Summary
BMPRIA and its high-affinity ligand BMP4 have recently been shown to be expressed in the β-cells of the pancreas. Here, we report the abnormalities of heterozygous mice for Bmpr1a in glucose metabolism during the course of intraperitoneal glucose tolerance test. The heterozygous mice had increased blood glucose levels throughout the first 2.5 h after the administration of glucose. Analysis of glucose-stimulated insulin secretion (GSIS) indicates that insulin secretion in the heterozygous mice is compromised, and induction of secreted insulin by stimulation is substantially lower compared with the wild-type controls. No apparent abnormalities in pancreas, thyroid, and liver were seen upon histological examination. Real-time PCR results of selected genes showed an increase in the mRNA level of Ins1 and Ins2 in the heterozygous group. These results indicate that the glucose-sensing pathway in these heterozygous mice is altered because of the heterozygosity in Bmpr1a. Together, our data suggest that BMP signaling through BMPRIA plays an important role in glucose metabolism and possibly working through the GSIS pathway. genesis 00:1–7, 2009.
Keywords: bone morphogenetic protein (BMP), bone morphogenetic protein receptor (BMPR), insulin, intraperitoneal glucose tolerance test (IPGTT), diabetes, heterozygous mutant mice, heterozygous phenotype, pancreas
INTRODUCTION
Diabetes is a chronic, debilitating, and costly disease that affects 20.8 million children and adults or 7% of the population in the United States. In type 2 diabetes, which accounts for most of the cases, either the pancreas does not produce enough insulin or the insulin is not properly utilized in maintaining the blood glucose level. Insulin is produced by the β-cells in the pancreas, allowing other cells in the body to utilize sugar molecules in the blood stream. The cause of diabetes is largely unknown, but genetic and environmental factors such as obesity and lack of exercise seem to play a major role in the development of this chronic disease. Many factors are involved in regulating the blood glucose levels through modulation of insulin secretion and/or its proper utilization in glucose metabolism. In addition to glucose-stimulated insulin secretion (GSIS) from pancreatic β-cells, specific amino acids may acutely and chronically regulate insulin secretion from the β-cell (Newsholme et al., 2007). Factors like free fatty acid and inflammatory adipocytokines play a critical role in the development and progression of diabetes (Pfeiffer, 2007). Decreased glucose tolerance in diabetes may lead to impaired renal function (Pessina, 2007). Increased blood glucose levels from elevated hepatic glucose production may happen because of diminished secretion of insulin, impaired hepatic sensitivity to insulin action, or a combination of both (Halter et al., 1985). Chronic inflammation and developing insulin resistance are the two hallmarks of type 2 diabetes. It has been reported that cytokines play important roles in glucose metabolism besides their immunoregulatory roles, and infusion of interleukin-6 in healthy humans is shown to increase glucose disposal by working like a hormone and to help increase fatty acid oxidation (Pedersen, 2007).
Other cytokines of interest include the TGF-β superfamily, and there is accumulating evidence that they play an important role in the development of endocrine pancreas as well as in insulin secretion (Gannon, 2007). TGF-β superfamily members, including TGF-β, activin, and bone morphogenetic proteins (BMPs), have been shown to be involved in islet morphogenesis and the establishment of β-cell mass in several gene ablation or overexpression studies in mice. Inhibition of TGF-β signaling resulted in reduced beta cell mass and poorly organized islets, which can be restored in overexpression studies using transgenic mice (Kim et al., 2000; Sanvito et al., 1994; Smart et al., 2006; Yamaoka et al., 1998). TGF-β signaling is involved in insulin production and secretion in adult mice, whereas inhibition of activin signaling resulted in significant decreases in insulin and glucagon expression. There is also a substantial decrease in the number and size of the islets in these mice (Gannon, 2007).
