Summary
Tumor suppressor p53 is activated upon genotoxic and oxidative stress and in turn inhibits cell proliferation and growth through induction of specific target genes. Cell growth is positively regulated by mTOR whose activity is controlled by the TSC1:TSC2 complex, a GTPase activating protein (GAP) for Rheb, the activator of mTOR. However, the mechanism by which p53 and genotoxic stress negatively control cell growth via the TSC1:TSC2-mTOR axis is not firmly established. We now demonstrate that the products of two p53 target genes, Sestrin1 and Sestrin2, activate the AMP-responsive protein kinase (AMPK) and target it to phosphorylate TSC2 and stimulate its GAP activity, thereby inhibiting mTOR. Correspondingly, Sestrin2-deficient mice fail to inhibit mTOR signaling upon genotoxic challenge. Sestrin1 and 2 therefore provide an important link between genotoxic stress, p53 and the mTOR signaling pathway.
Introduction
The major tumor suppressor p53 is a stress activated transcription factor that can either inhibit cell proliferation or induce apoptotic cell death (Levine, 1997). The different functions of p53 are mediated through numerous target genes, such as p21Waf1 which serves as a cell cycle inhibitor (Levine, 1997). In addition to cell proliferation, p53 also inhibits cell growth, a function that is important for preventing the wasteful buildup of proteins and other cellular building blocks during periods of stress (Vousden and Lane, 2007). Cell growth is regulated by the Target of rapamycin (TOR), an evolutionary conserved protein (280 kDa) that belongs to the phosphatidylinositol kinase-related kinase (PIKK) subfamily (Wullschleger et al., 2006). The mammalian TOR protein (mTOR) forms two distinct signaling complexes, called TORC1 and TORC2. The TORC1 complex, which in addition to mTOR consists of raptor, PRAS40 and mLST8, is responsible for control of cell growth and protein synthesis (Guertin and Sabatini, 2007; Yang and Guan, 2007). The TORC2 complex, consisting of mTOR, rictor, Sin1, PROTOR and mLST8, controls the actin cytoskeleton and cell spreading (Astrinidis et al., 2002; Sarbassov et al., 2004; Yang and Guan, 2007). Rapamycin, an inhibitor of cell growth, rapidly inhibits TORC1 through interaction with FKBP12, but after a while it may also inhibit TORC2 (Sarbassov et al., 2006). TORC1 activity is positively regulated by growth factors through the insulin/insulin-like growth factor (IGF1)-phosphoinositide-3’ kinase (PI3K)-AKT pathway, the Wnt-GSK3 pathway and the ERK-RSK kinase cascade (Wullschleger et al., 2006; Yang and Guan, 2007). TORC1 activity is inhibited by adverse environmental and metabolic conditions, including nutrient limitation, hypoxia and DNA-damage (Corradetti and Guan, 2006; Inoki et al., 2003a).
The positive and negative control of TORC1 activity is exerted through the TSC1:TSC2 complex, whose TSC2 subunit serves as a GAP for the small GTPase Rheb, which activates mTOR (Corradetti and Guan, 2006; Hay and Sonenberg, 2004; Wullschleger et al., 2006). TSC2 activity is regulated by several kinases, including AKT, ERK, RSK and AMP-activated protein kinase (AMPK) (Corradetti and Guan, 2006; Kwiatkowski and Manning, 2005). Genotoxic stress was suggested to inhibit TORC1 activity through p53-dependent upregulation of negative regulators such as PTEN, TSC2 and AMPKβ1 (Feng et al., 2007). p53 also increases the phosphorylation of the AMPKα subunit, leading to AMPK activation (Feng et al., 2005). However, the precise physiological mechanism by which p53 activates AMPK and inhibits mTOR is not established.
Two recently described p53 target genes genes are Sestrin1(Sesn1)/PA26 and Sestrin2(Sesn2)/Hi95 (Budanov et al., 2002; Peeters et al., 2003; Velasco-Miguel et al., 1999). Previous studies have shown that Sesn1 and Sesn2, whose expression is induced upon DNA damage and oxidative stress, may have a cytoprotective function based on regeneration of overoxidized peroxiredoxins (Budanov et al., 2004). We now demonstrate that Sesn1 and Sesn2 are negative regulators of mTOR signaling and that they execute this redox-independent function through activation of AMPK and TSC2 phosphorylation. We also provide in vivo evidence for the functional importance of Sesn2 in inhibition of mTOR signaling in mice treated with a p53-activating alkylating agent. Hence, Sesn1/2 link genotoxic stress, p53 and mTOR signaling.
Results
Sestrins inhibit mTOR-dependent S6K and 4E-BP1 phosphorylation
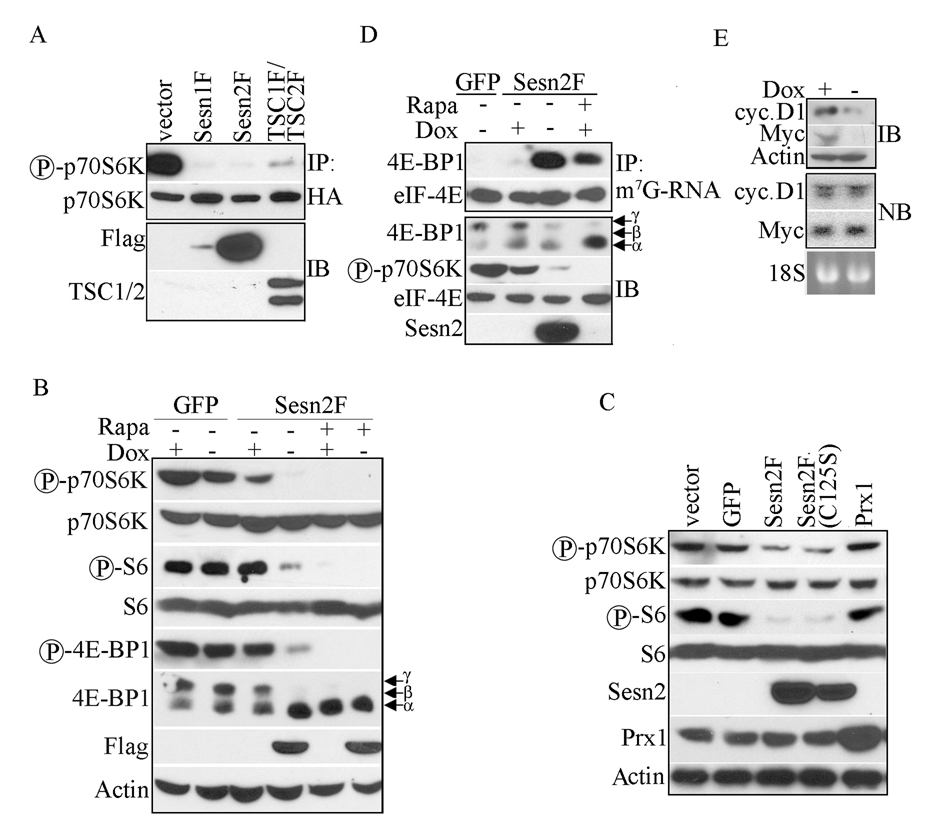
To examine whether Sestrins regulate mTOR we used p70S6K phosphorylation (Burnett et al., 1998; Corradetti and Guan, 2006) as a read-out. p70S6K activation can in turn be determined by phosphorylation of ribosomal S6 protein at S235/236 (Dufner and Thomas, 1999; Pende et al., 2004). We cotransfected HEK293 cells with HA-tagged p70S6K together with either Sesn1-Flag (F), Sesn2F, both TSC1F and TSC2F or GFP expression vectors. HA-p70S6K was immunoprecipitated and its phosphorylation examined with phospho-specific antibodies. Ectopic expression of either Sesn1F or Sesn2F dramatically inhibited T389 p70S6K phosphorylation, as did TSC1F:TSC2F overexpression (Figure 1A). Although Sesn1F was expressed less efficiently than Sesn2F, its inhibitory effect was pronounced (Figure 1A). However, when expressed at similar levels, no substantial differences in the inhibitory activity of the two Sestrins were observed (Figure S1A). To examine regulation of endogenous mTOR and p70S6K, we generated MCF7-tet OFF cells with doxycycline-regulated Sesn2F. To reduce possible toxicity due to Sesn2 overexpression we infected cells with low-titer virus and selected cells on puromycin as described (Budanov et al., 2002). After 24 hrs of doxycycline removal Sesn2F was expressed and considerable inhibition of p70S6K and S6 phosphorylation was observed (Figure 1B). This inhibitory effect of Sesn2 was seen as early as 6 hrs after doxycycline withdrawal, when its expression was barely detectible (Figure S1B). Another important target of mTOR is 4E-BP1, which is phosphorylated on many sites (Hay and Sonenberg, 2004). We examined 4E-BP1 phosphorylation using anti-phospho-S65 antibodies as well as changes in its electrophoretic mobility (Guan et al., 2007). Sesn2 induction decreased the abundance of the hyperphosphorylated γ form of 4E-BP1 and increased the abundance of the hypophosphorylated α form (Figure 1B). Similar results were seen upon infection of cells with lentiviral vectors expressing Sesn1 or Sesn2 (Figure S1C).
