Abstract
COX-2-derived PGs participate in a number of pathophysiological responses such as inflammation, carcinogenesis, and modulation of cell growth and survival. This study utilized complementary approaches of COX-2 transgenic and knockout mice models to evaluate the mechanism of COX-2 in Fas-induced hepatocyte apoptosis and liver failure, in vivo. We generated transgenic mice with targeted expression of COX-2 in the liver by using the albumin promoter-enhancer driven vector. The COX-2 transgenic (Tg), COX-2 knockout (KO), and wild type mice were treated with the anti-Fas antibody Jo2 (0.5 µg/g body weight) for 4–6 hours and the extent of liver injury was assessed by histopathology, serum transaminases, TUNEL staining and caspase activation. The COX-2 Tg mice showed resistance to Fas-induced liver injury when compared to the wild type mice, as reflected by the lower ALT and AST levels, less liver damage and less hepatocyte apoptosis (p<0.01). In contrast, the COX-2 KO mice showed significantly higher serum ALT and AST levels, more prominent hepatocyte apoptosis, and higher levels of caspase-8, 9, 3 activities than the wild type mice (p<0.01). The COX-2 Tg livers express higher levels of epidermal growth factor receptor (EGFR) than the wild type controls; the COX-2 KO livers express lowest levels of EGFR. Pretreatment with the COX-2 inhibitor (NS-398) or the EGFR inhibitor (AG1478) exacerbated Jo2-mediated liver injury and hepatocyte apoptosis. These findings demonstrate that COX-2 prevents Fas-induced hepatocyte apoptosis and liver failure at least in part through upregulation of EGFR.
Keywords: Cyclooxygenase-2, liver, Fas, apoptosis, epidermal growth factor receptor
INTRODUCTION
Cyclooxygenase-2 (COX-2) is a rate-limiting key enzyme that catalyzes the synthesis of prostaglandins (PGs) from arachidonic acid in a variety of tissues, including the liver. COX-2-derived PGs participate in a number of pathophysiological responses such as inflammation, carcinogenesis, and modulation of cell growth and survival. In the liver, COX-2 and PGs are implicated in hepatocyte growth and hepatocarcinogenesis. For example, liver regeneration following partial hepatectomy in mice and rats is associated with increases in the levels of PGs and the expression of COX-2, and the regenerative response is suppressed by treatment with COX inhibitors(1–7). COX-2 overexpression in hepatocytes has been shown to prevent apoptosis(8). PGs also stimulate DNA synthesis in primary cultures of neonatal and adult rat hepatocytes(9–13); these effects are exerted in synergism with epidermal growth factor (EGF), in a manner consistent with a co-mitogenic stimulation(12–14). The evidence for COX-2 and PGs in hepatocarcinogenesis is also compelling(15). For instance, the expression of COX-2 is increased in human and animal hepatocellular carcinomas (HCCs)(16–22). COX-2 overexpression or PGE2 treatment increases the growth and invasiveness of cultured HCC cells(21, 23). The cyclooxygenase inhibitors nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors prevent the growth of HCC cells in vitro and in vivo through both COX-dependent and -independent mechanisms(15). All of these findings underscore the importance of COX-2 and PG signaling in hepatocyte growth and liver tumorigenesis. It is of note that epidermal growth factor receptor (EGFR) has been shown to mediate COX-2 and PGE2 effects in cultured primary hepatocytes and HCC cells, in vitro(23, 24).
EGFR is a key receptor tyrosine kinase in the liver which plays an important role in liver regeneration and hepatocarcinogenesis(25–32). In human HCC cells, the COX-2- derived PGE2 transactivates (phosphorylates) EGFR in human HCC cells and this mechanism is important for HCC cell growth and invasion(23). In primary hepatocytes, PGE2 has also been found to enhance EGFR signaling through modulation of downstream mitogenic signaling pathways(24). The role of EGFR in hepatocyte growth is exemplified by the facts that EGFR ligands (transforming growth factor-α or EGF) enhance the growth of cultured hepatocytes in vitro(26, 27) and that conditional deletion of EGFR in mice impairs liver regeneration in vivo(28). However, to date, the role of EGFR in COX-2 and PG-mediated hepatic actions in vivo has not been addressed.
This study was designed to examine the mechanism of COX-2 in Fas-induced liver injury in vivo. We generated transgenic mice with targeted expression of COX-2 in the liver by using the albumin promoter-enhancer driven vector. The produced COX-2 transgenic mice, along with the COX-2 knockout and wild type mice, were injected with the anti-Fas antibody Jo2 to document the extent of liver injury. Our data show that the COX-2 Tg mice resist Fas-induced liver failure, as reflected by the lower serum transaminase levels, less liver damage, less apoptotic hepatocytes, less caspase activation, and less PARP cleavage. In contrast, the COX-2 KO mice showed more prominent liver injury than wild type mice under the same experimental condition. Furthermore, hepatic expression of EGFR is highest in COX-2 Tg mice, intermediate in wild type mice and lowest in COX-2 KO mice. Pretreatment with the COX-2 inhibitor (NS-398) or EGFR inhibitor (AG1478) exacerbated Jo2-mediated liver injury. These results demonstrate that COX-2 prevents Fas-induced liver failure at least in part through upregulation of EGFR.
MATERIALS AND METHODS
Animals
Transgenic mice with targeted expression of COX-2 in the liver were developed by using the well-established albumin promoter-enhancer driven vector, as described in a separate study(33). Briefly, the transgene containing full-length human COX-2 cDNA under the control of mouse albumin enhancer/promoter was micro-injected into mouse zygotes (B6SJL/F1 eggs). The COX-2 transgenic mice used in this study were derived from the transgenic line that was backcrossed to C57BL/6 wild type mice for more than five consecutive generations. The COX-2−/− mice and wild type C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, MA) and the colonies were maintained at the University of Pittsburgh Animal Facility. The COX-2−/− mice (obtained from the Jackson Laboratory)(34) were initially developed in the genetic background of C57BL/6 × 129Sv and have been backcrossed to C57BL/6 mice for more than 12 generations. The mice at the age of 8–10 weeks were utilized for experiments, with age- and sex-matched wild type C57BL/6 mice as controls. The animals were kept at 22°C under a 12-h light/dark cycle and received food and water ad libitum. The handling of mice and experimental procedures were conducted in accordance with experimental animal guidelines.
Experimental Protocol
Male C57BL/6 wild type mice, COX-2 Tg mice and COX-2 KO mice were used for experiments at the age of 8–10 weeks. The mice were administered intraperitoneally (i.p.) with 0.5µg/g body weight of Jo2 to induce acute fulminant hepatic failure (the reagents were dissolved in sterile nonpyrogenic saline solution). The animals were sacrificed at specific time points to obtain blood and liver tissues. The liver tissues were rapidly excised, and the specimens were immediately cut into small fragments and subjected to standard formalin fixation and paraffin-embedding for histological evaluation and TUNEL stain. The remaining liver samples were immediately frozen in liquid nitrogen and stored at −80°C for future preparation of tissue homogenates. The blood samples were centrifuged at 3000 rpm for 15 min, and the sera were collected and stored at −80°C. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were measured using an automatic analyzer at the University of Pittsburgh Medical Center Chemistry Department. All experimental animals used in this study were treated according to the protocol approved by the University of Pittsburgh Animal Care and Use Committee (Protocol # 0303501).
In another set of experiments, mice were pre-treated intraperitoneally with vehicle (DMSO), NS 398 (5 mg/kg), or AG1478 (25 mg/kg) for 30 min before Jo2 administration and the animals were sacrificed at 4 h after Jo2 administration to obtain blood and liver tissues.
Hematoxylin and Eosin Staining
For histological analysis, liver tissue was fixed in 10% neutral-buffered formalin and embedded in paraffin. Sections of 5-µm thickness were affixed to slides, deparaffinized, and stained with hematoxylin and eosin (H&E) to determine morphologic changes.
TUNEL Stain
The extent of hepatocyte apoptosis was detected by terminal deoxynucleotidy1-transferase (TdT)-mediated deoxyuridine triphosphate-digoxigenin (dUTP) nick-end labeling (TUNEL). TUNEL-positive cells were counted by randomly selecting high-power fields (400 x) distributed over 6 independent sections. The numbers of TUNEL-positive and -negative cells were compiled and the percentages of TUNEL-positive cells were calculated.
