Abstract
Oxidative stress has been implicated in the pathogenesis of several inflammatory lung disorders including chronic obstructive pulmonary disease (COPD) due to its effect on pro-inflammatory gene transcription. Cigarette smoke-mediated oxidative stress activates NF-κB-dependent transcription of pro-inflammatory mediators either through activation of inhibitor κB-α kinase (IKK) and/or the enhanced recruitment and activation of transcriptional co-activators. Enhanced NF-κB-co-activator complex formation results in targeted increase in chromatin modifications, such as histone acetylation leading to inflammatory gene transcription. NF-κB-dependent gene expression, at least in part, is regulated by changes in deacetylases such as histone deacetylases (HDACs) and sirtuins. Cigarette smoke and oxidants also alter the levels/activity of HDAC by post-translational modifications and in doing so further induces gene expression of pro-inflammatory mediators. In addition, cigarette smoke/oxidants can reduce glucocorticoid sensitivity by attenuating HDAC2 activity and expression, which may account for the glucocorticoid insensitivity in patients with COPD. Understanding the mechanisms of NF-κB regulation, and the balance between histone acetylation and deacetylation may lead to the development of novel therapies based on the pharmacological manipulation of IKK and deacetylases in lung inflammation and injury.
Keywords: COPD, reactive oxygen species, histone acetylation, HDAC, sirtuins
INTRODUCTION
Biological systems are continuously exposed to oxidants either generated endogenously by metabolic reactions (mitochondrial electron transport during respiration, during activating phagocytes) or exogenously (air pollutants or cigarette smoke). The great external surface area (1–2 m2) of the human airway epithelium (300 million alveoli), alveolar ducts (14 million), capillary segments (280 billion) plus its direct contact with the high-oxygen environment for gas exchange between air and blood (alveolar and alveolar-capillary surface area=40 to 80 square meters) makes the lung a major target for oxidative injury from reactive oxygen species (ROS) and free radicals (5,71,73). Inflammation is an important protective response to cellular/tissue injury. The purpose of this process is to destroy and remove the injurious agent and injured tissues, thereby promoting tissue repair. When this crucial and normally beneficial response occurs in an uncontrolled manner, the result is excessive cellular/tissue damage that results in chronic inflammation and destruction of normal tissue. ROS, such as superoxide anion (O2•-) liberated by phagocytes recruited to sites of inflammation, are proposed to be a major cause of the cell and tissue damage associated with many chronic inflammatory diseases such as asthma and chronic obstructive pulmonary disease (COPD) (58, 72). Increased levels of ROS have been implicated in initiating the lung inflammatory responses through the activation of redox sensitive transcription factor, nuclear factor-kappa B (NF-κB), and chromatin remodeling (histone acetylation/deacetylation), which regulate the gene expression of pro-inflammatory mediators (70) (Figure 1). In this review, we present an overview of redox regulation of NF-κB and describe the current evidence on involvement of histone deacetylase in oxidant-mediated lung inflammation.
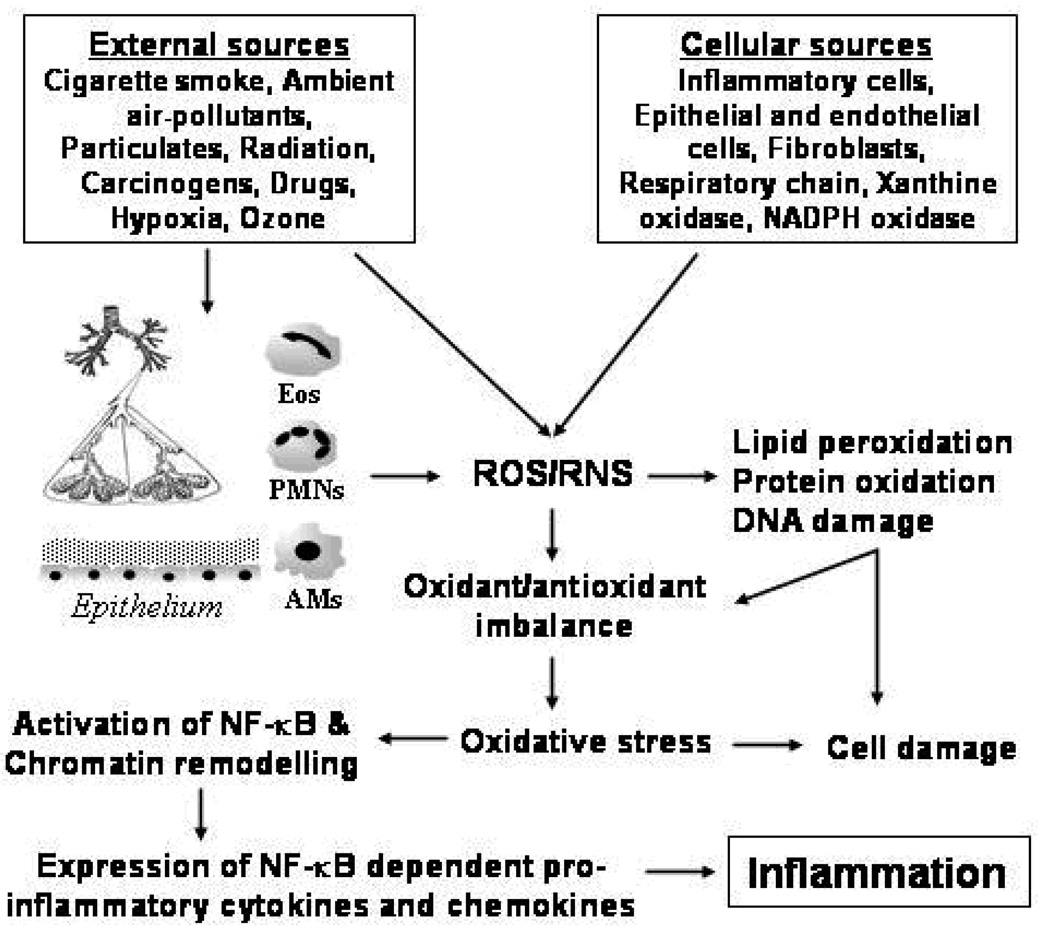
Figure 1. ROS-mediated inflammation in the lung.
Oxidative stress condition is produced in the lung by inhaled oxidants (external sources) and/or release of ROS by the cellular sources. Inflammatory cells such as activated neutrophils (PMNs), alveolar macrophages (AMs), eosinophils (Eos) are the most important cells involved in endogenous generation of ROS. Intra and extra cellular generation of ROS lead to oxidant/antioxidant imbalance and oxidation of biomolecules. ROS-mediated activation of NF-κB and chromatin remodeling leads to up-regulated transcription of pro-inflammatory chemokine and cytokine genes, and increased release of pro-inflammatory mediators which are involved in the inflammatory responses in patients with COPD. ROS: Reactive Oxygen Species; RNS: Reactive Nitrogen Species.
OXIDATIVE STRESS
Aerobic metabolism is the major source of ROS and reactive nitrogen (RNS) species, which are essentially the byproducts of normal oxygen metabolism. Biological systems are capable of forming highly reactive moieties, both free radicals and non-radicals. These biologically active species participate in normal cell functions by serve in host defense and also in cell signaling as messenger molecules of the autocrine or paracrine system (77), but their excess production may result in tissue injury and inflammation (38). To combat these unrelenting insults, the lungs have well coordinated and efficient endogenous antioxidant defense systems, which protect against the injurious effects of oxidants by electron transfer, enzymatic removal, scavenging and by keeping transition metal ions tightly sequestered. There are three major classifications in the endogenous antioxidant defenses. i) Primary defense: It can prevent radical formation. Transferrin and lactoferrin have this role in extracellular fluids. ii) Secondary defense: It removes or inactivates formed ROS. This may be enzymes such as superoxide dismutase, and glutathione peroxidase as well as non-enzymatic molecules such as reduced glutathione, α-tocopherol, ascorbic acid, β-carotene, albumin, mucin, glucoase, bilirubin and uric acid. iii) Tertiary defense: This system operates to remove and or repair oxidatively damaged molecules, and is particularly important for proteins and deoxyribonucleic acids (5). The lung has precise balance between the production/exposure of oxidants and endogenous antioxidants. Oxidative stress resulting from an imbalanced ratio between ROS production and detoxification plays an important role in cellular processes such as signal transduction and gene expression (25). During acute and chronic inflammations, ROS is produced at rates that overwhelm the capacity of the endogenous antioxidant defense system to remove it, resulting in oxidative stress (25). Increased ROS production and reduced endogenous antioxidant defense has been reported in several lung diseases including chronic obstructive pulmonary disease (52, 68, 73). This may results in oxidative stress condition, which appears involved in various chronic inflammatory lung disease pathogenesis.