BMPs were originally identified by their ability to induce ectopic bone formation (Urist, 1965). These secreted signaling molecules are involved in various cellular processes including proliferation, differentiation, and the determination of cell fate (Kishigami et al., 2004). BMP4 has been reported to promote the expansion of endocrine progenitor cells in the pancreas by blocking their differentiation, and hence, balancing between differentiation and expansion of pancreatic duct epithelial progenitors (Hua et al., 2006). The role of BMP2 in converting the acinar-like AR42J cells into insulin-secreting β-cells in the presence of exendin-4 (glucagons-like peptide 1) through the Smad pathway has been reported recently, suggesting a role of BMP signaling in β-cell formation (Yew et al., 2005).
BMPRIA (ALK3) is one of three type I receptors for BMPs and is expressed in many tissues during development as well as after birth (Kishigami and Mishina, 2005). The most studied ligands, BMP2 and BMP4, bind to BMPRIA with high affinity, whereas BMP7 binds only weakly to BMPRIA (Aoki et al., 2001) (Miyazono et al., 2005). Mice homozygous for Bmpr1a null allele fail to produce mesoderm and die at embryonic day 8.0 (E8.0) (Mishina et al., 1995). Recently, it has been shown that BMPRIA and its high-affinity ligand BMP4 are expressed in differentiating and adult β-cells in the pancreas. Attenuated BMPRIA signaling in the β-cells reduces the expression of key genes that are involved in glucose metabolism leading to the eventual onset of diabetes (Goulley et al., 2007). Here, we studied heterozygous knock out Bmpr1a to examine glucose metabolism. We found that the 6-month-old heterozygous mice showed increased blood glucose levels after intraperitoneal injection of glucose, despite their elevated levels of fasting plasma insulin. Glucose stimulation to these mice indicated that in fact, the mutant mice had impaired GSIS as observed during intraperitoneal glucose tolerance test (IPGTT). These results show that the heterozygous Bmpr1a mice are morphologically indistinguishable from their wild-type littermates, but manifested abnormalities in the glucose signaling pathway resulting in impaired insulin secretion upon glucose stimulation.
RESULTS
Abnormal Glucose Metabolism During IPGTT
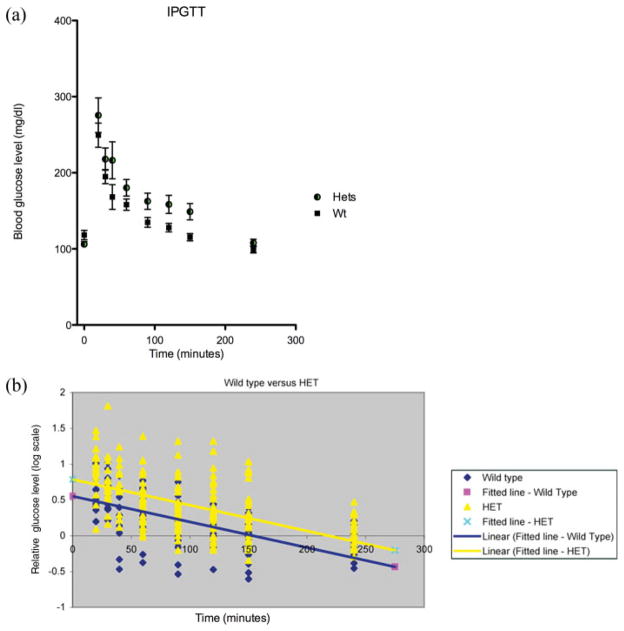
In multiple independent experiments, using different groups of 6-month-old heterozygous mutant male mice and their wild-type littermates, blood glucose values rose substantially higher in the heterozygous Bmpr1a mice (Fig. 1a). The increased blood glucose levels in the heterozygous mice were maintained for the first 2.5 h postglucose injection and gradually became insignificant at 4 h postinjection. Beyond the increase in initial glucose readings, 13 of the 33 heterozygous mutant mice (39%) showed some form of glucose rebounding and ineffective glucose control over a period of up to 2.5 h. This abnormality presented in different manners, sometimes as oscillations, other times it might increase gradually over the 2.5 h period. Only one control mouse in 22 (4.5%) showed even a mild form of abnormal glucose control. The same experiment performed on 3-month-old mice did not show any overt changes between heterozygous mice and control mice (data not shown). These results suggest that the loss of one copy of the gene causes the delay or reduction in the initial event involved in the metabolism of sugar molecules including the release of insulin in response to the demand.
