Abstract
Introduction:
Docetaxel chemotherapy prolongs survival in metastatic hormone-refractory prostate cancer (mHRPC), but many patients fail to respond to this therapy and there is potential for serious toxicity. Patients differ in their percent prostate-specific antigen (PSA) decline and rate of PSA decline following treatment. We propose that patients who achieve a rapid rate of PSA decline, measured as a shorter PSA half-life (PSAHL), may experience a longer overall survival (OS) than those who achieve a slower rate of PSA decline.
Methods:
A chart review of patients treated with docetaxel for mHRPC in Alberta from January 2000 to May 2006 was performed. At 42 days (after 2 cycles) and 84 days (after 4 cycles) following chemotherapy, PSA response and PSAHL were determined. PSAHL could only be determined in patients with a PSA drop from baseline. Optimal PSAHL values for OS stratification were determined using the log-rank chi-square statistic. Survival analysis was carried out using Kaplan-Meier curves and regression analysis.
Results:
There were 154 patients who fulfilled the inclusion criteria. Using 42-day postdocetaxel data, no associations with OS could be demonstrated. Using 84-day postdocetaxel data, patients stratified by PSAHL demonstrated a significant difference in OS (15 months vs. 25 months) and this relationship remained following multivariate analysis (hazard ratio 0.08 [0.021–0.34]).
Conclusion:
A more rapid rate of PSA decline (PSAHL <70 days) measured after 4 cycles of chemotherapy was associated with a longer OS. This result was independent of other known markers of survival and allowed for a greater survival differentiation than PSA response.
Résumé
Introduction :
La chimiothérapie par docetaxel prolonge la survie dans les cas de cancer métastatique de la prostate réfractaire aux traitements hormonaux, mais de nombreux patients ne répondent pas à cette chimiothérapie qui, par ailleurs, est associée à un risque de toxicité grave. Après ce traitement, le pourcentage de baisse du taux d’antigène prostatique spécifique (APS) et la vitesse de réduction des taux d’APS diffèrent d’un patient à l’autre. Nous avançons l’hypothèse que les patients qui présentent une baisse rapide des taux d’APS, se manifestant par une réduction de la demi-vie de l’APS, peuvent présenter une survie globale (SG) plus longue que les patients présentant une baisse plus lente des taux d’APS.
Méthodologie :
Nous avons procédé à un examen des dossiers de patients traités par le docetaxel en raison d’un cancer métastatique de la prostate réfractaire aux traitements hormonaux en Alberta entre janvier 2000 et mai 2006. Aux 42e (après 2 cycles) et 84e jours (après 4 cycles) de chimiothérapie, on a vérifié la réponse en fonction des taux d’APS et de la demi-vie de l’APS. La demi-vie de l’APS ne pouvait être mesurée que chez les patients ayant affiché une baisse des taux d’APS par rapport aux taux initiaux. Les valeurs optimales de la demi-vie de l’APS utilisées pour la stratification des patients en vue de l’évaluation de la SG ont été cernées à l’aide d’une méthode statistique reposant sur le test de Mantel-Haenzel. L’analyse des taux de survie a été effectuée à l’aide de courbes de Kaplan-Meier et d’une analyse de régression.
Résultats :
Cent cinquante-quatre (154) patients satisfaisaient aux critères d’inclusion. Selon les données recueillies après 42 jours de traitement par docetaxel, aucune corrélation avec la SG n’a pu être démontrée. Selon les données recueillies après 84 jours de traitement par docetaxel, les patients stratifiés en fonction de leur demi-vie de l’APS ont montré une différence significative quant à la survie globale (15 mois vs 25 mois), et cette corrélation a aussi été observée lors de l’analyse multivariée (RR 0,08 [0,021 à 0,34]).