ENDOGENOUS ROS PRODUCTION IN THE LUNG
Reactive oxygen and nitrogen species are generated by several inflammatory and structural cells of the airways. A common feature of all inflammatory lung diseases is involvement of an inflammatory-immune response, characterized by activation of epithelial cells, and resident macrophages, and the recruitment and activation of neutrophils, eosinophils, monocytes, and lymphocytes. Inflammatory cells once recruited in the airspace become activated and generate ROS in response to a sufficient level of a secretagogue stimulus (threshold condition). The activation of macrophages, neutrophils and eosinophils generates O2•-, which is rapidly converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD), and hydroxyl radical (•OH) formed non-enzymatically in the presence of Fe2+ as a secondary reaction. ROS can also be generated intracellularly from several sources such as mitochondrial respiration, the NADPH oxidase system and xanthine/xanthine oxidase (65). At sites of inflammation, increased free radical activity is associated with the activation of the neutrophil NADPH oxidase and/or the uncoupling of a variety of redox systems, including endothelial cell xanthine dehydrogenase (23, 53).
ENVIRONMENTAL OXIDANTS
The air is polluted with cigarette smoke, automobile exhaust, diesel soot, ozone, sulfur dioxide, nitrogen dioxide and varying degree of other pollutants produced by anthropogenic activities. Due to the unique structure and function of the lung, it is vulnerable to numerous pathogens, pollutants, oxidants, gases and toxicants that are inhaled continuously with ambient air. Inhalation of such toxic pollutants and microorganisms results in lung injury and generation of reactive oxygen/nitrogen species leading to cascades of signaling events triggering inflammatory influx, production of proinflammatory cytokines, chemokines, and other factors (21). Cigarette smoke is the major etiological factor in the pathogenesis of COPD (66). An increased oxidant burden in smokers derives from the fact that cigarette smoke contains an estimated 1017 oxidants/free radicals and 4,700 chemical compounds, including aldehydes and quinines per puff, and many of those are relatively long-lived such as tar-semiquinone that can generate •OH and H2O2 by Fenton reaction (14).
OXIDATIVE STRESS AND INFLAMMATION
Oxidative stress has been implicated in cell and tissue damage associated with many chronic inflammatory lung diseases such as asthma, COPD, idiopathic pulmonary fibrosis (IPF) and adult respiratory distress syndrome (ARDS) (1). Increased ROS production has been directly linked to oxidation of protein, DNA, and lipids which may cause direct lung injury or induce a variety of cellular responses through the generation of secondary metabolic reactive species. The pathogenesis of many forms of lung injury has implicated peroxidative breakdown of polyunsaturated fatty acids due to the effects on membrane function, inactivation of membrane-bound receptors and enzymes, and increased tissue permeability (67). There is increasing evidence that aldehydes, generated endogenously during the process of lipid peroxidation, are involved in many of the pathophysiological events associated with oxidative stress in cells and tissues (36). In addition to their cytotoxic properties, lipid peroxides are increasingly recognized as being important in signal transduction for a number of important events in the inflammatory response in the lungs (67).
ROS may alter the remodeling of extracellular matrix, apoptosis and mitochondrial respiration, cell proliferation, maintenance of surfactant and the antiprotease screen, effective alveolar repair responses and immune modulation in the lung (65, 66). ROS produced by phagocytes that have been recruited to sites of inflammation, are a major cause of the cell and tissue damage associated with many chronic inflammatory lung diseases such as asthma, COPD, IPF and ARDS. All these diseases involve the recruitment of immune and inflammatory cells to the lungs. These cells are activated and produce mediators of inflammation including ROS and cytokines, such as the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) (65).
OXIDATIVE STRESS AND CELL SIGNALING
There has been a paradigm shift in the belief that ROS may not always be detrimental and the concept that ROS can be produced deliberately and act on specific targets to modulate signaling pathways is gaining credence. In fact, the role of ROS as second messengers, in particular that of H2O2, is gaining acceptance, as the four characteristics of classical second messengers, i.e., regulated enzymatic production, degradation by specific enzymes, presence at low concentrations that can be transiently elevated to measurable amounts upon stimulation, and (at least for H2O2) ability to react at specific sites, such as metals and thiolates, are now getting support from studies with protein tyrosine phosphatases and other thiol-containing proteins.
The response of a cell or organism to an increase in ROS often involves the activation of numerous intracellular signaling pathways. These cytosolic pathways can, in turn, regulate a host of transcriptional changes that allow the cell to respond appropriately to the perceived oxidative stress. In addition to the regulation achieved by classical cytosolic signaling pathways, such as the family of mitogen-activated protein kinases, evidence suggests that certain transcription factors can directly or indirectly alter their activity, depending on cellular redox conditions (49). ROS such as hydrogen peroxide (H2O2) and oxygen free radicals are implicated in the regulation and pathogenesis of inflammation. There is growing body of evidence that ROS can be purposely made within cells to serve as signaling molecules (49, 67). Importantly, oxidants (ROS and RNS) produced by neutrophils participate as signaling molecules that regulate diverse physiological signaling pathways in neutrophils. They also serve as modulators of protein and lipid kinases, phosphatases, membrane receptors, ion channels, and transcription factors, including NF-κB. The latter regulates expression of key cytokines and chemokines that further modulate the inflammatory response (1).
Redox sensitive molecular targets usually contain highly conserved cysteine residues, and their oxidation, nitration, and formation of disulfide links are crucial events in oxidant/redox signaling. It is hypothesized that oxidation of those sulfide groups in signaling proteins causes structural modifications, resulting in the exposure of active sites and protein activation. Such molecular targets include transcription factors (NF-κB and AP-1), signaling molecules such as ras/rac or c-Jun N-terminal kinase (JNK), protein tyrosine phosphatases and p21ras. Thiol molecules such as intracellular reduced glutathione (GSH) and thioredoxin are of central therapeutic importance in the regulated control of such redox signaling pathways, by reducing disulfide bridges or oxidized cysteine residues (68). Recent studies have shown that in response to TNF-α and lipopolysaccharide (LPS), which are relevant stimuli for the inflammatory response in chronic inflammatory lung diseases, airway epithelial cells can concurrently produce increased amounts of intracellular ROS. This intracellular production of oxidants and the subsequent changes in intracellular redox status is important in the molecular events controlling the expression of genes for inflammatory mediators. The signaling pathways and activation of transcription factors in response to ROS is subject of rigorous investigation.
REDOX SIGNALING AND OXIDATIVE STRESS
At this juncture it would be prudent to differentiate redox signaling from oxidative stress. Redox signaling involves at least one reaction of reversible oxidation of a signaling molecule that is intended to modulate a signaling pathway, e.g. alteration of sulfhydryl group on protein phosphatases as discussed above. On the other hand oxidative stress is often characterized as an imbalance in the oxidant/antioxidant ratio, tilted toward a higher proportion of oxidants. The ultimate outcome of oxidative stress is cell type specific, which can not only elicit responses ranging from severe oxidative damage, loss of cell function and viability, to apoptosis and ultimately necrosis, but may also induce responses such as cell differentiation to cell cycle progression (2, 60). Since both redox signaling and oxidative stress may occur during different phases of inflammation, it is vital to understand the exact phenomenon that underlies a pathologic state before designing a therapeutic strategy to counter the aberrations. Also since cigarette smoke can elicit both redox and oxidative alterations (mainly carbonylation) in a given type of a cell, it would be very vital to systematically delineate as to which mechanistic and signaling pathway cigarette smoke alters. This knowledge would lead to better understanding of the pathology due to cigarette smoke and would also lead to development of a specific therapeutic agent.