FIG. 1.
Analysis of IPGTT data using the glucose response and Scatter plot. (a) Graph of glucose response to IPGTT. The heterozygous animals showed a larger response to the glucose challenge but tended to return back to resting levels at a normal rate. It is important to note that the erratic nature of many of the heterozygous individuals is lost in the averaging. (b) Scatter plot of log ratios, relative to baseline, of control (in blue) and heterozygous (in yellow), along with their respective linear regression lines. All the data points relative to the initial fasting glucose level from the wild-type control mice (Wt) and heterozygous mice (Het) were averaged to determine the significance of the values for intercept (a pink square and a cross on Y-axis) and the slope (a blue line and a yellow line) between the two genotypes.
Statistical Analysis of the IPGTT Data
For further comparison of glucose response over time relative to the baseline values, we divided the observed response at each time point by the response at the initial time point. Because we expected an exponential decay in response over time, we converted the data into log-scale. In doing so, we generated a linear response over time, and more importantly, the variability in response at each time point was approximately constant and was normally distributed (Fig. 1b). The two genotypes differed significantly in their intercepts (P < 0.0001), confirming that the heterozygous group had higher initial response to glucose, relative to the baseline, than the control group of animals. There was, however, no significant difference in the slopes between the two genotypes (P = 0.9337) (Table 1). Thus, the rate of decay in glucose levels over time, relative to the baseline values, did not differ significantly between the heterozygous and control group. These analyses highlight again that the heterozygous mice showed insufficient response during the first phase.
Table 1.
Statistical Analysis of the Intercept and the Slope
| Intercept (SE) | Slope (SE) | |
|---|---|---|
| Wt | 0.5512 (0.05978) | −0.00359 (0.000236) |
| Het | 0.7869 (0.05537) | −0.00361 (0.000247) |
| Difference | 0.2357 (0.08148) | −0.00002 (0.000342) |
| P-value | P < 0.0001 | P = 0.9337 |
Values for intercepts and slopes were obtained from Figure 1b. As shown in the table, there is no statistical significance between the Wt and Het slopes but the difference in the intercept is highly significant.
Abnormalities in Insulin Secretion in the Mutant Mice
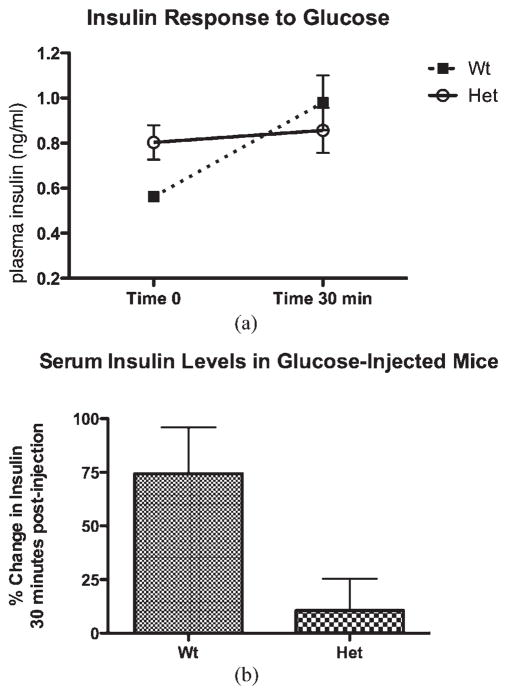
There was a slightly elevated plasma insulin level in the heterozygous mutant mice after an overnight fast compared with the wild-type littermates (see Fig. 2). This data indicate that the available insulin may not be utilized properly in mutant mice, reflecting the scenario of type 2 diabetes. Another likely possibility would be a defect in the GSIS pathway where the mutant mice do not secrete enough insulin to handle the surge of glucose in the plasma. To test this possibility, insulin levels were measured after glucose stimulation. The mutant mice had a much lower insulin release compared with the control animals after glucose challenge (Fig. 2a). The average percent change in insulin level between time 0 and 30-min postglucose injection was substantial. In control animals, the average percent change was 74%, whereas the mutant animals had only an 11% change with a P-value of 0.03 (P = 0.03) (Fig. 2b). These results indicated that mutant mice not only had a poor response to the glucose surge but also impaired GSIS.