Figure 1. Sesn1 and Sesn2 inhibit mTOR signaling.
(A) Ectopic Sesn1, Sesn2 or TSC1:TSC2 expression decreases p70S6K phosphorylation. HEK293 cells were co-transfected with HA-p70S6K together with either Sesn1F, Sesn2F, TSC1F plus TSC2F or GFP expression vectors. After 48 hrs HA-p70S6K was immunoprecipitated (IP) and its phosphorylation was examined by immunoblotting. Sesn1/2 and TSC1/2 expression in total lysates was examined by immunoblotting (IB). (B) Induction of Sesn2 inhibits mTOR signaling. Sesn2F was induced in MCF7-tet OFF Sesn2F cells by doxycycline (Dox) removal. Control cultures were left with doxycycline. After 22 hrs, rapamycin (Rapa; 20 ng/ml) was added to some of the cultures. Cell lysates were prepared 2 hrs later and expression and phosphorylation of the indicated mTOR pathway components was examined by immunoblotting. (C) Sesn2 redox activity is not required for inhibition of mTOR signaling. H1299 cells were infected with wt or mutant Sesn2F-lentiviruses as well as GFP- and Prx1-lentiviruses and analyzed for phosphorylation and expression of the indicated proteins as above. (D) Sesn2 increases association of 4E-BP1 and eIF-4E with m7G-RNA Cap structures. Sesn2 was induced as in B and cell lysates were incubated with m7G-Sepharose beads for 6 hrs and bead-bound proteins were examined by immunoblotting (upper panel). Expression levels and phosphorylation state of the indicated proteins in the total lysates were analyzed as in B (lower panel). (E) Sesn2 induction inhibits cyclin D1 and c-Myc protein expression, but does not affect their mRNAs. Sesn2F was induced by doxycycline withdrawal and expression of cyclin D1 and c-Myc was examined 24 hrs later by immunoblotting (IB). RNA from the same cells was analyzed by Northern blotting (NB) with cyclin D1 or c-Myc probes. 18S RNA was used as a loading control.
In an attempt to localize the protein domain responsible for inhibition of mTOR we expressed Sesn2 deletion mutants in H1299 cells. Deletion of either the N- or C-terminal portions completely blocked the ability of Sesn2 to inhibit mTOR signaling (Figure S1D). mTOR is redox sensitive (Dames et al., 2005; Sarbassov and Sabatini, 2005) and the Sestrins were reported to be involved in redox-mediated reactivation of peroxiredoxins (Budanov et al., 2004). However, a redox-inactive Sesn2(C125S) mutant (Budanov et al., 2004) was as effective as wt Sesn2 in inhibition of p70S6K and S6 phosphorylation (Figures 1C). Expression of peroxiredoxin 1 (Prx1) had no effect on p70S6K and S6 phosphorylation.
Non-phosphorylated 4E-BP1 binds and sequesters eIF-4E, thereby inhibiting formation of the eIF-4F complex involved in initiation of Cap-dependent translation of a subset of mRNAs that include cyclin D1 and c-Myc (Thomas, 2006; Averous and Proud, 2006; Mamane et al., 2004). Phosphorylated 4E-BP1 cannot interact with eIF-4E and does not inhibit eIF-4F formation (Richter and Sonenberg, 2005). To determine whether Sesn1/2 modulate the 4E-BP1 – eIF-4E interaction, we isolated Cap-binding complexes with 7-methyl-guanosine (m7G) -RNA- Sepharose beads from control and doxycycline-deprived MCF7-tet OFF Sesn2F cells. Expression of Sesn2F induced 4E-BP1:eIF-4E complex formation as effectively as rapamycin treatment (Figure 1D). Sesn2F induction inhibited expression of both cyclin D1 and c-Myc, but did not affect their mRNA amounts (Figure 1E).
TSC2 is required for Sestrin-mediated inhibition of mTOR signaling
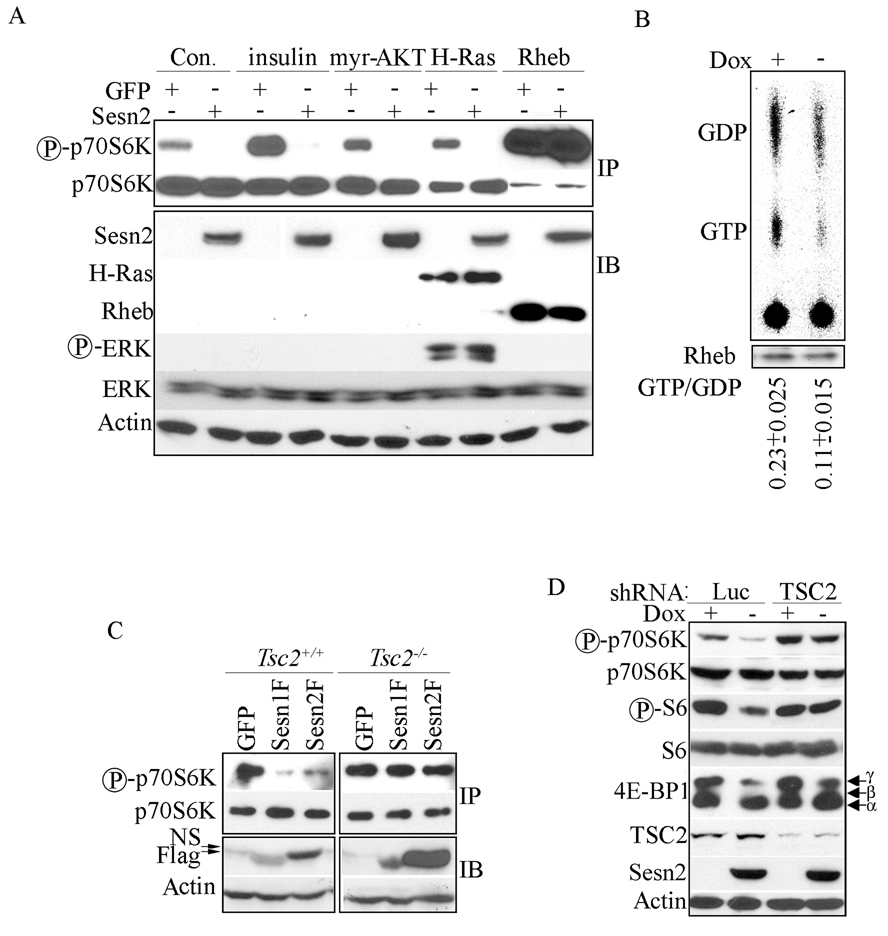
To elucidate how the Sestrins inhibit mTOR, we co-expressed either Sesn2 or GFP with HA-p70S6K and the transfected cells were treated with insulin to activate the PI3K-AKT-mTOR pathway. We also co-transfected cells with constructs expressing constitutively active H-Ras, myristoylated AKT (myr-AKT) or Rheb, which upon overexpression is no longer inhibited by endogenous TSC1:TSC2. Sesn2 inhibited p70S6K phosphorylation in all cases except when co-expressed with Rheb (Figure 2A) suggesting that Sesn2 affects mTOR signaling upstream of Rheb (Figure 2A).
Figure 2. Sestrins inhibit mTOR signaling upstream to the TSC1:TSC2 complex.
