Abstract
Protein kinases, which serve critical functions in signaling pathways in all cells, are popular therapeutic targets. At present, eight kinase inhibitors have been approved in the United States, each of which shows nanomolar potency. Although the initial goal was to generate inhibitors with a high degree of selectivity, recent experience has revealed that many of these approved compounds target more than one kinase. Surprisingly, this promiscuity is less problematic than one would have imagined; indeed, it opens new therapeutic opportunities. In this Perspective, we discuss the present status of Janus kinase inhibitors – a new class of immunosuppressive drugs – and the advantages and disadvantages of selectively inhibiting this class of kinase.
One benefit of fully understanding signal transduction in immune cells is the ability to identify appropriate targets for the development of new drugs. In principle, the ideal pharmacologic target would have discrete, but critical functions for immune cells and would be dispensable for the function of other cells. As a result, one could achieve immunosuppression without having detrimental effects on other critical organs. This notion is complicated by the fact that related molecules often serve different function in different cells and so presumably, the ideal drug presumably would be highly specific. Previously, it has been assumed that this is a desirable, indeed an essential attribute; but things aren’t always as they seem. It is timely in this regard to revisit the issue of specificity as it relates to kinase inhibitors, an important class of therapeutic compounds. Recent findings indicate that drugs that target multiple kinases may be less toxic than we would have imagined and there may even be downsides of having a high degree of selectivity. We will relate the lessons learned with other kinase inhibitors to particular family – Jak inhibitors, which are being used in the setting of autoimmune disease, transplant rejection and malignancy.
Kinase activity and structure
The importance of phosphorylation as a fundamental mechanism that controls cell physiology has been established since the pioneering work of Krebs and Fischer1. The completion of the human genome project confirmed that 1.7% of the human genome encodes for 518 kinases2,3. Protein kinases modify protein function by transferring phosphate groups from adenosine triphosphate (ATP) or guanosine triphosphate (GTP) to free hydroxyl groups of amino acids. Most protein kinases phosphorylate serine and threonine, but a subset of protein kinases selectively phosphorylates tyrosine residues. There are 90 protein tyrosine kinases (PTKs), many of which serve critical functions4–6. PTKs can be further divided into the two major subgroups, receptor tyrosine kinases (RTKs) and non-receptor or cytosolic tyrosine kinases. RTKs comprise an extracellular ligand-binding domain, transmembrane region and intracellular cytoplasmic kinase domain. They include the epidermal growth factor receptor (EGFR), fibroblast growth factor receptor (FGFR), insulin receptor and platelet-derived growth factor receptor (PDGFR). There are 32 cytoplasmic PTKs that are divided into nine sub-families including: the Src, Csk, Ack, Fak, Tec, Fes/Fer, Syk/Zap70, Abl, Jak families. Both the RTKs and cytosolic PTKs have a conserved kinase domain structure that consists of a N-terminal lobe (N-lobe) composed of five-stranded β sheets and a single α-helix connected to a larger C-terminal lobe (C-lobe) by a hinge region. The protein substrate binds to a surface groove formed by the α-helical C-lobe, whereas an ATP binding pocket is formed by the hinge region and the N- and C-lobes. The C-lobe contains the activation loop (A-loop), which becomes phosphorylated. This leads to conformational stabilization and activation, allowing the transfer of γ-phosphate from bound ATP to the bound substrate protein. Although the overall structure of protein kinases is similar, structural studies have led to the appreciation that the inactive state of the kinases is more distinct7. Ideally, a kinase inhibitor should bind to a single or limited number of kinases. Since all kinases bind ATP, a molecule that is abundant in every cell with intracellular concentrations up to 10 mM and most kinase inhibitors are competitive antagonists of ATP, the ability to specifically inhibit a selected PTK was not a foregone conclusion.