Conclusion :
Une chute plus rapide des taux d’APS (demi-vie de l’APS < 70 jours) mesurée après 4 cycles de chimiothérapie a été associée à une survie globale plus longue. Ce résultat s’est révélé indépendant des autres marqueurs connus de la survie et permet-tait une meilleure différenciation que la réponse de l’APS sur le plan de la survie.
Introduction
Advanced prostate cancer remains a therapeutic challenge for the clinical oncologist. Despite a constantly evolving understanding of the disease, the median overall survival (OS) for metastatic hormone refractory prostate cancer (mHRPC) is still under 2 years. Treatment of mHRPC with docetaxel in combination with prednisone has been shown to prolong survival by several months.1,2 Despite its adoption as a standard of care in men with mHRPC, there is still potential for serious toxicity to develop. This potential must be recognized, as only 36% to 48% of patients will experience a prostate-specific antigen (PSA) response (defined as a reduction in serum PSA of at least 50%) with this therapy.1,3–6 This is particularly important when considering that men may be treated with docetaxel chemotherapy for survival improvement while being asymptomatic. Unfortunately, there is a paucity of evidence to guide clinical decisions to continue or stop docetaxel treatment in asymptomatic patients once they start treatment.
Several prognostic factors in hormone-refractory prostate cancer (HRPC) have been reported, including initial PSA, tumour Gleason grade and stage, as well as baseline hemoglobin, performance status and PSA doubling time (PSADT).7,8 PSA response (defined as a 50% decline in baseline PSA) has also been proposed as a predictor of survival following chemotherapy for mHRPC.9 However, the magnitude of this benefit has been variable between studies and there is a need for better predictors of outcome from chemotherapy in mHRPC.10–12
After receiving docetaxel chemotherapy for mHRPC, patients differ not only in their percent PSA decline, but also in the rate at which they achieve this decline. We propose that patients who achieve a more rapid rate of PSA decline, measured as a shorter PSA half-life (PSAHL), may experience a longer OS than those who achieve a slower rate of PSA decline. Furthermore, we propose that the early rate of PSA decline, measured as PSAHL at 42 days (2 cycles) postchemotherapy, may also be associated with OS and allow for earlier prognostication than traditional measures obtained 84 days postdocetaxel (4 cycles).
Methods
Study design and data collection
After obtaining approval for the study through the Alberta Cancer Board (ACB) Research Ethics Board, patients having received docetaxel chemotherapy at a standard dose and schedule1 at the Cross Cancer Institute (Edmonton, AB, Canada) or Tom Baker Cancer Center (Calgary, AB, Canada) for prostate cancer from January 1, 2000, to November 1, 2006, were identified through the ACB Cancer Registry. Inclusion criteria for the study were: confirmation of mHRPC by 3 sequential rises in PSA with a castrate level of serum testosterone (<50 ng/dL) and metastatic disease on imaging (x-ray, computed tomography or bone scan).
A retrospective chart review was carried out on eligible patients. The data collected were: dates of initial prostate cancer diagnosis, HRPC diagnosis (defined as the date of the third consecutive PSA rise), metastatic prostate cancer diagnosis, chemotherapy failure (defined as a 50% rise from the nadir achieved on chemotherapy) and death. Laboratory results at the time of docetaxel initiation (including hemoglobin, alkaline phosphatase and lactate dehydrogenase) and all available PSA measurements were also collected. Finally, the dates and total number of cycles of docetaxel administered were obtained.
Overall survival from the date of diagnosis of HRPC to death or last follow-up was calculated irrespective of demonstrable metastatic disease on imaging. Overall survival from the date of initiation of docetaxel chemotherapy was calculated as the time from the first day of docetaxel administration to the date of death.
The PSA doubling time (PSADT) was determined retrospectively, using PSA data available immediately after the diagnosis of HRPC (Box 1).