REDOX REGULATION OF NF-κB
NF-κB is a family of seven structurally related transcription factors (p50, p105, p52, p100, RelA/p65, C-Rel and RelB) that play a central role in inflammation and stress response by controlling gene network expression (30). The members of the NF-κB/Rel family of transcription factors are known to activate expression of many pro-inflammatory genes involved in lung inflammation (71). The expression of inflammatory mediators can be regulated by the activation of redox-sensitive transcription factor, NF-κB, stimulated in response to ROS (66) (Figure 2). In addition to ROS, cellular redox status, particularly thiol status can be directly involved in activation of NF-κB, signal transduction and gene expression involved in cellular pathophysiologic activities (66). Based on earlier studies with antioxidants it was proposed that activation of NF-κB is redox-sensitive and is regulated by the changes in the oxidant/antioxidant balance, further suggesting that agonists activating NF-κB, in particular TNF-α, could stimulate the production of ROS (8, 62).
Figure 2. Mechanism of ROS-mediated activation of NF-κB and pro-inflammatory gene transcription.
NF-κB exists as a heterodimeric complex of p50 and p65/RelA subunits. In the absence of stimulation, NF-κB is found in the cytoplasm as an inactive, non-DNA binding form in association with inhibitory κB (IκB). ROS are involved in the activation of NF-κB by redox modulation of critical cysteine residue present on IκB kinase (IKK) subunit. Activation of NF-κB involves the phosphorylation, ubiquitination, and subsequent proteolytic degradation of the inhibitory protein IκB. Free/active NF-κB then translocate into the nucleus and binds with its consenses sites which leads to the expression of pro-inflammatory genes. p50 and p65: Subunits of NF-κB; ROS: Reactive Oxygen Species; P: Phosphate, Ub: Ubiquitin.
Many proteins in the NF-κB pathway have reactive cysteines that impact on NF-κB activation. Oxidation, nitration, and formation of disulfide links in cysteine residues of NF-κB are crucial events in oxidant/redox signaling. Oxidation of these sulfhydryl groups may cause structural modifications, resulting in the exposure of active site of the signaling protein and protein activation (69). In addition to cysteine, other amino acids particularly those containing aromatic rings, the thioester of methionine, and the protein backbone itself can also be oxidized, which may impact signaling modules, such as NF-κB activation (17). While NF-κB subunits themselves can be a target of oxidative regulation, upstream kinases of the NF-κB pathway are also subject to redox regulation, and may in fact determine whether NF-κB will be activated or inhibited in the presence of ROS. Many kinases (IKK, AKT/PI3K, MEKK-1 and various nuclear kinases) involved in direct or indirect activation of NF-κB are affected by oxidants and therefore, have the potential to alter NF-κB activity (62). Antioxidants, for instance, have been reported to block NF-κB activation in certain cell types, leading to the hypothesis the activation of this transcription factor is mediated by ROS in response to specific stimuli. A wide array of antioxidants that can detoxify ROS/RNS have been reported to suppress the activation of NF-κB signaling (37).
ROLE OF NF-κB in HISTONE MODIFICATIONS
The complex structure of chromatin consists of DNA wrapped around an octamer of core histones, which is composed of two molecules each of the histones H2A, H2B, H3, and H4. Nucleosomal DNA is dynamically packaged to varying degrees, resulting in different levels of chromatin compaction ranging from the 10-nm fiber to higher order structures such as the condensed mitotic chromosomes. Condensation of eukaryotic DNA in chromatin suppresses gene activity through the coiling of DNA on the surface of the nucleosome core and folding of nucleosome assemblies, thus decreasing the accessibility of transcriptional factors, such as NF-κB (71, 87). Alterations in the structure of chromatin are critical to the regulation of gene expression (83). Gene expression is regulated by modifications such as acetylation of core histones through the concerted action of coactivators such as CBP (cAMP-response element binding protein-binding protein)/adenoviral protein E1A (p300), CBP/p300 associated factor (P/CAF) and activating transcription factor-2 (ATF-2), which have intrinsic histone acetyltransferase (HAT) activity and are able to recruit other HAT enzymes. Acetylation of the ε-group on lysine (K) residues results in neutralization of positive charge on histone tails that weakens the electrostatic interaction between the histone and the DNA backbone and facilitates access to transcription factors (34). Increase in histone acetylation is therefore associated with increased gene expression. Recent studies postulates that histone tails themselves might participate directly in the binding of transcription factors, e.g., by virtue of modified amino acids recognized by these factors (39). In addition, phosphorylation of histone is known as early step in chromatin remodeling and has been shown to be important during chromosomal replication. Recently, this modification also has been found to be associated with rapidly inducible pro-inflammatory gene promoters (32).
CBP/p300, transcriptional coactivators with intrinsic HAT activity, is regulated by p38 MAP kinase pathway which is activated by oxidative stress condition (67). Kinase-mediated phosphorylation of CBP/p300 has local effects on the conformation and activity. The additional charge from the phosphate at Ser133 has been shown to increase the recruitment of CBP/p300 by forming hydrogen bonds with the Tyr658 and Lys662 residues in KIX domain (64, 96). Activation of CBP leads to acetylation of specific core histone lysine residues by an intrinsic HAT activity. Acetylation of the histone tails presumably destabilizes the nucleosome, thereby facilitating access by transcription factors and regulatory factors. Transcription coactivator, p300 and CBP are reported to involve in the regulation of various DNA-binding transcriptional factors (61). Like other transcription factors, NF-κB (RelA/p65) relies on the lysine acetylation of histones by HAT to initiate DNA uncoiling and allow the accessibility for binding (12). Thus histone acetylation via CBP/p300 has a significant role in the activation of NF-κB mediated pro-inflammatory gene expression.
Repression of genes is associated with histone deacetylation, a process controlled by histone deacetylases (HDACs). HDACs, conversely, act by catalyzing the removal of acetyl groups on amino-terminal lysine (K) residues of histones, producing DNA rewinding and a condensed chromatin structure, which results in transcriptional repression and gene silencing. Thus, HDACs play an important role in maintaining the balance of histone acetylation/deacetylation and/or transcriptional activity of pro-inflammatory genes in cells (67). Apart from the inhibition of gene transcription, HDACs also directly affect the nuclear activity of transcription factors such as NF-κB (12). HDACs not only deacetylate histones but also have the ability to deacetylate non-histone proteins such as NF-κB and thereby have the ability to regulate NF-κB dependent pro-inflammatory gene transcription (76). HDACs are known to regulate critical cellular processes such as cell cycle progression and differentiation while HDAC inhibitors are being studied as potential anti-cancer therapies since they induce apoptosis and cell cycle arrest in certain transformed cell lines (56).
There are 18 potential deacetylase enzymes, HDAC1 to HDAC11 and Silent Information Regulator (SIRT) SIRT1 to SIRT7, have been identified in humans (16,47). They are classified into four classes: Class I HDACs include HDACs 1, 2, 3, 8 and are almost exclusively localized to the nucleus depending on the presence or absence of a nuclear localization signal (NLS) and a nuclear export signal (NES) (18). Members of this class are related to the yeast RPD3 protein. Class II HDACs include HDACs 4–7, 9 and 10. These share similarities to the yeast HDAC1 and HDAC2 are thought to be able to shuttle between the nucleus and cytoplasm. However it is thought that class II HDACs unlike members of the class I family are expressed in a cell specific pattern suggesting they may play a role in specific cell functions. Class III HDACs include SIRT 1–7 NAD+ family. It is suggested they may play a role in aging, inflammation and cell senescence and possess non-histone protein substrates. Class IV HDACs include HDAC 11 (18).