Analysis of caspase activities
After Jo2 antibody challenge, caspase-3, 8 and 9 activities were measured in liver extracts from mice pretreated with or without inhibitors. Briefly, 30 µg of liver protein was incubated with 20 µM fluorogenic substrates Ac-DEVD-AFC, Ac-IETD-AFC, and Ac-LEHD-AFC for caspase-3, caspase-8, caspase-9 activity, respectively. The fluorescence signals were monitored by a fluorometer (Tecan, GENios) at excitation wavelengths of 400 nm and emission wavelengths of 510 nm and the background signals were corrected. The caspase activities were expressed as the fold changes over the control samples (from corresponding wild type mice).
RESULTS
Hepatic overexpression of COX-2 protects mice against Fas-induced liver failure
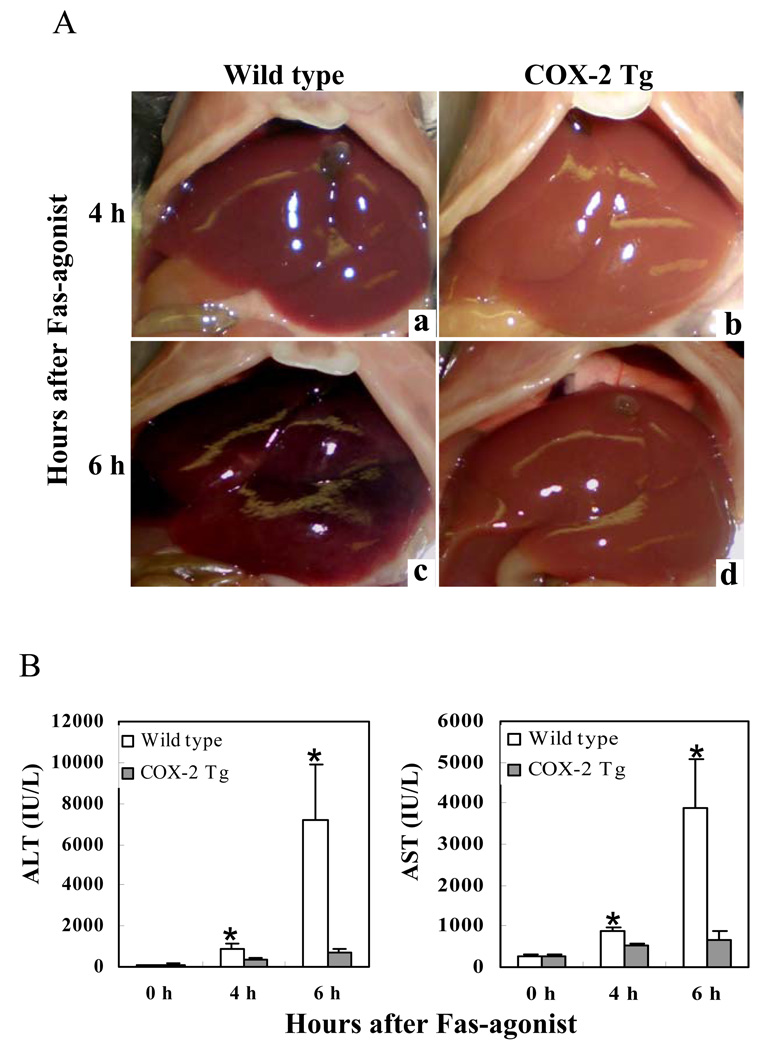
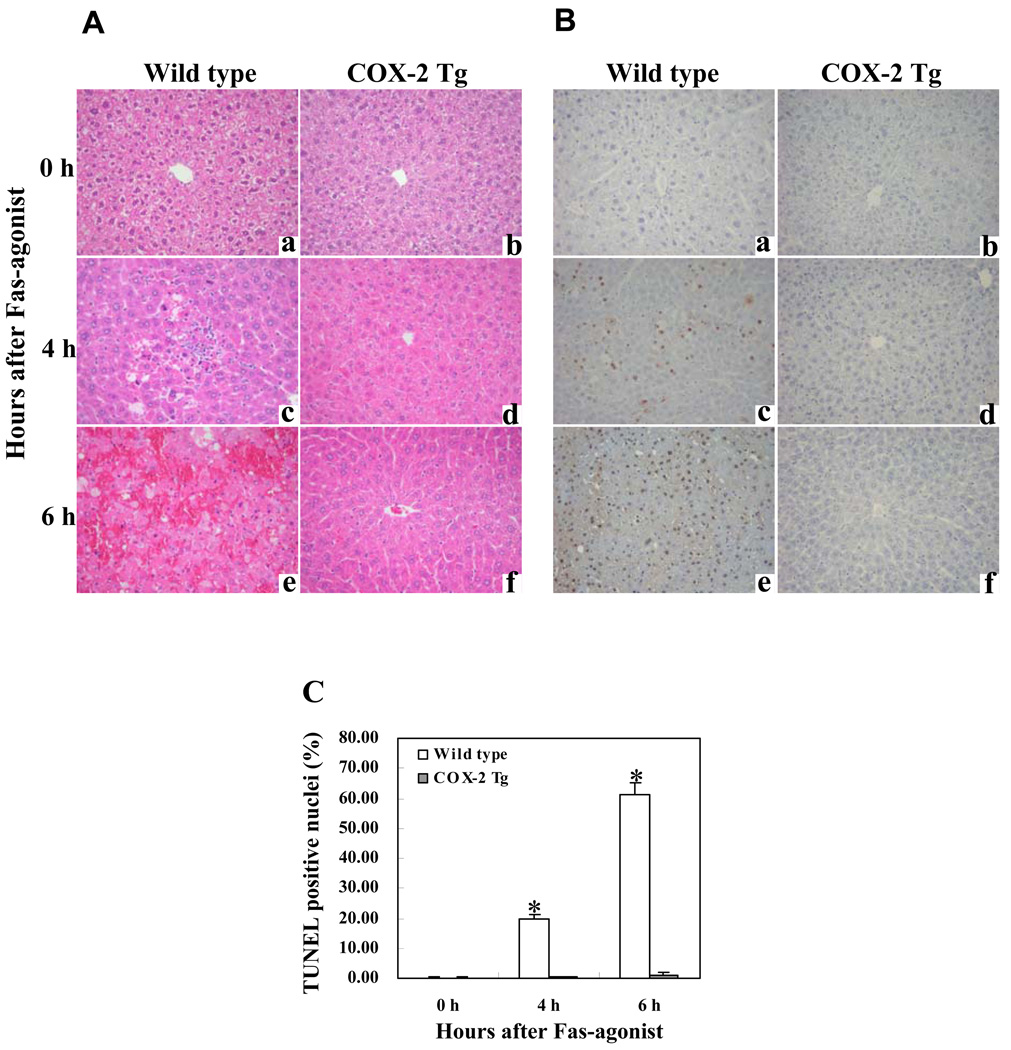
To investigate the effect of hepatocyte COX-2 in Fas-induced hepatocyte apoptosis, the COX-2 Tg mice and the age/sex-matched wild type mice were injected intraperitoneally with a single dose of purified hamster anti-mouse Fas monoclonal antibody Jo2 (0.5 µg/g body weight). The animals were sacrificed at specific time points (0, 4 and 6 h) after Jo2 administration to obtain blood samples and liver tissues for liver enzyme and tissue analyses. The livers of wild type mice turned to dark red after Jo2 injection because of massive hepatic hemorrhage, which was observed at 4 hour and became much more prominent at 6h. In contrast, the livers of COX-2 transgenic mice were completely normal at 4 h and became slightly red at 6 h (Fig. 1A). Accordingly, the wild type mice showed significantly higher serum alanine transaminase (ALT) and aspartate transaminase (AST) levels than COX-2 Tg mice at both 4 and 6 h time points (p<0.01) (Fig. 1B). Histological examination of the liver tissues revealed more prominent hepatocyte apoptosis and liver damage in the wild type mice than in COX-2 Tg mice (Fig. 2). In the wild type group, multifocal hepatocyte apoptosis was observed at 4 hours and massive hepatocyte apoptosis with hemorrhage was observed at 6 hours. In contrast, the livers from the COX-2 Tg mice showed no significant histological abnormalities at 4 h and only mild scattered apoptosis were observed in the COX-2 Tg mice at 6 h (Fig. 2A and B). The number of TUNEL-positive hepatocytes in the wild type mice is significantly higher than in the COX-2 transgenic mice at both 4 and 6 hour time points (p<0.01) (Fig. 2C). These results indicate that hepatic overexpression of COX-2 protects the liver from Fas-induced hepatocyte apoptosis and liver injury.
Figure 1. Hepatic overexpression of COX-2 prevents Fas-induced liver injury.