(A) Rheb abrogates Sesn2-induced inhibition of p70S6K phosphorylation. HEK293 cells were co-transfected with HA-p70S6K and either Sesn2 or GFP plasmids together with either myristoylated (myr)-AKT, H-Ras or Rheb constructs or were treated without (Con.) or with insulin for 1 hr as indicated. HA-p70S6K was immunoprecipitated (IP) with anti-HA antibody and its phosphorylation was examined by immunoblotting. Total cell lysates were immunoblotted (IB) with the indicated antibodies. (B) Sesn2 expression decreases Rheb GTP loading. Sesn2 was induced in MCF7-tet OFF Sesn2F cells for 24 hrs and cells were metabolically labeled with 32P for 4 hrs. Rheb was immunoprecipitated, guanine nucleotides were extracted, separated by thin layer chromatography, autoradiographed and quantified by QuantityOne software. Rheb recovery was examined by immunoblotting and used to normalize the calculated GTP/GDP ratio. The values shown are averages +/− S.D. of 3 experiments. (C) TSC2 is required for down-regulation of p70S6K phosphorylation by Sesn1/2. Tsc2+/+ and Tsc2−/− fibroblasts were co-transfected with HA-p70S6K together with Sesn1, Sesn2 or GFP expression vectors. HA-p70S6K was immunoprecipitated (IP) and its phosphorylation examined by immunoblotting. Sesn1/2 expression in total lysates was examined by immunoblotting (IB). NS - non-specific band. (D) Inhibition of mTOR signaling by Sesn2 requires TSC2. MCF7-tet OFF Sesn2F cells were infected with shTSC2- or shLuc-lentiviruses. After 48 hrs Sesn2F was induced by doxycycline removal and protein phosphorylation and expression was examined 24 hrs later.
To determine if Sesn2 modulates Rheb GTP loading, we induced its expression in MCF7-tet OFF cells that were labeled with 32P and immunoprecipitated endogenous Rheb. Inhibition of p70S6K phosphorylation by Sesn2F in these cells was confirmed by immunoblot analysis (Figure S2A). Rheb-associated guanine nucleotides were extracted and separated by thin-layer chromatography. Sesn2F expression induced a two-fold decrease in the relative amount of Rheb-associated GTP (Figure 2B). These results suggest that Sesn2 negatively regulates Rheb GTP loading.
Rheb GTP loading is controlled by the TSC1:TSC2 complex (Garami et al., 2003; Inoki et al., 2003b; Tee et al., 2003). To assess the role of this complex in inhibition of mTOR by Sesn1/2 we used Tsc2+/+ and Tsc2−/− cells. Whereas Sesn1 and Sesn2 inhibited p70S6K phosphorylation in wt cells, they failed to do so in TSC2-deficient cells (Figure 2C). To confirm the critical role of TSC1:TSC2 in the response to Sesn1/2 in human cancer cell lines, we knocked down TSC2 in both H1299 and MCF7-tet OFF Sesn2F cells. Down-regulation of TSC2 compromised Sestrin-induced inhibition of p70S6K, S6 and 4E-BP1 phosphorylation (Figure 2D, Figure S2B).
AMPK is important for Sestrin-induced mTOR inhibition
To study whether AMPK is involved in negative regulation of mTOR by Sesn1/2, we examined phosphorylation of AMPK and its target acetyl-CoA carboxylase (ACC) (Motoshima et al., 2006). Expression of Sesn1 or Sesn2 strongly enhanced AMPK phosphorylation and slightly increased ACC phosphorylation (Figure 3A, Figure S3A). By comparison to Sesn1/2 or campotecin treatment, activation of AMPK in response to 2-deoxyglucose (2-DG) resulted in much stronger ACC phosphorylation (Figure S3B). We considered whether the Sestrins activate AMPK through induction of metabolic stress by lowering of cellular ATP. We measured cellular ATP after Sesn2F induction and found no difference from control cells (data not shown). To further examine the role of AMPK in Sestrin-modulated mTOR inhibition, we used compound C, an AMPK inhibitor (Zhou et al., 2001). Compound C prevented inhibition of p70S6K phosphorylation by Sesn2F (Figure 3B). To rule out non-specific effects of compound C, we knocked-down AMPKα1, the major form of AMPK in MCF7 cells (data not shown), and found that this also prevented inhibition of p70S6K and S6 phosphorylation (Figure 3C). Similar results were obtained with another shRNA to AMPKα1 (data not shown).
Figure 3. Sestrin inhibit p70S6K phosphorylation via an AMPK-dependent mechanism.
(A) Sesn2 expression enhances AMPK phosphorylation. Sesn2F was induced in MCF7-tet OFF Sesn2F cells and phosphorylation of the indicated proteins was examined 24 hrs later. (B) Compound C abrogates Sestrin-induced inhibition of p70S6K phosphorylation. Sesn2F was induced in MCF7-tet OFF Sesn2F cells in the presence of compound C (CompC) or vehicle control (DMSO) and protein phosphorylation was examined 24 hrs later. (C) Knock-down of AMPKα1 abrogates Sesn2 inhibition of p70S6K and S6 phosphorylation. MCF7-tet OFF Sesn2F cells were infected with shAMPKα1- or shLuc-lentiviruses. Protein phosphorylation and expression were analyzed by immunoblotting 72 hrs after infection and 24 hrs after doxycycline removal.
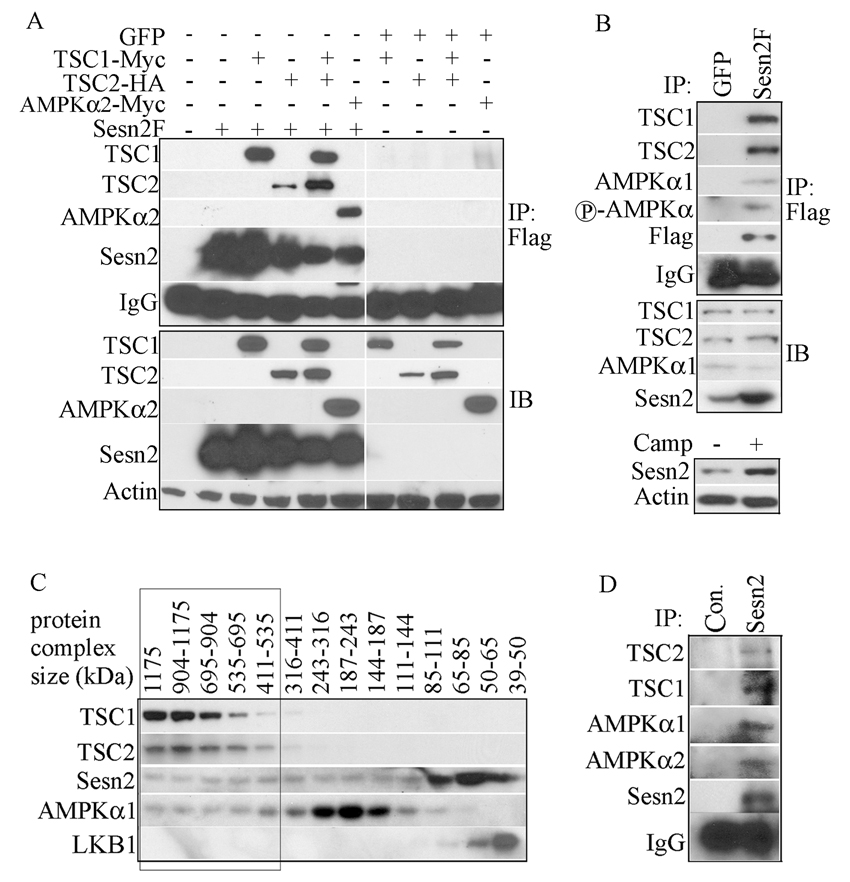
Sestrins interact with TSC1, TSC2 and AMPK
To determine whether the Sestrins can interact with TSC1, TSC2 or AMPK, we expressed TSC1-Myc, TSC2-HA and AMPKα2-Myc together with Sesn2F in HEK293 cells. Immunoprecipitation of Sesn2F resulted in co-precipitation of TSC1-Myc, TSC2-HA and AMPKα2–Myc (Figure 4A). None of these proteins was precipitated by the Flag antibody if they were co-expressed with GFP instead of Sesn2F. Reciprocal immunoprecipitation experiments confirmed the interaction of Sesn2 with TSC1, TSC2 and AMPKα2 (Figure S4A). Similar results were obtained with Sesn1 and AMPKα1 (Figure S4B,C). We also performed immunoprecipitation experiments with Sesn2 mutants lacking its N-terminal (ΔN), C-terminal (ΔC) or middle segments (ΔM) and found that the N-terminal segment was important for interaction with AMPKα2. However, all three mutants retained interaction with the TSC1:TSC2 complex (Figure S4D).