FIG. 2.
The average percent change in insulin level after glucose stimulation. (a) The average change of insulin level between the heterozygous (Het) and the control (Wt) mice between 0- and 30-min time period after glucose stimulation. (b) Percent change in serum insulin levels. The percent change in control mice (Wt) is fivefold higher than the heterozygous mice (Het) in that time period.
Histological and Blood Chemistry Analysis of Bmpr1a Heterozygous and Wild-Type Mice
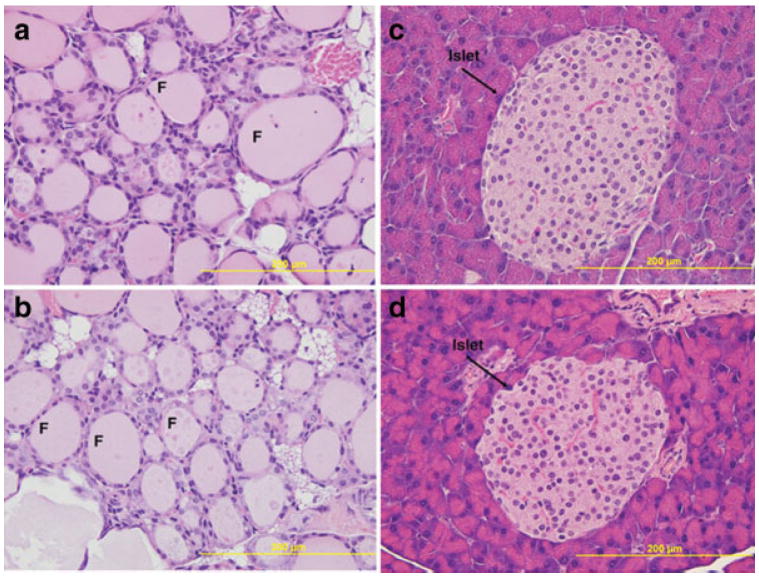
Histological analysis was conducted on primary organs involved in controlling blood glucose levels to determine whether there was any gross structural or organizational alteration. The pancreas showed islets that were well formed, of normal size and general morphology, and seemed to occur in a relatively normal quantity (Fig. 3a,b). Besides pancreas, we also performed the histological analyses on thyroid and liver. The oscillations in blood glucose values after glucose exposure prompted us to further analyze whether there is any difference in thyroid hormones. There were no significant differences in T3 (triiodothyronine) or T4 (thyroxine) values between the control and heterozygous mutant mice (1.00 vs. 1.11 ng/ml for T3 and 4.00 vs. 4.35 mg/ml for T4). T3 and T4 values in mice can vary substantially by strain and age, and many factors are known to interfere including autoantibodies with thyroid hormone assays. Thyroid also appeared unremarkable showing no overt phenotype (Fig. 3c,d). Liver was selected for its role in overall glucose homeostasis and glucose metabolism. Histological examination of the liver revealed no gross abnormalities in the mutant heterozygous mice (data not shown). It has been reported that abnormally high fructosamine concentration in serum correlates with suboptimal glycemic control in humans (Yahaya et al., 2006). The mice, we examined, however, showed no difference in fructosamine levels between the two genotypes (data not shown). Overall, no obvious differences between the heterozygous animals and the wild type littermates were observed in histological analysis of the major three organs involved in maintaining blood glucose levels or serum chemistry.
FIG. 3.
Histological analysis of pancreas and thyroid using H&E staining. Histology of thyroid (a, b) and pancreas (c, d), where panels a, c and b, d correspond to the wild type and heterozygous mice, respectively. Paraffin sections were stained with hematoxylin and eosin. No gross abnormalities were observed in either tissue. F, denotes the follicle and the bar represents 200 μm (×40 original magnification).