The COX-2 transgenic (Tg) mice and their age/sex-matched wild type mice were injected intraperitoneally with a single dose of purified hamster anti-mouse Fas monoclonal antibody Jo2 (0.5 µg/g body weight) to induce hepatocyte apoptosis. The animals were sacrificed at 4 hours and 6 hours after injection. The experiments include 6 mice per group. (A) Gross photographs of liver taken 4 hours (a and b) and 6 hours (c and d) after Jo2 injection. Note the livers of wild type mice turned to dark red after Jo2 injection because of massive hepatic hemorrhage, which was observed at 4 hour (a) and became much more prominent at 6 h (c). In contrast, the livers of COX-2 transgenic mice were completely normal at 4 h (b) and became slightly red at 6 h (d). (B) Serum levels of ALT and AST at 4 h and 6 h after Jo2 injection. Blood samples were collected and sera were separated for transaminase analysis. The COX-2 Tg mice show significantly lower serum ALT and AST levels than the wild type mice after Jo2 treatment. The data are expressed as mean ±SD from 6 mice (*p<0.01 vs. corresponding COX-2 Tg mice, Student’s t test).
Figure 2. Hepatic expression of COX-2 suppresses Fas-induced hepatocyte apoptosis and liver tissue damage.
The COX-2 Tg mice and their age/sex-matched wild type mice were administered intraperitoneally with saline or Jo2 ( 0.5 µg/g body weight). The animals (6 mice per group) were sacrificed at 0 (a and b), 4 (c and d) and 6 (e and f) hours after injection and the liver tissues were harvested for histological evaluation. Formalin-fixed and paraffin-embedded sections (5 µm thick) were stained with hematoxylin and eosin (H&E) (A), and terminal deoxynucleotidy1 – transferase-mediated deoxyuridine triphosphate-digoxigenin nick-end labeling (TUNEL) (B) (200X). After Jo2 administration, the livers of the wild type mice exhibit more prominent hemorrhage necrosis, hepatocyte apoptosis and degeneration (c and e), when compared to the livers of COX-2 transgenic mice (d and f). The number of TUNEL-positive hepatocytes in the wild type mice is significantly higher than in the COX-2 transgenic mice (*p<0.01) (the data are expressed as mean ±SD from 6 mice per group) (C).
Disruption of the COX-2 gene enhances Fas-induced liver injury
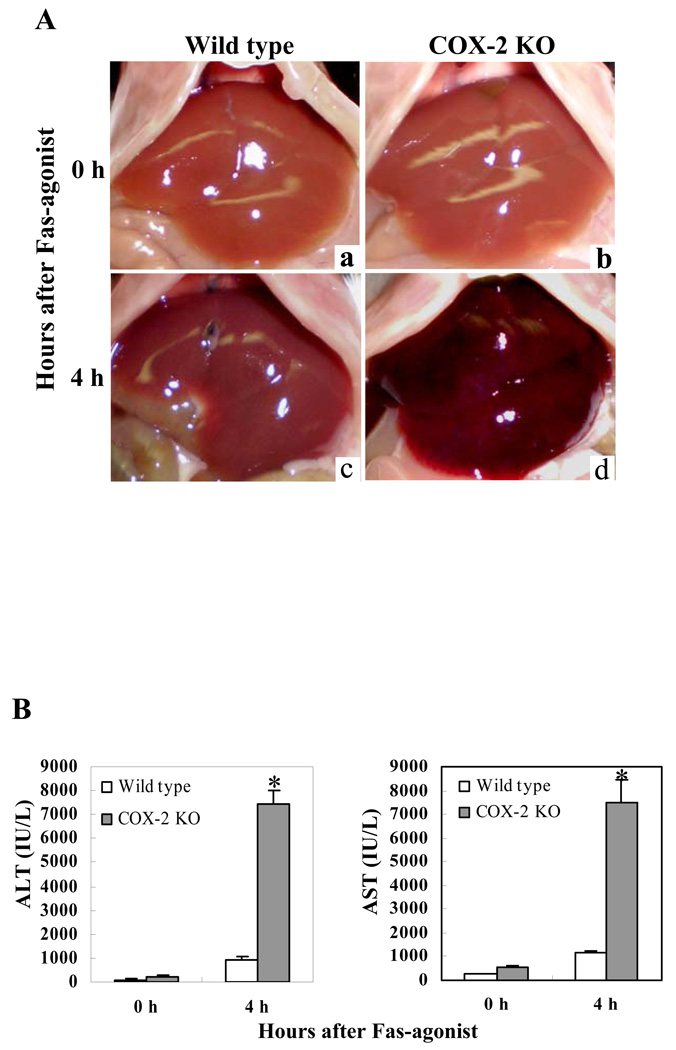
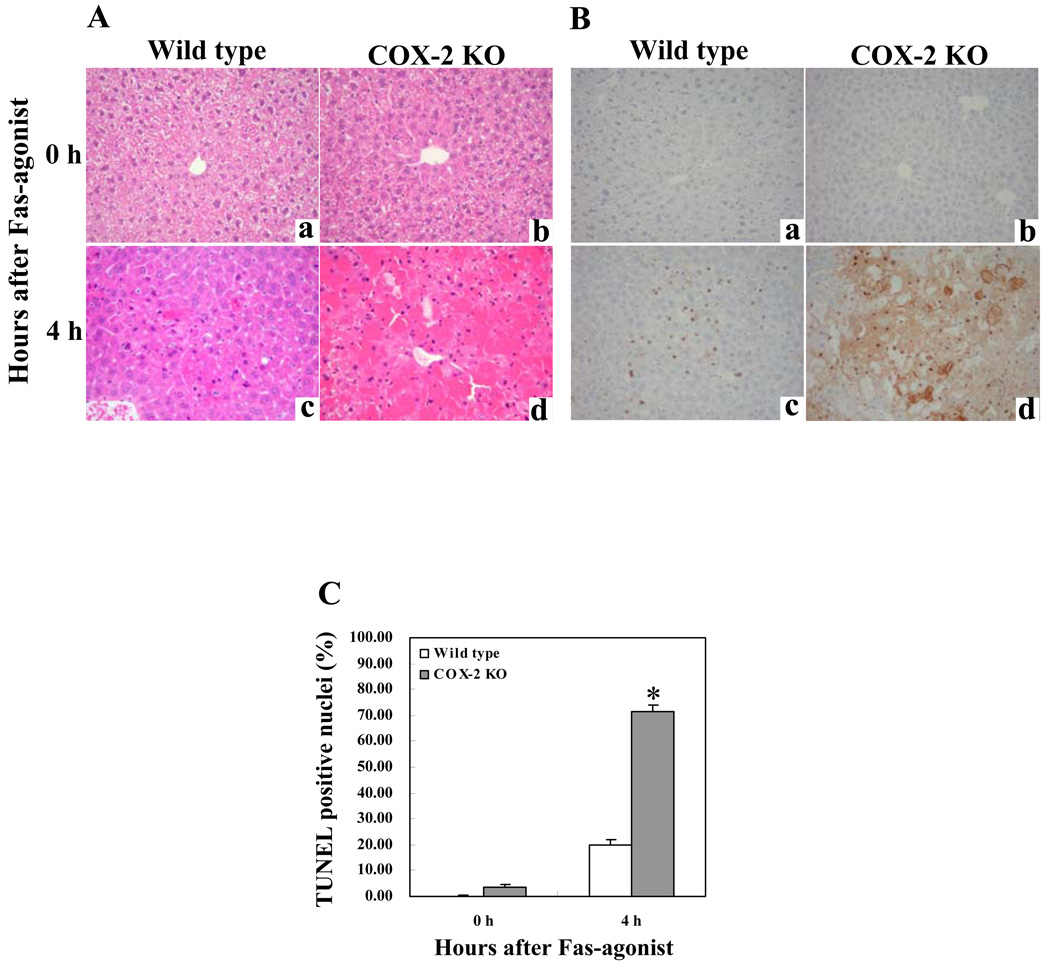
We next utilized the mice with homozygous deletion of COX-2 to determine their response to Jo2. The COX-2 knockout mice and the age/sex-matched wild type control mice were injected intraperitoneally with Jo2 (0.5 µg/g body weight) and the animals were sacrificed as described above. The livers from the COX-2 KO mice turned dark red in color and showed more injury than the wild type mice 4 hours after Jo2 injection (Fig. 3A). Accordingly, the COX-2 KO mice showed significantly higher serum ALT and AST levels than the wild type mice (p<0.01) (Fig. 3B). Histological examination of the liver tissues revealed more prominent hepatocyte apoptosis and liver damage in the COX-2 KO mice compared to the wild type mice (Fig. 4). The number of TUNEL-positive hepatocytes in the COX-2 KO mice is significantly higher than in the wild type mice after Jo2 challenge (p<0.01) (Fig. 4). These findings indicate that COX-2 inactivation augments Fas-induced hepatocyte apoptosis and liver injury, which is consistent with the observation that the COX-2 inhibitor, NS-398, enhances Fas-induced liver injury (see below).