Figure 4. Sestrins interact with TSC1:TSC2 and AMPK.
(A) Co-immunoprecipitation of TSC1, TSC2 and AMPKα2 with Sesn2. HEK293 cells were co-transfected with Sesn2F or GFP expression vectors along with tagged TSC1, TSC2, TSC1 plus TSC2, or AMPKα2 plasmids as indicated. Sesn2F was immunoprecipitated with anti-Flag and presence of the indicated proteins in the immunecomplexes (IP) and total lysates (IB) was examined by immunoblotting. (B) Co-immunoprecipitation of endogenous TSC1, TSC2 and AMPKα1 with Sesn2. Sesn2F or GFP were induced in MCF7-tet OFF Sesn2F or GFP cells by incubation in low doxycycline concentration (0.01 µg/ml). After 24 hrs the cells were lysed. Sesn2F was immunoprecipitated with anti-Flag and presence of the indicated proteins in the immunoprecipitates (IP) and original lysates (IB) was examined. For comparison, MCF7 cells were incubated with camptothecin (Camp) for 12 hrs to induce Sesn2 expression that was examined by immunoblotting of the same amount of cell lysates as above. (C) Sesn2 co-elutes with TSC1, TSC2 and AMPKα in high molecular weight fractions. Extracts of H1299 cells were separated on a Superdex 200 gel filtration column and analyzed by immunoblotting. The elution positions (in kDa) of molecular weight standards are indicated at the top. (D) Co-immunoprecipitation of TSC1, TSC2 and AMPKα with endogenous Sesn2. Two months old wt male mice were injected with DEN (100 mg/kg). After 24 hrs, the liver was removed, homogenized and filtered. The lysate was separated by gel filtration as above and the high molecular weight fractions were combined and immunoprecipitated with anti-Sesn2 or control anti-rabbit (Con.) IgG. Presence of the indicated proteins in the immuneprecipitates was analyzed by immunoblotting.
To study whether a complex containing Sesn2, TSC1, TSC2 and AMPK can form under more physiological conditions, we immunoprecipitated lysates of MCF7-tet OFF Sesn2F or MCF7-tet OFF GFP cells with the Flag antibody. The MCF7-tet OFF Sesn2F cells were kept on low concentration of doxycycline to achieve Sesn2F amounts similar to those seen in the same cells after camptothecin treatment (Figure 4B). Under these conditions, endogenous TSC1, TSC2, AMPKα1 and its phosphorylated form were co-precipitated along with Sesn2F (Figure 4B). To obtain further biochemical evidence for a complex containing TSC1, TSC2, AMPKα1 and Sesn2 we used gel filtration chromatography. TSC1, TSC2, AMPKα1 and Sesn2 co-eluted in several high molecular weight fractions ranging from 0.4 to 1.1 ×106 Da (Figure 4C). Only a portion of the total Sesn2 or AMPKα1 pool co-eluted with the TSC1:TSC2 complex. The AMPK activating kinase LKB1 eluted as a low molecular weight protein and was not present in the high molecular weight fractions (Figure 4C). As immunoprecipitation of endogenous Sesn2 with the available antibody from a variety of cell lines failed to detect the Sesn2:AMPK:TSC1:TSC2 complex, probably due to low antibody affinity or low Sesn2 amounts, we repeated the gel filtration experiment using extracts of livers isolated from mice injected with the hepatocarcinogen diethylnitrosamine (DEN; see below). As described above, a portion of Sesn2 eluted in fractions ranging from 0.3 to 1.15×106 Da (data not shown). We pooled these fractions and immunoprecipitated them with the Sesn2 antibody. This resulted in co-immunoprecipitation of AMPKα1, AMPKα2, TSC1 and TSC2 (Figure 4D).
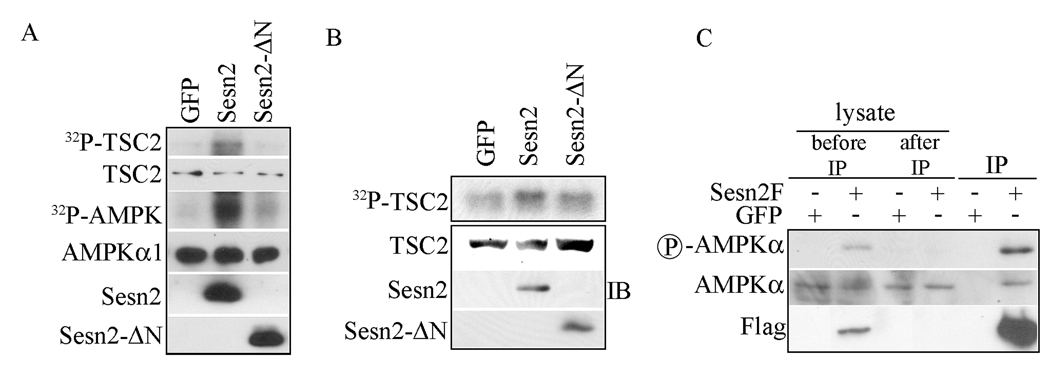
Sestrins activate AMPK and enhance TSC2 phosphorylation
To determine whether the Sestrins stimulate AMPK kinase activity we co-expressed Flag-tagged AMPKα1 with either GFP, wt Sesn2 or the Sesn2 –ΔN deletion mutant, which cannot interact with AMPK. The tagged AMPKα1 subunit was immunoprecipitated and the resulting immunecomplex was assayed for its ability to phosphorylate the TSC1:TSC2 complex isolated from transiently transfected HEK293 cells. Co-expression of wt Sesn2 strongly enhanced the ability of AMPK to phosphorylate TSC2 and undergo autophosphorylation (Figure 5A). No TSC1 phosphorylation was detected. The truncated Sesn2 protein had only a marginal effect on AMPK kinase activity. We also examined the ability of Sesn2 to induce TSC1 or TSC2 phosphorylation in live cells by metabolic labeling. Expression of Sesn2 in HEK293 cells resulted in a substantial increase in TSC2 phosphorylation (Figure 5B). No TSC1 phosphorylation could be detected and as above the truncated Sesn2-ΔN protein had little stimulatory effect on TSC2 phosphorylation. We also compared the phosphorylation of Sesn2-associated and Sesn2- unassociated AMPKα1. As shown in Figure 5C AMPKα1 that was coprecipitated with Sesn2 was more intensely phosphorylated at residue T172 within its activation loop than AMPKα1 that was not co-precipitated with Sesn2. Collectively, these results suggest that the Sestrins activate AMPK through direct interaction and stimulate its kinase activity towards TSC2, which is present in a complex with AMPK and Sesn1/2.
Figure 5. Sesn2 activates AMPK and induces TSC2 phosphorylation.
(A) Wt, but not N-terminally truncated, Sesn2 activates AMPK. HEK293 cells were cotransfected with AMPKα1F expression vector along with GFP, Sesn2F and Sesn2F-ΔN expression vectors. After 48 hrs the cells were lysed and AMPKα1F immune complexes were isolated and assayed for their ability to phosphorylate a TSC1:TSC2 complex isolated by immunoprecipitation from lysates of transiently transfected HEK293 cells. The amounts of AMPKα1F, Sesn2 and TSC2 in the kinase reaction were examined by immunoblotting (IB). (B) Sesn2 enhances TSC2 phosphorylation. HEK293 cells were co-transfected with TSC1Myc and TSC2F expression vectors along with either wt Sesn2F, N-terminally truncated Sesn2F or GFP expression vectors. After 48 hrs cells were metabolically labeled with 32P-orthophosphate and the TSC1:TSC2 complex was immunoprecipitated 4 hrs later, gel separated and autoradiographed. TSC2 and Sesn2 expression were examined by immunoblotting (IB). (C) Sesn2-associated AMPKα is more extensively phosphorylated than AMPK that is not associated with Sesn2. Sesn2F or GFP were induced by doxycycline withdrawl in MCF7-tet cells. After 24 hrs cells were lysed and the lysates were subjected to immunoprecipitation with anti-Flag antibody. The supernatant of the immunoprecipitation reaction, the immune complexes and the total infractionated lysate were examined for AMPKα phosphorylation and content by immunoblotting.