The first successful PTK inhibitors
Chronic myelogenous leukemia (CML) is a myeloproliferative disorder (MPD) characterized by the clonal expansion of myeloid cells carrying the Philadelphia chromosome, initially described as a ‘minute chromosome’ by Nowell and Hungerford in 1960. This represents reciprocal translocation between the long arms of chromocomes 9 and 22, which generates a fusion protein consisting of the abl tyrosine kinase and the breakpoint cluster region (bcr) genes. The resulting protein, BCR-Abl, is a constitutively active PTK that is responsible for the aberrant proliferation and cell survival in CML and expression of BCR-Abl is sufficient to induce a CML-like disease in experimental mice8. Because BCR-Abl is an artificial, transforming kinase expressed in all patients with CML, it represented an ideal therapeutic target to generate a selective PTK inhibitor. In fact, a highly successful inhibitor, imatinib, was generated that revolutionized the treatment of CML9. This drug established that targeting PTKs is safe and is even tolerated better than conventional anti-neoplastic therapies10,11.
Some solid tumors exhibit high expression of RTKs like EGFR (ErbB1) and HER2/neu (ErbB2). The EGFR inhibitors gefitinib and erlotinib were first approved for non-small-cell lung cancer (NSCLC). Patients with pancreatic tumors also benefit from erlotinib and EGFR antagonism is under evaluation for colorectal cancer, malignant glioma, squamous cell cancer of head and neck, breast cancer and other tumors. The PTK inhibitor lapatinib is used in patients with advanced breast cancer that expresses HER2/Neu12.
Lack of specificity: maybe not so bad after all
While imatinib was designed to be a selective BCR-Abl inhibitor, we now know that this drug has activity against multiple kinases including the RTKs PDGFR and KIT, as well as CSF1R and LCK13 (Fig. 1). This was fortuitous in that, analogous to CML, an RTK fusion protein underlies hypereosinophilic syndrome (FIP1L1- PDGFRA) and a fusion protein of PDGFRB ligand (COL1A1-PDGF-B) is associated with dermatofibrosarcoma protuberans14,15. Similarly, systemic mastocytosis is associated with mutations of kit and gastrointestinal stromal tumors over express KIT. Imatinib has benefit in these settings. PDGF is also thought to be involved in the pathogenesis of systemic sclerosis; consequently, imatinib is also being tested in clinical trials in this disorder16. Very recently, imatinib has been reported to improve type I autoimmune diabetes in experimental mice, apparently by inhibiting PDGFR17. At present there are more than 100 clinical trials underway using imatinib in: non-Hodgkin’s lymphoma, ovarian, prostate, breast and lung cancer, glioblastoma multiforme, malignant mesothelioma, melanoma and chronic graft-versus-host disease.
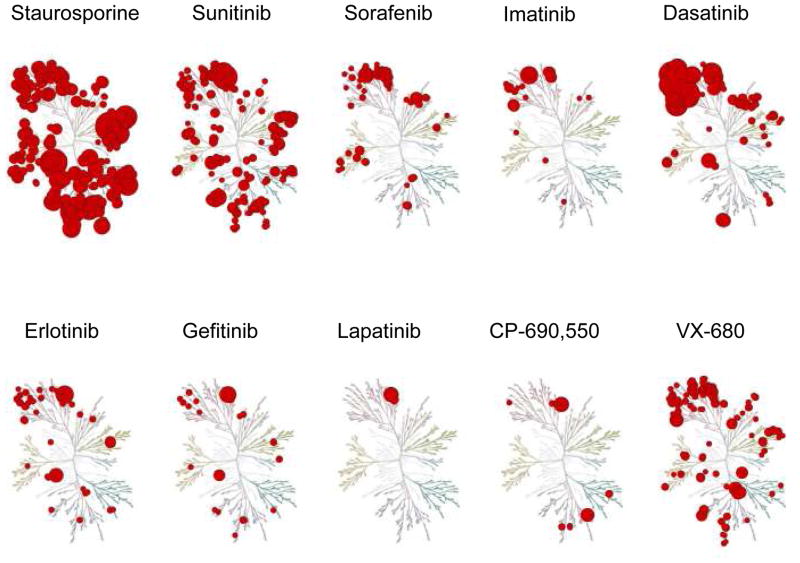
Figure 1.