Box 1. Calculation of PSA doubling time.
| PSADT = (0.693 × t) / [In (PSA2) – In (PSA1)] |
| Where: “t” is the time (in days) between PSA measurements, “PSA2” is the PSA defining HRPC (third rising PSA), “PSA1” is the next available PSA level |
PSA = prostate-specific antigen; PSADT = prostate-specific antigen doubling time; HRPC = hormone-refractory prostate cancer
Percent PSA decline and PSAHL were determined after 2 cycles of chemotherapy (day 42) and after 4 cycles of chemotherapy (day 84). The baseline PSA for both of these calculations was the PSA immediately prior to docetaxel initiation. The PSAHL calculation is expanded in Box 2.
Box 2. Calculation of percent PSA decline and PSAHL.
| PSAHL = (0.693 × t) / [In (PSA2) – In (PSA1)] |
| Where: “t” is the time (in days) between PSA measurements, “PSA2” is the PSA 42 days or 84 days after docetaxel initiation, “PSA1” is the PSA immediately prior to docetaxel initiation |
PSA = prostate-specific antigen; PSAHL = prostate-specific antigen half-life
Patients who experienced a rise in PSA relative to their baseline at 42 days (29 patients) or at 84 days (33 patients) after chemotherapy onset were excluded from PSAHL determination, as the calculation would yield a negative number.
Statistical analysis
Kaplan-Meier curves were constructed to estimate OS from diagnosis of HRPC and from the first administration of docetaxel. Overall survival was also analyzed after stratifying patients by PSA response (50% decline from baseline) and by PSAHL at 42 days postdocetaxel and at 84 days post-docetaxel initiation. The log-rank chi-square statistic was applied using different PSAHL values to stratify data for OS. The optimal PSAHL stratification value was the value that provided the greatest survival differentiation. This optimal PSAHL stratification value was determined independently using the 42-day postdocetaxel data and the 84-day postdocetaxel data.
Univariate and multivariate Cox-regression analyses were then performed using known prognostic factors in HRPC (initial PSA, Gleason score, stage, age, hemoglobin, alkaline phosphatase and lactate dehydrogenase), in addition to PSA decline and PSAHL 42 days (2 cycles) and 84 days (4 cycles) postdocetaxel initiation.
Results
Patient characteristics
A total of 154 patients fulfilled the criteria for mHRPC. The mean number of cycles of docetaxel administered was 6 (range 1 to 26). The clinical and pathological characteristics, biochemistry, PSA and treatment variables are summarized in Table 1.
Table 1.
Baseline demographics, biochemistry and cancer characteristics
| No. | % | Range | |
|---|---|---|---|
| Demographics | |||
| No. of patients | 154 | ||
| Age at docetaxel initiation, yrs | 69 | (44–87) | |
| Cancer characteristics at original diagnosis | |||
| Tx N0 M0 | 69 | 45 | |
| N1–2 or M1 | 71 | 46 | |
| Unknown TNM stage | 14 | 9 | |
| Gleason ≤6 | 68 | 44 | |
| Gleason 7 | 23 | 15 | |
| Gleason 8–10 | 63 | 41 | |
| PSA (μg/L) | 238 | (2–3672) | |
| Pre-chemotherapy biochemistry | |||
| Hemoglobin (mmol/L) | 127 | (73–178) | |
| Alkaline phosphatase (U/L) (normal <350 U/L) | 309 | (57–2975) | |
| LDH (U/L) (normal <150 U/L) | 127 | (108–9353) | |
| PSA (μg/L) | 298 | (1–3212) | |
Empty cells do not have any applicable data associated with them. Tx = tumour; N0 = no regional lymph node metastasis; M0 = no distant metastasis; N1 = metastasis with a single lymph node mass 2 cm or less in greatest dimension, or multiple lymph nodes 2 cm or less in greatest dimension; N2 = metastasis with a single lymph node mass larger than 2 cm, but no more than 5 cm in greatest dimension, or multiple lymph nodes no more than 5 cm in greatest dimension; M1 = distant metastasis; TNM = tumour, node, metastasis; PSA = prostate-specific antigen; LDH = lactate dehydrogenase.