Sestrins play a physiologically important role in mTOR regulation
To study the role of endogenous Sestrins in mTOR regulation, we knocked-down Sesn1 or Sesn2 using shRNA in H1299 cells (Budanov et al., 2004). Silencing of Sesn1 had a marginal effect in this cell line, whereas the knock-down of Sesn2 increased phosphorylation of both p70S6K and S6 (Figure S6A). Two different Sesn1 and Sesn2 shRNAs exerted similar effects (data not shown). Because we suspected that Sesn1 does not significantly contribute to mTOR regulation in the p53-negative H1299 cells, we examined its function in p53-positive 3T3 fibroblasts. Silencing of Sesn1 with two different lentiviral-delivered shRNAs increased p70S6K and S6 phosphorylation, indicating mTOR activation (Figure 6A). Furthermore, Sesn1 knock-down accelerated the proliferation of these cells (Figure 6B).
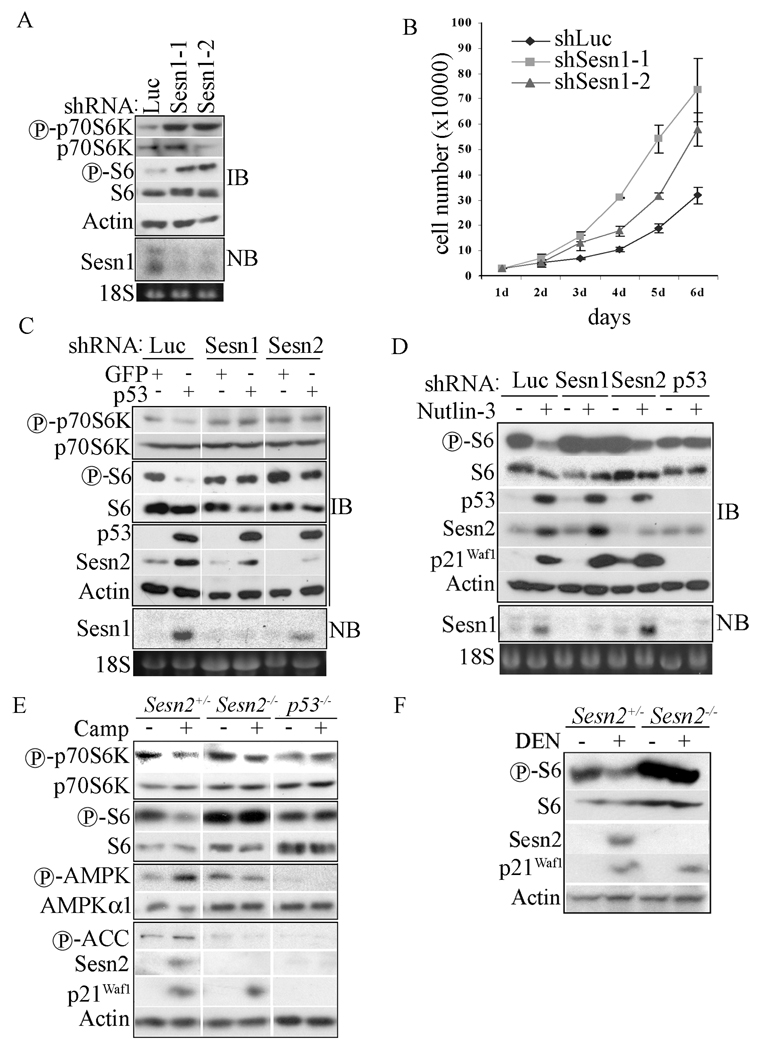
Figure 6. Sesn1/2 are required for negative regulation of mTOR under basal and stressed conditions.
(A) Sesn1 negatively regulates mTOR signaling in mouse fibroblasts. 3T3 cells were infected with shLuciferase (shLuc)-, shSesn1-1- or shSesn1-2-lentiviruses, selected with puromycin and analyzed 48 hrs later for protein phosphorylation (IB) and Sesn1 mRNA expression by Northern blotting (NB). 18S RNA was used to control for RNA loading. (B) Cells (30,000) from (A) were plated onto 6 well plates and counted every 24 hrs for 6 days. (C) Sestrins are required for p53-induced mTOR inhibition. H1299 cells were infected with shLuciferase (shLuc)-, shSesn1- or shSesn2- lentiviruses and 48 hrs later were infected with p53- or GFP-lentiviral vectors. After 48 additional hrs the cells were lysed and analyzed by immunoblotting (IB) for p70S6K and S6 phosphorylation and p53 and Sesn2 expression. Sesn1 expression was examined by Northern blotting (NB). (D) Sesn1/2 are required for down-regulation of S6 phosphorylation in Nutlin-3 treated cells. U2OS cells were infected with shLuciferase (shLuc)-, shSesn1-, shSesn2- or shp53-lentiviruses. After 48 hrs the cells were incubated or not with Nutlin-3 (6 µM) for 12 hrs as indicated to induce p53 accumulation. Expression of p53, Sesn2, and p21Waf1 and S6 phosphorylation were examined by immunoblotting (IB). Sesn1 mRNA was analyzed by Northern blotting (NB). (E) Sesn2 and p53 are required for inhibition of mTOR signaling during camptothecin-induced genotoxic stress. 3T3 cells of the indicated genotypes were treated with camptothecin (Camp, 20 µM) for 12 hrs and protein expression and phosphorylation were analyzed by immunoblotting. (F) Sesn2 is required for DEN-induced inhibition of S6 phosphorylation in mouse liver. Two months old Sesn2+/− and Sesn2−/− male mice were injected with DEN (100 mg/kg). After 24 hrs, protein expression and phosphorylation in liver homogenates were analyzed as above.
p53 activation can down-regulate mTOR signaling (Feng et al., 2005; Horton et al., 2002). To examine the role of Sesn1 and 2 in this response, we silenced their expression in p53-negative H1299 cells and re-infected the silenced cells with GFP- or p53-lentiviral vectors. As expected (Sablina et al., 2005), p53 induced expression of both Sesn1 and Sesn2 (Figure 6C). Importantly, the ability of p53 to inhibit mTOR signaling, as measured by p70S6K and S6 phosphorylation, was attenuated by silencing of either Sesn1 or Sesn2 (Figure 6C). A similar abrogation of p53-induced inhibition of S6 phosphorylation was observed upon knock-down of either TSC2 or AMPKα1 (Figure S6B). To further examine the role of Sesn1/2 in p53-induced mTOR-inhibition, we treated U2OS osteosarcoma cells which contain amplified MDM2, a negative regulator of p53 (Florenes et al., 1994), with a specific inhibitor of the MDM2-p53 interaction, Nutlin-3 (Vassilev et al., 2004). Nutlin-3 induced p53 accumulation and expression of p21Waf1, Sesn1 and Sesn2 and down-regulation of S6 phosphorylation (Figure 6D). Knock-down of Sesn1 and to a lesser extent Sesn2 compromised the inhibition of S6 phosphorylation but had no effect on accumulation of p53 and induction of p21Waf1, both of which were abolished by silencing p53, which also prevented the inhibition of S6 phosphorylation.
Genotoxic stress can inhibit mTOR signaling through activation of p53 (Feng et al., 2005). To study the role of Sestrins in this response in vivo, we generated Sesn2 knock-out mice using gene trap ES cells from Bay Genomics. The Sesn2-deficient ES cells contain a pGT0Lxf expression vector with a strong splice acceptor site integrated into the third intron of the mouse Sesn2 gene, which results in expression of a truncated mRNA and no Sesn2 protein (Figure S6C). Mice generated from these cells were indeed Sesn2 null (Figure 6E, F). We treated Sesn2+/−, Sesn2−/− and p53−/− MEFs immortalized by the 3T3 procedure with camptothecin, a topoisomerase I inhibitor, to activate p53. Camptothecin treatment inhibited p70S6K and S6 phosphorylation while enhancing AMPK and ACC phosphorylation in Sesn2+/− cells but not in Sesn2−/− or p53−/− cells (Figure 6E). Silencing of Sesn1 in Sesn2+/− cells also compromised the inhibitory effect of camptothecin on mTOR (Figure S6D). Sesn2, however, had no effect on activation of AMPK by AICAR (Figure S6E).
