1. Summary
Dendritic cells (DCs) differentiated in the presence of IL-10 preferentially induce regulatory T-cells and tolerance. Whether the tolerogenic properties displayed by these DCs (Tol-DCs) can be overcome has not been fully explored. Here we show for the first time that Tol-DCs express higher levels of TLR5 mRNA, but not TLR4 or TLR9 mRNA relative to DCs differentiated with GM-CSF and IL-4 (BM-DCs). In response to flagellin, a natural TLR-5 ligand, Tol-DCs produced IL-12 but not IL-10. Unlike Tol-DCs stimulated with LPS, which produce high levels of IL-10 and fail to generate a cognate inflammatory response in responder CD4+ T-cells, flagellin-stimulated Tol-DCs promoted the differentiation of CD4+ T cells with a T-helper 1 phenotype. The divergent T-cell outcomes induced by Tol-DCs in response to different TLR-ligands highlights not only their plasticity, but also points to TLR5 ligation as a potential strategy to overcome tolerance in environments that are otherwise conducive to immune unresponsiveness.
2. Introduction
DCs represent a heterogeneous population of professional antigen-presenting cells (APCs) with the unique ability to prime naïve T cells [1]. The activation status of the DC at the time of antigen presentation is central in determining the functional outcome of antigen-specific T-cells [2]. DCs displaying high levels of co-stimulatory molecules and producing pro-inflammatory cytokines direct T-cell differentiation towards effector cells. Conversely, non-inflammatory DCs by displaying lower levels of co-stimulatory molecules, low production of inflammatory cytokines and/or increased production of anti-inflammatory cytokines preferentially induce T-cell anergy [3]. DCs with such tolerogenic properties (Tol-DCs) have been indeed generated following DC's exposure to environments rich in IL-10 or TGF-β [4].
Engagement of Toll-like receptors (TLRs) by microbial products is one of the most efficient ways to activate DCs and induce productive T-cell responses [5]. Following TLR stimulation of bona-fide DCs, both pro- and anti-inflammatory cytokines are produced. Among them, IL-12 and IL-10 shape the magnitude and duration of the immune response elicited by TLR ligation [6]. Little is known however, about the function of Tol-DCs following their stimulation with TLR ligands. Here, we show that Tol-DCs produced IL-12 but not IL-10 when stimulated with flagellin. More importantly, antigen presentation by flagellin-treated Tol-DCs led to CD4+ T-cells that produce IFN-γ but not IL-10, indicating a polarization towards a Th-1 phenotype.
3. Materials and Methods
3.1 Mice
Six to ten week old male BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). TCR transgenic mice expressing an αβ T-cell receptor specific for amino acids 110-120 from influenza hemagglutinin presented by I-Ed were a generous gift from H. von Boehmer [7]. All experiments were performed in accordance with protocols approved by the Animal Care and Use Committees of the University of South Florida.
3.2 TLR ligands
Endotoxin-free S. typhimurium flagellin was from InVivoGen (InVivoGen, San Diego, CA) and LPS from Alexis (Alexis Corporation, Lausen, Switzerland). TLR ligands were used as indicated in the text.
3.3 Generation of DCs
Bone marrow (BM) was collected from femurs and tibias of BALB/c mice. Red blood cells were lysed in ACK buffer and T-cells depleted using complement-mediated lysis. Cells were cultured for 7 days in media supplemented with 20 ng/ml GM-CSF plus 20 ng/ml IL-4 (bona fide DCs or BM-DCs) or 20 ng/ml GM-CSF plus 40 ng/ml IL-10 plus 2.5 ng/ml TNF-α (Tol-DCs) as previously described [8]. After 7 days, differentiated DCs were harvested. CD11c expression was determined by staining with an APC-conjugated anti-CD11c MoAb (eBioscience Clone N418). MHC class II (I-A/IEd) was determined by staining with the PE-conjugated MoAb M5/114.15.2. and B7.2 expression with the FITC-conjugated anti-CD86 MoAb, clone GL1 (both from BD Pharmingen, San Diego, CA). Ten thousand gated events were collected on a FACScan (Beckton Dickinson, San Jose, CA) and analyzed using Flow-Jo software (Treestar, Inc).
3.4 Quantification of cytokine production by DCs
One × 105 DCs were plated by triplicate in 96 well plates and treated with TLR ligands for 24 hours unless otherwise specified. Supernatants were harvested and kept at -70°C until ELISA for IL-10 or IL12p40-70 (BD Pharmingen, San Diego, CA) was performed following manufacturer's instructions.