PSA response and PSA half-life
The mean PSA decline 42 days after docetaxel initiation was 17% (range, −951% to 99%). Seventy-one percent of patients (84/119) with available PSA measurements at this time point, achieved a PSA decline. PSAHL was determined in these patients and ranged from 6 days (very rapid rate of PSA decline) to 12 734 days (very slow rate of PSA decline), with a mean PSAHL of 404 days. By applying the log-rank chi-square statistic using different PSAHL values to stratify data, the optimal PSAHL stratification for OS at this time point (42 days postdocetaxel) was 71 days. Using this cutoff, 55 patients achieved a rapid rate of PSA decline (PSAHL ≤71 days) and 35 patients achieved a slow rate of PSA decline (PSAHL >71 days) after 42 days of docetaxel.
The mean PSA decline 84 days after docetaxel initiation was 10% (range, −1841% to 100%), with 92/126 patients (73%) with available PSA measurements achieving a PSA decline at this time point. PSAHL was determined in these patients and ranged from 13 days (very rapid rate of PSA decline) to 3151 days (very slow rate of PSA decline), with a mean of 136 days. By applying the log-rank chi-square statistic using different PSAHL values to stratify data, the optimal PSAHL stratification for OS at this time point (84 days postdocetaxel) was 70 days. Using this cutoff, 47 patients achieved a rapid rate of PSA decline (PSAHL ≤70 days) and 45 patients achieved a slow rate of PSA decline (PSAHL >70 days) after 84 days of docetaxel.
Kaplan-Meier survival analysis
During the follow-up period, 54% of patients died and disease progression was documented in 74% of all patients. The median OS from diagnosis of HRPC was 49 months and the median OS from initiation of docetaxel was 16 months for the entire group. For those patients with a PSA rise at 42 days, the median OS was 12.1 months; for those patients with a PSA rise at 84 days, the median OS was 11.5 months.
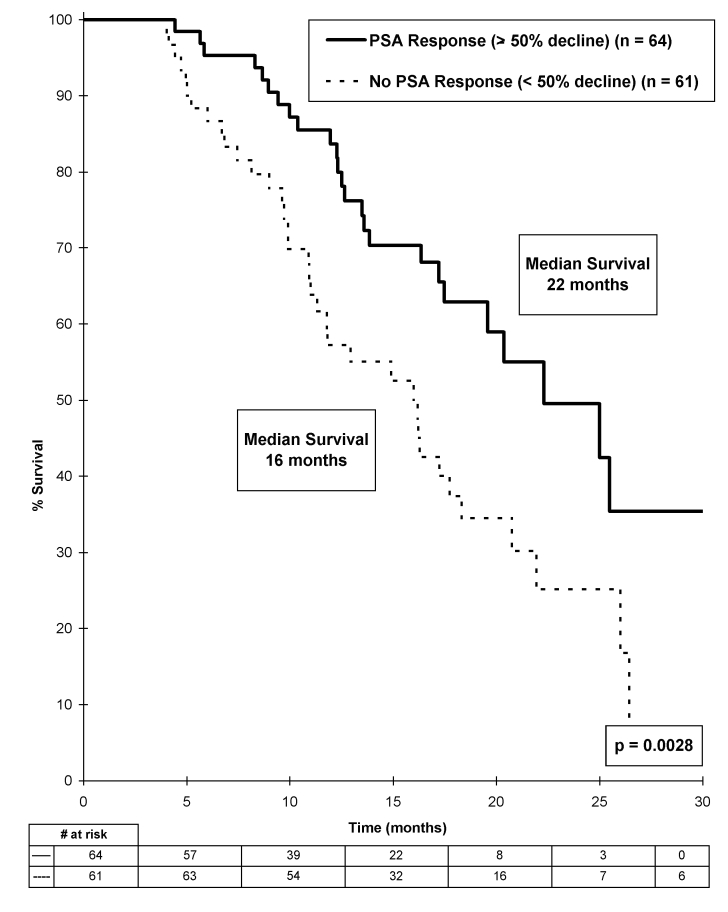
Stratifying patients by those having no or any PSA decline (≥0%) at 84 days after docetaxel initiation did not predict OS (p = 0.84). However, stratifying patients by PSA response, as defined as a ≥50% PSA decline, correlated with OS, with a median survival of 22 months in patients with a PSA response compared with 16 months in those without a PSA response (p = 0.0028) (Fig. 1). After excluding patients with a PSA rise following docetaxel, PSA response was no longer associated with OS.