We treated 2 months old Sesn2+/− and Sesn2−/− mice with DEN, which upon metabolic activation in zone 3 hepatocytes becomes a potent alkylating agent (Buhler et al., 1992; Verna et al., 1996) that causes p53 activation and induction of p53 target genes, such as Sesn1, Sesn2 and p21Waf1 (Figure S6F). DEN also led to inhibition of S6 phosphorylation (Figure S6D), an effect that was not seen in Sesn2−/− mice (Figure 6G). Immunohistochemistry revealed that inhibition of S6 phosphorylation was restricted to zone 3 hepatocytes, the main site of DEN metabolic activation (Figure S6G). This effect was much weaker in Sesn2−/− mice. Using hepatocyte-specific p53 knock-out mice, created by crossing p53F/F mice (Jonkers et al., 2001) with Alb-Cre mice (Postic et al., 1999), we confirmed that induction of Sesn2 and p21Waf1 by DEN was p53-dependent (Figure S6H).
The growth inhibitory activity of Sestrins is mediated, in part, through inhibition of mTOR signaling
mTOR is a critical regulator of cell growth and proliferation (Fingar et al., 2004; Wullschleger et al., 2006). To examine whether Sestrins negatively regulate cell growth and proliferation, we expressed Sesn1 and Sesn2 in HEK293 cells and measured cell size and cell cycle distribution. Either Sesn1 or Sesn2 decreased cell size (similar to the effect of rapamycin), especially in G1 cells whose fraction was increased upon Sesn1/2 expression (Figure 7A, B). To examine whether p53 regulates cell size in a Sestrin-dependent manner, we infected H1299 cells with shSesn2- or shLuc-lentiviruses and re-infected the cells with either p53- or GFP-lentiviruses. p53 expression decreased cell size, but this effect was abrogated in Sesn2-silenced cells (Figure S7). We also found that Sesn2 truncation mutants, that were incapable of mTOR inhibition and AMPK activation (Figure S8A), failed to inhibit clonogenic growth of HEK293 cells, which was readily inhibited by wt Sesn1 or Sesn2 (Figure S8B). The redox-defective Sesn2(C125S) mutant also inhibited clonogenic growth but not as effectively as wt Sesn2, suggesting that some of the growth inhibitory activity of Sesn2 may be redox-dependent and mTOR-independent. To examine whether the Sestrin effect on cell size and proliferation is TSC2-dependent, we infected Tsc2+/+ and Tsc2−/− cells with a Sesn2- or GFP- lentiviruses and measured cell size and clonogenic growth. Sesn2 expression decreased cell size and inhibited clonogenic growth of Tsc2+/+ but not Tsc2−/− cells (Figure 7C, D).
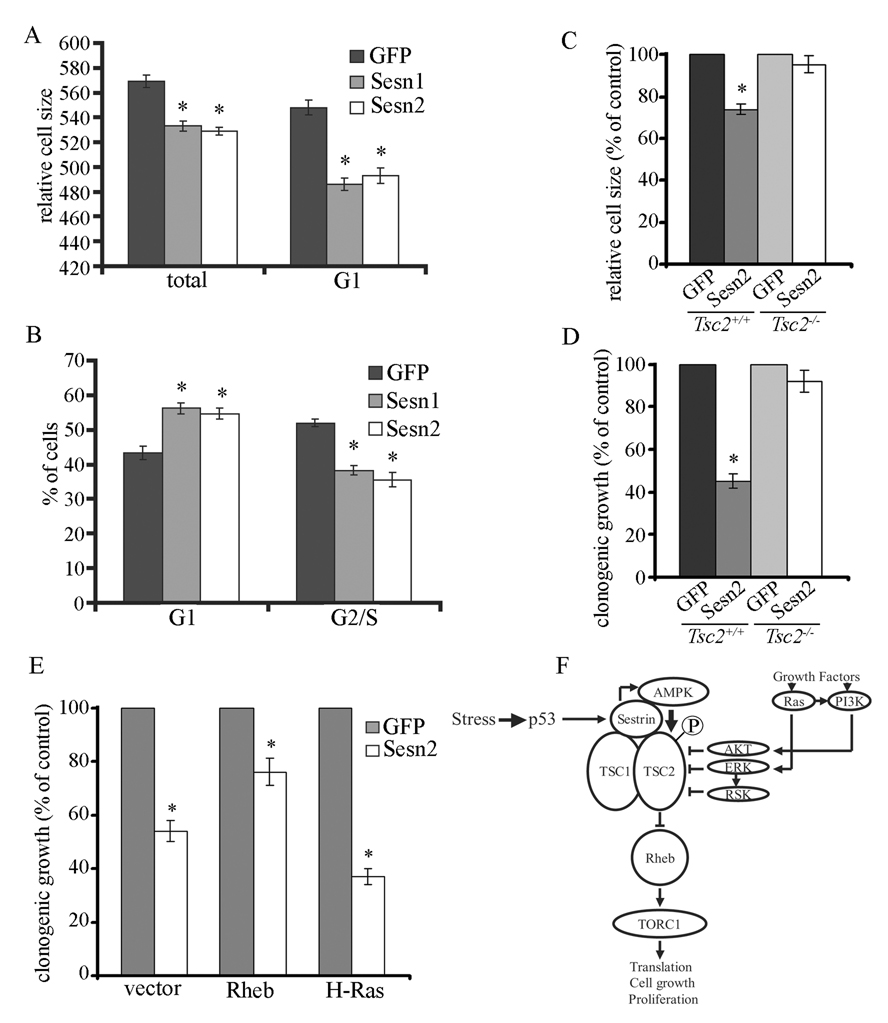
Figure 7. Sestrins inhibit cell growth and proliferation via mTOR.
(A,B) Sesn1/2 expression decreases cell size and inhibits cell proliferation at G1. HEK293 cells were infected with Sesn1-, Sesn2- or GFP-lentiviruses. After 48 hrs cell size (A) and cell cycle distribution (B) were analyzed by flow cytometry. (C, D) Sesn2 expression decreases cell size and inhibits clonogenic growth in Tsc2+/+ but not Tsc2−/− cells. Tsc2+/+ and Tsc2−/− cells were infected with Sesn2- or GFP-lentiviruses. After 48 hrs cell size (C) was analyzed by flow cytometry. Colony formation (D) was analyzed 14 days after infection. (E) Sesn2 inhibits clonogenic growth upstream to Rheb. MCF7 cells were transfected with Sesn2 or GFP expression vectors along with Rheb, H-Ras or an empty expression vector. The cells were selected in puromycin-containing medium and colony formation was quantitated after 3 weeks. Results in (A, B, C, D, E) are averages ±S.E. * − p ≤ 0.05 by Student’s t test. (F) A model explaining our results. Sesn1/2 are induced upon stress-triggered p53 activation. Sesn1/2 dimers interact with TSC1:TSC2 and AMPKα and activate AMPK by induced proximity-dependent autophosphorylation. This results in TSC2 phosphorylation and stimulation of its GAP activity leading to inhibition of mTOR signaling, which is positively regulated by growth factors via Ras and PI3K.
Ectopic Sesn2 expression inhibits cell growth and proliferation (Budanov et al., 2002). To determine the role of mTOR in this effect, we co-transfected MCF7 cells with Sesn2 or GFP expression vectors together with Rheb or H-Ras constructs. Sesn2 expression inhibited clonogenic growth by about 50% and co-expression of Rheb, but not H-Ras, attenuated this effect (Figure 7E). In fact, H-Ras slightly potentiated the growth-inhibitory activity of Sesn2.
Discussion
The mTOR signaling pathway is a central regulator of cell growth and survival (Corradetti and Guan, 2006; Fingar and Blenis, 2004; Guertin and Sabatini, 2005; Hay and Sonenberg, 2004; Soliman, 2005; Thomas, 2006; Wullschleger et al., 2006). It is therefore not surprising that adverse environmental conditions negatively regulate cell growth by inhibiting mTOR (Corradetti and Guan, 2006). In addition to nutrient limitation, mTOR activity is negatively regulated by genotoxic stress and hypoxia, conditions that activate tumor suppressor p53. The ability of p53 to inhibit mTOR signaling is in line with its function as a negative regulator of cell growth and proliferation (Levine et al., 2006). The results described above strongly suggest that the ability of p53 to inhibit mTOR signaling depends on two of its target genes: Sesn1 and Sesn2.