The kinome binding maps of seven of the eight FDA-approved kinase inhibitors (sunitinib, sorafenib, imatinib, dasatinib, erlotinib, gefitinib and lapatinib), compared to the pan-inhibitor staurosporine (which affects more than 250 kinases) and to investigational protein kinase inhibitors CP-690,550 and VX-680. Even though none of the approved protein kinase inhibitors are absolutely selective, toxicity is limited. The diameter of the red circle is indirectly correlated with the affinity with which the inhibitor binds to the kinase; the cluster at the upper left of the dendogram represents the tyrosine kinases. (Modified from Karaman et al.13)
Even though imatinib is effective in the majority of patients with CML, leukemic cells can become resistant to imatinib therapy due to mutations of BCR-Abl that make it less sensitive to imatinib18. In other tumors, mutation of ‘gatekeeper’ residues in EGFR, KIT or PDGFRA result in stabilization of the active kinase conformation and enzymes with reduced access for ATP-competitive PTK inhibitors19. This had led to the development of other PTK inhibitors for drug-resistant patients. Second generation BCR-Abl inhibitors like dasatinib also inhibit PDGFR, KIT, CSF1R but also Src family PTK, Tec, p38α. Sorafenib and sunitinib are approved for the treatment of renal cell cancer; they bind more than 15 and 50 kinases, respectively13 (Fig. 1). Consequently, several studies are testing the efficacy of sorafenib in a wide range of tumors. Despite the broad actions of sorafenib, toxicity is acceptable - at least in the setting of treatment for malignancy. Similarly, sunitinib, like imatinib has been reported to be useful in a model of type I diabetes17. Several phase 1 and 2 clinical trials are even investigating the safety and efficacy of 7-hydroxystaurosporine (UCN-01), an analog of staurosporine, which binds to more than 250 kinases, in patients with advanced, metastatic or refractory cancers such as pancreatic cancer, kidney cancer, melanoma, lymphoma.
It seems contrary to expectations that, in contrast to traditional chemotherapeutic agents, whose efficacy is limited by toxicity, an inhibitor with such a broad spectrum of targets would not have overwhelming toxicity. However, the threshold of acceptable toxicity is higher in drug trials involving oncology patients than in trials involving patients with chronic inflammatory disease. Although the US Food and Drug Administration (FDA-)approved kinase inhibitors mentioned here have acceptable toxicity profiles, several candidate compounds failed early drug trials. Compounds that have been especially disappointing are the p38 MAPK inhibitors. p38 MAPK was discovered through the use of kinase inhibitors and initial work suggested that this kinase is an ideal target in the treatment of inflammatory disease20. However despite the best attempts of the pharmaceutical industry and multiple candidate compounds, attempts at bringing a p38 MAPK inhibitor to market have failed due to drug toxicity.
Targeting Jaks
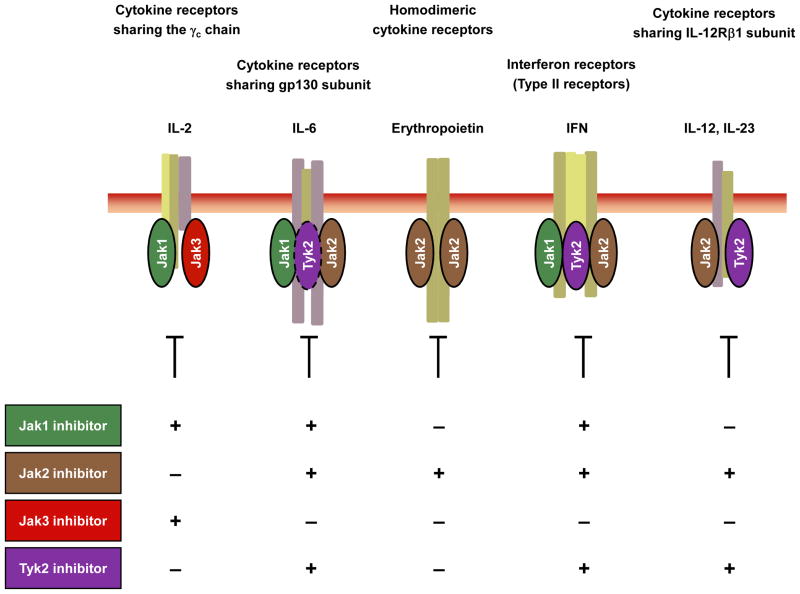
Cytokines are crucial for development, survival, proliferation and differentiation of hematopoietic cells. Type I and type II cytokine receptors lack receptor-intrinsic tyrosine kinase activity but rather transmit their signals through receptor-associated Janus kinases (Jak1, Jak2, Jak3 and Tyk2). In principle, all 4 Jaks might be considered useful targets; however, germline targeting of Jak1 and Jak2 resulted in perinatal and embryonic lethality respectively. Hormone-like cytokines (growth hormone, prolactin, erythropoietin (Epo), and thrombopoietin), GM-CSF, IL-3 and IL-5 use Jak2. IL-6, IL-10, IL-11, IL-19, IL-20 