Fig 1.
Overall survival stratified by prostate-specific antigen (PSA) response (PSA decline ≥50%) 84 days after starting docetaxel.
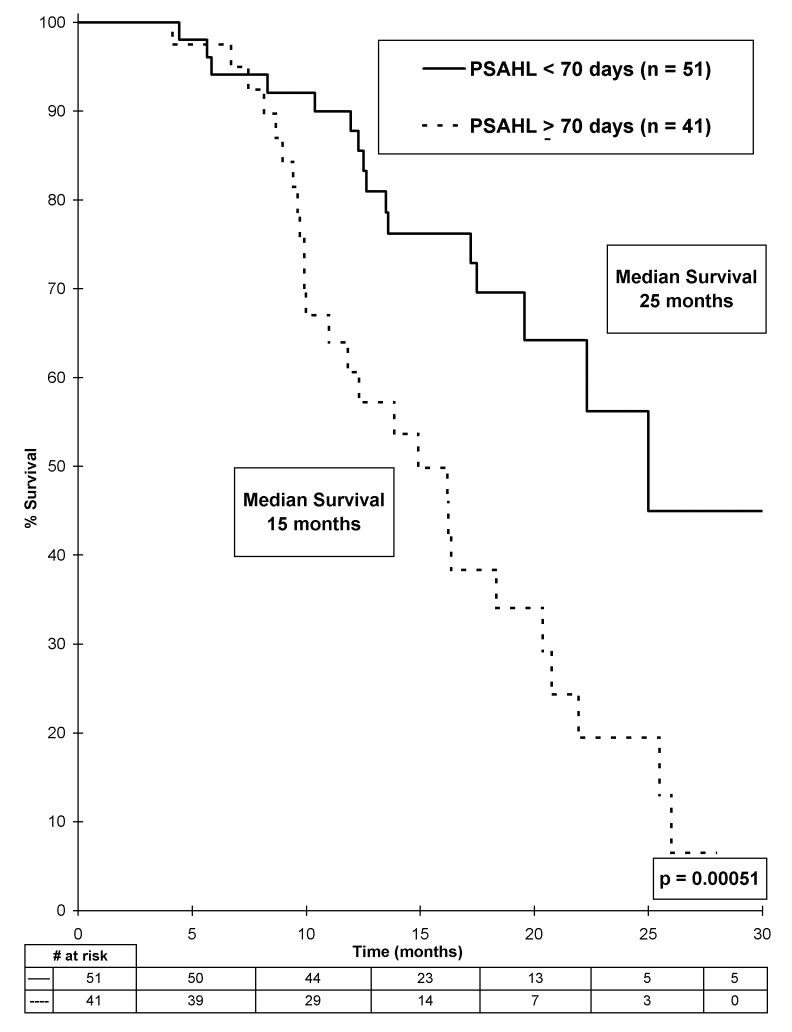
Using early PSAHL data (determined 42 days postdocetaxel initiation), it was not possible to demonstrate an association with survival, even with the optimal PSAHL stratification of 71 days (p = 0.20). Using PSAHL data determined at 84 days postdocetaxel initiation, we found a strong association with OS was demonstrated, with a median survival of 25 months in patients with a rapid rate of PSA decline (PSAHL <70 days) compared with a median survival of 15 months in patients with a slow rate of PSA decline (PSAHL ≥70 days) (p = 0.00051) (Fig. 2).