The Sestrins belong to a small and evolutionary conserved family composed of 3 members in mammals, of which Sesn1 and 2 are stress inducible and p53 regulated (Budanov et al., 2002; Velasco-Miguel et al., 1999). The ability of Sesn1/2 to inhibit cell growth and proliferation was attributed to their redox activity (Budanov et al., 2004). The present work, however, demonstrates that Sesn1/2 are potent inhibitors of mTOR signaling, acting in a manner that does not depend on their redox activity, which only makes a partial contribution to their growth inhibitory activity. Sesn1 and 2 inhibit TORC1 activity towards p70S6K and 4E-BP1 in a variety of human and mouse cell lines, as well as in mouse liver. Notably, the ability of the hepatocarcinogen DEN to inhibit S6 phosphorylation is restricted to zone 3 hepatocytes, which are the main site in which it undergoes metabolic activation to become a potent alkylating agent (Buhler et al., 1992), and this inhibitory activity is Sesn2-dependent. By inhibiting 4E-BP1 phosphorylation, Sesn2 enhances its interaction with eIF-4E and inhibits expression of growth regulatory proteins, such as cyclin D1 and c-Myc, whose translation is eIF-4E-dependent and sensitive to 4E-BP1 phosphorylation (Averous et al., 2007; Averous and Proud, 2006; Mamane et al., 2004).
The Sestrins impact TORC1 activity through the TSC1:TSC2 complex. Being a GAP for Rheb, the direct activator of TORC1, the TSC1:TSC2 complex is a central regulator of mTOR signaling (Corradetti and Guan, 2006; Kwiatkowski and Manning, 2005). Sesn2 expression decreases Rheb GTP loading and the ability of both Sesn1 and Sesn2 to inhibit mTOR signaling is TSC2-dependent. One way to regulate TSC1:TSC2 GAP activity is through TSC2 phosphorylation, but other modes of regulation may also exist (Corradetti and Guan, 2006). Although the Sestrins have no effect on ERK and its target RSK or GSK3β, which can all serve as TSC2 kinases, they stimulate the activity of AMPK, a major TSC2 kinase (Corradetti and Guan, 2006). Furthermore, Sestrin expression enhanced TSC2 phosphorylation in live cells and this effect required the N-terminus of Sesn2, which mediates AMPKα binding. Sesn2 did not stimulate TSC1 phosphorylation and Sesn2-activated AMPK did not phosphorylate TSC1.
Importantly, the mTOR inhibitory activity of Sesn1/2 depends on AMPKα, whose phosphorylation at the activation loop was enhanced upon Sestrin expression. Inhibition of AMPK using compound C as well as shRNA silencing of AMPKα1 attenuated the ability of Sesn2 to inhibit mTOR signaling. Co-immunoprecipitation and gel filtration analyses revealed an interaction between Sesn2 and AMPKα, suggesting that Sestrins are engaged in formation of a large protein complex containing AMPK and TSC1:TSC2. We propose that Sesn1/2 induction in response to genotoxic stress results in binding of Sestrins, most likely as dimers (data not shown), to AMPK and TSC1:TSC2, as well as auto-activation of AMPK through a mechanism based on induced proximity. In addition to activation of AMPK the Sestrins recruit it to phosphorylate TSC2 (Figure 7F). Phosphorylation of TSC2 correlates with enhancement of its GAP activity that leads to inhibition of Rheb and mTOR.
Importantly, we obtained ample and clear evidence that Sesn1/2 are critical mediators of p53’s ability to inhibit mTOR signaling. Using shRNA-mediated silencing we found that both Sesn1 and Sesn2 participate in mTOR inhibition upon p53 activation in human cancer cells. Furthermore, disruption of the Sesn2 gene in mice attenuated the inhibition of p70S6K activity by the DNA-damaging agents: camptothecin in fibroblasts and DEN in hepatocytes. In both cases inhibition of p70S6K was p53-mediated, but unlike the p53 deficiency, the absence of Sesn2 has no effect on induction of p21Waf1, another p53 target gene. Thus, Sesn2 (and presumably Sesn1) seems to mediate only one aspect of p53 signaling – inhibition of mTOR. Correspondingly, the growth-inhibitory activity of Sesn2 is not as strong as that of p53, which has additional targets with anti-proliferative activity, such as p21Waf1.
p53 deficiency and activation of mTOR signaling are hallmarks of human cancer (Levine, 1997; Sabatini, 2006; Thomas, 2006). Several mechanisms account for mTOR activation in cancer, including activation of Ras, PI3K and AKT and inactivation of tumor suppressors that negatively regulate these molecules: PTEN, TSC1, TSC2 and LKB1 (Corradetti and Guan, 2006; Sabatini, 2006; Thomas, 2006). Although p53 can induce expression of several negative regulators of mTOR, including PTEN, TSC2, AMPKβ1 and IGF-BP3 in a cell type-dependent manner (Feng et al., 2007), our results demonstrate that p53-mediated inhibition of mTOR depends mainly on Sesn1 and 2 in mouse fibroblasts and certain human cancer cell lines and on Sesn2 in mouse liver.
Inhibition of mTOR suppresses cell growth and proliferation (Fingar et al., 2004; Wullschleger et al., 2006). Sesn2 was known to inhibit cell proliferation (Budanov et al., 2002), but its mechanism of action was heretofore unknown. Our results strongly suggest that Sesn1 and Sesn2 exert their growth inhibitory effect via mTOR and may cooperate with other anti-proliferative p53 targets, such as p21Waf1. Interestingly, the SESN1 (6q21) and SESN2 (1p35) loci are frequently deleted in a variety of human cancers (Velasco-Miguel et al., 1999); (Ragnarsson et al., 1999; Schwab et al., 1996), suggesting they harbor one or more tumor-suppressors. We found that Sesn2 deficiency renders murine fibroblasts more susceptible to oncogenic transformation and this effect may depend on mTOR inhibition (Figure S9). Hence, SESN1 and SESN2 may indeed be important components of the tumor suppressor network activated by p53.
In summary, while more remains to be learned about Sestrin biology and mechanism of action, our results establish these proteins as critical links between p53 and mTOR that enable p53 to inhibit cell growth.
Experimental Procedures
Mice
Sesn2−/− mice on a mixed C57BL/129 background were generated from ES cells obtained from Bay Genomics. These cells were created by a random gene trap approach and contain a pGT0Lxf construct within exon 3 of the Sesn2 gene with a strong splice acceptor site expressing a β-gal-Neo fusion protein that disrupts the Sesn2 ORF. p53Δhep mice were generated by crossing p53F/F mice on a mixed FVB/129 (Jonkers et al., 2001) with Alb-Cre mice (Postic et al., 1999) on a C57BL6 background for at least 5 generations. Efficient and specific deletion of p53 in hepatocytes was confirmed by PCR analysis. All mice were maintained in filter topped cages on autoclaved food and water at UCSD according to NIH guidelines.
Cell culture, transfection, infection and treatment
Tsc2+/+ and Tsc2−/− 3T3 fibroblasts were a gift from D. Kwiatkowsky. MCF7-tet OFF cells were from Clontech. Sesn2+/− and Sesn2−/− 3T3 fibroblasts were derived using the 3T3 protocol from MEFs. All cells were cultured in high-glucose DMEM supplemented with 10% FCS, L-glutamine, penicillin and streptomycin. For the MCF7-tet OFF system doxycycline was added at 0.5 µg/ml. Lipofectamine-Plus (Invitrogen) was used as a transfection reagent. Infection with retroviral vectors was described (Budanov et al., 2004; Budanov et al., 2002). For rapamycin treatment, cells were usually incubated with 20 ng/ml rapamycin. For camptothecin treatment cells were incubated 12 hrs after plating with camptothecin (20 µM) for 12 more hrs. For Nutlin-3 treatment cells were incubated with 6 µM of Nutlin-3. For insulin and IGF1 treatment cells were kept in serum-free DMEM for 12 hrs and incubated with the growth factors for 1 hr. For colony formation assays cells were transfected with the different plasmids as described (Budanov et al., 2002), cultured for 14–21 days under selective pressure, fixed and stained with methylene blue.
Metabolic labeling
HEK293 cells were transfected with TSC1-Myc plus TSC2-Flag along with GFP, wt or N-terminally trancated Sesn2 expression vectors. After 48 hrs the growth medium was replaced with phosphate free DMEM supplemented with dialysed serum and 1mCi of orthophosphate. After 4 hrs cells were lysed and the TSC1:TSC2 complex was immunoprecipitated using anti-Flag antibody.
AMPK kinase assay
HEK293 cells were co-transfected with AMPKα1-Flag along with GFP, wt or N-terminally truncated Sesn2 expression vectors. After 48 hrs the cells were lysed and AMPKα1F was immunoprecipitated using anti-Flag agarose (Sigma). Kinase activity was examined in AMPK reaction buffer (20mM HEPES-NaOH, pH 7.5, 0.4mM DTT, 18mM MgCl2, 125 µM unlabeled ATP, 6 µM β-glycerophosphate, 1 mM EGTA and 0.25 mM Na3VO4) containing 5 µCi [γ-32P]ATP.