IL-22 and interferon (IFN)-γ use Jak1 and Jak2 (Fig. 2). In contrast, humans and mice lacking Jak3 present with a distinct primary immunodeficiency disorder designated T−B+NK− severe combined immunodeficiency (SCID)21,22. Jak3 is the only Jak family member that associates with just one cytokine receptor - the common γ (γc) chain, which is exclusively used by the receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 (Fig. 2). These cytokines are critically involved in T and NK cell development and B cell function. By contrast, γc-dependent cytokines are not important for the development or function of organs or tissue outside the immune system. Thus, a specific Jak3 inhibitor should have limited but precise effects on immune system cells and should leave other cell types unaffected.
Figure 2.
Targeting cytokine signaling pathways using Jak inhibitors. Jak3 has been viewed as an ideal target, as a truly specific Jak3 inhibitor would inhibit only γc-chain-containing cytokine receptors. However, whether such a high degree of selectivity is essential, or even beneficial, remains to be determined. Compounds selectively directed against Jak1, Jak2 or Tyk2 would affect a broader range of cytokine receptors.
Given its highly specific functions, one might predict that Jak3 antagonist might be superior to cyclosporine, tacrolimus or corticosteroids that act on targets present in all cells. Calcineurin inhibitors, cyclosporine and tacrolimus, are selective, targeting an important signaling pathway. However, this is an ubiquitous pathway that is not specific for immune cells and these drugs are hampered by extra immunological side effects. In particular, calcineurin inhibitors are nephrotoxic, a considerable problem in the setting of renal transplantation. Similarly, corticosteroids are highly effective immunosuppressants, but have multiple side effects including diabetes, hypertension and osteoporosis. In principle, many of these adverse events could be potentially avoided by therapeutics that selectively target PTK involved in immune cell signaling. Biologics like TNF antagonists are highly selective and effective in rheumatoid arthritis and inflammatory bowel disease. They are reasonably well tolerated in most patients, but their use is associated with increased incidence of serious infections and can exacerbate some autoimmune diseases. Furthermore, not all patients respond and the drugs are quite costly. An oral effective drug might be cheaper and more convenient. Thus, Jak3 is conceivably an ideal target and an efficient and safe selective Jak3 inhibitor could conceivably revolutionize the therapy of autoimmune diseases and transplant rejection, as imatinib did for CML.
Tyk2 has broader functions than Jak3, as it binds to a number of cytokine receptor subunits (Fig. 2). Tyk2-deficient mice are viable but have reduced sensitivity to interferons (IFNs), IL-12 and IL-2323. This results in increased susceptibility to some microbial pathogens. As of yet, only one patient with a Tyk2 deficiency has been described, whose cells were found to have impaired signaling in response to type I IFNs, IL-12, IL-23, IL-10 and IL-6. This patient had a primary immunodeficiency disorder characterized as a hyper-IgE-like syndrome with multiple, opportunistic infections with viruses, bacteria and fungi24. We now know that IL-23 receptor gene variants are strongly associated with the susceptibility to Crohn’s disease, ankylosing spondylitis and psoriasis25,26. Accordingly, ustekinumab, which targets IL-12/IL-23p40, seems to have efficacy in these diseases27. Thus, a Tyk2 inhibitor could conceivably be very effective in treating diseases influenced by IL-23.
