Abstract
Neuromuscular control strategies may change with age and predispose elderly to knee joint injury. The purposes of this study were to determine if long latency responses (LLR), muscle activation patterns, and movement accuracy differs between the young and elderly during a novel single limb squat (SLS) task. Ten young and ten elderly subjects performed a series of resistive SLS (~0–30 degrees) while matching a computer-generated sinusoidal target. The SLS device provided a 16% body weight resistance to knee movement. Both young and elderly showed significant overshoot error when the knee was perturbed (p < 0.05). Accuracy of the tracking task was similar between the young and elderly (p=0.34), but the elderly required more muscle activity compared to the younger subjects (p < 0.05). The elderly group had larger long latency responses (LLRs) than the younger group (p < 0.05). These results support that neuromuscular control of the knee changes with age, and may contribute to injury.
Keywords: Neuromuscular Control, Long Latency Responses, Elderly, Motor Control
INTRODUCTION
Neuromuscular control is believed to be fundamental to minimizing undue stresses and strains to tissues that stabilize the knee (Riemann & Lephart, 2002; Williams, Chmielewski, Rudolph, Buchanan, & Snyder-Mackler, 2001). Because of incorrect central nervous system (CNS) programming, in preparation for a weight-bearing load, non-contact knee injuries are prevalent during weight bearing tasks (Griffin et al., 2000). Functional activities, which include climbing stairs, stepping off curbs, standing from sitting, descending stairs, and squatting to pick an item up from the floor, are everyday tasks that, if programmed incorrectly via the CNS, may contribute to injury with age. Accordingly, understanding age-associated changes in the central nervous system during unexpected weight bearing perturbations may assist in developing new methods to prevent knee injury with age.
A natural consequence of aging is a loss of muscle mass and strength (Deschenes, 2004; Nikolic, Bajek, Bobinac, Vranic, & Jerkovic, 2005). Thus, everyday tasks that normally require low levels of motor unit recruitment are associated with higher levels of CNS drive in order to generate the necessary muscle forces. Increased CNS drive may lead to excessive reflex responses when coupled with an unexpected perturbation of a freely moving limb segment (Matthews, 1986). Previous studies suggest that the elderly have prolonged reflex latencies, and enhanced amplitudes during perturbations induced during quiet stance (Kawashima et al., 2004; Nardone, Siliotto, Grasso, & Schieppati, 1995). However, to our knowledge, no previous report has quantified the long latency responses during a weight-bearing task with co-activation of lower extremity muscles in the elderly.
One common method to assess the neuromuscular response to a perturbation is to examine the muscles’ long latency responses (LLRs) that occur before the volitional reaction time (Etty Griffin, 2003; Hewett, Paterno, & Myer, 2002). Long latency responses of the quadriceps have central and non-central contributions and occur between 50 and 200 ms after the perturbation (Mrachacz-Kersting, Grey, & Sinkjaer, 2006). Muscle spindle, visual input, and vestibular sensory systems “tune” long latency responses during perturbations (Horak, Nashner, & Diener, 1990; Lewis, Polych, & Byblow, 2004; Petersen, Christensen, Morita, Sinkjaer, & Nielsen, 1998). Thus, the LLR during a weight bearing task may be context specific (Bonnard, de Graaf, & Pailhous, 2004; Burleigh & Horak, 1996; Horak, Diener, & Nashner, 1989). The resistive single limb task, as developed for this study, enables us to assess task competency (accuracy), and perturb the limb during active muscle contractions during weight bearing. Because we provide visual feedback and adequate time, we expect both the young and elderly to attain a similar level of accuracy with this tracking task. The accuracy of the tracking task provides a method to “normalize conditions” prior to the perturbation to minimize individuals from developing a “stiffness” strategy common in unexpected perturbation studies. We previously demonstrated that increased co-activation of the quadriceps and hamstrings during this task is associated with poor accuracy (Madhavan & Shields, 2007). Thus, employing a similar accuracy strategy would enable us to compare the triggered long latency responses as they would be triggered in “real life” conditions. In addition, the single limb weight bearing task is also a standard exercise used during rehabilitation programs to teach improved neuromuscular control (Escamilla et al., 1998; Toutoungi, Lu, Leardini, Catani, & O’Connor, 2000; Wilk, Reinold, & Hooks, 2003).
The purposes of this study are to determine if long latency responses, muscle activation patterns, and movement accuracy differs between the young and elderly during a weight-bearing task. We hypothesized that, 1) the elderly will show elevated long latency responses of the quadriceps during unexpected perturbations; 2) the elderly will recruit a higher percentage of muscle to perform the weight adjusted single limb squat exercise; and 3) the elderly and young will achieve comparable levels of accuracy in performing this task.
MATERIALS AND METHODS
Subjects
Ten healthy right-handed young males (ages 24.2 ± 1.6 years) and ten healthy right-handed elderly males (ages 67.4 ± 8.2 years) participated in this study (Table 1). Subjects were excluded if there was a history of neurological deficits, musculoskeletal disorders, degenerative joint diseases, cardiovascular diseases, previous knee injury or surgery, previous fractures of the lower extremity, patellar dislocations, past or current knee pain during activity or rest and inability to ascend at least a flight of 10 stairs. All subjects were recreationally active but were not under any regular physical training program during the time of the study. Following a brief description of the experimental protocol, subjects signed an informed consent document approved by the University of Iowa’s human subjects review board. In addition to filling out a medical questionnaire, subjects also completed the Short Form Medical Outcome Questionnaire (SF 36) to evaluate their health related quality of life and perceived functional status (Ware, 1993). Subjects also performed the single leg stance balance test (standing on the dominant leg for a maximum of 30 seconds) with eyes open and closed to measure balance impairments (Balogun, Ajayi, & Alawale, 1997).
Table 1.