Cell lysis, immunoprecipitation and immunoblot analyses
For ordinary immunoblot analysis cells were lysed in RIPA-SDS buffer (Budanov et al., 2002). For immunoprecipitation analysis cells or livers were lysed in NP40 buffer (20 mM Tris-HCl, 5% glycerol, 138 mM NaCl, 2.7 mM KCl, 1% NP40, 20 mM NaF, 18 µM pepstatin, 5 mM EDTA, 1 mM Na3VO4, 20 µM leupeptin, 2.5 mM Na pyrophosphate and 1 mM β-glycerophosphate). Cell lysates were incubated with the indicated antibodies for 1 hr followed by incubation with 1:1 mixture of protein A- and protein G-Sepharose beads. For Cap-binding assay cell lysates were incubated with m7GRNA-Sepharose beads (Amersham) for 6 hrs. After centrifugation the beads were washed 4 times in lysis buffer. Total and immunoprecipitated proteins were resolved by SDS-PAGE, transferred onto PVDF membranes and probed with the relevant antibodies (Budanov et al., 2004).
Analysis of Rheb GTP/GDP content
Cells were metabolically labeled with 32P-orthophosphate, immunoprecipitated with anti-Rheb antibody (Santa Cruz, CA), guanine nucleotides were extracted and resolved by thin layer chromatography (Garami et al., 2003).
Gel filtration analysis
Gel Filtration analysis was performed on a Superdex 200 column (Amersham Pharmacia Biotech Inc.) using a FPLC system. The column buffer was PBS. The column was calibrated with protein markers ranging from 29 to 2000 kDa (Sigma).
Supplementary Material
Supplemental data including 8 figures and Supplemental Experimental Procedures can be found with this article online at…
Acknowledgements
We thank D.J. Kwiatkowski (Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School) for Tsc2−/− and Tsc2+/+ fibroblasts, E. Feinstein (Quark Biotech Inc.) for Sesn2 antibodies, N. Sonenberg (McGill University), K.L. Guan (UCSD), P.M. Chumakov (Lerner Research Institute, The Cleveland Clinic Foundation), J. Blenis (Department of Cell Biology, Harvard Medical School), G. Thomas (Genome Research Institute, University of Cincinnati), N. Hay (Department of Biochemistry and Molecular Genetics, University of Illinois at Chicago), M.J. Birnbaum (Howard Hughes Medical Institute and Department of Medicine, University of Pennsylvania School of Medicine), S.W. Lowe (Cold Spring Harbor Laboratory), R.J. Shaw for gifts of plasmids and antibodies. We also thank W.E. Naugler for protein and cDNA samples from DEN-treated mice, J. Wang for p53 knock-out mice, P.H. Tseng for help with gel filtration experiments and V. Temkin for help with FACS analysis. Work was supported by grants from the Tobacco Related Disease Research Program (TRDRPW; 15RT-0197), the National Institute of Environmental Health Science (5RO1 ES06376 and 5R37 ES04151) and the Superfund Basic Research Program (5P42 ES010337). A.B. was supported by a postdoctoral fellowship from the TRDRP.
Reference List
- Astrinidis A, Cash TP, Hunter DS, Walker CL, Chernoff J, Henske EP. Tuberin, the tuberous sclerosis complex 2 tumor suppressor gene product, regulates Rho activation, cell adhesion and migration. Oncogene. 2002;21:8470–8476. doi: 10.1038/sj.onc.1205962. [DOI] [PubMed] [Google Scholar]
- Averous J, Fonseca BD, Proud CG. Regulation of cyclin D1 expression by mTORC1 signaling requires eukaryotic initiation factor 4E-binding protein 1. Oncogene. 2007 doi: 10.1038/sj.onc.1210715. [DOI] [PubMed] [Google Scholar]
- Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423–6435. doi: 10.1038/sj.onc.1209887. [DOI] [PubMed] [Google Scholar]
- Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- Budanov AV, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, Gorodin S, Fishman A, Chajut A, Einat P, et al. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 2002;21:6017–6031. doi: 10.1038/sj.onc.1205877. [DOI] [PubMed] [Google Scholar]
- Buhler R, Lindros KO, Nordling A, Johansson I, Ingelman-Sundberg M. Zonation of cytochrome P450 isozyme expression and induction in rat liver. Eur J Biochem. 1992;204:407–412. doi: 10.1111/j.1432-1033.1992.tb16650.x. [DOI] [PubMed] [Google Scholar]
- Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- Dames SA, Mulet JM, Rathgeb-Szabo K, Hall MN, Grzesiek S. The solution structure of the FATC domain of the protein kinase target of rapamycin suggests a role for redox-dependent structural and cellular stability. J Biol Chem. 2005;280:20558–20564. doi: 10.1074/jbc.M501116200. [DOI] [PubMed] [Google Scholar]
- Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res. 1999;253:100–109. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ. The Regulation of AMPK {beta}1, TSC2, and PTEN Expression by p53: Stress, Cell and Tissue Specificity, and the Role of These Gene Products in Modulating the IGF-1-AKT-mTOR Pathways. Cancer Res. 2007;67:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florenes VA, Maelandsmo GM, Forus A, Andreassen A, Myklebost O, Fodstad O. MDM2 gene amplification and transcript levels in human sarcomas: relationship to TP53 gene status. J Natl Cancer Inst. 1994;86:1297–1302. doi: 10.1093/jnci/86.17.1297. [DOI] [PubMed] [Google Scholar]
- Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- Guan L, Song K, Pysz MA, Curry KJ, Hizli AA, Danielpour D, Black AR, Black JD. PKC-mediated down-regulation of cyclin D1 involves activation of the translational repressor 4E-BP1 via a PI3K/Akt-independent, PP2A-dependent mechanism in intestinal epithelial cells. J Biol Chem. 2007 doi: 10.1074/jbc.M610513200. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the Role of mTOR in Cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Horton LE, Bushell M, Barth-Baus D, Tilleray VJ, Clemens MJ, Hensold JO. p53 activation results in rapid dephosphorylation of the eIF4E-binding protein 4E-BP1, inhibition of ribosomal protein S6 kinase and inhibition of translation initiation. Oncogene. 2002;21:5325–5334. doi: 10.1038/sj.onc.1205662. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003a;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003b;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14:R251–R258. doi: 10.1093/hmg/ddi260. Spec No. 2. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E--from translation to transformation. Oncogene. 2004;23:3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters H, Debeer P, Bairoch A, Wilquet V, Huysmans C, Parthoens E, Fryns JP, Gewillig M, Nakamura Y, Niikawa N, et al. PA26 is a candidate gene for heterotaxia in humans: identification of a novel PA26-related gene family in human and mouse. Hum Genet. 2003;112:573–580. doi: 10.1007/s00439-003-0917-5. [DOI] [PubMed] [Google Scholar]
- Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5’-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- Ragnarsson G, Eiriksdottir G, Johannsdottir JT, Jonasson JG, Egilsson V, Ingvarsson S. Loss of heterozygosity at chromosome 1p in different solid human tumours: association with survival. Br J Cancer. 1999;79:1468–1474. doi: 10.1038/sj.bjc.6690234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Sabatini DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem. 2005;280:39505–39509. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- Schwab M, Praml C, Amler LC. Genomic instability in 1p and human malignancies. Genes Chromosomes Cancer. 1996;16:211–229. doi: 10.1002/(SICI)1098-2264(199608)16:4<211::AID-GCC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Soliman GA. The mammalian target of rapamycin signaling network and gene regulation. Curr Opin Lipidol. 2005;16:317–323. doi: 10.1097/01.mol.0000169352.35642.06. [DOI] [PubMed] [Google Scholar]
- Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- Thomas GV. mTOR and cancer: reason for dancing at the crossroads? Curr Opin Genet Dev. 2006;16:78–84. doi: 10.1016/j.gde.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Velasco-Miguel S, Buckbinder L, Jean P, Gelbert L, Talbott R, Laidlaw J, Seizinger B, Kley N. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene. 1999;18:127–137. doi: 10.1038/sj.onc.1202274. [DOI] [PubMed] [Google Scholar]
- Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 1996;71:57–81. doi: 10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data including 8 figures and Supplemental Experimental Procedures can be found with this article online at…