3.5 Real time (RT)-PCR analysis
Total RNA was extracted using TriZol reagent (Qiagen,Valencia,CA) and cDNA obtained with the iScript cDNA synthesis kit (Bio-Rad,Hercules,CA). Target mRNA was quantified using MyIQ single color real time PCR detection system (Bio-Rad) and iQ SYBR green Supermix (Bio-Rad,Hercules,CA). TLR4 primers (left oligo GTGCCAATTTCATGGGTCT, right oligo CATCGAAGTCAATTTTGGTGTT), TLR5 primers (left oligo GCATAGCCTGAGCCTGTTTC, right oligo AAGTTCCGGGGAATCTGTTT), TLR9 primers (left oligo CTGTGGTCCCAGCCTTCTT, right oligo ATCACAATGGGCCCAAACC) and GAPDH primers (left oligo ATGGCCTTCCGTGTTCCTAC, right oligo CAGATGCCTGCTTCACCAC) were used for PCR amplification (cycling parameters 3 min 95°C, 15 seconds 95 °C, 30 seconds 60°C 40 reps, 1 min 95°C). Single product amplification was confirmed by melting curve analysis and primer efficiency was near 100% in all the experiments performed. Quantification is expressed in arbitrary units and target mRNA levels were normalized to GAPDH expression.
3.6 Isolation of naïve CD4+ T cells and in vitro priming and differentiation
Naïve anti-HA CD4+ T cells were purified from splenocytes and lymph node cells from HA+/- TCR transgenic mice using magnetic column separation selecting positively for CD4+ cells. T-cells (5×104) were then cultured for 7 days with BM-DCs or Tol-DCs (1×104) in 96-well plates in the presence of 12.5μg/mL cognate HA-peptide110-120 and additionally, when indicated, of specific TLR ligands. Cells were washed and transferred to a new plate where they were re-stimulated with HA-peptide110-120 using fresh splenocytes as APCs. After 48 hours, supernatants were collected and IFN-γ, IL-10 or IL-4 production was determined by ELISA. After 72 hours, [3H]-thymidine (1 μCi/well, NEN Life Science, MA) was added to the cultures. Eighteen hours later, cells were harvested and thymidine incorporation was measured.
4. Results and Discussion
4.1 Tolerogenic BM-derived APCs lack the capacity to stimulate T cells
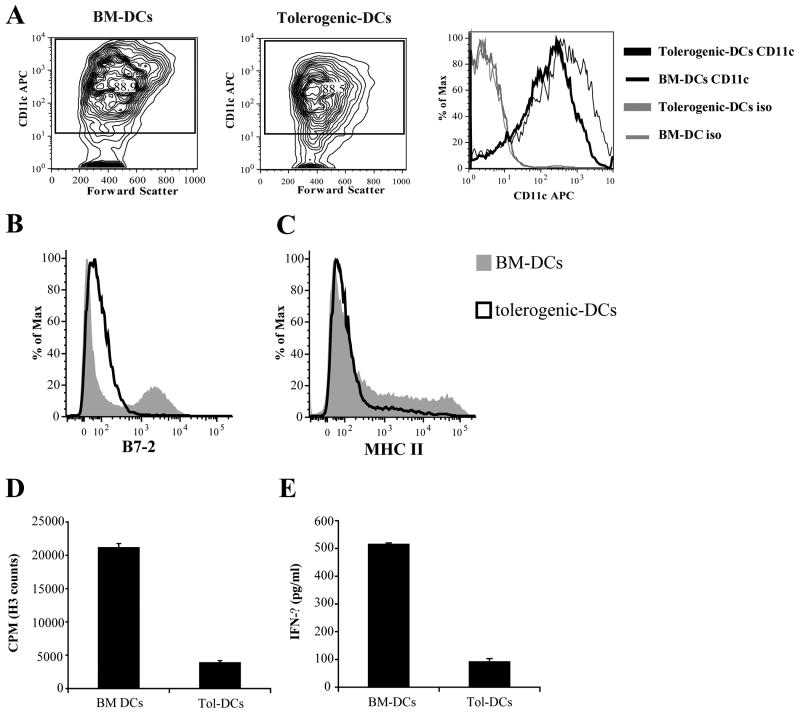
A cursory evaluation of cell surface marker expression allows for a functional means of differentiating Tol-DCs versus non-tolerized BM-DCs. Reminiscent of prior studies [8], in vitro differentiation of BM cells in the presence of GM-CSF plus IL-10 (Tol-DCs) or GM-CSF plus IL-4 (BM-DCs) resulted in similar yield of CD11c-positive cells (Figure 1A, left and middle panel: BM-DCs: 88.9% and Tol-DCs: 88.5%). However, surface expression levels of CD11c (Figure 1A- right panel) were distinctly lower in the Tol-DC cell population as compared to BM-DCs. Furthermore, B7.2 and MHC class II surface expression (Fig. 1B-C) were comparatively diminished in the Tol-DCs, suggestive of a cell with inhibited T cell-stimulating capacity. And consistent with this altered cell surface antigen pattern, Tol-DCs are much less efficient than BM-DCs at priming cognate T cell responses. CD4+ T-cells incubated with BM-DCs display a strong antigen-specific proliferation (Figure 1D, BM-DCs) and have acquired a full effector function given their significant production of IFN-γ in response to cognate HA (Figure 1E, BM-DCs). In sharp contrast, CD4+ T cells encountering cognate peptide in Tol-DCs are inadequately induced given their minimal antigen-specific proliferation (Fig. 1D, Tol-DCs) and IFN-γ production (Fig. 1E, Tol-DCs).