ROLE OF HDAC2 IN THE REGULATION OF NF-κB DEPENDENT GENES
Several reports have shown that HDACs 1–3 can also be associated with inactive RelA/p65 and play a role in the regulation of NF-κB-mediated gene transcription (3, 12, 95) (Table 1). Thus, changes in HDAC activity associated with RelA/p65 can enhance or repress NF-κB-mediated gene expression (95). Among several types of HDACs, HDAC2 is well characterized and reported to play a role in the regulation of inflammation and has been implicated in the dysregulation in smokers and patients with COPD (4, 44, 78). HDAC2 expression and activity is decreased in smokers, COPD subjects, and mild asthma patients and that there is a good correlation between cytokine production and HDAC activity in alveolar macrophages from smokers and non-smokers (1, 4, 44). Decreased levels/activities of HDAC2, i.e. disruption of the acetylation:deacetylation balance, may lead to sustained transcription of pro-inflammatory gene controlled by NF-κB, resulting in a chronic inflammatory response. Cigarette smoke extract-mediated reduction in HDAC2 was associated with increased RelA/p65, and indicated RelA/p65 interacts with HDAC2 and RelA/p65 becomes available or retained in the nucleus for pro-inflammatory gene transcription when HDAC2 is decreased (88). In addition, we also demonstrated HDAC3, but not HDAC1, interacted with RelA/p65, suggesting an important role of specific HDACs such as HDAC2 and HDAC3 in regulation of NF-κB signaling pathway particularly in response to cigarette smoke (88). The binding of NF-κB to HDAC2 presumably reflects the recruitment of HDAC2 to specific NF-κB target promoters. The domains of HDAC2 and NF-κB that interact remain to be identified. It is unlikely that this interaction involves the DNA binding domain of NF-κB, because trichostatin A (TSA) treatment did not significantly interfere with DNA binding (93).
Table 1.
Intracellular localization and functions of some of the deacetylases discussed in this review
| Deacetylase | Intracellular Localization | Target | Function | Ref. |
|---|---|---|---|---|
| HDAC1 | Nucleus | Histone H1, and p50 and RelA/p65 subunits of NF-κB | •Involved in cell proliferation | (75) |
| •Interacts with p50 and RelA/p65 | (12, 95) | |||
| HDAC2 | Cytoplasm and nucleus | Histones, glucocorticoid receptor, RelA/p65 and CBP/p300 | •Recruited by glucocorticoid on pro-inflammatory genes | (4, 43) |
| •Post-translationally modified by oxidants/nitric oxide/carbonyls | (41, 88) | |||
| •Interacts with RelA/p65 to inhibit NF-κB transactivation | (88) | |||
| •Activated by theophylline | (15, 45) | |||
| •Phosphorylated and degraded | (28, 48, 82) | |||
| HDAC3 | Nucleus | Histone H4 and RelA/p65 | •Interacts with RelA/p65 and inhibits its transactivation | (12) |
| SIRT1 | Cytoplasm and nucleus | RelA/p65, p53 and FOXO3 | •Interacts with RelA/p65 | (89, 91) |
| •Involved in regulation of pro-inflammatory mediators (cytokines) and MMPs | (57, 89) | |||
| •Calorie restriction | (6, 35) | |||
| •Regulation of p53, FOXO3, DNA repair, cell proliferation, cell cycle, senescence and aging | (9, 20, 47, 51, 59, 81, 84) |
Activity of HDAC1, HDAC2 and HDAC3 is inhibited by trichostain A, sodium butyrate, valproic acid and suberoylanilide hydroxamic acid, whereas SIRT1 activity is inhibited by sirtinol and nicotinamide adenine dinucleotide (NAD+), and activated by resveratrol.
HDAC2 has also been implicated in the anti-inflammatory effects of corticosteroids as ligand bound steroid receptors recruit HDAC2 to promoters of pro-inflammatory genes leading to deacetylation and transcriptional repression. However, a small proportion of severe asthmatics who smoke and severe COPD patients exhibit unresponsiveness towards high doses of oral corticosteroids. The current hypothesis indicates that cigarette smoke-induced oxidative stress alters the basal HDAC2 response via post-translational modifications and an induced net loss of HDAC2. The absence of HDAC2 or the presence of a defective HDAC2 protein may thus explain the abnormal inflammatory response and corticosteroid inefficiency in patients with COPD. Decreased HDAC2 protein expression and deacetylase activity observed in the macrophage of COPD patients (15, 42) is implicated in an impaired ability of dexamethasone to inhibit basal cytokine release in alveolar macrophages from COPD patients compared to casual smokers (16) (Figure 3). However while many studies have focused on establishing a link between decreased HDAC2 expression and increased susceptibility to persistent inflammation, little is known on how HDAC2 expression is regulated in response to cigarette smoke-induced oxidative stress.
Figure 3. Proposed mechanism of glucocorticoid resistance in COPD inflammation and the effect of theophylline.
Cigarette smoke-mediated oxidative stress activates the NF-κB pathway leading to acetylation of chromatin on pro-inflammatory genes thus increasing the accessibility of transcription factors leading to increased pro-inflammatory gene expression. Deacetylation results in DNA rewinding around histone proteins, decreasing gene transcription. CBP/p300 function as intrinsic histone acetyltransferases (HATs). Activated corticosteroid receptors recruit HDAC2 into the transcriptome complex promoting histone deacetylation, chromatin condensation and expulsion of DNA polymerases, shutting off gene expression. Cigarette smoke/oxidants inhibit HDAC2 activity (by post-translational modifications) as well as activating NF-κB, facilitating histone acetylation by the transcriptome complex even in the presence of activated glucocorticoid receptor. Theophylline, a methyl xanthine, inhibits pro-inflammatory gene expression via restoring transrepressive pathways through the activation of HDAC2. GC: Glucocorticoid; Ac: Acetylation; 4-HNE: 4-hydroxy-2-nonenol; NO2: Nitric oxide; P: Phosphate; CBP: CREB (cAMP response element binding protein)-binding protein.
POST-TRANSLATIONAL MODIFICATIONS OF HDAC2 AND ITS IMPACT ON TRANCRIPTIONAL REGULATION
Post-translational modifications of HDAC2 have recently been thought to be the primary mechanism involved in decreased activity of HDAC2 particularly as observed in alveolar macrophages and peripheral lung tissue from healthy smokers and patients with COPD as compared to healthy non-smokers (42, 54, 88). The decrease in HDAC2 activity correlated with increased production of inflammatory cytokines in the lung (42, 88). Decreased HDAC2 activity was also observed in vitro experiments in monocytic cell lines treated with cigarette smoke extract showing modifications by aldehydes and by protein nitration, and restoration of HDAC2 activity by pre-treating the cells with GSH monoethyl ester (88). Synthetic nitric oxide (NO)/peroxynitrite donors also increases nitrosylation of tyrosine residues on HDAC2 leading to a decrease in deacetylase activity (41, 54). Moreover, alveolar macrophages from COPD patients show a marked increase in nitrosylation of HDAC2 (15). The results suggested that oxidative stress may play a role in reduced activity of HDAC2 by post-translational modification. Thus, several post-translational modifications to HDAC2 such as nitrosylation and α-β unsaturated aldehyde-adduct formation (protein carbonyl-adducts) have been implicated in the loss of deacetylase activity and as possible mechanisms for pro-inflammatory response in patients with COPD (Figure 4).
Figure 4. Oxidant-mediated degradation of deacetylases (HDAC2/SIRT1) leading to increased expression of pro-inflammatory cytokines.
DNA is coiled around the histone proteins and form chromatin structure. Acetylation of chromatin (on pro-inflammatory genes) increasing the accessibility of transcription factors and there by increasing gene transcription. Deacetylation results in DNA rewinding around histone proteins, and decreasing gene transcription. Thus, HDACs involved in the maintenance of histone acetylation and deacetylation balance and thereby control the gene expression. HDAC and/or SIRT1 may be post-translationally modified by phosphorylation, carbonylation, aldehyde adducts formation and nitration leading to increased ubiquitination and subsequent degradation of HDACs. Decreased HDACs leads to increased histone acetylation/deacetylation ratio which cause increased acetylation of chromatin, chromatin remodulation and increased transcription of pro-inflammatory mediators. HAT: Histone acetyltransferase, HDAC: Histone deacetylase; P: phosphate; Ac: Acetylation; 4-HNE: 4-hydroxynonenol; NO2: Nitric oxide.
