 |
Δ = [during-HD – pre-HD] or [post-HD – pre-HD].
P values compare difference from baseline [Δ] between single-dose or double-dose with control protocols.
P values compare difference from baseline [Δ] between single-dose and double-dose protocols.
Figure 4.
Box-and-whisker plots of changes from baseline (during-HD – pre-HD, and post-HD – pre-HD) are depicted for whole-body protein synthesis (A), whole-body protein breakdown (B), and whole-body net protein balance (C). Open boxes represent control group, light gray boxes represent Pro-Stat single-dose group, and dark gray boxes represent Pro-Stat double-dose group. Units are mg/kg fat-free mass per minute. P values were obtained from general linear models with bootstrap covariance to account for correlated measures within a patient.
Discussion
In this study, we compared the metabolic response to a high nitrogen-containing collagen protein supplement administered at time of HD at two different doses with the metabolic response in a nonintervention control group. Our results are consistent with the hypothesis that both regimens induce a positive net protein anabolic effect, and further show that the higher dose regimen resulted in a more robust net protein anabolic response. These effects were evident in both the whole-body and skeletal muscle compartments. The data indicate that selective protein supplementation during HD is effective, and that the administration of this nutritional supplementation should be tailored according to the needs of the patients.
Clear-cut evidence indicates that HD is a protein catabolic event.10 Based on data that the administration of nonselective nutritional supplementation containing all macronutrients (amino acid, lipid, and carbohydrate) during HD counteracts this adverse effect, we sought to examine the efficacy of an oral supplementation containing amino acids alone. The rationale for this strategy was several-fold, and included mechanistic and practical aspects. It is generally believed that amino-acid supplementation is the primary driving force for net protein synthesis. A plethora of studies in healthy individuals and subjects with chronic disease states showed that, if given at adequate doses, acute amino-acid supplementation improves skeletal muscle protein balance.6 This effect is thought to be primarily mediated by increased substrate delivery to the skeletal muscle. The beneficial effects of amino-acid supplementation are more readily observed in the setting of decreased plasma levels, as observed during HD.3,11 On the other hand, adequate energy in the form of carbohydrates was suggested as necessary to optimize the anabolic effects of amino acids. The release of insulin in response to carbohydrate administration is one proposed mechanism for this additive effect.12 To date, no study has examined the isolated effects of amino-acid supplementation in CHD patients. Our data show that amino-acid supplementation, without any additional carbohydrate or lipid supplementation, is feasible and effective in terms of promoting net protein anabolism during HD.
In addition to its obvious anabolic effects, the use of amino acids alone has certain practical advantages, especially when one considers the limited tolerability of commonly used oral supplementations. First, a significantly lower volume is administered with the current regimen. Second, complaints of gastrointestinal discomfort, such as bloating and diarrhea, are highly likely because of the high carbohydrate and fat content of the earlier regimens; these effects were not experienced by any of our study subjects. However, this particular study was limited to only onetime administration, and repetitive administrations over time may lead to some inconveniences.
Another unique aspect of our study was its attempt to examine the dose response with varying levels of amino-acid supplementation. Our data indicate that the single-dose regimen, as applied in this study, is capable of counteracting the acute catabolic effects of HD, and can be effectively used as a preventive measure of HD-associated catabolism. On the other hand, this regimen, in general, fell short of inducing a robust positive anabolic response, such that higher doses may be necessary for the treatment of overt uremic protein energy wasting. Indeed, we observed a more robust net protein anabolic effect with the administration of double-dose amino-acid supplementation, indicating that this is a reasonable strategy. Further evidence in line with the dose-response relationship in nutritional supplementation comes from previous data from our laboratory showing an even stronger anabolic response, both in skeletal muscle and whole-body net protein anabolism, when higher amounts of amino acids were supplemented.4 Taken together, the limited effectiveness of many earlier published (and possibly unpublished) studies aimed at treating uremic protein-energy wasting could be explained by the nonstandardization of nutrition protocols.13 It is possible that the amount of supplementation provided in these studies is adequate only to counteract HD-associated catabolism. Future studies exploring the efficacy of nutritional interventions other than PO supplements should include low-dose intradialytic oral nutritional supplementation, to rule out the catabolic effects of HD.
The results presented here are interesting, but should be interpreted with certain caveats. First, our study was designed to examine acute metabolic response, and cannot be extrapolated to indicate long-term beneficial effects. On the other hand, these data provide a strong rationale for long-term studies exploring the long-term effects of these regimens, with or without additional anabolic interventions. Second, comparable to any substrate kinetic study in the literature, we studied a small number of subjects, which limits the generalizability of our results. Although readers are encouraged to take this into account when interpreting our results, we believe that basic demographic characteristics are unlikely to influence the data. On the other hand, certain clinical conditions, such as acute or chronic inflammation, congestive heart failure, coronary artery disease, or diabetes mellitus, may have a significant impact on the efficacy of nutritional supplementation. Clearly, similar studies in these high-risk populations are necessary.