Demographic data of young and elderly subjects depicting age, height, weight, single leg stance time and scores of the Physical Function domain of the SF 36 questionnaire. Values are means (standard deviations).
| Young | Elderly | |
|---|---|---|
| Age (yrs) | 24.2 (1.7) | 67.5 (8.3) |
| Height (cm) | 178.8 (5.0) | 180.1 (4.6) |
| Weight (kg) | 77.1 (8.5) | 84.6 (7.8) |
| Single leg stance Eyes Open (seconds) | 30 (0.0) | 25.4 (8.4) |
| Single leg stance Eyes Closed (seconds) | 24.6 (7.6) | 8.64 (11.0) |
| SF-36 Physical Function | 100 (0.0) | 90.4 (10.4) |
Justification for Age Differences
The young and older groups differed significantly on the Physical Function domain of the SF 36 questionnaire (p < 0.001); suggesting that the elderly group perceived limitations due to their health in performing physical activities (Table 1). A significant difference was also found between the two groups in the single leg eyes closed balance test on their right leg (p < 0.001). On average, the young could maintain their balance for 24.6 seconds while the elderly were able to stand on one leg with eyes closed for just 8.64 seconds. These two tests suggest that although the elderly represented a healthy subject group, they still differ from the younger group in terms of perceived functional status and measurable neurological tests. We tested standing balance on the dominant leg, a different limb than that being tested in this study, in order to get an index of CNS differences between the groups. It is tempting to equate the single limb stance balance test to the single limb squat task implemented in this study. However, pilot data supports that body sway (center of pressure) during single limb stance with eyes closed is reduced nearly 80% when subjects are attached to the weight-bearing tracking system designed for this study. This is an important consideration because the device is designed to study perturbations in weight-bearing in subjects who otherwise may have balance impairments. The degrees of freedom when attached to this instrumentation are reduced because only saggital plane motion is permitted. Accordingly, the limited degrees of freedom permit the safe delivery of perturbations in weight bearing in an intact system (visual, vestibular, somato-sensory integration) without an emphasis on maintaining balance.
Experimental Task
The main task was for the subjects to perform a single leg squat (SLS) exercise on the left (non-dominant) leg. We chose the non-dominant leg because we wanted the task to magnify changes that may occur with age. It is possible that the dominant leg maintains neural control with age longer than a limb that is non-dominant. The subjects used a custom mechanical device that enabled the subject’s to perform the task while following a sinusoidal target (0.4 Hz, T = 2500 ms) projected on a computer screen in front of them. Resistance (16 % body weight (BW)), to both the knee flexion and extension phase of movement required that the subjects increased their central drive to complete the task.
Instrumentation
We custom designed a SLS testing device for the purposes of this study (Shields & Madhavan, 2005). This system includes a rack and pinion gear and associated braking device to resist knee flexion and extension (Figure 1). We measured linear displacement of the knee with a potentiometer attached to the shaft of the brake. The brake was under current control from a microcomputer-controlled digital to analog output. A touch support force system provides for monitoring fingertip forces during SLS tasks. For this study, no subject exceeded 2 N.
Figure 1.
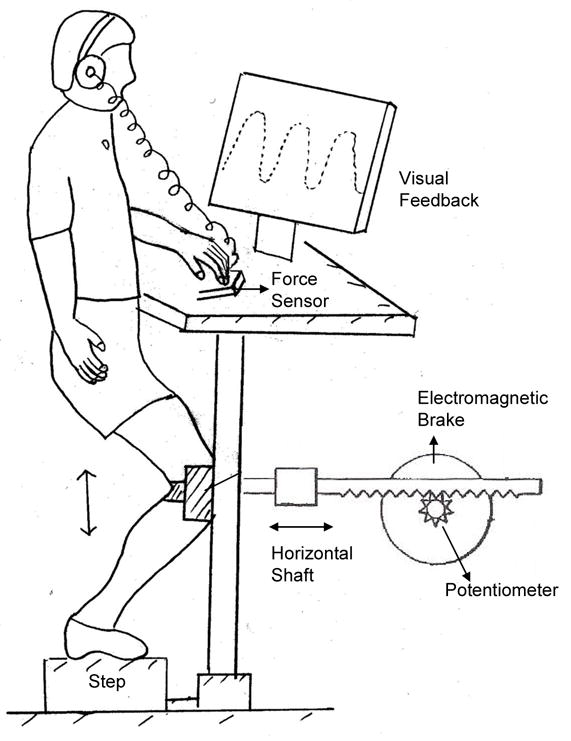
Schematic diagram of the single leg squat testing system
The correlation between knee joint angle and horizontal linear displacement was high in a separate unpublished pilot study using video analysis of knee angle (0.98). For ease of experimentation, all trials in this study were completed without kinematic assessment via video recording and marking of bony landmarks. For this reason the data will be presented in terms of linear displacement (cm), recognizing that this displacement is closely associated with knee angle as previously established.
We displayed the linear motion of the knee on a computer screen directly in front of the subjects. The subject’s goal was to follow a sinusoidal target also projected on the screen. The peak-to-peak amplitude of the target corresponded to 15 cm of linear horizontal displacement, which was approximately 30 degrees of knee flexion.
We recorded muscle activity of the rectus femoris (RF), vastus medialis oblique (VM), vastus lateralis (VL), lateral hamstrings (LH) and medial hamstrings (MH) of the exercised limb using surface electromyography (EMG). We placed bipolar silver-silver chloride EMG electrodes (8 mm in diameter) according to the landmarks described by Cram et al (Cram, Kasman, & Holtz, 1998). Before the start of the SLS exercise we obtained the EMG activity during maximum voluntary isometric contractions (MVIC) of each muscle, and presented all EMG activity during the SLS as a percentage of MVIC. Maximum contractions were performed with subjects seated on the chair of a Kin-Com isokinetic dynamometer (Kin-Com 125E+; Chattex Corp.; Chattanooga, TN) with the knee joint positioned in 30° of flexion. Subjects performed 3 maximum isometric contractions in extension followed by 3 maximum contractions in flexion. The trial with the highest recorded peak EMG was used to normalize the activity of each muscle during the resisted SLS task. While this method does not identically normalize each muscle based on its length during the dynamic single limb test, it does provide a relative measure of muscle activation for normalization across muscles and subjects.
Experimental Protocol
Prior to the start of the experimental protocol, subjects performed a standardized warm up protocol on an exercise bike for 5 minutes. All subjects performed five sets of the SLS exercise to get familiar with the platform and task. Each set consisted of 10 repetitions of the single leg squat. Previous studies have shown that motor learning of this task (tracking the sine wave pattern with knee displacement) is achieved within 5 sets of 10 repetitions (Madhavan & Shields, 2007). Learning during these trials allowed both young and elderly to achieve a similar level of competency (within 3 cm of the target). We excluded three elderly subjects who did not reduce their tracking error to within 3 cm of the target after training. The subjects performed the task with the left leg and were instructed to relax their right leg and let it hang freely during the entire set.