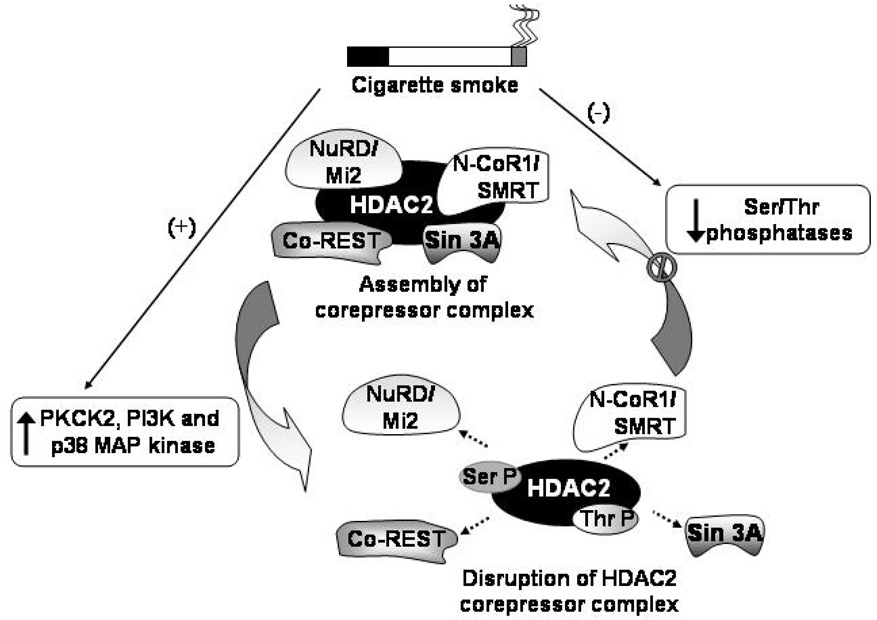
With the exception of mammalian HDAC8, recombinant HDACs are not enzymatically active (40) and require a co-repressor complex that involves complex protein-protein interactions that enhance their deacetylase activities or mediate their association with histones or DNA since HDACs have no ability to bind DNA. HDAC2 is known to associate with three different co-repressor complexes; Sin3, NuRD/Mi2 and the CoRest complexes (Figure 5). Both the Sin3 and Mi2 complexes contain a core group of four proteins HDAC1, HDAC2, RbAp46 and RbAp48 including the distinct mSin3A protein in the Sin3 complex and Mi2 in the NuRD/Mi2 complex (76). RbAp48 is known to selectively bind to histone H4 in vivo (85), thus serving to recruit HDAC2 to DNA. Since histone deacetylases where first discovered to be phosphoproteins (82), an increasing body of evidence is elucidating mechanisms by which HDAC2 is regulated by hyperphosphorylation. Galasinski et al first showed that HDAC2 exists in three forms; the unphosphorylated, basally phosphorylated and hyperphosphorylated forms (28). Hyperphosphorylation of HDAC2 induced by inhibiting cellular serine phosphatase activity increased disruption of the HDAC2-mSin3A interaction (28); oxidative and nitrative stress was also shown to reduce the Mi2/mSin3A interaction with HDAC2 without a reduction in protein expression (29). In vitro phosphorylated flag-tagged HDAC2 showed a reduction in deacetylase activity (82) indicating that hyperphosphorylation of co-repressor complex may be one plausible explanation for decreased HDAC2 deacetylase activity in response to oxidative stress. However the observation that hypophosphorylated HDAC2 disrupts interaction with mSin3A/Mi2 (82) is further evidence of the complex mechanisms by which phosphorylation regulates HDAC2. Site directed mutations of Ser421 and Ser423 phosphorylation sites in HDAC1 completely abolishes co-repressor complex formation (76). Basal phosphorylation is required for its deacetylase activity, however, hyperphosphorylation of HDAC2 has been shown to cause a dissociation of the co-repressor complex required for deacetylase activity (28) (Figure 5).
Figure 5. Proposed model for the regulation of HDAC2 co-repressor complex via phosphorylation/dephosphorylation.
HDAC2 can be phosphorylated by phospho kinase CK2 (PKCK2) and possibly by other kinases, such as phosphoinositide-3 kinase (PI3K) and members of the mitogen-activated protein (MAP) kinase family, and dephosphorylated by ser/thr phosphatase-dependent mechanisms. Alteration in phosphorylation/dephosphorylation status can modify HDAC2 activity, particularly in response to cigarette smoke/oxidants exposure. The activity of HDAC2 relies on its association with co-repressor complexes (Sin 3A, NuRD/Mi2, N-CoR1/SMRT and Co-REST). Post-translational modifications of HAC2 may have ramifications in sustained inflammatory responses by the disruption of HDAC2 co-repressor complex. The mechanisms that underlie cigarette smoke-mediated reductions in HDAC2 levels are not known, although an attractive hypothesis is that it involves alterations in HDAC2 phosphorylation or inhibition of ser-thr phosphatases may lead to subsequent ubiquitination and degradation of HDAC2 by the 26S proteasome. A similar mechanism may be possible for SIRT1 and other HDAC members. Ser-P: Phosphoserine; Thr-P: Phosphothreonine; ---→: disruption of HDAC2 complex.
Current theories on HDAC2 degradation has focused mainly on a possible role of the ubiquitin-proteasomal pathway. Proteolytic degradation regulates the basal turnover HDAC2 and it is currently hypothesized that hyperphosphorylation of HDAC2 may increase ubiquitination and eventual net loss of HDAC2. Valproic acid, a potent inhibitor of HDACs, increases the polyubiquitination of HDAC2, while the over-expression of the E2 ubiquitin ligase Ubc8 causes a decrease in HDAC2 protein expression (48). It remains to be seen whether oxidative stress-induced hyperphosphorylation may regulate HDAC2 in a similar fashion. While protein kinase A (PKA) is known to phosphorylate HDAC1, only casein kinase 2 (CK2) is known to phosphorylate HDAC2 in vivo and in vitro (82). Oxidative stress-induced phosphoinositide-3 kinase (PI3K) activation has been shown to be involved in reducing HDAC2 activity (22); other studies have shown a decreased inflammatory response to cigarette smoke exposure in the PI3Kγ knock out mouse model (33) while PI3Kδ knock-in restored steroid function in a mouse model of cigarette smoke exposure (53). However no direct link between PI3K and hyperphosphorylation of HDAC2 has been established. Cigarette smoke is known to cause increased HDAC2 phosphorylation. Since valproic acid, an HDAC2 inhibitor induces HDAC2 ubiquitination and proteasomal degradation (48), it is thought that cigarette smoke might induce degradation of HDAC2 via the ubiquitin-proteasome pathway and that hyperphosphorylation might be the trigger for this event. This contention of cigarette smoke-mediated HDAC2 regulation by ubiquitin-proteasome pathway requires further investigation.
The loss of HDAC2 levels in the lung of COPD patients may also be a result of observed reduction in HDAC2 mRNA expression (42). A recent study using a luciferase reporter system linked to the HDAC2 promoter showed diminished promoter activation in response to hypoxia and oxidative stress which was rescued by corticosteroids (10). Possible mechanisms may involve deacetylation of critical histones in the promoter region or increased methylation of DNA which has been linked to gene silencing. However, very little is known about HDAC2 promoter regulation that may prove to be an important link to how cells respond to oxidative stress.
ROLE OF HDAC1 and HDAC3 IN THE REGULATION OF NF-κB
HDAC1 (class I HDAC) is crucial in suppressing NF-κB dependent gene expression, including and silencing of pro-inflammatory cytokine (IL-8) expression (3, 95). Moreover, HDAC1 directly associates with the Rel homology domain of NF-κB p65 to alter its NF-κB activity in cultured cells. HDAC2 may also regulate NF-κB activity through its association with HDAC1 (3). Recruitment of HDAC1 results in local histone H3 deacetylation, which facilitates the closed chromatin complex formation and prevents the association of RNA polymerase II with the promoter, leading to its repression (86). On stimulation, NF-κB with RelA/p65 phosphorylated possibly by PKA recruits CBP with intrinsic HAT activity and translocates to the nucleus where it displaces the HDAC1/p50 complex to increase gene transcription (95). Oxidative stress-mediated phosphorylation of RelA/p65 may lead to dissociation from HDAC1 and can then associate with CBP/p300 (88, 95) which leads to expression of NF-κB mediated pro-inflammatory gene expression. Oxidative stress may also cause post-translational modifications of HDAC1 and prevent it from efficiently binding unstimulated RelA/p65, this may predispose the cell to a higher NF-κB activation than would normally be possible.