Gene Expression Analysis
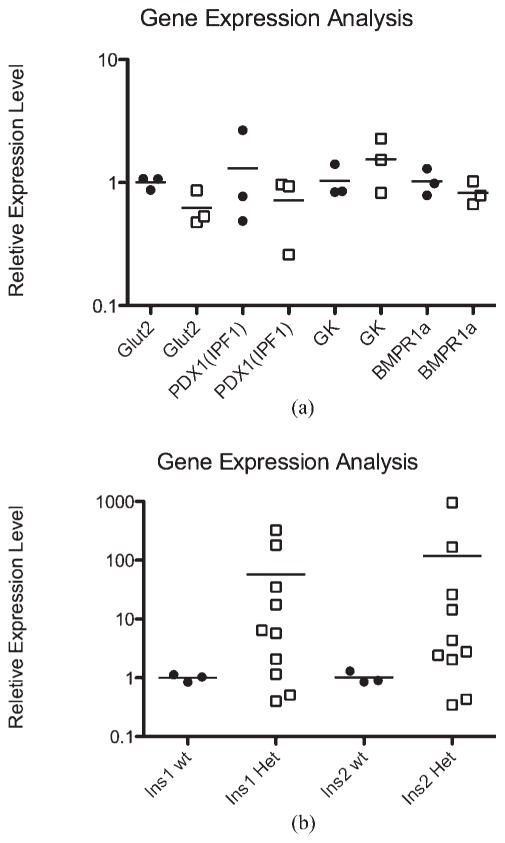
Several genes involved in glucose regulation and metabolism on both the liver and pancreas were selected and tested whether their expression levels were altered in the heterozygous mice. In the liver, Irs2 (insulin receptor substrate-2), Srebf1 (Sterol regulatory element binding transcription factor 1), Srebp-1a, Srebp-1c), Gck (glucokinase), and Gcg (glucagon) were all tested and showed no significant changes in the heterozygous animals (data not shown). In the pancreas, we tested Pdx1 (pancreatic and duodenal homeobox 1, Ipf1), Ins1 (insulin 1), Ins2 (insulin 2), Slc2a2 [solute carrier family 2 (facilitated glucose transporter), member 2, Glut2], and Gck (Fig. 4a); although most showed an insignificant difference, the average Ins1 was increased over 50-fold in the heterozygous mice, and Ins2 increased over 100-fold. (Fig. 4b).
FIG. 4.
Real-time RT PCR analysis. Real-time analysis of selected genes revealed that most of the tested genes were not modified in a substantial way (a). The only genes showing any significant changes were Ins1 and Ins2, with average increases over 50- and 100-fold, respectively (b). Relative expression of genes shown in fold increase on a log10 scale. λ = WT, □= Hets.
DISCUSSION
We report here an observed abnormality in glucose metabolism in Bmpr1a heterozygous mice. The elevated glucose response observed in 6-month-old heterozygous male mice was consistent among different independent experiments, which were performed using heterozygous and wild-type mice. The higher glucose response in the heterozygous mice was maintained for the first 2.5 h after glucose injection, and it became statistically insignificant thereafter in a 4-h experimental period. This indicates that the initial signaling mechanism required to initiate the glucose metabolism pathway may have been impaired in the heterozygous mice. If insulin resistance in these mice is a possibility, that would make it a mouse model similar to type 2 diabetes in humans.