Fig 2.
Overall survival stratified by prostate-specific antigen half-life, determined 84 days after starting docetaxel.
Univariate and multivariate Cox-regression analysis
Hemoglobin, alkaline phosphatase, PSA response and PSAHL (determined 84 days after docetaxel initiation) correlated with OS in the univariate analysis. After correcting for these factors in multivariate analysis, only PSA response and PSAHL determined 84 days after docetaxel initiation correlated to OS (hazard ratio 0.08 [0.021–0.34]) (Table 2).
Table 2.
Univariate and multivariate Cox-regression analysis for survival using known prognostic factors in hormone refractory prostate cancer, prostate-specific antigen and prostate-specific antigen half-life
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Variable | No. | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value |
| Gleason at initial diagnosis | |||||
| ≤6 | 68 | 1.0 | – | 1.0 | – |
| 7 | 23 | 1.20 (0.62–2.33) | 0.58 | 3.85 (0.71–20.77) | 0.12 |
| 8–10 | 63 | 0.99 (0.62–1.58) | 0.96 | 0.77 (0.20–2.75) | 0.68 |
| PSA at initial diagnosis | |||||
| <20 | 40 | 1.0 | – | 1.0 | – |
| ≥20 | 75 | 0.79 (0.48–1.30) | 0.35 | 1.02 (0.33–3.10) | 0.97 |
| Stage at initial diagnosis | |||||
| Tx | 69 | 1.0 | – | 1.0 | – |
| N1-2 or M1 (5–7) | 71 | 0.71 (0.45–1.11) | 0.13 | 0.41 (0.10–1.62) | 0.21 |
| Age at start of docetaxel | |||||
| <70 | 70 | 1.0 | – | 1.0 | – |
| ≥70 | 84 | 1.29 (0.83–2.00) | 0.26 | 0.35 (0.09–1.27) | 0.11 |
| Hb at start of docetaxel | |||||
| Normal | 105 | 1.0 | – | 1.0 | – |
| Low (<120) | 47 | 2.61 (1.67–4.10) | <0.0001 | 0.67 (0.20–2.31) | 0.53 |
| Alk phos at start of docetaxel | |||||
| Normal | 69 | 1.0 | – | 1.0 | – |
| High (>150) | 78 | 2.59 (1.60–4.20) | 0.0001 | 2.43 (0.79–7.47) | 0.12 |
| LDH at start of docetaxel | |||||
| Normal | 54 | 1.0 | – | 1.0 | – |
| High (>350) | 89 | 1.18 (0.74–1.88) | 0.49 | 1.92 (0.57–6.46) | 0.29 |
| Early pre-chemotherapy PSADT | |||||
| >70 days | 55 | 1.0 | – | 1.0 | – |
| ≤70 days | 93 | 0.69 (0.44–1.09) | 0.12 | 0.76 (0.29–1.98) | 0.58 |
| PSA response 84 days after docetaxel | |||||
| <50% decline | 70 | 1.0 | – | 1.0 | – |
| ≥50% decline | 55 | 0.38 (0.22–0.67) | 0.0007 | 0.16 (0.05–0.48) | 0.001 |
| PSAHL 42 days after docetaxel | |||||
| >71 days | 56 | 1.0 | – | 1.0 | – |
| ≤71 days | 33 | 0.66 (0.36–1.23) | 0.2 | 0.86 (0.23–1.67) | 0.34 |
| PSAHL 84 days after docetaxel | |||||
| >70 days | 47 | 1.0 | – | 1.0 | – |
| ≤70 days | 45 | 0.34 (0.18–0.64) | 0.009 | 0.08 (0.021–0.34) | 0.0005 |
CI = confidence interval; PSA = prostate-specific antigen; Tx = tumour; N0 = no regional lymph node metastasis; M0 = no distant metastasis; N1 = metastasis with a single lymph node mass 2 cm or less in greatest dimension, or multiple lymph nodes, 2 cm or less in greatest dimension; N2 = metastasis with a single lymph node mass larger than 2 cm, but no more than 5 cm in greatest dimension, or multiple lymph nodes, no more than 5 cm in greatest dimension; M1 = distant metastasis; LDH = lactate dehydrogenase; Hb = hemoglobin; Alk phos = alkaline phosphatase; PSADT = prostate-specific antigen doubling time; PSAHL = prostate-specific antigen half-life.