Following the practice trials, subjects performed the perturbation trials. These trials consisted of five sets of 10 repetitions. Two of the 5 sets were selected randomly to have perturbations delivered. Two perturbations were delivered randomly within each of the two sets (of 10 perturbations). A perturbation consisted of the release of brake resistance from 16% of BW to 0% BW. Each release lasted for 500 ms and started when the linear knee displacement crossed the 250 ms mark of the target during the knee flexion phase. This was approximately at 25% of the knee flexion phase of the squat. Thus, the release occurred under identical knee displacement positions for all experiments. We instructed the subjects to continue following the target pattern as accurately as possible even when they felt a change in resistance level. A minimum of one-minute rest, as necessary, occurred between each set. After every set, subjects were asked to report their rating of perceived exertion (RPE) of their quadriceps on the Borg Scale (Borg, 1982). If they perceived any fatigue of the quadriceps, they took additional time to rest. This scale is found to be sensitive to perceived levels of exertion in isolated muscle (Hunter, Critchlow, & Enoka, 2004; Hunter, Lepers, MacGillis, & Enoka, 2003). Subjects reported their RPE to be very light throughout the entire session supporting that the subjects’ perception was that they were not fatigued. This finding was confirmed by the EMG which showed no significant change from the 5th to the 10th set. Some changes in EMG during the first 5 sets are attributed to changes in motor control strategy with learning (Madhavan & Shields, 2007).
Data Analysis
Electromyography (EMG), displacement, and target trajectories were collected and analyzed using Datapac II software (RUN Technologies, CA). We sampled the EMG at 2000 Hz and the displacement at 1000 Hz. We determined task accuracy by calculating the absolute error (absolute value of tracking signal minus target signal) and averaging the error in 10% bins of the flexion and extension cycles. We defined the error during the perturbations as the difference between the greatest flexion displacements relative to the distal target of the sinusoid target for knee flexion. In the event of a perturbation, the overshoot should be extensive if the subject did not expect the perturbation. Repeatability data supports that the perturbation induced similar effects regardless of whether it was the first or the second perturbation delivered during any set of 10 flexion displacements.
We normalized all EMG data as a percentage of each subject’s MVIC in sitting with the knee at 30 degrees. We averaged each muscle’s EMG activation during the flexion and extension cycle, respectively.
To compare muscle responses to the perturbations, we determined the peak long latency response (LLR) of each muscle by calculating the peak EMG value during 50 – 150 ms following the perturbation and subtracting the peak EMG of the unperturbed trials, corresponding to the 50 – 150ms timeframe when a perturbation could have occurred.
We also normalized the peak long latency responses of the quadriceps muscles, 50 – 150 ms after the perturbation according to the following formula:
The time of the peak EMG activity in the 50 – 150 ms following the perturbation was calculated and reported as the latency of the Peak LLR.
Statistical Analysis
We used a Split Plot Repeated measures ANOVA to analyze for significant differences between the young and elderly groups for all the dependant variables. We considered the flexion phase and extension phase of the muscles separately in order to highlight the effect of the perturbation. In the event of a significant interaction between age and perturbation condition, we performed a simple effects analysis. The criterion for significance for each test was 0.05.
RESULTS
Effectiveness of Perturbation Trials
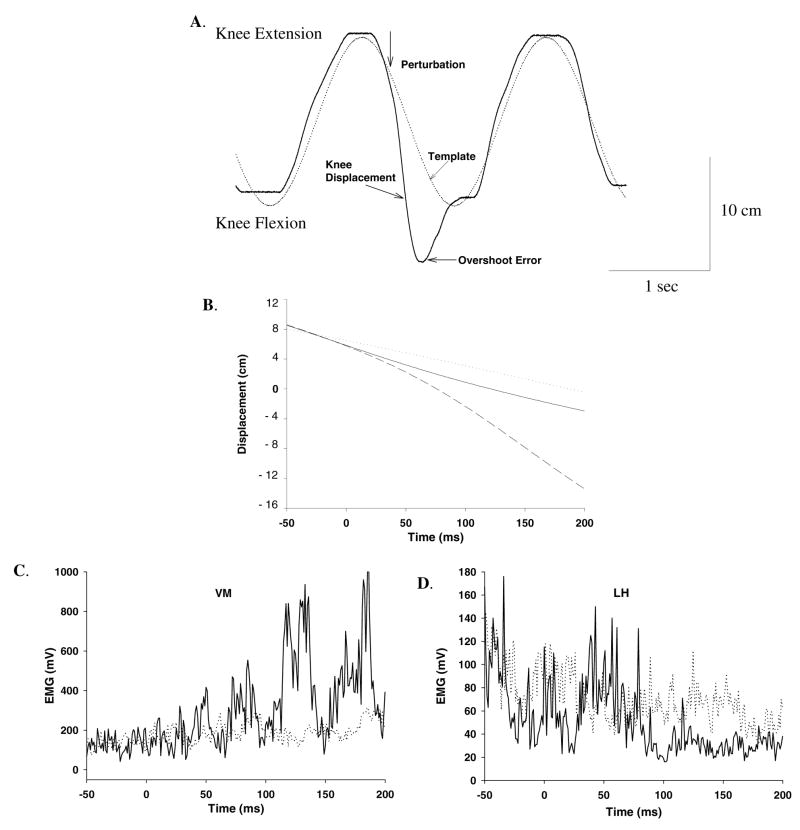
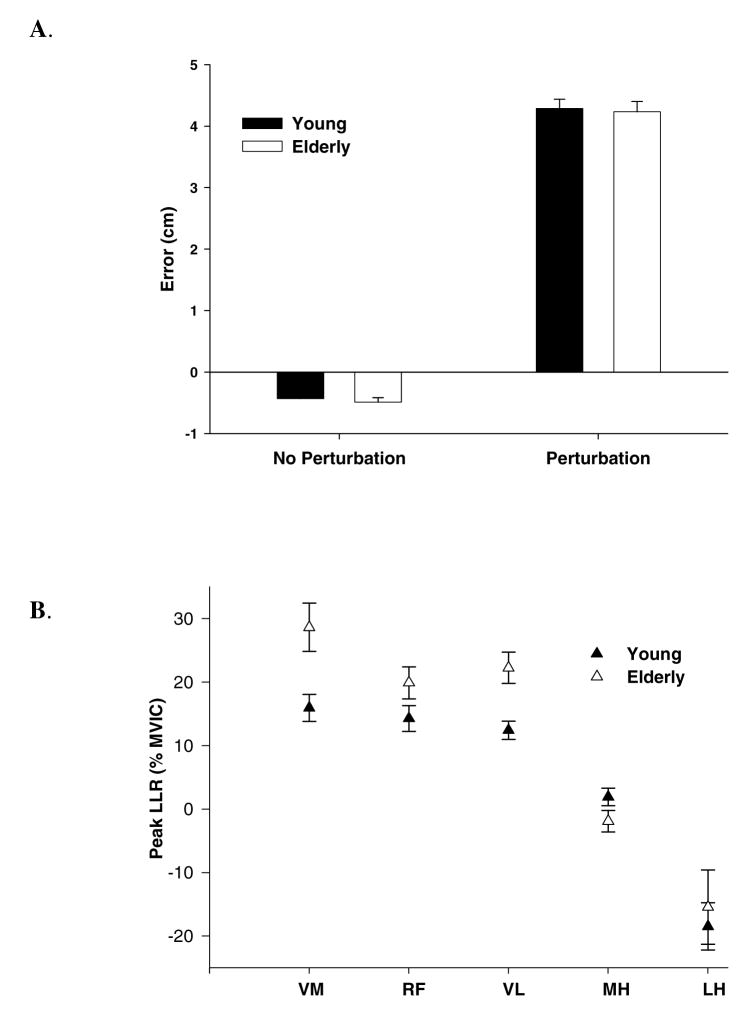
The consistent direction of the error induced during a perturbation trial supports that the subjects did not anticipate the perturbation as shown in this single subject (Figure 2A and 2B). An overlay of the EMG for a perturbed and non-perturbed condition displays the magnitude of the long latency response during the perturbation for a representative subject (Figure 2C and 2D). Note the rapid movement into knee flexion displacement (when the brake was released) resulting in a significant error that overshot the target by ~ 4.5 cm as compared to the non-perturbation condition (p<0.05) (Figure 3A). There was no difference in error between the younger and older groups during the perturbation trials indicating a similar degree of knee displacement (p=0.56).