Another possible explanation for this abnormality may lie in GSIS where there may not be enough insulin available in the plasma for glucose disposal. Subsequent analysis of GSIS in these mice determined that the insulin secretion pathway is impaired in the heterozygous animals. The initial insulin level in the heterozygous animals was elevated after an overnight fast. The increase in insulin after the glucose challenge, however, was much lower in the heterozygous mice. Also of importance was the notable inability of some mice to control their glucose level after the initial response. Analysis of the individual results showed that 39% of the heterozygous group had some form of glucose rebounding or instability, this would seem a considerable and significant portion. This could possibly relate back to disrupted timing in the secretions of the β-cells. Islets do secrete insulin in bursts, and can vary, and even be modified in their frequency (Ritzel et al., 2003). It is therefore possible that the frequency of insulin secretion in heterozygous mice is altered and perhaps inconsistent. Much of the difference in the two groups of mice came from this phenotypic 39%. In the only significant gene expression results, Ins1 and Ins2 were increased over 50- and 100-fold, respectively, in the heterozygous mice. Closer examination of the data showed that of the 10 heterozygous mice tested for Ins1 and Ins2, none was in the normal range for both, and four (40%) showed an enormous increase (>10-fold) of Ins1 and Ins2. However, the simple averages do not do justice to this data. The range of results was remarkable in there own right, ranging from 0.40 to 324 fold induction in Ins1 and 0.34 to 958 fold induction in Ins2. This would seem to point to another compounding factor involved in this phenotype.
The consequence of increased Ins1 and Ins2 found in insulinomas (Asfari et al., 1992) generally results in a hypoglycemic state with increased insulin levels. In the case of Bmpr1a heterozygous mice, despite higher Ins1 and Ins2 expression and elevated resting levels of plasma insulin, they fail to respond to the increase in blood glucose. These facts reinforce the idea that the mutant mice cannot respond properly to a glucose challenge. There are significant examples linking the Tgf-β family to INS, showing that Tgf-β1 increases insulin transcription and release (Sayo et al., 2000). However, there are examples that some human insulinomas show a decrease of Tgf-β1 that results in a decrease of its target genes including TGFBI and NMMT (Nabokikh et al., 2007), suggesting relations between growth factor signaling and insulin expression would be context dependent. Despite accumulating reports describing that BMPs stimulate differentiation of pancreatic cells (Jiang and Harrison, 2005; Jiang et al., 2002), the only study directly assessing Bmpr1a signaling on INS is found in the pancreas-specific knockout study of Bmpr1a (Goulley et al., 2007), which shows a reduction in resting insulin secretion. The increase in Ins1 and Ins2 and resting insulin after a reduction of Bmpr1a in heterozygous mutant mice seems counter to these previous reports. This would suggest that either the cause of the abnormal blood glucose response in the Bmpr1a heterozygous mutant mice is located away from the pancreas, or, more likely, we have a situation where the phenotype is not simply dose dependent, and in fact it is the reduced Bmpr1a expression in the pancreas that is responsible. It would seem very possible that an increase of such magnitude in Ins-1 and Ins-2 could cause a subsequent reduction of a similar magnitude in the insulin receptor substrates, or their downstream targets, resulting in the inability to respond to the increase in glucose. Further in vitro and in vivo work will elucidate the exact cause of this inconsistency in insulin secretion.
To determine whether the heterozygous mice suffer any form of hypothyroidism, we tested T3 and T4 levels in these animals. T3 and T4 values in the heterozygous animals were not different from those of the control animals and fell in the normal range. The heterozygous mice showed no difference in fructosamine level from that of the controls as well.
Overall, these heterozygous mice could well be used for further study to reveal the molecular mechanisms involved in this glucose intolerance, and would be interesting to explore how BMPRIA signaling is linked to normal glucose metabolism. Although the broad ranging and sometimes inconsistent phenotypes of heterozygous mice make them difficult to study, their potential application to human conditions seems to demand that we look further in this area.
METHODS
Generation of Mutant Bmpr1a Mutant Mice
Generation of the mutant Bmpr1a mouse line was described previously (Mishina et al., 1995). Heterozygous mice were maintained in mixed 129SvEv and C57BL/6 background. Animal protocols were approved by the Institutional Animal Care and Use Committee at the National Institute of Environmental Health Sciences, NIH.
Intraperitoneal Glucose Tolerance Test
Mice were subjected to an overnight fast (greater than 6 h) with access to drinking water. Mice were weighed the next morning and a fasting glucose level was obtained. Blood was collected from the lateral marginal vein in the hind leg, and glucose levels were measured using a Glucometer (Accu-Chek, Roche, NJ). Glucose (2,000 mg/kg) was injected intraperitoneally according to the weight of the individual mouse, and blood glucose levels were measured at either 20, 40, 60, 90, 120, 150, and 240 min or 30, 60, 90, 120, 150, and 240 min postinjection.