Experience with Jak3 inhibitors
The appeal of inhibiting Jak3 was widely appreciated, but one compound, CP-690,550, has been most intensively studied in mouse and man. Initial studies showed this drug was remarkably efficacious in transplant rejection in animal models28. Subsequent studies in inflammatory diseases showed that CP-690,550 was effective in arthritis and asthma models. Use of the drug was associated with a reduction in NK cell numbers and IFN-γ and IL-6 production, but did not result in significant reduction in T or B cells. In preclinical models, the drug was also noted to cause anemia.
On the basis of the efficacy in animals, phase 1 and 2 clinical trials with CP-690,550 have commenced in rheumatoid arthritis, psoriasis, Crohn’s disease and renal transplant rejection, and preliminary data show efficacy with limited toxicity. In a phase 2 study in rheumatoid arthritis involving hundreds of subjects, approximately two-thirds had some response (20% improvement in the American College of Rheumatology (ACR) criteria) and one-quarter of patients had a strong response (70% ACR improvement)29. Of note, this response was achieved in patients that failed to prior therapies including methotrexate and TNF antagonists. Additionally, it appears that CP-690,550 can be used with the standard therapies in RA like methotrexate and glucocorticosteroids30. Efficacy was also observed in psoriasis and no graft loss was reported in the setting of kidney transplantation31,32. Consistent with preclinical data, Jak3 inhibition resulted in a decrease of NK cell numbers with no reduction in CD4+ or CD8+ T cells32. Use of CP-690,550 seems to be associated with increased incidence of infections, especially when it is used with other immunosuppressive drugs. Additionally, anemia has been observed in humans treated with CP-690,550 as has increases in serum lipids.
The reduction in NK cells is presumably related to antagonism of IL-15, a cytokine that is important for NK cell homeostasis. Increased incidence of infection is an expected effect of any potent immune suppressive drug. But what about anemia? Jak2 signaling is essential for the actions of erythropoietin and the most likely explanation for the anemia seen in these trials is the inhibitory effects of CP-690,550 on Jak233. Although the drug was initially thought to be selective for Jak3, a recent study has called this conclusion into question13. Compared to most other PTK inhibitors, CP-690,550 has a very restricted number of targets within the human kinome. However, the data also argued that CP-690,550 binds Jak3 and Jak2 equivalently (2.2 nM and 5 nM, respectively)13. This study did not include Jak1 in the screen, but it raises the possibility that some of the efficacy of CP-690,550 might be due to inhibition of Jak1 or Jak2 as well Jak3. CP-660,550 is apparently not a potent inhibitor of Tyk2.
Jak2 and polycythemia vera
The lethality associated with germline deletion of Jak2 was certainly grounds for caution in developing compounds that might interfere with Jak2. However, the dramatic discovery of a subset of diseases associated with Jak2 mutations, provided a setting in which such compounds could be rationally considered. Polycythemia vera (PV) and essential thrombocytosis (ET) are myeloproliferative disorders (MPD), which typically run an indolent course with a proportion developing into myelofibrosis or acute myeloblastic leukemia. Remarkably, more than 95% of cases of PV and 50% of cases of ET and myelofibrosis are associated with point mutations of Jak234–37. The most common mutation, V617F, is located within the JH2 pseudokinase domain and results in a constitutively active kinase that mediates cytokine-independent growth of hematopoietic cells 38,39. Presently, there are at least fifteen clinical trials in MPD using various PTK inhibitors that inhibit Jak2. Several reportedly selective JAK2 inhibitors are being studied. Interestingsly, lestaurtinib, dasatinib and the aurora kinase inhibitor MK-0457 (VX-680) have also been reported to have activity against mutated Jak2 and are under current evaluation for PV. Even imatinib which apparently has little in vitro activity towards JAK2 is also being tested in PV. Contrasting the data obtained from in vitro screens, a recent report has indicated that CP-690, 550 has relatively little activity against unmutated Jak2, but has more impressive activity in cells with mutated Jak240. Another compound, INCB018424 has been reported to be a potent JAK2 and JAK1 antagonist and is being tested in MPD, but is also being studied in other cancers in which Jak2 mutations have not been identified including: prostate cancer, multiple myeloma, AML and CML. From all these trials we will learn much about whether it is safe to inhibit Jak2 in humans.