Figure 3. COX-2-deficientcy enhances liver injury induced by Fas-agonist.
The COX-2 knockout (KO) mice and their age/sex-matched wild type mice were injected intraperitoneally with Jo2 (0.5 µg/g body weight) and the animals were followed up for 4 hours (6 mice per group). (A) Liver appearance after Jo2 challenge. The COX-2 KO mice (b and d) show more prominent liver hemorrhage and injury than the wild type mice (a and c). (B) Serum ALT and AST levels after Jo2 treatment. The COX-2 KO mice show higher levels of serum ALT and AST than the wild type mice after Jo2 treatment (*p<0.01 vs. the corresponding wild type mice; the ata are expressed as mean ±SD from 6 mice).
Figure 4. COX-2-deficiency enhances Fas-induced hepatocyte apoptosis and liver tissue damage.
The COX-2 knockout (KO) mice and their age/sex-matched wild type mice (6 mice per group) were administered intraperitoneally with saline or Jo2 (0.5 µg/g body weight). The animals were sacrificed at 0 (a and b) and 4 hours (c and d) after Jo2 injection and the liver tissues were harvested for histological evaluation. Formalin-fixed and paraffin-embedded sections (5 µm thick) were stained with hematoxylin and eosin (H&E) (A), and terminal deoxynucleotidy1 – transferase-mediated deoxyuridine triphosphate-digoxigenin nick-end labeling (TUNEL) (B) (200X). The livers of COX-2 knockout mice exhibit more prominent hepatocyte apoptosis and hemorrhage (d) when compared to the livers of wild type mice (c). The number of TUNEL-positive hepatocytes in COX-2 knockout mice is significantly higher than in wild type mice (*p<0.01; the data are expressed as mean ±SD from 6 mice) (C).
COX-2 prevents Fas-induced caspase activation
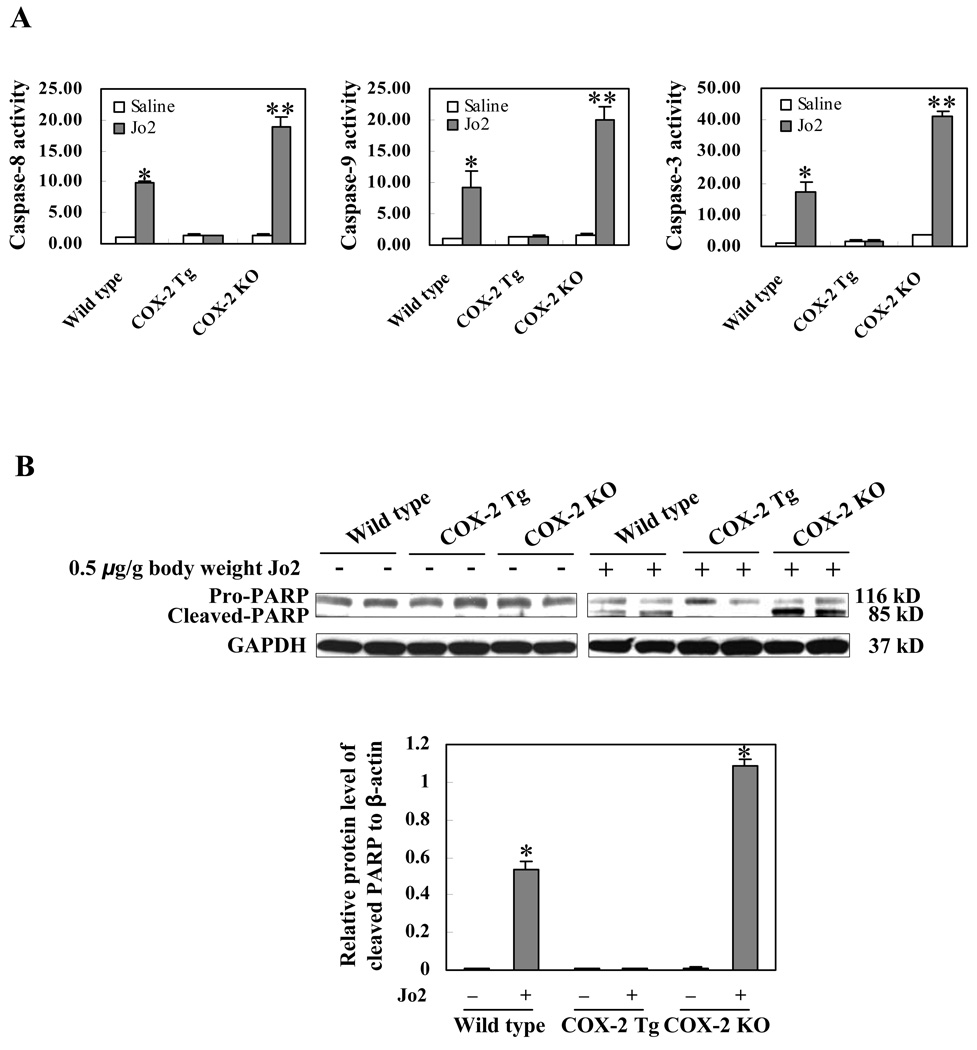
Fas, followed by binding of its ligand (e.g. Jo2), undergoes trimerization to form the DISC complex, which recruits initiator caspase-8 via the adaptor protein FADD and then activates caspase-8. Activated caspase-8 stimulates the caspase cascade leading to the activation of caspase-9 and caspase-3. Given the involvement of caspase 3, 8, and 9 in Fas-induced apoptosis, we next measured their activities in the COX-2 transgenic and knockout livers. As shown in Fig. 5A, although Jo2 injection increased the levels of hepatic caspase 3, 8, and 9 activities in the COX-2 KO and wild type mice (p<0.01 compared to the corresponding saline treatment groups), the same concentration of Jo2 did not alter caspase activities in the COX-2 Tg mice. Moreover, the hepatic caspase 3, 8, and 9 activities in Jo2-treated COX-2 knockout mice are significantly higher than in Jo2-treated wild type mice (p<0.01). Since PARP is one of the main cleavage targets of caspase-3 in vivo, we next performed western blot analysis to determine the levels of cleaved PARP in the liver tissues. As shown in Fig. 5B, Jo2 treatment for 4 hours induced the cleavage of PARP in the COX-2 KO and wild type mice, but not in the COX-2 Tg mice. It is noteworthy that more prominent PARP cleavage was observed in the COX-2 KO mice than in wild type mice. These results demonstrate that COX-2 signaling prevents Fas-induced caspase activation in the liver.
Figure 5. COX-2 protects the liver from Fas-induced apoptosis.
The COX-2 transgenic (Tg), COX-2 knockout (KO), and their matched wild type mice were administered intraperitoneally with saline or Jo2 (0.5 µg/g body weight) (6 mice per group). The animals were sacrificed at 4 hours after the injection and the liver tissues were harvested and homogenized for subsequent caspase activity assay and western blotting for PARP. (A) The levels of caspases-3, 8, and 9 activities in liver homogenates. Caspase 3, 9, and 8 activities were measured by fluorometric assay with Ac-DEVD-AFC, Ac-LEHD-AFC, and Ac-IETD-AFC as the substrates, respectively. The results are expressed as mean ±SD of fold changes over wild type livers (*p<0.01 compared to the corresponding wild type mice, **p<0.01 compared to the corresponding COX-2 KO mice, n = 6 for each group). (B) Western blot analysis to detect PARP cleavage. Liver homogenates from the COX-2 Tg, COX-2 KO and wild type livers were subjected to SDS-PAGE and Western blot analysis to determine the protein level of proform and cleaved PARP. Western blot for GAPDH was used as the loading control. Jo2 treatment for 4 hours induced PARP cleavage in the COX-2 KO and wild type mice, but not in the COX-2 Tg mice (more prominent PARP cleavage was observed in the COX-2 KO mice than in wild type mice). *p<0.01 compared to the corresponding mice without Jo2 treatment.