In conclusion, we report that the protein anabolic response associated with intradialytic oral nutritional supplementation is dependent on the characteristics of the administered regimen. Our data indicate that a low dose of amino-acid supplementation (single-dose Pro-Stat) can adequately counteract HD-associated catabolism. On the other hand, a higher dose of amino-acid supplementation not only completely attenuates the protein catabolic effects of HD, but induces a robust net anabolic response. We recommend that these data should be taken into account during clinical decision-making (e.g., when considering prevention versus treatment in protein energy wasting of kidney disease), and when designing clinical trials exploring the nutritional and other potential beneficial effects of intradialytic parenteral or oral nutritional supplementation, with or without concurrent anabolic interventions.
Acknowledgments
The authors express their appreciation to the patients and staff of the Outpatient Dialysis Unit at Vanderbilt University Medical Center for their participation in the study. The excellent technical assistance of Phyllis Egbert, Cindy Booker, Suzan Vaughan, Feng Sha, Mu Zheng, Wanda Snead, and the nursing staff at the Vanderbilt General Clinical Research Center is appreciated.
This study was supported in part by National Institutes of Health grants R01-DK45604 and K24-DK62849, by Diabetes Research Training Center Grant DK-20593 from the National Institute of Diabetes, Digestive and Kidney Diseases, by grant UL1 RR024975 from the National Center for Research Resources, and by an unrestricted grant from Medical Nutrition USA, Inc. M.B.S. is supported in part by a grant from the National Kidney Foundation Council of Renal Nutrition. K.L.C. is supported by grant K23 K23-DK080952 from the National Institute of Diabetes, Digestive and Kidney Diseases.
Footnotes
The authors declare no conflict of interest with regard to involvement with the commercial entities that supplied nutritional supplements.
References
- 1.Ikizler TA, Hakim RM. Nutrition in end-stage renal disease. Kidney Int. 1996;50:343–357. doi: 10.1038/ki.1996.323. [DOI] [PubMed] [Google Scholar]
- 2.Kopple JD. Effect of nutrition on morbidity and mortality in maintenance dialysis patients. Am J Kidney Dis. 1994;24:1002–1009. doi: 10.1016/s0272-6386(12)81075-4. [DOI] [PubMed] [Google Scholar]
- 3.Pupim LB, Flakoll PJ, Brouillette JR, et al. Intradialytic parenteral nutrition improves protein and energy homeostasis in chronic hemodialysis patients. J Clin Invest. 2002;110:483–492. doi: 10.1172/JCI15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pupim LB, Majchrzak KM, Flakoll PJ, Ikizler TA. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J Am Soc Nephrol. 2006;17:3149–3157. doi: 10.1681/ASN.2006040413. [DOI] [PubMed] [Google Scholar]
- 5.Cano NJ, Fouque D, Roth H, et al. Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: a 2-year multicenter, prospective, randomized study. J Am Soc Nephrol. 2007;18:2583–2591. doi: 10.1681/ASN.2007020184. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe RR. Protein supplements and exercise. Am J Clin Nutr. 2000;72(Suppl):551S–557S. doi: 10.1093/ajcn/72.2.551S. [DOI] [PubMed] [Google Scholar]
- 7.Ikizler TA, Pupim LB, Brouillette JR, et al. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am J Physiol Endocrinol Metab. 2002;282:E107–E116. doi: 10.1152/ajpendo.2002.282.1.E107. [DOI] [PubMed] [Google Scholar]
- 8.Pupim LB, Flakoll PJ, Brouillette JR, et al. Intradialytic parenteral nutrition improves protein and energy homeostasis in chronic hemodialysis patients. J Clin Invest. 2002;110:483–492. doi: 10.1172/JCI15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfe R. Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. Wiley-Liss; New York: 1992. pp. 283–316. [Google Scholar]
- 10.Ikizler TA, Pupim RB, Brouillette JR, et al. Hemodialysis stimulates muscle and whole-body protein loss and alters substrate oxidation. Am J Physiol Endocrinol Metab. 2002;282:E107–E116. doi: 10.1152/ajpendo.2002.282.1.E107. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H, Borsheim E, Anthony TG, et al. Reduced amino acid availability inhibits muscle protein synthesis and decreases activity of initiation factor eIF2B. Am J Physiol Endocrinol Metab. 2003;284:E488–E498. doi: 10.1152/ajpendo.00094.2002. [DOI] [PubMed] [Google Scholar]
- 12.Flakoll PJ, Carlson M, Cherrington AC. Physiological action of insulin. In: Leroith DTS, Olefsky J, editors. Diabetes mellitus: a fundamental and clinical text. 2nd ed. Williams & Wilkins; Philadelpia: 2000. pp. 148–161. [Google Scholar]
- 13.Stratton RJ, Bircher G, Fouque D, et al. Multinutrient oral supplements and tube feeding in maintenance dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2005;46:387–405. doi: 10.1053/j.ajkd.2005.04.036. [DOI] [PubMed] [Google Scholar]