Selective inhibition of Jaks: good or bad?
Whereas avoiding activity against Jak1 and Jak2 would have been considered essential in the early days of designing Jak inhibitors, inhibition of Jak1 and Jak2 is no longer viewed as an obstacle and might even be considered beneficial. INCB018424 reportedly had efficacy in preclinical models of arthritis and is now being studied in phase 2 studies for rheumatoid arthritis. In preliminary results, ten patients (83%) treated with INCB018424 had some response (a 20% improvement in ACR criteria)41. The efficacy of such a drug is perhaps not surprising as a Jak1 and Jak2 inhibitor would target γc-, gp130-, common-β–chain-cytokine receptors (GM-CSF receptor, IL-3R, IL-5R), as well as receptors for IL-12 and IL-23, IFNs and other type II cytokines, colony stimulating factors and hormone-like cytokines. This drug reportedly has little activity against Jak3, but functionally this should make little difference as all receptors that use Jak3, also use Jak1 for signaling. Curiously though, no cytopenias were reported in this small study – a remarkable, but surely unanticipated observation. The comparative efficacy and toxicity of the various Jak inhibitors will be important to follow in longer term studies.
Another compound, R348, has also entered Phase 1 trials in RA and psoriasis. R348 is a Jak3 inhibitor, but it also inhibits Syk, which is structurally related to Zap70 and has two SH2 domains, Through these SH2 domains, Syk binds to immunoreceptors including Fc and NK receptors, integrin receptors, and Dectin-1. Syk inhibitors such as R788 are also under clinical investigation in phase 2 trials for rheumatoid arthritis. Preliminary results show efficacy, but also dose-dependent neutropenia42. Conceivably, there may be considerable benefit in inhibiting members of two classes of immunologically relevant kinases; however, the relative toxicity of a more selective Jak inhibitor versus a Jak/Syk inhibitor will need to be ascertained. At this point, the jury is still out.
The future of kinase inhibition for immunological disease
Eight PTK inhibitors have now been approved by the FDA. Initially, it seemed essential to generate highly selective compounds to accomplish efficacy without profound toxicity. However drugs with remarkably little selectivity are being routinely used in the treatment of cancer and the lack of selectivity is considered an attribute. Moreover, in certain oncological settings the combination of kinase inhibitors with different targets may be more beneficial to achieve better response rates and to overcome resistance due to the activation of multiple oncogenic signaling pathways. Two approaches are conceivable - the simultaneous use of more than one kinase inhibitor or the intentional generation of inhibitors targeting more than one kinase with nanomolar potency; the latter would represent a first step toward targeted polypharmacology43,44.
At present, we cannot “cure” autoimmune diseases, and as a result these diseases require lifelong treatment with immunosuppressants. It is worth recalling that drugs used to treat autoimmune disease, such as methotrexate and cyclophosphamide, were initially used in the setting of malignancy, albeit at higher doses than that commonly used in rheumatology patients. To what extent we will be able to use “broad spectrum” kinase inhibitors in patients with immune-mediated disease remains to be determined. It is tempting to speculate that we may soon see the use of drugs like dasatinib and sunitinib for the treatment of human autoimmune disease. Regardless, we are witnessing a sea change with respect to the need for selectivity, and it will be fascinating to see where the pendulum comes to rest.