Enzyme-Linked Immunosorbent Assay
Serum insulin levels were measured by ELISA (Linco Research, Charles, MO) following the protocol supplied by the manufacturer. Three wild type and five heterozygous mice each had ~20 μl of blood drawn by submandibular puncture at time 0- and 30-min postglucose injection. Blood was allowed to clot and spun at 2,500g for 15 min at 4°C in hemostat tubes to separate serum.
Histology
Tissue samples were collected from animals after standard CO2 euthanasia. Liver, pancreas, and thyroid glands were removed, fixed in 10% formalin, and embedded in paraffin for sectioning. Tissue was sectioned at 8 μm and stained with standard H&E protocol.
Gene Expression
Three wild type and 3–10 heterozygous individuals were selected for RNA extraction and real-time analysis. RNA was collected from both the liver and pancreas using Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was then made using superscript II (Invitrogen, Carlsbad, CA). Real-time PCR was done using Power SYBR Green from Applied Biosystems (Foster City, CA). All cycles were run with an annealing temperature of 58°C, and all samples were run in at least triplicate and compared using the 2DDt method (Livak and Schmittgen, 2001).
Statistical Methods
Because repeated measurements are recorded on each animal, we expect the responses within animals to be correlated. To account for this correlation, we used mixed effects models by treating animals as random effects. Using PROC MIXED (statistical software package SAS, version 9.1.3), we fitted a linear regression model with an intercept and a slope for each genotype.
Acknowledgments
The authors thank Ms. Page Myers and Mr. James Clark in the Comparative Medicine Branch at the NIEHS for helping them with the IPGTT experiment and tissue collection, Ijeoma Nwosu, Gloria MacDonald, and Tonya Miller for their excellent technical assistances. They also thank Drs. Debbie Stumpo, and Yuan Wang, for critical reading of this manuscript.
Contract grant sponsor: Intramural Research Program of the NIH, National Institute of Environmental Health Sciences
LITERATURE CITED
- Aoki H, Fujii M, Imamura T, Yagi K, Takehara K, Kato M, Miyazono K. Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J Cell Sci. 2001;114:1483–1489. doi: 10.1242/jcs.114.8.1483. [DOI] [PubMed] [Google Scholar]
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- Gannon M. BuMP-ing up insulin secretion by pancreatic β cells. Cell Metab. 2007;5:157–159. doi: 10.1016/j.cmet.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. BMP4-BMPR1A signaling in β cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007;5:207–219. doi: 10.1016/j.cmet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Halter JB, Ward WK, Porte D, Jr, Best JD, Pfeifer MA. Glucose regulation in non-insulin dependent diabetes mellitus. Interaction between pancreatic islets and the liver. Am J Med. 1985;79:6–12. doi: 10.1016/0002-9343(85)90579-0. [DOI] [PubMed] [Google Scholar]
- Hua H, Zhang YQ, Dabernat S, Kritzik M, Dietz D, Sterling L, Sarvetnick N. BMP4 regulates pancreatic progenitor cell expansion through Id2. J Biol Chem. 2006;281:13574–13580. doi: 10.1074/jbc.M600526200. [DOI] [PubMed] [Google Scholar]
- Jiang FX, Harrison LC. Convergence of bone morphogenetic protein and laminin-1 signaling pathways promotes proliferation and colony formation by fetal mouse pancreatic cells. Exp Cell Res. 2005;308:114–122. doi: 10.1016/j.yexcr.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Jiang FX, Stanley EG, Gonez LJ, Harrison LC. Bone morphogenetic proteins promote development of fetal pancreas epithelial colonies containing insulin-positive cells. J Cell Sci. 2002;115:753–760. doi: 10.1242/jcs.115.4.753. [DOI] [PubMed] [Google Scholar]