Discussion
Prostate-specific antigen half-life is a simple tool to deter-mine the rate of decline of PSA following chemotherapy. The only parameters required to calculate it are the baseline PSA (before chemotherapy), the PSA after chemotherapy and the number of days between the 2 PSA determinations (i.e., 42 or 84 days). Prostate-specific antigen half-life assumes that tumour killing after docetaxel is logarithmic (as opposed to PSA response, which assumes it to be linear). This idea is somewhat analogous to PSADT, which assumes that tumour growth is logarithmic. In this multi-centre, retrospective analysis of mHRPC, we have demonstrated that when applied to patients having received 4 cycles of chemotherapy, the survival differentiation between a low PSAHL (<70 days) and a high PSAHL (≥70 days) is greater than the survival differentiation between a PSA response and no PSA response. This result was independent of other known markers of survival and was a better predictor of survival than PSA response. Interestingly, we did not find an association between PSADT and survival following doc-etaxel chemotherapy in our study, even if previous investigators have noted such an association.8 This finding may be explained by the fact that our PSADT data was determined retrospectively, and included PSA measurements determined at different time points following treatment.
The relatively large survival difference (10 months) seen in patients with a rapid rate of PSA decline is particularly compelling given that patients who experienced a rise in PSA 84 days after starting docetaxel were excluded from PSAHL analysis. Exclusion of these nonresponders was necessary because, mathematically, it is not possible to calculate a PSAHL for a patient whose PSA is rising (a negative number of days for PSAHL would result). Exclusion of this high-risk group is expected to bias the PSAHL results, resulting in a more homogeneous cohort (with a longer survival). However, we found that segregating these patients according to PSAHL (even after excluding the high-risk patients) actually yielded groups with more distinct OS compared with a PSA response-based segregation. If patients with a PSA rise on chemotherapy had been similarly excluded prior to stratifying patients based on PSA response, the survival difference between patients achieving a PSA response and those without a PSA response would no longer be significant.
A recent subgroup analysis of the TAX 327 study has suggested that the optimal PSA decline cutoff to stratify patients based on survival is a PSA decline of 30%.13 In this analysis, a survival difference of 8.6 months (21.6 months vs. 13.0 months) was demonstrated between groups with a PSA decline of 30% versus those without a PSA decline of 30%. In our study, stratifying patients based on PSAHL was associated with an even greater survival difference (10 months). Furthermore, PSAHL analysis requires that patients with a rising PSA be excluded from analysis, which should result in a more homogenous group, as previously discussed, yet this method still stratified patients to a greater degree than a 30% PSA decline.
We also studied the relationship between early PSAHL (determined after only 42 days or 2 cycles of docetaxel) and OS. The potential advantage of finding an association at this earlier time point would be to allow oncologists to predict survival earlier in the course of treatment and tailor the subsequent therapy accordingly. Unfortunately, neither PSAHL nor PSA response determined 42 days postdocetaxel correlated to survival in Kaplan-Meier analysis or Cox-regression analysis. This finding may be a result of a delayed PSA response to docetaxel noted in some patients such that a minimum number of cycles must be administered before a conclusion can be drawn about the effectiveness of the treatment. Thus, if patients are tolerating therapy, a minimum of 4 cycles of treatment should be given before making decisions about the effectiveness of treatment.