Figure 2.
A. Representative example of one perturbation trial of a single subject displaying knee displacement, target template, and error following perturbations. B, C and D show ensemble average of 10 perturbations superimposed over trials in which no perturbation was delivered. In B, the dotted line represents the template, the dark line represents knee displacement during no perturbation and dashed line shows knee displacement when perturbed. In C and D, muscular responses of the vastus medialis and lateral hamstrings are shown. The dotted trace shows the trials in which no perturbation was given and the dark trace is the average of trials in which perturbations were delivered. Note the enhanced quadriceps response and inhibition of the hamstrings in response to the perturbation
Figure 3.
A. shows the errors of the perturbation and non-perturbation trials for the young and elderly subjects. The white bars represent the young and the dark bars represent the older group. The Y-axis depicts error in centimeters. Error bars represent standard error (± SE).
B. shows the peak long latency responses of the vastus medialis (VM), rectus femoris (RF), vastus lateralis (VL), medial hamstrings (MH) and lateral hamstring (LH). The Y-axis measures the difference of non-perturbation peak EMG activity from perturbation long latency peak EMG activity, as a percent of maximum voluntary isometric contraction (MVIC). The filled symbols represent the young and open symbols represent the older group.
Long Latency Responses
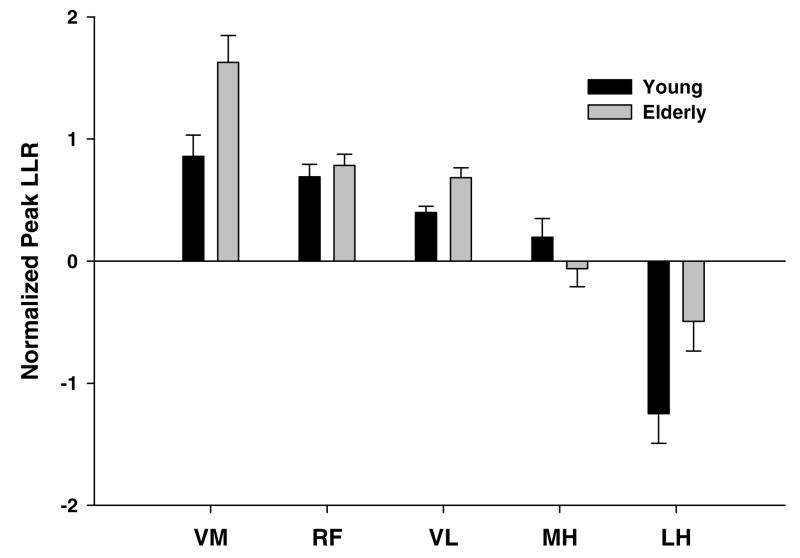
The elderly showed an average 47%, 25% and 45% increase in the peak LLR for the VM, RF and VL muscles, respectively (p<0.05) as compared to the younger group (Figure 3B). A significant inhibition (10–20% MVIC) of the hamstrings (MH and LH) in both groups was present during the enhanced quadriceps long latency response, but this inhibition was not different between the young and the old. When we normalized the long latency reflex response to the background EMG activity of the non-perturbation trials, the elderly showed an average 50% and 40% increase in long latency reflex response for the VM and VL, respectively (p< 0.05) (Figure 4). Less inhibition of the LH was also present (p < 0.05) (Figure 4).
Figure 4.
Normalized peak long latency responses (difference in peak EMG activity between perturbed and unperturbed trials/mean EMG of the unperturbed trial) of the vastus medialis (VM), rectus femoris (RF) and vastus lateralis (VL), medial hamstrings (MH) and lateral hamstring (LH) during 50 – 150 ms following the onset of the perturbation. The white bars represent the young and the dark bars represent the older group.
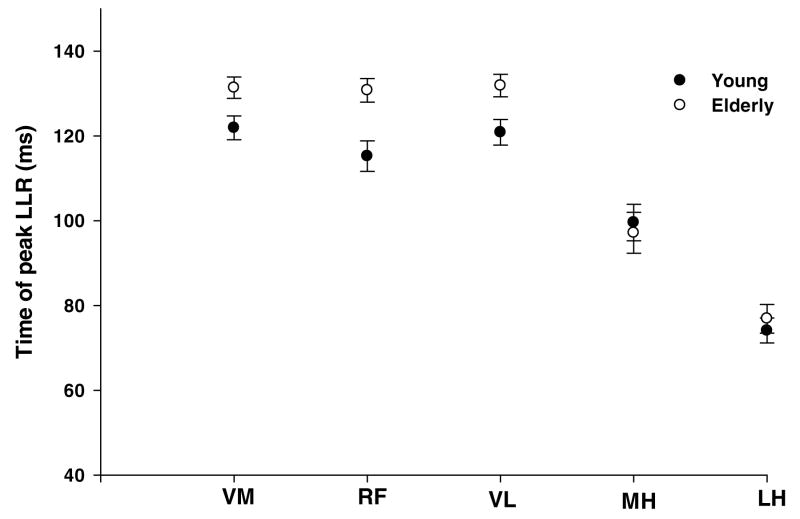
The time latency of the peak response was, on average, 120 ms for the quadriceps in the younger group. The elderly group showed a delay in the peak response to 133 ms as compared to the younger group (p< 0.05) (Figure 5).