HDAC3 functions as a co-repressor for many sequence-specific transcription factors, including NF-κB. Through a physical interaction with transcription factors, HDAC3 is recruited to specific promoters, where it brings about transcriptional repression through histone deacetylation. In addition to this local function, HDAC3 is also important for global genome-wide histone deacetylation and specific inactivation of HDAC3 leads to an increase in global histone acetylation (31, 94). HDAC3 associated with the acetyltransferases p300 and p300/CBP-associated factor (PCAF) to reverse autoacetylation. Reversible deacetylation of the inducible transcription factor NF-κB RelA by HDAC3 promotes effective binding to IκBα and nuclear export of the complex to regulate target gene transcription (12).
Post-translational modification of histone proteins in chromatin architecture plays a central role in the epigenetic regulation of transcription (1). Cigarette smoke is reported to decrease the activity of HDAC and the levels of HDAC 1, 2 and 3, which are associated with the significant increase in 4-hydroxy-2-nonenal (4-HNE) and nitrotyrosine post-translational modification of HDAC proteins (41, 88). This decline in HDAC1–3 protein was however restored by pre-treating with GSH monoethyl ester suggesting a role for oxidative stress in reduced HDAC activity (88). Since HDACs involved in the repression of pro-inflammatory genes, activation of HDAC may have therapeutic potential, and theophylline has been shown to have this property, resulting in marked potentiation of the anti-inflammatory therapy (45). However, it is not known whether theophylline bypasses oxidative stress modifications or inhibits phosphorylation of HDAC2 to restore steroid efficacy. Similarly, we have recently shown that curcumin, a dietary polyphenolic antioxidant, reversed the steroid efficacy by attenuating the CSE-induced reduction of HDAC2 in monocytes. This effect was not due to curcumin’s antioxidant effect.
ROLE OF SIRTUIN (CLASS III HDAC) IN THE REGULATION OF NF-κB
Sirtuins (SIRT, Silent information regulator 2, Sir2 family of enzymes) belong to class III HDACs that regulate gene expression in a variety of organisms by deacetylation of modified lysine residues on histones, transcription factors and other proteins. SIRTs are widely distributed in all phyla and are implicated in aging, cell cycle regulation, apoptosis, metabolism and inflammation. SIRT include five homologues in yeast (ySir2 and Hst1–4) and seven in humans (SIRT1–7, classes I – IV and U) (26). Unlike class I and II HDACs, which require water molecule for deacetylation, sirtuins require NAD+ as a co-substrate. The stoichiometry between NAD+ and the substrate (acetylated protein) is 1:1 and the products of the reaction are: 2-O-acetyl-ADP-ribose (ADPR), nicotinamide and deacetylated protein. The deacetylating activity of sirtuins depends on NAD+ and is inhibited by the reaction product, nicotinamide (IC50 <50 µM) (6). The other reaction product, O-acetyl-ADP-ribose is now hypothesized to have signaling function (7).
Sirtuin1 (SIRT1) is an important mammalian sirtuin, predominantly present in the nucleus and is homologous to yeast Sir2α(55). SIRT1 histone deacetylases bind directly to one or more constituents in the chromatin complex resulting in structural re-organization, and therefore has the ability to establish silent chromatin domains (24, 79). The involvement of NAD+ in the deacetylation reaction is also thought to link sirtuin deacetylase activity to metabolism (19). SIRT1 control the energy metabolism and mediate the longevity effect of dietary caloric restriction by activating gluconeogenesis and repressing glycolysis in the liver via deacetylation of PPARγ coactivator-1 (PGC1). In addition, SIRT1 inhibits fat storage and increases fat release in white adipose tissue via repression of PPARγ (35, 63, 74). In humans, SIRT1 deacetylates transcription factors such as drosophila forkhead transcription factor (FOXO), p53 and NF-κB, which mediates stress resistance, apoptosis, and inflammatory responses that participate in physiological responses to toxicity (57, 90). Since the transcriptional activity of FOXO3 is regulated by phosphorylation and acetylation, SIRT1 mediated deacetylation of FOXO3 leads to its activation and induction of cell cycle arrest. Thus high SIRT1 activity would promote cell survival (59, 92). SIRT1 also upregulates stress-positive pathways via deacetylation of FOXO3 transcription factor leading to increased transcription of GADD45 (DNA repair) and MnSOD (reactive oxygen detoxification) (9, 47). Interestingly, SIRT1 interacts with p53 and deacetylates the C-terminal regulatory domain (84), whereas reduction of SIRT1 leads to increased of acetylation of p53 thereby increasing its pro-apoptotic and cell senescence function (50, 84). Oxidative stress accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion (27). Nuclear SIRT1 levels were decreased in vivo and in vitro in response to cigarette smoke exposure (89), but it is not known if SIRT1-mediated regulation of FOXO3 (acetylation) plays a role in cigarette smoke-mediated apoptosis and senescence.
Recently, it has been shown that endogenous and exogenous SIRT1 were both able to partially localize in cytoplasm in certain cell lines. Cytoplasm-localized SIRT1 enhances apoptosis (46), whereas the nuclear form suppressed the oxidative stress-mediated apoptosis. Thus, nucleocytoplasmic shuttling is important in the regulatory mechanism of SIRT1, which may involve in differentiation and in inhibition of cell death (80). Evidence indicates that sirtuins have evolved to mediate signaling initiated by stress condition such as nutrient deprivation to produce adaptation to improve organism survival (35). SIRT1 regulates NF-κB-dependent transcription and cell survival in response to TNF-α (11, 84, 89, 91). SIRT1 protein associated directly with RelA/p65 subunit of NF-κB and deacetylates Lys310 of RelA/p65, a site that critical for NF-κB transcriptional activity, and thereby inhibit the activity of NF-κB (13, 91). Genetic ablation of SIRT1 leads to increased NF-κB activation and pro-inflammatory cytokine release in mouse lung suggesting that SIRT1 knockdown leads to lung inflammation and pulmonary edema (55). Similar to HDAC2, we have recently shown that decreased level of SIRT1 in response to cigarette smoke extract was associated with increased activation of RelA/p65 subunit of NF-κB and pro-inflammatory mediator release in human monocytic cell lines (89). This may be partially explained on the basis that cigarette smoke-derived oxidants caused post-transcriptional modification by reactive aldehydes/reactive oxygen species/reactive nitrogen species, thereby disrupting SIRT1-RelA/p65 complex and increased the release of pro-inflammatory mediators (89). Such oxidative/aldehyde/nitrosative modifications may in turn render SIRT1 ineffective for interaction with other signaling components. Recently, Yang et al have shown that resveratrol, a polypenolic antioxidant which induces SIRT1 activity, attenuated CSE-induced pro-inflammatory cytokine release in monocytes (89). Overall, sirtuin is a redox-sensitive protein and prone to alterations by oxidants and/or free radicals, which in turn linked with the abnormal regulation of various pro-inflammatory transcription regulatory pathways.
CONCLUSIONS
ROS are critical in the pro-inflammatory response to cigarette smoke or other environmental oxidants through the activation of NF-κB dependent redox-sensitive transcription factors and alteration in histone acetylation/deacetylation balance (chromatin remodeling). ROS alters/affects deacetylases such as HDAC 1,2,3 and SIRT1 (increased HAT/HDAC ratio) which leads to acetylation of RelA/p65, disruption of HDAC-RelA/p65 complex and opening of chromatin culminating in NF-κB dependent gene transcription. Oxidative stress-mediated post-translational modifications (nitration, carbonylation or adduct formation) and phosphorylation of HDACs and SIRT1 resulting in ubiquitination and proteosomal degradation. These post-translational modifications will also lead to disruption of co-repressor complex, which is essential for enzyme activity of HDAC2 and SIRT1. Since HDAC2 and its co-repressor complex are involved in steroid efficacy, decreased HDAC2 or disruption of co-repressor complex will cause steroid resistance which is seen in severe asthmatics and in patients with COPD. Understanding the redox regulation of HDACs and their signaling mechanisms through NF-κB will lead to better understanding of therapeutic targets and development of therapies in chronic inflammatory lung diseases where oxidative stress occurs. Furthermore, these mechanisms might be a key to overcome the limitations of glucocorticoid treatment in COPD patients.