Figure 1. Dendritic cells differentiated in the presence of IL-10 (Tol-DCs) display an impaired ability to present cognate antigen to antigen-specific CD4+ T-cells.
BM-DCs and Tol-DCs were generated from the bone marrow of BALB/c mice. The expression levels of CD11c (A), B7.2 (B) and MHC class II molecules (C) were determined by flow cytometric analysis. Numbers in A (right and middle panel) represent the percent of CD11c positive DCs. % of Max is the intensity of each curve relative to the 100% value of the histogram with the highest number of analyzed cells as assigned by the Flow-Jo software. Shown is a representative experiment of three independent experiments with similar results. In parallel experiments, APCs were evaluated for their ability to stimulate cognate T cell responses, as described in Materials & Methods. Functional capacity of naïve anti-HA CD4+ T cells (CD4+ CD62L+) from TCR transgenic HA +/- mice were determined based on HA-specific proliferation (D) and IFN-γ production (E). Data represent mean ± s.d. of triplicate cultures. Shown is a representative experiment of two independent experiments with similar results.
4.2 TLR5 ligation in tolerized BM-DCs promotes reengagement of T cell stimulatory capacity
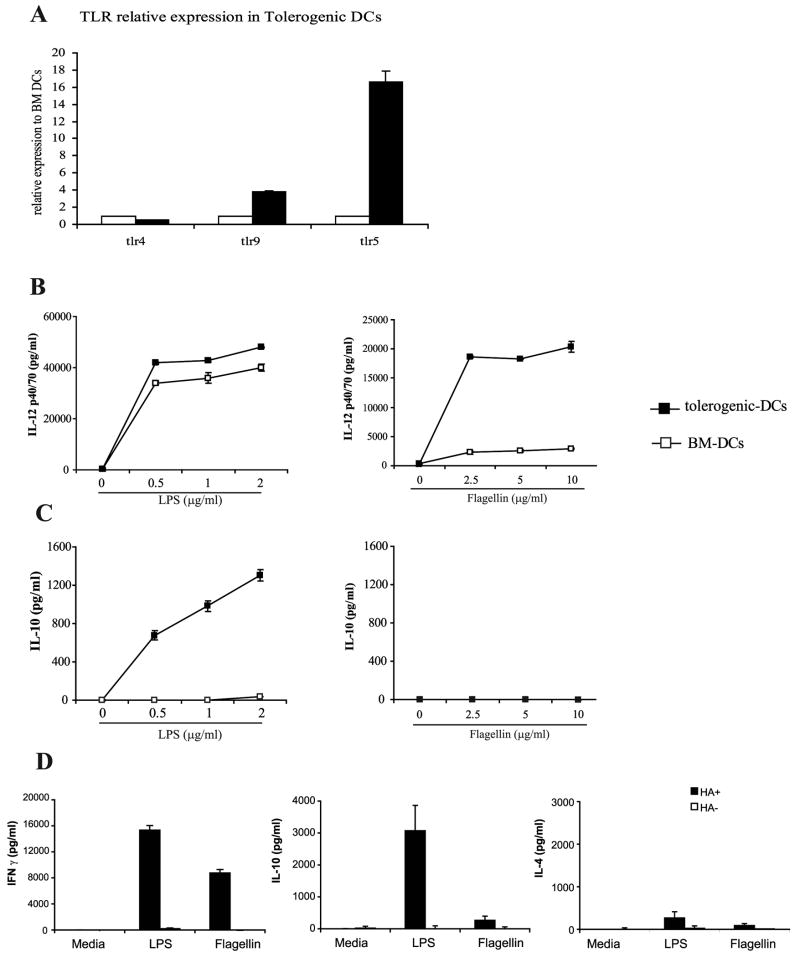
Prior studies performed in our laboratory have demonstrated the ability of flagellin via TLR5 interactions to revert tolerogenic macrophages to immunogenic APCs [9], but we questioned whether we would see similar results in DCs, especially in light of the fact that others have shown a lack of responsiveness in BM-DCs to flagellin [10]. To determine whether the tolerogenic properties of Tol-DCs can be overcome by TLR-ligation, we assessed first the expression of different TLRs in BM-DCs and Tol-DCs. As shown in Fig. 2A, TLR5 expression was greatly enhanced in Tol-DCs relative to its expression in BM-DCs. No significant differences in the expression levels of TLR4 or TLR9 was observed, suggesting that differentiation of DCs in the presence IL-10 might specifically enhance TLR5 expression. Next, we evaluated the production of cytokines by Tol-DCs and BM-DCs in response to specific TLR-ligands. As shown in Figure 2B-left, both DC populations produce similar levels of IL-12 in response to stimulation with LPS. However, in response to flagellin, Tol-DCs produce higher levels of IL-12 than BM-DCs (Figure 2B-right) whereas, similar to prior reports, BM-DCs produce minimal IL-12 [10]. Assessment of their production of IL-10 revealed more striking differences. Consistent with our prior data in macrophages [9], stimulation of Tol-DCs with LPS resulted in significant production of IL-10 (Figure 