Figure 5.
Time latency of the peak long latency responses of the vastus medialis (VM), rectus femoris (RF), vastus lateralis (VL), medial hamstrings (MH) and lateral hamstring (LH) during the perturbed trials. The Y-axis depicts time (in milliseconds) at which the peak of the long latency response was seen. The filled symbols represent the young and open symbols represent the older group.
Muscle Activation Patterns
All three quadriceps muscles (VM, VL, RF) showed greater EMG activity for the elderly group as compared to the younger group during the knee extension phase of the SLS (p<0.05)(Table 2). Overall, during the extension phase, the elderly showed 61%, 21% and 22% greater EMG activity of the VM, RF and VL, respectively, compared to the younger group (Table 2). There were no differences in quadriceps EMG activity between the young and elderly for the flexion phase of the SLS (p=0.42). However, the elderly subjects showed approximately 42% and 29% greater EMG activity of the MH and LH, respectively, as compared to the younger group during the flexion phase (p< 0.05). There was significantly increased activity of only the LH (42%) during the knee extension phase in the older group (p<0.05)
Table 2.
Average muscle synergistic activity (expressed as % MVIC) of the young and elderly groups during the performance of the single leg squat task. Values are means (standard deviations) for the vastus medialis (VM), rectus femoris (RF), vastus lateralis (VL), medial hamstrings (MH) and lateral hamstrings (LH).
| Muscle (% MVIC) Mean (SD) | Flexion | Extension | ||
|---|---|---|---|---|
| Young | Elderly | Young | Elderly | |
| VM | 23.1 (23.9) | 27.9 (20.7) | 26.2 (18.3) | 68.4 (52.0) |
| RF | 18.4 (25.8) | 10.5 (9.0) | 19.7 (17.2) | 24.7 (15.3) |
| VL | 35.5 (20.1) | 28.7 (17.2) | 51.9 (21.2) | 66.1 (22.2) |
| MH | 15.8 (13.6) | 26.2 (23.0) | 11.2 (10.2) | 9.5 (8.1) |
| LH | 71.3 (42.3) | 99.4 (51.8) | 35.3 (28.8) | 59.4 (45.3) |
Accuracy of Performance
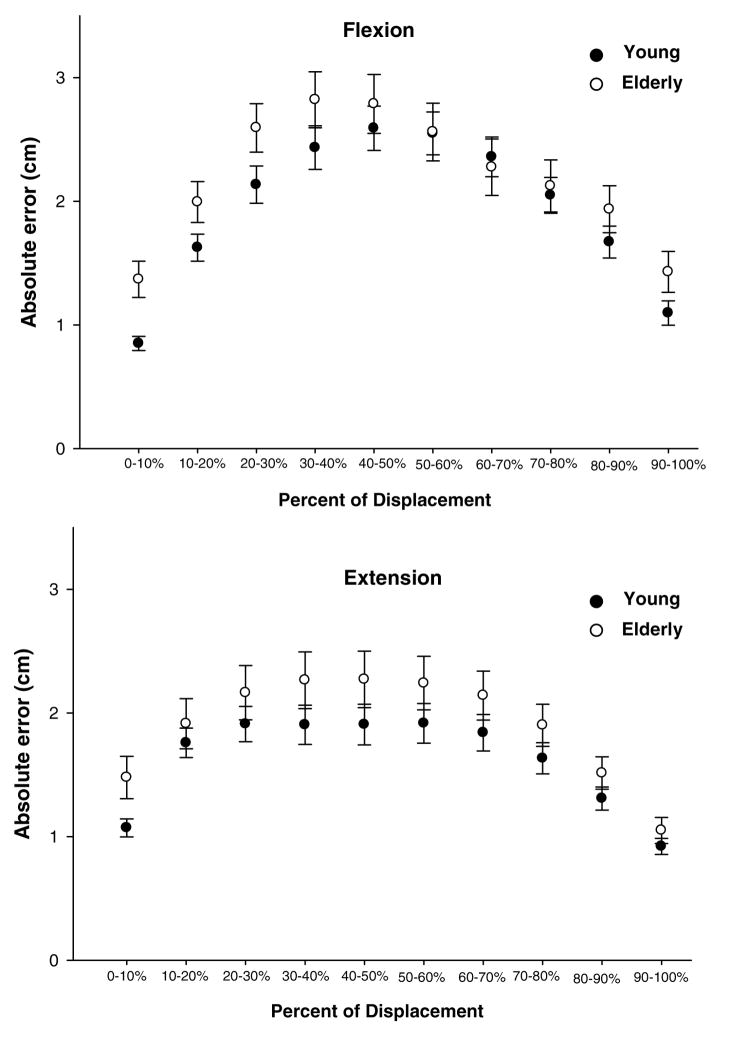
The young and elderly groups showed similar trends in performance of the SLS task with the peak errors being midway between knee flexion and extension (Figure 6). There was no overall difference in absolute error between the younger and older groups (p=0.34).
Figure 6.
Absolute error (centimeters) for young and elderly subjects during the learning sessions when no perturbation was given. Flexion displacements and extension displacements are divided into 10% increments of each respective cycle. The dark circles represent the younger subjects and the open circles represent the elderly subjects. Data points represent the mean of all subjects and error bars are standard errors.
DISCUSSION
A primary challenge for the central nervous system (CNS) is to coordinate muscle activity in an effort to promote joint stability during functional activities. Joint stability requires a complex interaction between the neural control system and the musculoskeletal system. Aging is associated with a significant musculoskeletal loss as well as a decline in the visual, vestibular and somatosensory systems (Nikolic et al., 2005; G. E. Stelmach, Phillips, Difabio, & Teasdale, 1989). Decline in central processing and slowing of performance in speed tasks are other consequences of age that challenge the motor control system (Lassau-Wray & Parker, 1993; Salthouse & Somberg, 1982). The various changes that occur with age may predispose the elderly to injury.