ACKNOWLEDGEMENTS
This work was supported by the NIEHS Environmental Health Sciences Center (ES01247), NIEHS Toxicology Training Program Grant (ES07026), and National Institute of Health grant (R01-HL085613).
ABBREVIATIONS
- AP-1
activator protein-1
- AKT
protein kinase B/serine-threonine kinase
- ATF-2
activating transcription factor-2
- CBP
cAMP-response element binding protein-binding protein
- COPD
chronic obstructive pulmonary disease
- CoRest
co-repressor for element-1-silencing transcription factor
- FOXO
fork-head box, class O protein
- GADD45
growth arrest and DNA-damage-inducible protein 45
- GSH
reduced glutathione
- H2O2
hydrogen peroxide
- HAT
histone acetyl transferase
- HDAC
histone deacetylase
- HNE
4-hydroxyl-2-nonenol
- IKK
inhibitor κB-α kinase
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAP
mitogen-activated protein
- MEKK-1
extracellular signal-regulated kinase kinase
- MnSOD
manganese superoxide dismutase
- NAD
nicotinamide adenine dinucleotide
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa B
- NuRD
nucleosome remodeling/histone deacetylase
- O2•-
superoxide radical
- •OH
hydroxyl radical
- PI3K
phosphoinositide-3 kinase
- PKA
protein kinase A
- PPARγ
peroxisome proliferator-activated receptor-γ
- RbAp
retinoblastoma-associated protein
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- SIRT
silent information regulator
- TNF-α
tissue necrosis factor-α
REFERENCES
- 1.Adcock IM, Ford P, Barnes PJ, Ito K. Epigenetics and airways disease. Respir Res. 2006;7:21. doi: 10.1186/1465-9921-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- 3.Ashburner BP, Westerheide SD, Baldwin AS., Jr The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001;21:7065–7077. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PJ, Ito K, Adcock IM. Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase. Lancet. 2004;363:731–733. doi: 10.1016/S0140-6736(04)15650-X. [DOI] [PubMed] [Google Scholar]
- 5.Bast A, Haenen GR, Doelman CJ. Oxidants and antioxidants: state of the art. Am J Med. 1991;91:2S–13S. doi: 10.1016/0002-9343(91)90278-6. [DOI] [PubMed] [Google Scholar]
- 6.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 7.Borra MT, O'Neill FJ, Jackson MD, Marshall B, Verdin E, Foltz KR, Denu JM. Conserved enzymatic production and biological effect of O-acetyl-ADP-ribose by silent information regulator 2-like NAD+-dependent deacetylases. J Biol Chem. 2002;277:12632–12641. doi: 10.1074/jbc.M111830200. [DOI] [PubMed] [Google Scholar]
- 8.Bowie A, O'Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 9.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 10.Charron C, Adcock I, Barnes P, Ito K. HDAC2 promoter activation is diminished under hypoxia and oxidative stress but enhanced by corticosteroid. American Journal of Respiratory and Critical Care Medicine. 2007;175:A112. [Google Scholar]
- 11.Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 13.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. Embo J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosio BG, Tsaprouni L, Ito K, Jazrawi E, Adcock IM, Barnes PJ. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J Exp Med. 2004;200:689–695. doi: 10.1084/jem.20040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Culpitt SV, Rogers DF, Shah P, De Matos C, Russell RE, Donnelly LE, Barnes PJ. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:24–31. doi: 10.1164/rccm.200204-298OC. [DOI] [PubMed] [Google Scholar]
- 17.Davies MJ. The oxidative environment and protein damage. Biochim Biophys Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 18.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denu JM. Linking chromatin function with metabolic networks: Sir2 family of NAD(+)-dependent deacetylases. Trends Biochem Sci. 2003;28:41–48. doi: 10.1016/s0968-0004(02)00005-1. [DOI] [PubMed] [Google Scholar]
- 20.Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 2003;23:3173–3185. doi: 10.1128/MCB.23.9.3173-3185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emmendoerffer A, Hecht M, Boeker T, Mueller M, Heinrich U. Role of inflammation in chemical-induced lung cancer. Toxicol Lett. 2000;112–113:185–191. doi: 10.1016/s0378-4274(99)00285-4. [DOI] [PubMed] [Google Scholar]
- 22.Failla M, To Y, Ito M, Adcock I, Barnes P, Ito K. Oxidative stress-induced PI3-kinase activation reduces HDAC activity and is inhibited by theophylline. Am J Respir Crit Care Med. 2007;175:A45. [Google Scholar]
- 23.Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42:153–164. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 24.Fraga MF, Esteller M. Epigenetics and aging: the targets and the marks. Trends Genet. 2007 doi: 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Fridovich I. Fundamental aspects of reactive oxygen species, or what's the matter with oxygen? Ann N Y Acad Sci. 1999;893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 26.Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem. 2007;20:45–54. doi: 10.1159/000104152. [DOI] [PubMed] [Google Scholar]
- 28.Galasinski SC, Resing KA, Goodrich JA, Ahn NG. Phosphatase inhibition leads to histone deacetylases 1 and 2 phosphorylation and disruption of corepressor interactions. J Biol Chem. 2002;277:19618–19626. doi: 10.1074/jbc.M201174200. [DOI] [PubMed] [Google Scholar]
- 29.Getting S, Adcock I, Barnes P, Ito K. Reduced association of co-repressors under oxidative stress reduces HDAC2 activity in airway epithelial cells. Am J Respir Crit Care Med. 2007;175:A175. [Google Scholar]
- 30.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 31.Glaser KB, Li J, Staver MJ, Wei RQ, Albert DH, Davidsen SK. Role of class I and class II histone deacetylases in carcinoma cells using siRNA. Biochem Biophys Res Commun. 2003;310:529–536. doi: 10.1016/j.bbrc.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 32.Gloire G, Horion J, El Mjiyad N, Bex F, Chariot A, Dejardin E, Piette J. Promoter-dependent effect of IKKalpha on NF-kappa B/p65 DNA binding. J Biol Chem. 2007 doi: 10.1074/jbc.M610728200. [DOI] [PubMed] [Google Scholar]
- 33.Grumelli S, Lu B, Shapiro S, Gerard C. Decreased inflammation in the smoking model of PI3K knock-out mice. Am J Respir Crit Care Med. 2007;175:A648. [Google Scholar]
- 34.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 35.Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- 37.Haddad JJ. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal. 2002;14:879–897. doi: 10.1016/s0898-6568(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 38.Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 39.Hildmann C, Riester D, Schwienhorst A. Histone deacetylases--an important class of cellular regulators with a variety of functions. Appl Microbiol Biotechnol. 2007;75:487–497. doi: 10.1007/s00253-007-0911-2. [DOI] [PubMed] [Google Scholar]
- 40.Hu E, Chen Z, Fredrickson T, Zhu Y, Kirkpatrick R, Zhang GF, Johanson K, Sung CM, Liu R, Winkler J. Cloning and characterization of a novel human class I histone deacetylase that functions as a transcription repressor. J Biol Chem. 2000;275:15254–15264. doi: 10.1074/jbc.M908988199. [DOI] [PubMed] [Google Scholar]
- 41.Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun. 2004;315:240–245. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 42.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, Barnes PJ. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352:1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 43.Ito K, Jazrawi E, Cosio B, Barnes PJ, Adcock IM. p65-activated histone acetyltransferase activity is repressed by glucocorticoids: mifepristone fails to recruit HDAC2 to the p65-HAT complex. J Biol Chem. 2001;276:30208–30215. doi: 10.1074/jbc.M103604200. [DOI] [PubMed] [Google Scholar]
- 44.Ito K, Lim S, Caramori G, Chung KF, Barnes PJ, Adcock IM. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. Faseb J. 2001;15:1110–1112. [PubMed] [Google Scholar]