- Kim SK, Hebrok M, Li E, Oh SP, Schrewe H, Harmon EB, Lee JS, Melton DA. Activin receptor patterning of foregut organogenesis. Genes Dev. 2000;14:1866–1871. [PMC free article] [PubMed] [Google Scholar]
- Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Yoshikawa S, Castranio T, Okazaki K, Furuta Y, Mishina Y. BMP signaling through ACVRI is required for left-right patterning in the early mouse embryo. Dev Biol. 2004;276:185–193. doi: 10.1016/j.ydbio.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−δ δ C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Nabokikh A, Ilhan A, Bilban M, Gartner W, Vila G, Niederle B, Nielsen JH, Wagner O, Base W, Luger A, Wagner L. Reduced TGF-β1 expression and its target genes in human insulinomas. Exp Clin Endocrinol Diabetes. 2007;115:674–682. doi: 10.1055/s-2007-984477. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Bender K, Kiely A, Brennan L. Amino acid metabolism, insulin secretion and diabetes. Biochem Soc Trans. 2007;35:1180–1186. doi: 10.1042/BST0351180. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. IL-6 signaling in exercise and disease. Biochem Soc Trans. 2007;35(Part 5):1295–1297. doi: 10.1042/BST0351295. [DOI] [PubMed] [Google Scholar]
- Pessina AC. Target organs of individuals with diabetes caught between arterial stiffness and damage to the microcirculation. J Hypertens Suppl. 2007;25:S13–S18. doi: 10.1097/01.hjh.0000271504.62325.a4. [DOI] [PubMed] [Google Scholar]
- Pfeiffer AF. Adipose tissue and diabetes therapy: Do we hit the target? Horm Metab Res. 2007;39:734–738. doi: 10.1055/s-2007-990271. [DOI] [PubMed] [Google Scholar]
- Ritzel RA, Veldhuis JD, Butler PC. Glucose stimulates pulsatile insulin secretion from human pancreatic islets by increasing secretory burts mass: Dose-response relationships. J Clin Endocrinol Metab. 2003;88:742–747. doi: 10.1210/jc.2002-021250. [DOI] [PubMed] [Google Scholar]
- Sanvito F, Herrera PL, Huarte J, Nichols A, Montesano R, Orci L, Vassalli JD. TGF-β 1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development. 1994;120:3451–3462. doi: 10.1242/dev.120.12.3451. [DOI] [PubMed] [Google Scholar]
- Sayo Y, Hosokawa H, Imachi H, Murao K, Sato M, Wong NC, Ishida T, Takahara J. Eur J Biochem. 2000;267:971–978. doi: 10.1046/j.1432-1327.2000.01080.x. [DOI] [PubMed] [Google Scholar]
- Smart NG, Apeiqvist AA, Gu X, Harmon EB, Tropper JN, McDonald RJ, Kim SK. Conditional expression of Smad7 in pancreatic β cells disrupts TGF-β signaling and induces reversible diabetes mellitus. PLoS Biol. 2006;4:200–209. doi: 10.1371/journal.pbio.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist MR. Bone: Formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Yahaya IA, Isah HS, Anaja PO. Serum fructosamine in the assessment of glycemic status in patients with sickle cell anaemia. Niger Postgrad Med J. 2006;13:95–98. [PubMed] [Google Scholar]
- Yamaoka T, Idehara C, Yano M, Matsushita T, Yamada T, Ii S, Moritani M, Hata J, Sugino H, Noji S, Itakura M. Hypoplasia of pancreatic islets in transgenic mice expressing activin receptor mutants. J Clin Invest. 1998;102:294–301. doi: 10.1172/JCI2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew KH, Hembree M, Prasadan K, Preuett B, McFall C, Benjes C, Crowley A, Sharp S, Tulachan S, Mehta S, Tei E, Gittes G. Crosstalk between bone morphogenetic protein and transforming growth factor-β signaling is essential for exendin-4-induced insulin-positive differentiation of AR42J cells. J Biol Chem. 2005;280:32209–32217. doi: 10.1074/jbc.M505465200. [DOI] [PubMed] [Google Scholar]