The goal of this study was to examine how young and elderly adults control the knee during a weight bearing activity and to examine differences in their non-volitional responses to unexpected perturbations. The results supported our hypotheses that the elderly showed greater long latency responses to unexpected perturbations; that elderly utilize greater muscle activation than the young to perform certain parts of the weight-adjusted exercise, and those elderly and young showed similar levels of competency (accuracy) in performing the SLS exercise, given adequate time for learning and visual feedback.
Weight bearing exercises have been shown to improve lower limb strength, balance and mobility in older individuals who are healthy and those with impairments (Bean et al., 2002; Hauer et al., 2001). In the present study, we assessed neuromuscular control of the lower extremity by using a target-tracking task during a weight bearing activity (single leg squat). The absolute error of both groups followed a similar pattern throughout the SLS. The highest error occurred while the subject was accelerating towards the midpoint of the flexion and extension movement. The lowest absolute error occurred at the beginning and end of the flexion and extension cycle.
Although both groups used similar muscle activation patterns, the elderly were activating their quadriceps muscles at a higher percentage of MVIC compared to young individuals during the extension phase of the SLS. In addition, and as important, the elderly used their hamstrings at a higher percentage of MVIC during the flexion phase. Thus, the primary agonists for this novel SLS resistive task are the hamstrings during the flexion phase and the quadriceps during the extension phase, both of which were elevated in the elderly. Given the natural atrophy with age, it is not surprising that the elderly required a higher percentage of these muscles in order to perform the flexion and extension phases of the SLS task. Interestingly, the antagonists (quadriceps) during the flexion phase were not different between the young and elderly groups. The similarity in antagonist activity (quadriceps) between the young and the elderly provided a unique opportunity to contrast the LLRs with and without normalization of background EMG activity.
Reflex activation, including long latency responses, elicited by perturbations of a moving or stationary limb segment or by support surface perturbations have been examined to further understand neuromuscular control of the human body (Chmielewski, Hurd, & Snyder-Mackler, 2005; Horak et al., 1989; Shultz et al., 2000). The involvement of a transcortical component (Petersen et al., 1998) and the adaptability of these responses with training (Horak et al., 1989) has made the long latency responses a focus of many rehabilitation programs. Long latency responses are mediated by the spindle group Ia afferents through a supra-spinal pathway in response to rapid stretch of an active muscle (Chan, 1983). These triggered muscle responses which occur within 50 – 200 ms of a perturbation and are influenced by the perturbation duration and velocity (Lewis, Perreault, & MacKinnon, 2005), amplitude (Nardone, Giordano, Corra, & Schieppati, 1990), type of task performed (Bawa & Sinkjaer, 1999), previous experience (Horak et al., 1989), environmental context (Burleigh & Horak, 1996) and age (Nardone et al., 1995). Numerous studies provide valuable information about the effect of aging on long latency responses, but these perturbations are associated with a primary balance task and involves the displacement of the standing support surface during quiet stance, with low levels of agonist-antagonist co-activation. Moreover, when the balance is disturbed in elderly, they often develop an “increased stiffness” strategy which is different from their everyday activation. Studies have noted an increased latency of leg muscle responses to perturbations, altered temporal sequence of muscle activation and an increase in the amplitude of responses (Nardone et al., 1995; G. Stelmach & Worringham, 1985; Woollacott, Shumway-Cook, & Nashner, 1986), but prior perturbation (central set) is known to influence these responses. In the present study, we demonstrated that the quadriceps LLRs are over 40% greater in the elderly, even when adjusted for muscle EMG background activity. This increase is present on the first and all subsequent perturbations, confirming that the goal of accuracy prevents the adoption of a “stiffness strategy” and keeps the central set fixed even though the subjects were fooled. We suggest, for the first time, that excessive LLRs under these types of conditions may lead to soft tissue injury during everyday unexpected perturbations in the elderly.
As indicated previously, we believe the required accuracy was an important component of this study. A common strategy in whole body perturbation research is to stiffen the leg for all subsequent trials after the delivery of the first unexpected perturbation. However, stiffening the limb would compromise the performance accuracy during this task, as was apparent in two of the elderly subjects who could not perform the task accurately following the first perturbation. The consistent overshoot error in both groups during the perturbations support that the unexpected release of the brake fooled the subjects. The subjects focus on accuracy simulated a condition that is more “life like” during the experimental protocol. Indeed, it is common in an experimental condition to set the CNS in a default mode that strives to maintain stiffness of a limb, especially following the first unexpected perturbation. However, the requirement of maintaining accuracy at the time of a perturbation provides new insights into how the CNS responds when the goal of accuracy is included in the context of the task. Our data suggests that the greater concentration given to task accuracy, the easier it is for repeated random perturbations to “fool” the nervous system. We also believe, for these reasons, this task translates into real life situations. For example, we know that injury commonly occurs when a person steps down (off a curb) and the CNS programs incorrectly for the impending distance to landing because they were preoccupied with another task. The extent to which the CNS can assume a “default” mode that is more accommodating to unexpected perturbations is the focus of many rehabilitation scientists. Training paradigms that optimize CNS control may some day be instrumental in preventing musculoskeletal injury in young as well as an aging society.
Subjects in both groups demonstrated significantly increased quadriceps and inhibitory hamstrings LLRs during the 50 – 150 ms following the perturbation, compared with the corresponding time of the non-perturbation trials. Similar to some previous studies, the amplitude of the long latency response was enhanced in the elderly subjects (Nardone et al., 1990; Steffen, Hacker, & Mollinger, 2002). There was no difference in the background EMG of the vastus medialis and vastus lateralis during flexion between the young and elderly groups. Thus, the long latency response was task dependent rather than just automatic gain compensation from background central drive. The underlying mechanisms for this enhancement of these long latency responses are not clear. However, age associated alteration in neurological mechanisms like impairment in spindle discharge rate, excitability of the motor neurons and possibly an amplified supraspinal set with age are plausible explanations (Chung et al., 2005; Kawashima et al., 2004; Mynark & Koceja, 2002). Long latency responses have been suggested to make effective contributions in protecting the limb against dynamic unpredictable force changes (Marsden, Rothwell, & Day, 1983). The older individuals seem to increase their descending drive to the antagonist muscles (hamstrings), which may influence the agonist LLR gain compensation for this task. Accordingly, it appears that the context of the task, and, in this case, the background activity of the muscle not being stretched (hamstrings) appear predictive of the amplitude of the quadriceps LLR during this novel weight bearing test. We need further studies to verify this effect across various perturbation amplitudes in both young and elderly groups.