Hepatic overexpression of COX-2 induces antiapoptotic molecules
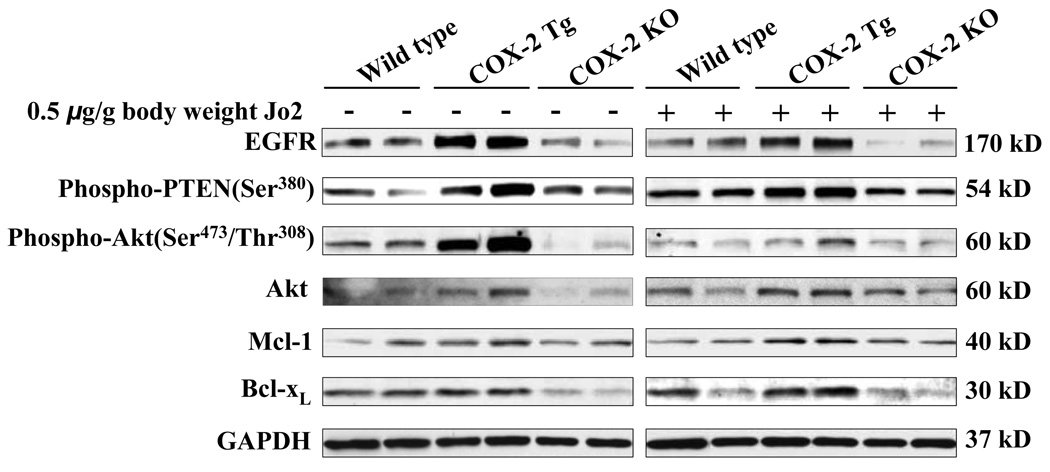
To address the mechanisms by which COX-2 protects against Fas-induced hepatocyte apoptosis in our system, we further performed western blot analyses to determine the levels of several molecules that are implicated in hepatocyte survival. Given that EGFR has been shown to play a key role in COX-2-mediated growth of transformed hepatocytes(23), we first investigated the levels of EGFR in the COX-2 transgenic and knockout mice. As shown in Figure 6 and Supplemental Figure S1, the level of EGFR is increased in the COX-2 transgenic liver and decreased in the COX-2 knockout liver. This pattern of alteration is observed in mice with or without Jo2 treatment. Since EGFR is known to phosphorylate and activate Akt and this process is facilitated by PTEN phosphorylation, we next examined the phosphorylation level of Akt and PTEN in the COX-2 Tg and knockout livers. As shown in Figure 6, the phosphorylation of Akt and PTEN is increased in the liver of COX-2 Tg mice and decreased in the COX-2 knockout mice. The levels of Mcl-1 and Bcl-xL, two downstream targets of Akt, are slightly increased in the COX-2 Tg mice and slightly reduced in the COX-2 knockout mice (Figure 6). These findings suggest a potential role of EGFR and related signaling in COX-2 mediated hepatocyte survival, in vivo.
Figure 6. Changes of EGFR and related signaling molecules in mice with altered expression of COX-2.
The COX-2 Tg, COX-2 KO and wild type mice were injected intraperitoneally with saline or Jo2 (0.5 µg/g body weight). The livers were harvested 4 hours after the injection and the liver tissues were then homogenized. The obtained cellular proteins were subjected to SDS-PAGE and Western blot analysis to determine the protein levels of EGFR and its related signaling molecules, including phospho-PTEN, phospho-Akt, Akt, Mcl-1, and Bcl-xL. Western blot for GAPDH was shown as the loading control. The blots in this figure were obtained from two individual mice for each group.
Inhibition of COX-2 and EGFR exacerbates Fas-induced hepatocyte apoptosis and liver injury
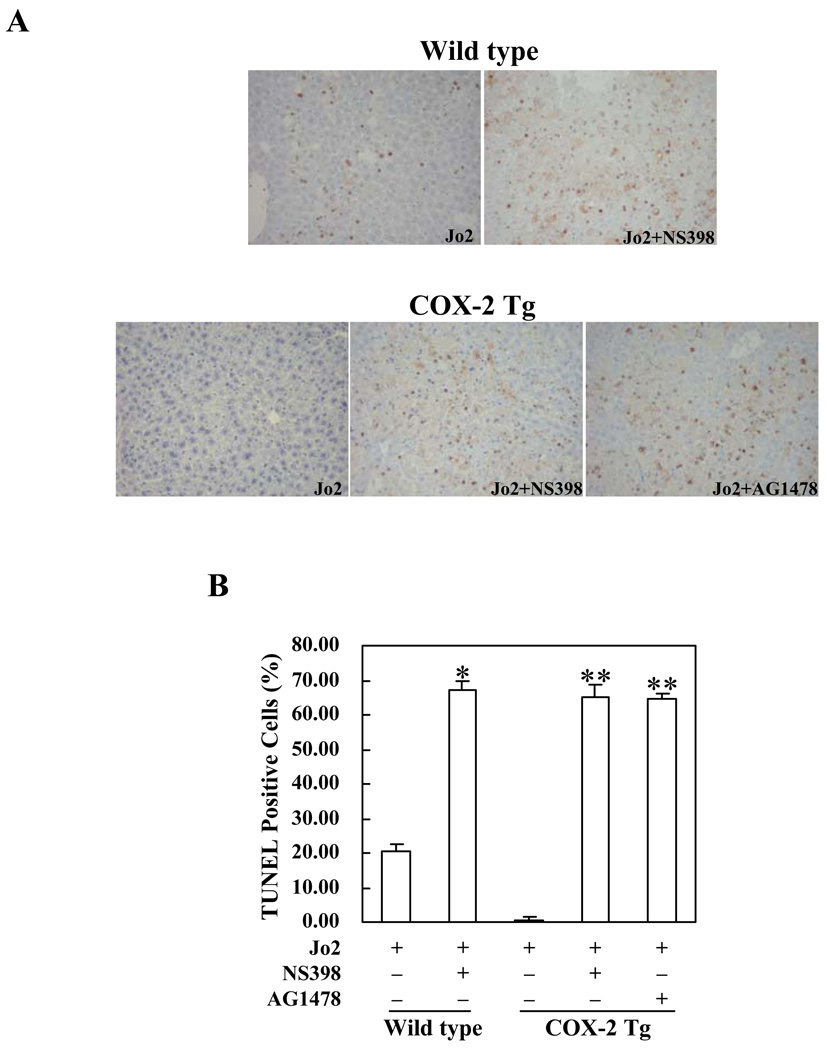
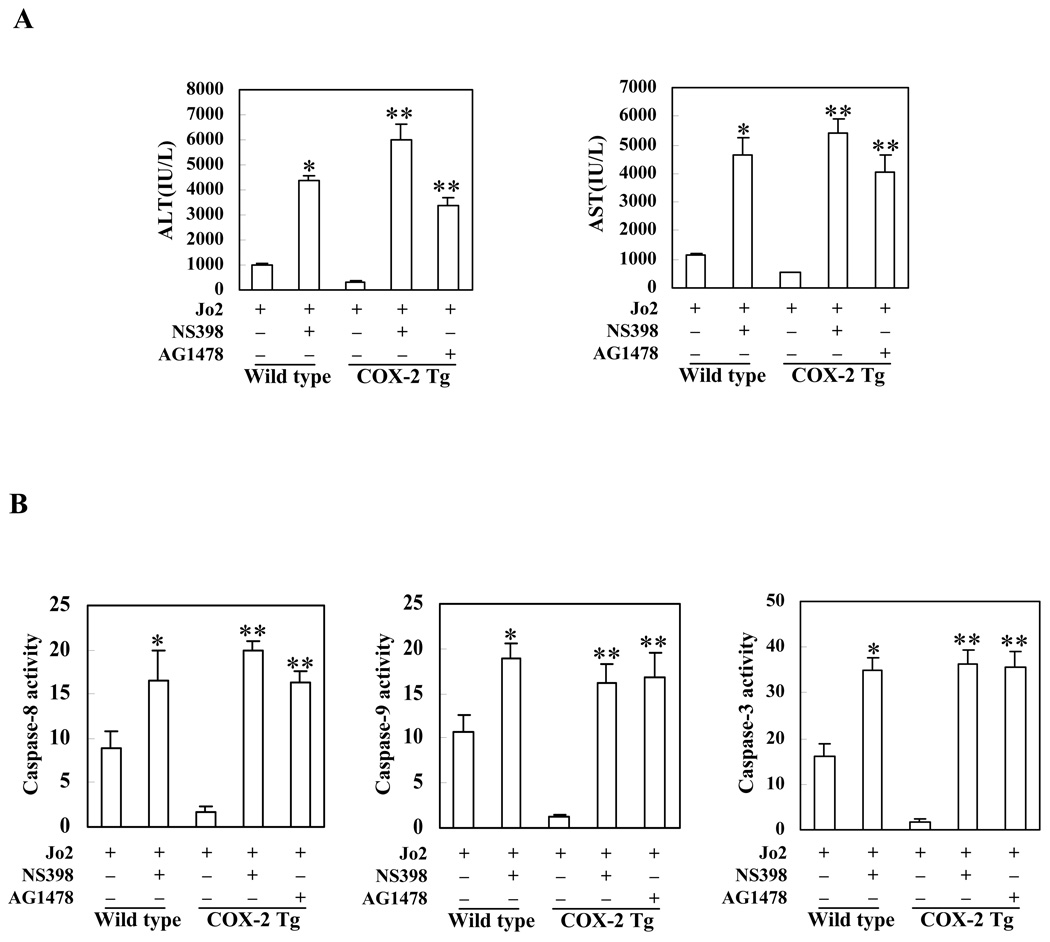
To further investigate the role of COX-2 and EGFR in Fas-induced hepatocyte apoptosis, we next employed pharmacological inhibitors of COX-2 and EGFR in our animal models. For the COX-2 inhibitor protocol, wild type mice and COX-2 Tg mice received intraperitoneal injection of the COX-2 inhibitor, NS-398 (5 mg/kg body weight), 30 minutes before administration of Jo2 (0.5 mg/kg body weight); the animals were sacrificed 4 hours after Jo2 injection. As shown in Figure 7, pretreatment with NS-398 resulted in more prominent hepatocyte apoptosis in response to Jo2 challenge; this phenomenon is observed in both wild type and COX-2 Tg mice. The number of TUNEL-positive hepatocytes in the wild type and COX-2 Tg mice pretreated with NS398 is significantly higher than in the mice pretreated with vehicle control (p<0.01) (Figure 7). Pretreatment of wild type and COX-2 Tg mice with NS-398 induced significantly higher serum ALT and AST levels when compared to pretreatment with vehicle control (p<0.01) (Figure 8A). Furthermore, pretreatment with NS-398 also induced more caspase-8, 9, 3 activation than pretreatment with vehicle in both wild type and COX-2 Tg mice (p<0.01) (Figure 8B).