- 45.Ito K, Lim S, Caramori G, Cosio B, Chung KF, Adcock IM, Barnes PJ. A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression. Proc Natl Acad Sci U S A. 2002;99:8921–8926. doi: 10.1073/pnas.132556899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin Q, Yan T, Ge X, Sun C, Shi X, Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol. 2007 doi: 10.1002/jcp.21091. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med. 2005;16:237–243. [PubMed] [Google Scholar]
- 48.Kramer OH, Zhu P, Ostendorff HP, Golebiewski M, Tiefenbach J, Peters MA, Brill B, Groner B, Bach I, Heinzel T, Gottlicher M. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. Embo J. 2003;22:3411–3420. doi: 10.1093/emboj/cdg315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 50.Luo J, Li M, Tang Y, Laszkowska M, Roeder RG, Gu W. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci U S A. 2004;101:2259–2264. doi: 10.1073/pnas.0308762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 52.MacNee W, Rahman I. Oxidants and antioxidants as therapeutic targets in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S58–S65. doi: 10.1164/ajrccm.160.supplement_1.15. [DOI] [PubMed] [Google Scholar]
- 53.Marwick J, Stevenson C, Koremu M, Barnes P, Ito K, Adcock I, Kirkham P. PI-3K-delta dead knock-in restores steroid function in smoking mouse model. Am J Respir Crit Care Med. 2007;175:A696. [Google Scholar]
- 54.Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, Macnee W, Rahman I. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol. 2004;31:633–642. doi: 10.1165/rcmb.2004-0006OC. [DOI] [PubMed] [Google Scholar]
- 55.McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLaughlin F, Finn P, La Thangue NB. The cell cycle, chromatin and cancer: mechanism-based therapeutics come of age. Drug Discov Today. 2003;8:793–802. doi: 10.1016/s1359-6446(03)02792-2. [DOI] [PubMed] [Google Scholar]
- 57.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morcillo EJ, Estrela J, Cortijo J. Oxidative stress and pulmonary inflammation: pharmacological intervention with antioxidants. Pharmacol Res. 1999;40:393–404. doi: 10.1006/phrs.1999.0549. [DOI] [PubMed] [Google Scholar]
- 59.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 60.Nakajima Y, Aoshiba K, Yasui S, Nagai A. H2O2 induces apoptosis in bovine tracheal epithelial cells in vitro. Life Sci. 1999;64:2489–2496. doi: 10.1016/s0024-3205(99)00206-4. [DOI] [PubMed] [Google Scholar]
- 61.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 62.Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal. 2006;8:1791–1806. doi: 10.1089/ars.2006.8.1791. [DOI] [PubMed] [Google Scholar]
- 63.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Radhakrishnan I, Perez-Alvarado GC, Parker D, Dyson HJ, Montminy MR, Wright PE. Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell. 1997;91:741–752. doi: 10.1016/s0092-8674(00)80463-8. [DOI] [PubMed] [Google Scholar]
- 65.Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanisms. Cell Biochem Biophys. 2005;43:167–188. doi: 10.1385/CBB:43:1:167. [DOI] [PubMed] [Google Scholar]
- 66.Rahman I. Oxidative stress, chromatin remodeling and gene transcription in inflammation and chronic lung diseases. J Biochem Mol Biol. 2003;36:95–109. doi: 10.5483/bmbrep.2003.36.1.095. [DOI] [PubMed] [Google Scholar]
- 67.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 68.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- 69.Rahman I, MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radic Biol Med. 2000;28:1405–1420. doi: 10.1016/s0891-5849(00)00215-x. [DOI] [PubMed] [Google Scholar]
- 70.Rahman I, MacNee W. Role of transcription factors in inflammatory lung diseases. Thorax. 1998;53:601–612. doi: 10.1136/thx.53.7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol. 2004;68:1255–1267. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 72.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154:1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- 73.Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am J Respir Crit Care Med. 1997;156:341–357. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- 74.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 75.Senese S, Zaragoza K, Minardi S, Muradore I, Ronzoni S, Passafaro A, Bernard L, Draetta GF, Alcalay M, Seiser C, Chiocca S. Role for histone deacetylase 1 in human tumor cell proliferation. Mol Cell Biol. 2007;27:4784–4795. doi: 10.1128/MCB.00494-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sengupta N, Seto E. Regulation of histone deacetylase activities. J Cell Biochem. 2004;93:57–67. doi: 10.1002/jcb.20179. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 78.Szulakowski P, Crowther AJ, Jimenez LA, Donaldson K, Mayer R, Leonard TB, Macnee W, Drost EM. The effect of smoking on the transcriptional regulation of lung inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:41–50. doi: 10.1164/rccm.200505-725OC. [DOI] [PubMed] [Google Scholar]
- 79.Takata T, Ishikawa F. Human Sir2-related protein SIRT1 associates with the bHLH repressors HES1 and HEY2 and is involved in HES1- and HEY2-mediated transcriptional repression. Biochem Biophys Res Commun. 2003;301:250–257. doi: 10.1016/s0006-291x(02)03020-6. [DOI] [PubMed] [Google Scholar]
- 80.Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 81.Trapp J, Jung M. The role of NAD+ dependent histone deacetylases (sirtuins) in ageing. Curr Drug Targets. 2006;7:1553–1560. doi: 10.2174/1389450110607011553. [DOI] [PubMed] [Google Scholar]
- 82.Tsai SC, Seto E. Regulation of histone deacetylase 2 by protein kinase CK2. J Biol Chem. 2002;277:31826–31833. doi: 10.1074/jbc.M204149200. [DOI] [PubMed] [Google Scholar]
- 83.Urnov FD, Wolffe AP. Chromatin remodeling and transcriptional activation: the cast (in order of appearance) Oncogene. 2001;20:2991–3006. doi: 10.1038/sj.onc.1204323. [DOI] [PubMed] [Google Scholar]
- 84.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 85.Vermaak D, Wade PA, Jones PL, Shi YB, Wolffe AP. Functional analysis of the SIN3-histone deacetylase RPD3-RbAp48-histone H4 connection in the Xenopus oocyte. Mol Cell Biol. 1999;19:5847–5860. doi: 10.1128/mcb.19.9.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wooten-Blanks LG, Song P, Senkal CE, Ogretmen B. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. Faseb J. 2007 doi: 10.1096/fj.07-8621com. [DOI] [PubMed] [Google Scholar]
- 87.Wu C. Chromatin remodeling and the control of gene expression. J Biol Chem. 1997;272:28171–28174. doi: 10.1074/jbc.272.45.28171. [DOI] [PubMed] [Google Scholar]
- 88.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 89.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- 90.Yang T, Sauve AA. NAD metabolism and sirtuins: metabolic regulation of protein deacetylation in stress and toxicity. Aaps J. 2006;8:E632–E643. doi: 10.1208/aapsj080472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.You H, Mak TW. Crosstalk between p53 and FOXO transcription factors. Cell Cycle. 2005;4:37–38. doi: 10.4161/cc.4.1.1401. [DOI] [PubMed] [Google Scholar]
- 93.Yu Z, Zhang W, Kone BC. Histone deacetylases augment cytokine induction of the iNOS gene. J Am Soc Nephrol. 2002;13:2009–2017. doi: 10.1097/01.asn.0000024253.59665.f1. [DOI] [PubMed] [Google Scholar]
- 94.Zhang X, Wharton W, Yuan Z, Tsai SC, Olashaw N, Seto E. Activation of the growth-differentiation factor 11 gene by the histone deacetylase (HDAC) inhibitor trichostatin A and repression by HDAC3. Mol Cell Biol. 2004;24:5106–5118. doi: 10.1128/MCB.24.12.5106-5118.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 96.Zor T, Mayr BM, Dyson HJ, Montminy MR, Wright PE. Roles of phosphorylation and helix propensity in the binding of the KIX domain of CREB-binding protein by constitutive (c-Myb) and inducible (CREB) activators. J Biol Chem. 2002;277:42241–42248. doi: 10.1074/jbc.M207361200. [DOI] [PubMed] [Google Scholar]