Many studies have measured muscle response characteristics under resting conditions or when the joint is unloaded (Shultz et al., 2000; Wojtys & Huston, 1994). The novelty of this study is we used a dynamic weight-bearing task under loaded conditions, which also emphasized accuracy to examine neuromuscular control of the lower extremity. The accuracy of the task was similar between the two subject groups prior to the perturbation. We view this as a strength of the study because it appeared that the central set was similar across subjects prior to the perturbation. Introducing random perturbations during a single leg squat may allow the injured and/or elderly individual to learn motor patterns that increase knee stability to apply to novel situations of everyday life. Training programs using perturbations of the support surface have been shown to reduce the risk of continued episodes of the knee giving away and allowing participants to maintain their functional status for longer periods of time (Fitzgerald, 2000). Thus, the application of destabilizing forces to the knee during treatment may enhance neuromuscular responses, which may lead to increased function for the individual. Further research is needed to carefully analyze the adaptive effects of practice on the LLRs and determine if training is associated with improvements in performance during unexpected perturbations.
In summary, we found that elderly individuals require greater muscle activity and have similar error when performing a novel weight-bearing task. Furthermore, the elderly trigger larger long latency responses of the quadriceps muscle group despite similar levels of background activity of the quadriceps, suggesting pure gain compensation (background quadriceps activity) is not the mechanism. Future studies to determine if long latency responses are trainable with practice and influence knee injury with age is of prime interest to motor control and rehabilitation scientists.
Acknowledgments
This work was supported in part by NIH-R01NR010285.
References
- Balogun JA, Ajayi LO, Alawale F. Determinants of single limb stance balance performance. African Journal of Medicine and Medical Sciences. 1997;26(3–4):153–157. [PubMed] [Google Scholar]
- Bawa P, Sinkjaer T. Reduced short and long latency reflexes during voluntary tracking movement of the human wrist joint. Acta Physiological Scandinavica. 1999;167(3):241–246. doi: 10.1046/j.1365-201x.1999.00608.x. [DOI] [PubMed] [Google Scholar]
- Bean J, Herman S, Kiely DK, Callahan D, Mizer K, Frontera WR, et al. Weighted stair climbing in mobility-limited older people: a pilot study. Journal of the American Geriatrics Society. 2002;50(4):663–670. doi: 10.1046/j.1532-5415.2002.50160.x. [DOI] [PubMed] [Google Scholar]
- Bonnard M, de Graaf J, Pailhous J. Interactions between cognitive and sensorimotor functions in the motor cortex: evidence from the preparatory motor sets anticipating a perturbation. Reviews in the Neurosciences. 2004;15(5):371–382. doi: 10.1515/revneuro.2004.15.5.371. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise. 1982;14(5):377–381. [PubMed] [Google Scholar]
- Burleigh A, Horak F. Influence of instruction, prediction, and afferent sensory information on the postural organization of step initiation. Journal of Neurophysiology. 1996;75(4):1619–1628. doi: 10.1152/jn.1996.75.4.1619. [DOI] [PubMed] [Google Scholar]
- Chan C. Segmental versus Suprasegmental Contributions to Long-Latcny Stretch Rsponses in Man. In: Desmedt JE, editor. Motor Control Mechanisms in Health and Disease. New York: Raven Press; 1983. pp. 509–539. [Google Scholar]
- Chmielewski TL, Hurd WJ, Snyder-Mackler L. Elucidation of a potentially destabilizing control strategy in ACL deficient non-copers. Journal of Electromyography and Kinesiology. 2005;15(1):83–92. doi: 10.1016/j.jelekin.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Chung SG, Van Rey EM, Bai Z, Rogers MW, Roth EJ, Zhang LQ. Aging-related neuromuscular changes characterized by tendon reflex system properties. Archives of Physical Medicine and Rehabilitation. 2005;86(2):318–327. doi: 10.1016/j.apmr.2004.04.048. [DOI] [PubMed] [Google Scholar]
- Cram JR, Kasman GS, Holtz J. Introduction to surface electromyography. Gaithersburg: Aspen Publishers Inc; 1998. [Google Scholar]
- Deschenes MR. Effects of aging on muscle fibre type and size. Sports Medicine. 2004;34(12):809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- Escamilla RF, Fleisig GS, Zheng N, Barrentine SW, Wilk KE, Andrews JR. Biomechanics of the knee during closed kinetic chain and open kinetic chain exercises. Medicine and Science in Sports and Exercise. 1998;30(4):556–569. doi: 10.1097/00005768-199804000-00014. [DOI] [PubMed] [Google Scholar]
- Etty Griffin LY. Neuromuscular training and injury prevention in sports. Clinical Orthopaedics and Related Research. 2003;409:53–60. doi: 10.1097/01.blo.0000057788.10364.aa. [DOI] [PubMed] [Google Scholar]
- Fitzgerald G, Kelley The Efficacy of Perturbation Training in Nonoperative Anterior Cruciate Ligament Rehabilitation Programs for Physically Active Individuals. Physical Therapy. 2000;80(2):128. [PubMed] [Google Scholar]
- Griffin LY, Agel J, Albohm MJ, Arendt EA, Dick RW, Garrett WE, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. The Journal of the American Academy of Orthopaedic Surgeons. 2000;8(3):141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- Hauer K, Rost B, Rutschle K, Opitz H, Specht N, Bartsch P, et al. Exercise training for rehabilitation and secondary prevention of falls in geriatric patients with a history of injurious falls. Journal of the American Geriatrics Society. 2001;49(1):10–20. doi: 10.1046/j.1532-5415.2001.49004.x. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Paterno MV, Myer GD. Strategies for enhancing proprioception and neuromuscular control of the knee. Clinical Orthopaedics and Related Research. 2002;402:76–94. doi: 10.1097/00003086-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Horak FB, Diener HC, Nashner LM. Influence of Central Set on Human Postural Responses. Journal of Neurophysiology. 1989;62(4):841–853. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- Horak FB, Nashner LM, Diener HC. Postural Strategies Associated with Somatosensory and Vestibular Loss. Experimental Brain Research. 1990;82(1):167–177. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Enoka RM. Influence of aging on sex differences in muscle fatigability. Journal of Applied Physiology. 2004;97(5):1723–1732. doi: 10.1152/japplphysiol.00460.2004. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Lepers R, MacGillis CJ, Enoka RM. Activation among the elbow flexor muscles differs when maintaining arm position during a fatiguing contraction. Journal of Applied Physiology. 2003;94(6):2439–2447. doi: 10.1152/japplphysiol.01038.2002. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Nakazawa K, Yamamoto SI, Nozaki D, Akai M, Yano H. Stretch reflex excitability of the anti-gravity ankle extensor muscle in elderly humans. Acta Physiologica Scandinavica. 2004;180(1):99–105. doi: 10.1046/j.0001-6772.2003.01230.x. [DOI] [PubMed] [Google Scholar]