Figure 7. Effects of the COX-2 inhibitor, NS-398, and the EGFR inhibitor, AG1478, on Fas-induced hepatocyte apoptosis in COX-2 Tg or wild type mice.
The animals were injected intraperitoneally with the COX-2 inhibitor, NS-398 (5 mg/kg body weight) or the EGFR inhibitor, AG1478 (25 mg/kg body weight), 30 minutes before intraperitoneal administration of Jo2 (0.5 mg/kg body weight). The animals were sacrificed 4 hours after Jo2 injection and the liver tissues were harvested for histopathological examination (6 mice per group). (A) Representative TUNEL stains (200X) of the liver tissues from mice pretreated with or without inhibitors (all the mice received Jo2 injection). (B) Quantitation of TUNEL-positive hepatocytes in mice pretreated with or without inhibitors (*p<0.01 compared to the corresponding wild type mice without inhibitor pretreatment; **p<0.01 compared to the corresponding COX-2 Tg mice without inhibitor pretreatment).
Figure 8. Effects of the COX-2 inhibitor, NS-398, and the EGFR inhibitor, AG1478, on serum transaminase activity and liver caspase activity levels.
The COX-2 Tg and wild type mice were injected intraperitoneally with the COX-2 inhibitor, NS-398 (5 mg/kg body weight) or the EGFR inhibitor, AG1478 (25 mg/kg body weight), 30 minutes before intraperitoneal administration of Jo2 (0.5 mg/kg body weight). The animals were sacrificed at 4 hours after Jo2 injection (6 mice per group). Upon sacrifice the blood samples were collected for serum transaminase analysis, whereas the liver tissues were harvested and homogenated for caspase activity analysis. (A) Serum ALT and AST levels in mice with or without inhibitor pretreatment (all the mice received Jo2 injection). *p<0.01 compared to the corresponding wild type mice without inhibitor pretreatment; **p<0.01 compared to the corresponding COX-2 Tg mice without inhibitor pretreatment (n = 6). (B) Liver caspases 3, 8, and 9 activities in mice with or without inhibitor pretreatment (all the mice received Jo2 injection). The liver tissue homogenates were analyzed for caspase-3, 9, and 8 activities by fluorometric assay with Ac-DEVD-AFC, Ac-LEHD-AFC, and Ac-IETD-AFC as the substrates, respectively. The data are expressed as mean ±SD of changes over wild type livers (n = 6 for each group). *p<0.01 compared to the wild type mice without inhibitor pretreatment; **p<0.01 compared to the corresponding COX-2 Tg mice without inhibitor pretreatment (n = 6).
For the EGFR inhibitor protocol, the COX-2 Tg mice were pretreated with the EGFR inhibitor, AG1478 (25 mg/kg body weight), or vehicle 30 minutes before Jo2 injection. Histological examination of the liver tissues harvested 4 hours after Jo2 injection showed more prominent hepatocyte apoptosis in AG1478 pretreated mice (Figure 7). The number of TUNEL-positive hepatocytes in the AG1478 pretreated group is significantly higher than in the control group (p<0.01) (Figure 7). The mice pretreated with AG1478 showed significantly higher serum ALT and AST levels (Figure 8A) and higher caspase-8, 9, 3 activities (Figure 8B) (p<0.01), when compared with the mice pretreated with vehicle. These findings further support the role of EGFR in COX-2-mediated protection against Fas-induced liver injury, in vivo.
Activation of NF-kB is a potential mechanism for COX-2-induced EGFR expression in the liver
Given that the EGFR promoter contains NF-κB binding site that is necessary for the transcriptional activity of EGFR and that inhibition of NF-κB decreases EGFR promoter activity(35), we sought to document NF-κB activation and binding to EGFR promoter in mice with altered expression of COX-2. As shown in Supplemental Figure S2, the COX-2 transgenic livers have higher level of p65 NF-κB as well as phosphorylated p65 NF-κB than wild type livers. Moreover, the liver tissues from the COX-2 transgenic mice show increased binding of NF-κB to its response element in the EGFR promoter (Supplemental Figure S3). These findings suggest that COX-2 upregulates and phosphorylates NF-κB, leading to its nuclear translocation and binding to the EGFR promoter. Thus, NF-κB activation may represent a potential mechanism for COX-2-mediated EGFR expression in the liver.
DISCUSSION
In this study, we present evidences for the involvement of COX-2 in hepatocyte survival by complementary genetic and pharmacological approaches. In the COX-2 transgenic model, targeted expression of COX-2 in the liver was found to protect against Fas-induced liver injury. This is highlighted by the observation that Jo2 treated COX-2 transgenic mice have lower transaminase levels, less liver tissue damage, fewer apoptotic hepatocytes, lower caspase-8, 9, 3 activities and less PARP cleavage, when compared to Jo2 treated wild type mice. These findings indicate that COX-2 in hepatocytes is an important survival molecule that prevents Fas-induced hepatocyte apoptosis and liver injury. This conclusion is corroborated by the data from the COX-2 KO mouse model showing that deletion of COX-2 exacerbates Fas-induced liver injury. The role of COX-2 in hepatocyte survival is further supported by the pharmacological approach showing that pretreatment with the COX-2 inhibitor, NS-398, enhanced Jo2-induced liver injury. All of these findings demonstrate a protective effect of COX-2 against Fas-induced liver injury, in vivo. Furthermore, our western blot analyses reveal that the COX-2 Tg livers express higher level of EGFR than the wild type controls; the COX-2 KO livers express lowest level of EGFR. The direct involvement of EGFR in COX-2 actions is reflected by the observation that pretreatment with the EGFR inhibitor, AG1478, augmented Jo2-induced liver injury. These results suggest that upregulation of EGFR may be an important mechanism for COX-2-mediated resistance to Fas-induced liver injury.
EGFR is a receptor tyrosine kinase that controls a wide variety of biological responses such as proliferation, migration and modulation of apoptosis(36). Our previous studies have shown that the COX-2-derived PGE2 transactivates EGFR in cultured human HCC cells(23), a phenomenon also documented in other transformed cell lines including cholangiocarcinoma cells(37) and colon cancer cells(38–40). However, in cultured primary hepatocytes PGE2 does not appear to induce EGFR phosphorylation; instead, PGE2 acts in synergism with EGF by modulating mitogenic mechanisms downstream of EGFR(24). It remains unclear why COX-2-derived PGE2 transactivates EGFR in HCC cells but not in cultured primary hepatocytes. This scenario appears to coincide with the notion that G protein coupled receptors (including prostaglandin receptors) more often elicit EGFR transactivation in transfected or malignant cells as compared to normal cells(41, 42). In the current study we show that COX-2 upregulates hepatic expression of EGFR, in vivo.