2C-left), these same Tol-DCs do not produce this inhibitory cytokine when stimulated with flagellin (Figure 2C-right). It is plausible therefore that the enhanced expression of TLR5 in Tol-DCs might account for their response to flagellin resulting in IL-12 but not IL-10 production by these cells. However, the findings that both BM-DCs and Tol-DCs produced less IL-12 and IL-10 when stimulated with flagellin as compared to LPS also raise the possibility that the observed difference could be a reflection of flagellin being a weaker TLR agonist for DCs.
Figure 2. Flagellin-treated but not LPS-treated Tol-DCs lack IL-10 production and preferentially induce IFN-γ producing CD4+ T-cells.
(A) TLR4, TLR5 and TLR9 mRNA were quantified by real time RT-PCR using GAPDH as an internal control. Displayed are the levels of TLR expression in tolerogenic-DCs (black bars) relative to the levels expressed in BM-DCs (open bars). In a parallel experiment, BM-DCs (white squares) or tolerogenic-DCs (black squares) were cultured in the presence of increasing concentrations of LPS (left) or flagellin (right). After 24 hours supernatants were collected and the levels of IL-12 p40-70 (B) or IL-10 (C) were determined by ELISA. Shown is a representative experiment of three independent experiments with similar results. (D) Tol-DCs were treated with either LPS (1μg/ml) or flagellin (5μg/ml) and cultured with naïve anti-HA transgenic CD4+ T-cells to assess the ability of treated Tol-DCs and BM-DCs to elicit a cognate T cell response. Levels of IFN-γ (left), IL-10 (middle) or IL-4 (right) were determined by ELISA. Data represent mean ± S.D. of triplicate cultures. Shown is a representative experiment of three independent experiments with similar results.
The divergent cytokine production among LPS and flagellin-treated Tol-DCs prompted us to investigate whether these differences could influence antigen-specific CD4+ T cell responses. Antigen presentation by LPS-stimulated Tol-DCs resulted in T-cells that produce IFN-γ, high levels of IL-10 and low levels of IL-4. (Figure 2D-LPS). CD4+ T-cells with a similar phenotype were originally described by Roncarolo's group and termed IL-10 secreting type-1 regulatory T cells or Tr1 cells [11]. In sharp contrast, antigen-specific CD4+ T-cells that encountered cognate HA-antigen in flagellin-stimulated Tol-DCs produced IFN-γ but very low levels of IL-10 and IL-4 (Figure 2D-Flagellin). This decreased production of IL-10 suggest that Tr-1 differentiation is prevented in T-cells encountering antigen in flagellin-stimulated Tol-DCs. Instead, their preferential production of IFN-γ indicate that these T-cells display a Th-1 character.