- Lassau-Wray ER, Parker AW. Neuromuscular responses of elderly women to tasks of increasing complexity imposed during walking. European Journal of Applied Physiology and Occupational Physiology. 1993;67(5):476–480. doi: 10.1007/BF00376466. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Perreault EJ, MacKinnon CD. The influence of perturbation duration and velocity on the long-latency response to stretch in the biceps muscle. Experimental Brain Research. 2005;163(3):361–369. doi: 10.1007/s00221-004-2182-9. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Polych MA, Byblow WD. Proposed cortical and sub-cortical contributions to the long-latency stretch reflex in the forearm. Experimental Brain Research. 2004;156(1):72–79. doi: 10.1007/s00221-003-1767-z. [DOI] [PubMed] [Google Scholar]
- Madhavan S, Shields RK. Weight-bearing Exercise Accuracy Influence Muscle Activation Strategies of the Knee. Journal of Neurologic Physical Therapy. 2007;31(1):15–19. doi: 10.1097/01.npt.0000260569.69863.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Rothwell JC, Day BL. Long-Latency Automatic Responses to Muscle Stretch in Man: Origin and Function. In: Desmedt JE, editor. Motor Control Mechanisms in Health and Disease. New York: Raven Press; 1983. pp. 509–539. [PubMed] [Google Scholar]
- Matthews PB. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. Journal of Physiology. 1986;374:73–90. doi: 10.1113/jphysiol.1986.sp016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Grey MJ, Sinkjaer T. Evidence for a supraspinal contribution to the human quadriceps long-latency stretch reflex. Experimental Brain Research. 2006;168(4):529–540. doi: 10.1007/s00221-005-0120-0. [DOI] [PubMed] [Google Scholar]
- Mynark RG, Koceja DM. Down training of the elderly soleus H reflex with the use of a spinally induced balance perturbation. Journal of Applied Physiology. 2002;93(1):127–133. doi: 10.1152/japplphysiol.00007.2001. [DOI] [PubMed] [Google Scholar]
- Nardone A, Giordano A, Corra T, Schieppati M. Responses of leg muscles in humans displaced while standing. Effects of types of perturbation and of postural set. Brain. 1990;113(Pt 1):65–84. doi: 10.1093/brain/113.1.65. [DOI] [PubMed] [Google Scholar]
- Nardone A, Siliotto R, Grasso M, Schieppati M. Influence of aging on leg muscle reflex responses to stance perturbation. Archives of Physical Medicine and Rehabilitation. 1995;76(2):158–165. doi: 10.1016/s0003-9993(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Bajek S, Bobinac D, Vranic TS, Jerkovic R. Aging of human skeletal muscles. Collegium Antropologicum. 2005;29(1):67–70. [PubMed] [Google Scholar]
- Petersen N, Christensen LO, Morita H, Sinkjaer T, Nielsen J. Evidence that a transcortical pathway contributes to stretch reflexes in the tibialis anterior muscle in man. Journal of Physiology. 1998;512(Pt 1):267–276. doi: 10.1111/j.1469-7793.1998.267bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann BL, Lephart SM. The Sensorimotor System, Part I: The Physiologic Basis of Functional Joint Stability. Journal of Athletic Training. 2002;37(1):71–79. [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Somberg BL. Isolating the age deficit in speeded performance. Journal of Gerontology. 1982;37(1):59–63. doi: 10.1093/geronj/37.1.59. [DOI] [PubMed] [Google Scholar]
- Shields RK, Madhavan S. Neuromusuclar Control of the Knee during a Resisted Single Limb Squat Exercise. American Journal of Sports Medicine. 2005;33(10):1520–1526. doi: 10.1177/0363546504274150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SJ, Perrin DH, Adams JM, Arnold BL, Gansneder BM, Granata KP. Assessment of neuromuscular response characteristics at the knee following a functional perturbation. Journal of Electromyography and Kinesiology. 2000;10(3):159–170. doi: 10.1016/s1050-6411(00)00002-x. [DOI] [PubMed] [Google Scholar]
- Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Physical Therapy. 2002;82(2):128–137. doi: 10.1093/ptj/82.2.128. [DOI] [PubMed] [Google Scholar]
- Stelmach G, Worringham C. Sensorimotor deficits related to postural stability. Implications for falling in the elderly. Clinics in Geriatric Medicine. 1985;1(3):679–694. [PubMed] [Google Scholar]
- Stelmach GE, Phillips J, Difabio RP, Teasdale N. Age, Functional Postural Reflexes, and Voluntary Sway. Journals of Gerontology. 1989;44(4):B100–B106. doi: 10.1093/geronj/44.4.b100. [DOI] [PubMed] [Google Scholar]
- Toutoungi DE, Lu TW, Leardini A, Catani F, O’Connor JJ. Cruciate ligament forces in the human knee during rehabilitation exercises. Clinical Biomechanics. 2000;15(3):176–187. doi: 10.1016/s0268-0033(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Ware JE. SF-36 Health Survey. Manual and Interpretation Guide. Nimrod Press; 1993. [Google Scholar]
- Wilk KE, Reinold MM, Hooks TR. Recent advances in the rehabilitation of isolated and combined anterior cruciate ligament injuries. Orthopedic Clinics of North America. 2003;34(1):107–137. doi: 10.1016/s0030-5898(02)00064-0. [DOI] [PubMed] [Google Scholar]
- Williams GN, Chmielewski T, Rudolph K, Buchanan TS, Snyder-Mackler L. Dynamic knee stability: current theory and implications for clinicians and scientists. The Journal of Orthopaedic and Sports Physical Therapy. 2001;31(10):546–566. doi: 10.2519/jospt.2001.31.10.546. [DOI] [PubMed] [Google Scholar]
- Wojtys EM, Huston LJ. Neuromuscular performance in normal and anterior cruciate ligament-deficient lower extremities. American Journal of Sports Medicine. 1994;22(1):89–104. doi: 10.1177/036354659402200116. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Shumway-Cook A, Nashner LM. Aging and posture control: changes in sensory organization and muscular coordination. International Journal of Aging and Human Development. 1986;23(2):97–114. doi: 10.2190/VXN3-N3RT-54JB-X16X. [DOI] [PubMed] [Google Scholar]