Casado et al(8) has reported a protective effect of COX-2 against Fas-induced hepatocyte apoptosis using a mouse model of targeted expression of COX-2 in the liver under the control of ApoE promoter; this part of study is confirmed in our transgenic model with targeted expression of COX-2 expression under the control of albumin promoter. In the current study, we further utilized the COX-2 knockout mice as a complementary model to validate the effect of COX-2. More importantly, the present study provides novel mechanistic insight into COX-2 actions in the liver. Our results show that EGFR upregulation plays a key role in COX-2-mediated protection against Fas-induced liver injury. Furthermore, our data suggest the involvement of NF-κB in COX-2-mediated EGFR expression in the liver.
Given that EGFR is able to phosphorylate and activate Akt and this process is facilitated by PTEN phosphorylation, we also examined the phosphorylation level of Akt and PTEN in the COX-2 Tg and knockout livers. Our data indicate that the levels of p-Akt and p-PTEN are increased in the COX-2 Tg livers but reduced in the COX-2 knockout livers. Additionally, the levels of Mcl-1 and Bcl-xL, two downstream targets of Akt, are slightly increased in the COX-2 Tg mice and slightly decreased in the COX-2 knockout mice. These findings suggest a potential role of EGFR and related signaling in COX-2 mediated hepatocyte survival, in vivo.
Reinehr and colleagues have shown that Fas activation induces the phosphorylation and activation of EGFR which in turn triggers Fas-tyrosine phosphorylation in hepatocytes; this process is proposed to be a prerequisite for Fas oligomerization and microtubule-dependent translocation of Fas to the plasma membrane leading to subsequent FADD and caspase 8 recruitment and apoptosis induction(43, 44). Therefore, the role of EGFR in hepatocytes is complex and involves both cell survival and proapoptotic actions. However, the exact reason for such a paradoxical effect of EGFR in hepatocyte survival is not known. Reinehr and colleagues have shown that phosphorylation of Fas by EGFR is mediated by phospho-EGFR-Tyr845 and phospho-EGFR-Tyr1173 but not by phospho-EGFR-Tyr1045(45). On the other hand, activation of EGFR by its ligand (epidermal growth factor and transforming growth factor-α) induces autophosphorylation of EGFR at distinct and overlapping tyrosine residues (including Tyr992, Tyr1068, Tyr1086, Tyr1148, and Tyr1173)(46, 47); these phosphorylated tyrosine residues serve as docking sites for a range of adaptor proteins, whose recruitment leads to activation of the downstream cell survival signaling cascade including the Akt pathway. Therefore, EGFR may influence cell survival or Fas-mediated apoptosis depending on its phosphorylation at specific tyrosine residues. Further studies are needed to define the role of specific forms of phospho-EGFR in hepatocyte growth control and their interaction with other key signaling molecules.
Our experimental findings suggest that COX-2 is not a general cytoprotective mediator in the liver. The data presented in the current study show that COX-2 protects the liver from Fas-induced apoptosis via up-regulation of EGFR and activation of its downstream Akt signaling pathway. A separate study from our laboratory has shown that after LPS/D-galactosamine injection the COX-2 transgenic mice exhibit more prominent liver tissue damage than wild-type mice and the effect involves activation of JNK2(33). Our unpublished data indicate that CCl4-induced acute liver injury is not significantly different between the COX-2 transgenic and wild type groups. Thus, hepatic COX-2 may mediate different responses depending on the context of liver injuries. Consequently, when or where to inhibit or enhance these pathways therapeutically requires careful consideration of the underlying cause of liver injuries as well as the potential benefit and/or risk associated with the intervention.
In summary, this study discloses an important role of EGFR in COX-2-mediated protection against Fas-induced hepatocyte apoptosis and liver failure, in vivo. Given the importance of COX-2-derived prostaglandins in several key aspects of liver pathobiology including hepatocyte survival, liver regeneration, chronic hepatitis, liver injury and hepatocarcinogenesis, further investigations are warranted to detail the mechanisms of COX-2 and prostaglandin actions in different liver diseases and animal models.
Supplementary Material
Acknowledgments
This work is partially supported by the National Institutes of Health R01 grants CA106280, CA102325 and DK077776 (to T.W.). G.L. is supported in part by the China Scholarship Council
ABBREVIATIONS
- COX-2
cyclooxygenase-2
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- HCC
hepatocellular carcinoma
- PG
prostaglandin
- NSAID
nonsteroidal anti-inflammatory drug
Footnotes
No conflicts of interest exist.
REFERENCES
- 1.MacManus JP, Braceland BM. A connection between the production of prostaglandins during liver regeneration and the DNA synthetic response. Prostaglandins. 1976;11:609–620. doi: 10.1016/0090-6980(76)90064-2. [DOI] [PubMed] [Google Scholar]
- 2.Miura Y, Fukui N. Prostaglandins as possible triggers for liver regeneration after partial hepatectomy. A review. Cell Mol Biol. 1979;25:179–184. [PubMed] [Google Scholar]
- 3.Rixon RH, Whitfield JF. An early mitosis-determining event in regenerating rat liver and its possible mediation by prostaglandins or thromboxane. J Cell Physiol. 1982;113:281–288. doi: 10.1002/jcp.1041130216. [DOI] [PubMed] [Google Scholar]
- 4.Callery MP, Mangino MJ, Flye MW. Kupffer cell prostaglandin-E2 production is amplified during hepatic regeneration. Hepatology. 1991;14:368–372. [PubMed] [Google Scholar]
- 5.Tsujii H, Okamoto Y, Kikuchi E, Matsumoto M, Nakano H. Prostaglandin E2 and rat liver regeneration. Gastroenterology. 1993;105:495–499. doi: 10.1016/0016-5085(93)90725-r. [DOI] [PubMed] [Google Scholar]
- 6.Casado M, Callejas NA, Rodrigo J, Zhao X, Dey SK, Bosca L, Martin-Sanz P. Contribution of cyclooxygenase 2 to liver regeneration after partial hepatectomy. Faseb J. 2001;15:2016–2018. doi: 10.1096/fj.01-0158fje. [DOI] [PubMed] [Google Scholar]
- 7.Rudnick DA, Perlmutter DH, Muglia LJ. Prostaglandins are required for CREB activation and cellular proliferation during liver regeneration. Proc Natl Acad Sci U S A. 2001;98:8885–8890. doi: 10.1073/pnas.151217998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casado M, Molla B, Roy R, Fernandez-Martinez A, Cucarella C, Mayoral R, Bosca L, et al. Protection against Fas-induced liver apoptosis in transgenic mice expressing cyclooxygenase 2 in hepatocytes. Hepatology. 2007;45:631–638. doi: 10.1002/hep.21556. [DOI] [PubMed] [Google Scholar]
- 9.Andreis PG, Whitfield JF, Armato U. Stimulation of DNA synthesis and mitosis of hepatocytes in primary cultures of neonatal rat liver by arachidonic acid and prostaglandins. Exp Cell Res. 1981;134:265–272. doi: 10.1016/0014-4827(81)90425-0. [DOI] [PubMed] [Google Scholar]
- 10.Skouteris GG, Ord MG, Stocken LA. Regulation of the proliferation of primary rat hepatocytes by eicosanoids. J Cell Physiol. 1988;135:516–520. doi: 10.1002/jcp.1041350321. [DOI] [PubMed] [Google Scholar]
- 11.Skouteris GG, McMenamin M. Transforming growth factor-alpha-induced DNA synthesis and c-myc expression in primary rat hepatocyte cultures is modulated by indomethacin. Biochem J. 1992;281(Pt 3):729–733. doi: 10.1042/bj2810729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Refsnes M, Thoresen GH, Dajani OF, Christoffersen T. Stimulation of hepatocyte DNA synthesis by prostaglandin E2 and prostaglandin F2 alpha: additivity with the effect of norepinephrine, and synergism with epidermal growth factor. J Cell Physiol. 1994;159:35–40. doi: 10.1002/jcp.1041590106. [DOI] [PubMed] [Google Scholar]