Previous studies have shown that differentiation of naïve CD4+ T-cells into Tr-1 regulatory CD4+ T-cells is dependent upon the presence of IL-10 acting mainly on the APC and to a lesser extent over the responding T-cell [11-13]. Our studies showed that a difference among Tol-DCs stimulated with either LPS or flagellin lies precisely in their production of IL-10. In the absence of this cytokine, a scenario that typifies how naïve CD4+ T-cells encountered cognate antigen in flagellin-stimulated Tol-DCs in vitro, differentiation of naïve antigen-specific CD4+ T-cells is directed towards a predominant Th-1 response (Figure 2D). A similar Th-1 like orientation of the immune response has been recently described in mice immunized with flagellin acting as a carrier for a p27 mycobacterium tuberculosis antigen [14].
Several studies have demonstrated that the immunosuppressive cytokine IL-10 is detected at steady-state conditions in the gut and lungs where it plays a fundamental role in the prevention of immune responses against commensal microorganisms [15-17]. Interestingly, DCs from the gut stimulated with microbial products or with CD40L produced higher levels of IL-10 than splenic's DCs [18]. Furthermore, Uematsu et al. recently demonstrated that murine DCs present in the intestinal lamina propria display high levels of TLR5 and, when stimulated with flagellin produced IL-12 but not IL-10 [19]. It is important to note that the tolerogenic DCs we have generated in vitro display striking similarities with the phenotypic and functional characteristics of the DCs described in those studies, suggesting that DC's differentiation in immunosuppressive environments rich in IL-10 is conducive to cells programmed to induce tolerance rather than immune activation. Supporting this notion, in tumor bearing hosts, tumor antigen encounter by DCs usually occurs in a microenvironment rich in immunosuppressive factors, such as IL-10 and TGF-β leading to the generation of DCs with tolerogenic properties [20, 21]. Our findings that TLR5 ligation can convert DCs from tolerogenic into activating APCs provide therefore the framework for further evaluating flagellin as an adjuvant to break the barrier that tolerance has imposed to cancer immunotherapy.
Finally, our data provide a word of caution to the current use of other TLR ligands as immune adjuvants in cancer. Their beneficial pro-inflammatory properties might be diminished if they also induce IL-10 production in the target APC, an effect that could lead to suppression of immune responses through the expansion of T-cells with a regulatory phenotype.
Acknowledgments
We thank Drs. J. Pinilla and I. Borrello for helpful discussions and review of the manuscript. This work was supported by PHS Grants CA100850 (E.M.S.) and CA87583 (E.M.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guermonprez P, et al. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 3.Sato K, et al. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18(3):367–79. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 4.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108(5):1435–40. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 6.Napolitani G, et al. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirberg J, et al. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180(1):25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakkach A, et al. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18(5):605–17. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 9.Vicente-Suarez I, Takahashi Y, Cheng F, Horna P, Wang HW, Wang HG, Sotomayor EM. Identification of a novel negative role of flagellin in regulating IL-10 production. Eur J Immunol. 2007;37:3164–3175. doi: 10.1002/eji.200737306. [DOI] [PubMed] [Google Scholar]
- 10.Didierlaurent A, Ferrero I, Otten LA, Dubois B, Reinhardt M, Carlsen H, Blomhoff R, Akira S, Krachenbuhl JP, Siraud JC. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type responses. J Immunol. 2004;172:6922–30. doi: 10.4049/jimmunol.172.11.6922. [DOI] [PubMed] [Google Scholar]
- 11.Roncarolo MG, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 12.Moore KW, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 13.Levings MK, et al. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105(3):1162–9. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 14.Le Moigne V, Robreau G, Mahana W. Flagellin as a good carrier and potent adjuvant for Th1 response: Study of mice immune response to the p27 (Rv2108) Mycobacterium tuberculosis antigen. Mol Immunol. 2008;45(9):2499–2507. doi: 10.1016/j.molimm.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn R, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez S, et al. Inhibition of IL-10 receptor function in alveolar macrophages by Toll-like receptor agonists. J Immunol. 2004;172(4):2613–20. doi: 10.4049/jimmunol.172.4.2613. [DOI] [PubMed] [Google Scholar]
- 17.Novak N, Bieber T. 2. Dendritic cells as regulators of immunity and tolerance. J Allergy Clin Immunol. 2008;121(2 Suppl):S370–4. doi: 10.1016/j.jaci.2007.06.001. quiz S413. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190(2):229–39. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uematsu S, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7(8):868–74. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 20.Jarnicki AG, et al. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177(2):896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 21.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive Strategies that are Mediated by Tumor Cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]