Conclusion
For men who are taking docetaxel therapy to palliate cancer symptoms, improvements in quality of life should supersede laboratory values in determining whether or not to continue therapy. However, due to survival improvements seen in randomized clinical trials, many men are offered chemotherapy to improve survival, even if they are asymptomatic. In these patients, early determination of who will benefit most from chemotherapy before toxicity arises would be very beneficial. We hope that PSAHL will help oncologists and their patients to decide whether to carry on with or discontinue docetaxel after 4 cycles of treatment. In particular, we feel that PSAHL may be of use in patients experiencing treatment-related side effects.
The major limitation of our study is the retrospective nature of the analysis; this limits the data collection to what is available in the charts and electronic records. Larger, prospective studies are required to further validate this new clinical tool. A larger study will also allow for a more definitive analysis of PSAHL determination at earlier time points after docetaxel initiation.
Footnotes
This paper has been peer-reviewed.
Competing interests: None declared.
References
- 1.Tannock IF, de Wit R, Berry WR, et al. Docetaxel and prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Beer TM, Pierce WC, Lowe BA, et al. Phase II study of weekly docetaxel in symptomatic androgen-independent prostate cancer. Ann Oncol. 2001;12:1273–9. doi: 10.1023/a:1012258723075. [DOI] [PubMed] [Google Scholar]
- 4.Berry W, Dakhil S, Gregurich MA, et al. Phase II trial of single-agent weekly docetaxel in hormone-refractory, symptomatic, metastatic carcinoma of the prostate. Semin Oncol. 2001;28(Suppl 15):8–15. doi: 10.1016/s0093-7754(01)90149-6. [DOI] [PubMed] [Google Scholar]
- 5.Friedland D, Cohen J, Miller R, Jr, et al. A phase II trial of docetaxel (Taxotere) in hormone-refractory prostate cancer: correlation of antitumour effect to phosphorylation of Bcl-2. Semin Oncol. 1999;26(Suppl 17):19–23. [PubMed] [Google Scholar]
- 6.Picus J, Schultz M. Docetaxel (Taxotere) as monotherapy in the treatment of hormone-refractory prostate cancer: preliminary results. Semin Oncol. 1999;26(Suppl 17):14–8. [PubMed] [Google Scholar]
- 7.Cho D, Di Blasio CJ, Rhee AC, et al. Prognostic factors for survival in patients with hormone-refractory prostate cancer after initial androgen deprivation therapy. Urol Oncol. 2003;21:282–91. doi: 10.1016/s1078-1439(03)00057-7. [DOI] [PubMed] [Google Scholar]
- 8.Semeniuk RC, Venner PM, North S. Prostate-specific antigen doubling time is associated with survival in men with hormone-refractory prostate cancer. Urology. 2006;68:565–9. doi: 10.1016/j.urology.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 9.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: Recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 10.Kelly WK, Scher HI, Mazumdar M, et al. Prostate-specific antigen as a measure of disease outcome in metastatic hormone-refractory prostate cancer. J Clin Oncol. 1993;11:607–15. doi: 10.1200/JCO.1993.11.4.607. [DOI] [PubMed] [Google Scholar]
- 11.Smith DC, Dunn RL, Strawderman MS, et al. Change in serum prostate-specific antigen as a marker of response to cytotoxic therapy for hormone-refractory prostate cancer. J Clin Oncol. 1998;16:1835–43. doi: 10.1200/JCO.1998.16.5.1835. [DOI] [PubMed] [Google Scholar]
- 12.Sridhara R, Eisenberger MA, Sinibaldi VJ, et al. Evaluation of prostate-specific antigen as a surrogate marker for response of hormone-refractory prostate cancer to suramin therapy. J Clin Oncol. 1995;13:2944–53. doi: 10.1200/JCO.1995.13.12.2944. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong AJ, Garrett-Mayer E, Ou Yang YC, et al. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2007;25:3965–70. doi: 10.1200/JCO.2007.11.4769. [DOI] [PubMed] [Google Scholar]