- 13.Refsnes M, Dajani OF, Sandnes D, Thoresen GH, Rottingen JA, Iversen JG, Christoffersen T. On the mechanisms of the growth-promoting effect of prostaglandins in hepatocytes: the relationship between stimulation of DNA synthesis and signaling mediated by adenylyl cyclase and phosphoinositide-specific phospholipase C. J Cell Physiol. 1995;164:465–473. doi: 10.1002/jcp.1041640304. [DOI] [PubMed] [Google Scholar]
- 14.Michalopoulos GK. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990;4:176–187. [PubMed] [Google Scholar]
- 15.Wu T. Cyclooxygenase-2 in hepatocellular carcinoma. Cancer Treat Rev. 2006;32:28–44. doi: 10.1016/j.ctrv.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Kondo M, Yamamoto H, Nagano H, Okami J, Ito Y, Shimizu J, Eguchi H, et al. Increased expression of COX-2 in nontumor liver tissue is associated with shorter disease-free survival in patients with hepatocellular carcinoma. Clin Cancer Res. 1999;5:4005–4012. [PubMed] [Google Scholar]
- 17.Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, et al. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688–696. doi: 10.1002/hep.510290355. [DOI] [PubMed] [Google Scholar]
- 18.Shiota G, Okubo M, Noumi T, Noguchi N, Oyama K, Takano Y, Yashima K, et al. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999;46:407–412. [PubMed] [Google Scholar]
- 19.Bae SH, Jung ES, Park YM, Kim BS, Kim BK, Kim DG, Ryu WS. Expression of cyclooxygenase-2 (COX-2) in hepatocellular carcinoma and growth inhibition of hepatoma cell lines by a COX-2 inhibitor, NS-398. Clin Cancer Res. 2001;7:1410–1418. [PubMed] [Google Scholar]
- 20.Rahman MA, Dhar DK, Yamaguchi E, Maruyama S, Sato T, Hayashi H, Ono T, et al. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin Cancer Res. 2001;7:1325–1332. [PubMed] [Google Scholar]
- 21.Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology. 2003;38:756–768. doi: 10.1053/jhep.2003.50380. [DOI] [PubMed] [Google Scholar]
- 22.Cheng AS, Chan HL, To KF, Leung WK, Chan KK, Liew CT, Sung JJ. Cyclooxygenase-2 pathway correlates with vascular endothelial growth factor expression and tumor angiogenesis in hepatitis B virus-associated hepatocellular carcinoma. Int J Oncol. 2004;24:853–860. [PubMed] [Google Scholar]
- 23.Han C, Michalopoulos GK, Wu T. Prostaglandin E(2) receptor EP(1) transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J Cell Physiol. 2006;207:261–270. doi: 10.1002/jcp.20560. [DOI] [PubMed] [Google Scholar]
- 24.Dajani OF, Meisdalen K, Guren TK, Aasrum M, Tveteraas IH, Lilleby P, Thoresen GH, et al. Prostaglandin E2 upregulates EGF-stimulated signaling in mitogenic pathways involving Akt and ERK in hepatocytes. J Cell Physiol. 2008;214:371–380. doi: 10.1002/jcp.21205. [DOI] [PubMed] [Google Scholar]
- 25.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 26.Michalopoulos GK, Bowen WC, Mule K, Luo J. HGF-, EGF-, and dexamethasone-induced gene expression patterns during formation of tissue in hepatic organoid cultures. Gene Expr. 2003;11:55–75. doi: 10.3727/000000003108748964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalopoulos GK, Bowen WC, Zajac VF, Beer-Stolz D, Watkins S, Kostrubsky V, Strom SC. Morphogenetic events in mixed cultures of rat hepatocytes and nonparenchymal cells maintained in biological matrices in the presence of hepatocyte growth factor and epidermal growth factor. Hepatology. 1999;29:90–100. doi: 10.1002/hep.510290149. [DOI] [PubMed] [Google Scholar]
- 28.Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci U S A. 2007;104:17081–17086. doi: 10.1073/pnas.0704126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyaki M, Sato C, Sakai K, Konishi M, Tanaka K, Muraoka M, Kikuchi-Yanoshita R, et al. Malignant transformation and EGFR activation of immortalized mouse liver epithelial cells caused by HBV enhancer-X from a human hepatocellular carcinoma. Int J Cancer. 2000;85:518–522. doi: 10.1002/(sici)1097-0215(20000215)85:4<518::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Schiffer E, Housset C, Cacheux W, Wendum D, Desbois-Mouthon C, Rey C, Clergue F, et al. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005;41:307–314. doi: 10.1002/hep.20538. [DOI] [PubMed] [Google Scholar]
- 31.Huether A, Hopfner M, Sutter AP, Schuppan D, Scherubl H. Erlotinib induces cell cycle arrest and apoptosis in hepatocellular cancer cells and enhances chemosensitivity towards cytostatics. J Hepatol. 2005;43:661–669. doi: 10.1016/j.jhep.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 32.Hopfner M, Sutter AP, Huether A, Schuppan D, Zeitz M, Scherubl H. Targeting the epidermal growth factor receptor by gefitinib for treatment of hepatocellular carcinoma. J Hepatol. 2004;41:1008–1016. doi: 10.1016/j.jhep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Han C, Li G, Lim K, DeFrances MC, Gandhi CR, Wu T. Transgenic expression of cyclooxygenase-2 in hepatocytes accelerates endotoxin-induced acute liver failure. J Immunol. 2008;181:8027–8035. doi: 10.4049/jimmunol.181.11.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 35.Tao YG, Tan YN, Liu YP, Song X, Zeng L, Gu HH, Tang M, et al. Epstein-Barr virus latent membrane protein 1 modulates epidermal growth factor receptor promoter activity in a nuclear factor kappa B-dependent manner. Cell Signal. 2004;16:781–790. doi: 10.1016/j.cellsig.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Bogdan S, Klambt C. Epidermal growth factor receptor signaling. Curr Biol. 2001;11:R292–R295. doi: 10.1016/s0960-9822(01)00167-1. [DOI] [PubMed] [Google Scholar]
- 37.Han C, Wu T. Cyclooxygenase-2-derived prostaglandin E2 promotes human cholangiocarcinoma cell growth and invasion through EP1 receptor-mediated activation of the epidermal growth factor receptor and Akt. J Biol Chem. 2005;280:24053–24063. doi: 10.1074/jbc.M500562200. [DOI] [PubMed] [Google Scholar]
- 38.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 39.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 40.Shao J, Lee SB, Guo H, Evers BM, Sheng H. Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Res. 2003;63:5218–5223. [PubMed] [Google Scholar]
- 41.Shah BH, Baukal AJ, Shah FB, Catt KJ. Mechanisms of extracellularly regulated kinases 1/2 activation in adrenal glomerulosa cells by lysophosphatidic acid and epidermal growth factor. Mol Endocrinol. 2005;19:2535–2548. doi: 10.1210/me.2005-0082. [DOI] [PubMed] [Google Scholar]
- 42.Snider AC, Meier KE. Receptor transactivation cascades. Focus on “Effects of alpha1D–adrenergic receptors on shedding of biologically active EGF in freshly isolated lacrimal gland epithelial cells”. Am J Physiol Cell Physiol. 2007;292:C1–C3. doi: 10.1152/ajpcell.00364.2006. [DOI] [PubMed] [Google Scholar]
- 43.Reinehr R, Schliess F, Haussinger D. Hyperosmolarity and CD95L trigger CD95/EGF receptor association and tyrosine phosphorylation of CD95 as prerequisites for CD95 membrane trafficking and DISC formation. FASEB J. 2003;17:731–733. doi: 10.1096/fj.02-0915fje. [DOI] [PubMed] [Google Scholar]
- 44.Reinehr R, Becker S, Hongen A, Haussinger D. The Src family kinase Yes triggers hyperosmotic activation of the epidermal growth factor receptor and CD95. J Biol Chem. 2004;279:23977–23987. doi: 10.1074/jbc.M401519200. [DOI] [PubMed] [Google Scholar]
- 45.Reinehr R, Becker S, Eberle A, Grether-Beck S, Haussinger D. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J Biol Chem. 2005;280:27179–27194. doi: 10.1074/jbc.M414361200. [DOI] [PubMed] [Google Scholar]
- 46.Batzer AG, Blaikie P, Nelson K, Schlessinger J, Margolis B. The phosphotyrosine interaction domain of Shc binds an LXNPXY motif on the epidermal growth factor receptor. Mol Cell Biol. 1995;15:4403–4409. doi: 10.1128/mcb.15.8.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Batzer AG, Rotin D, Urena JM, Skolnik EY, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994;14:5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.