References
- 1.Fischer EH, Krebs EG. Relationship of structure to function of muscle phosphorylase. Fed Proc. 1966;25:1511–1520. [PubMed] [Google Scholar]
- 2.Venter JC, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 3.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 4.Hunter T. A thousand and one protein kinases. Cell. 1987;50:823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- 5.Pawson T. Protein-tyrosine kinases. Getting down to specifics. Nature. 1995;373:477–478. doi: 10.1038/373477a0. [DOI] [PubMed] [Google Scholar]
- 6.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 7.Schindler T, et al. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 8.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 9.Druker BJ, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien SG, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 11.Druker BJ, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 12.Geyer CE, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 13.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 14.Cools J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 15.Simon MP, et al. Deregulation of the platelet-derived growth factor B-chain gene via fusion with collagen gene COL1A1 in dermatofibrosarcoma protuberans and giant-cell fibroblastoma. Nat Genet. 1997;15:95–98. doi: 10.1038/ng0197-95. [DOI] [PubMed] [Google Scholar]
- 16.Baroni SS, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354:2667–2676. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 17.Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, Zhu S, Weiss A, Bluestone JA. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA. 2008;105:18895–18900. doi: 10.1073/pnas.0810246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorre ME, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 19.Azam M, Seeliger MA, Gray NS, Kuriyan J, Daley GQ. Activation of tyrosine kinases by mutation of the gatekeeper threonine. Nat Struct Mol Biol. 2008;15:1109–1118. doi: 10.1038/nsmb.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 21.Macchi P, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 22.Russell SM, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 23.Karaghiosoff M, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–560. doi: 10.1016/s1074-7613(00)00054-6. [DOI] [PubMed] [Google Scholar]
- 24.Minegishi Y, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–755. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Duerr RH, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burton PR, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonardi CL, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 28.Changelian PS, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 29.Kremer JM, Bloom BJ, Breedveld FC, et al. A randomized, double-blind placebo-controlled trial of 3 dose levels of CP-690,550 versus placebo in the treatment of active rheumatoid arthritis. Arthritis Rheum. 2006;54:4116. [Google Scholar]
- 30.Wilkinson B, Chow V, LaBadie R, Zwillich SH, Cohen S. Co-administration of an oral JAK inhibitor CP-690,550 and methotrexate is well tolerated in patients with rheumatoid arthritis. Arthr Rheumatism. 2008;58:S297. doi: 10.1111/j.1365-2125.2009.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan G, Cunshan W, Boy M, Chow V, Herron J. Dose-dependent reduction in psoriasis severity as evidence of immunosuppressive activity of an oral Jak3 inhibitor in humans. Am J Transplant. 2006;6:S87. [Google Scholar]
- 32.van Gurp E, et al. Phase 1 dose-escalation study of CP-690 550 in stable renal allograft recipients: preliminary findings of safety, tolerability, effects on lymphocyte subsets and pharmacokinetics. Am J Transplant. 2008;8:1711–1718. doi: 10.1111/j.1600-6143.2008.02307.x. [DOI] [PubMed] [Google Scholar]
- 33.Parganas E, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 34.Baxter EJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 35.James C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 36.Kralovics R, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 37.Levine RL, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 38.Geron I, et al. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell. 2008;13:321–330. doi: 10.1016/j.ccr.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Wernig G, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008;13:311–320. doi: 10.1016/j.ccr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Manshouri T, et al. The JAK kinase inhibitor CP-690,550 suppresses the growth of human polycythemia vera cells carrying the JAK2V617F mutation. Cancer Sci. 2008;99:1265–1273. doi: 10.1111/j.1349-7006.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams W, Scherle P, Shi J, Newton R, McKeever E, Fridman J, Burn T, Vaddi K, Levy R, Moreland L. A randomized placebo-controlled study of INCB018424, a selective Janus kinase1&2 (JAK1&2) inhibitor in rheumatoid arthritis (RA) Arthr Rheumatism. 2008;58:S431. [Google Scholar]
- 42.Weinblatt ME, et al. Treatment of rheumatoid arthritis with a syk kinase inhibitor: A twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 2008;58:3309–3318. doi: 10.1002/art.23992. [DOI] [PubMed] [Google Scholar]
- 43.Engelman JA, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apsel B, et al. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol. 2008;4:691–699. doi: 10.1038/nchembio.117. [DOI] [PMC free article] [PubMed] [Google Scholar]