Abstract
In this double-blind, cross-over study physiological (i.e. blood pressure, heart rate, plasma catecholamine concentrations, plasma cortisol concentrations) and subjective (i.e. McGill Pain Questionnaire, positive affect, distress) response to a cold pressor task was assessed in 19 subjects one hour after the administration of 50 mg naltrexone and after placebo. Significant differences in plasma catecholamine concentrations were found. Plasma epinephrine concentrations increased during the one hour period after naltrexone administration but remained largely unchanged after placebo administration. A significant treatment × period effect was also found for plasma norepinephrine concentrations. No significant differences were found for other measures assessed. Further research is necessary to determine the subpopulations in which these effects are of greatest magnitude and the long term safety implications of these effects.
INTRODUCTION
When one is subjected to a stressor, activation of the sympathetic system and the HPA axis occurs (Strike and Steptoe 2003; Velasco et al 1997). The magnitude of response to stressors has been studied as it relates to areas including cardiovascular disease and substance abuse (al’Absi 2006; Sinha et al 2006; Treiber et al 2003). To more fully understand mechanisms by which stress response influences disease outcomes, it is important to study the various pathways by which stress response is regulated, of which the endogenous opioid system is one.
Opioid receptor antagonists are being studied in the treatment of substance use disorders (i.e. alcohol dependence, nicotine dependence, opioid abuse) (O’Malley et al 2006; Roozen et al 2006) and impulse control disorders (i.e. pathological gambling) (Kim et al 2001). As use of these agents increases, more fully understanding their neuroendocrine effects is necessary. Since the endogenous opioid system is involved in the regulatory response to physiological and pain stressors, evaluation of opioid antagonists needs to be conducted both under resting and stress conditions. Additionally, testing the neuroendocrine and sympathetic response of opioid antagonists during stress can increase understanding of the endogenous opioid system in modulating stress response.
The goal of the described study is to evaluate the effect of naltrexone, an opioid antagonist, on cardiovascular response (i.e. blood pressure, heart rate, plasma catecholamine concentrations), neuroendocrine response (i.e. plasma cortisol concentrations), pain and distress measures during a cold pressor task. We hypothesized that opioid blockade would reverse the expected effects of endogenous opioids leading to an enhanced cardiovascular and neuroendocrine response and increased pain response.
Methods
Subjects
We recruited adults (≥ 18 years of age) in generally good health. Eligibility was determined at a screening visit at which a medical examination was performed and the absence of contraindications to naltrexone determined. Medical and psychiatric history was obtained by subject report. Subjects were excluded if they reported a current chronic disease (e.g. hypertension, renal or hepatic disease, cardiovascular disease, diabetes, respiratory disorders), reported current opiate dependence or use of narcotic medication within 3 days before the study, reported a history of a major psychiatric disorder, were pregnant or reported routine use of any prescription medication. This study was approved by the University of Minnesota Institutional Review Board and written informed consent was obtained from all subjects.
Study Design / Procedures
In this randomized, double-blind, cross-over study, physiological and subjective response to a cold pressor task (CPT) was assessed in subjects 1 hour after a single 50 mg naltrexone dose and a matching placebo. One hour corresponds to the time at which peak plasma concentrations typically occur (Gonzalez and Brogden 1988). Each subject attended two laboratory sessions separated by a minimum of 3 days with all laboratory sessions beginning at approximately the same time of day (i.e. 12:00 –1:00 PM).
The procedures for the two laboratory session were identical except for the medication that was administered (Figure 1). The order of medication administration was randomized between subjects. During an initial thirty minute baseline rest period blood pressure (BP) and heart rate (HR) were measured at 5 minute intervals and blood was drawn through an indwelling catheter at the conclusion of this baseline period. Subjects then took either a 50 mg naltrexone or matching placebo capsule. A 60 minute rest period followed during which BP and HR were measured at 10 minute intervals; blood was drawn and subjects completed a mood state questionnaire at 20 minute intervals. This questionnaire covered 2 factors (Positive Affect and Distress) as described previously (al’Absi and Petersen 2003). The CPT was then administered using previously described methodology (al’Absi et al 2000). In brief, subject placed one hand into an ice-water slurry for 90 seconds with pain ratings occurring at 15 second intervals. After removing their hand from the ice water, pain ratings continued during a 90 second recovery period. One BP and HR measurement was obtained during the CPT and one immediately after subjects removed their hand from the ice water. The mood states and McGill Pain Questionnaire (Melzack 1975) were completed after the cold stressor task. Blood was drawn during completion of the questionnaires. A final 60 minute rest period then began during which BP and HR were measured at 10 minute intervals; mood states questionnaires was completed and blood was drawn at 20 minute intervals.
Figure 1.
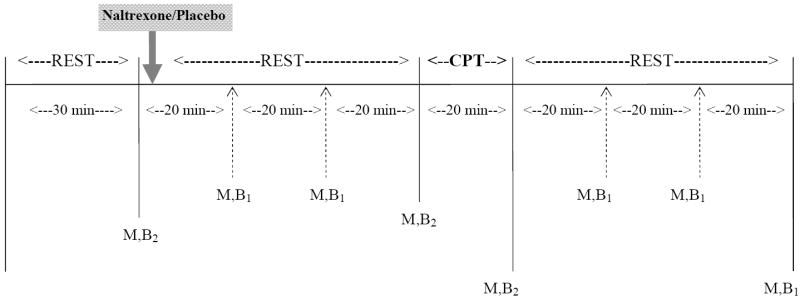
Outline of each visit at which cold pressor test was administered. CPT = cold pressor test; M = mood questionnaire; B1 = blood draw assayed for plasma cortisol concentrations; B2 = blood draw assayed for plasma cortisol, epinenphrine and norepinephrine concentrations
All blood samples were analyzed using enzyme immunoassay to assess plasma cortisol concentrations and three of the samples (i.e. baseline, immediately prior to, immediately following CPT) were analyzed using high performance liquid chromatography with electrochemical detection to determine plasma epinephrine and norepinephrine concentrations. For samples with epinephrine concentrations lower than the lower limit of quantitation (10 pg/ml), a value of 5 pg/ml was used for purposes of analysis. The lower limit of quantitation for the plasma cortisol assay is 0.5 mcg/dl.
Statistical Analysis
The average BP and HR were determined for each of four periods (predrug baseline, postdrug rest, during CPT, during recovery). Plasma catecholamine concentrations were measured during 3 periods and plasma cortisol concentrations and mood states questionnaire were measured during 8 periods (as described previously). Pain ratings were determined for each of the two periods (during CPT, after CPT) by averaging ratings obtained during that period. The McGill Pain Questionnaire was administered after the CPT.
Drug order effects were assessed via a series of preliminary analyses of variance comparing the 2 drug orders with no effects of drug order found on any of the measures of interest. Data were then collapsed across drug conditions for subsequent analyses.
Cardiovascular data were analyzed using 2 (Drug Condition) × 4 (Period) repeated ANOVAs. Plasma catecholamine concentrations were analyzed using 2 (Drug Condition) × 3 (Period) ANOVAs. Plasma cortisol concentrations and mood states questionnaire data were analyzed using 2 (Drug Condition) × 8 (Period) ANOVAs. Pain ratings were analyzed using 2 (Drug Condition) × 2 (Period) ANOVAs and McGill Pain Questionnaire scores were analyzed using a 2 (Drug Condition) × 1 (Period) ANOVAs.
RESULTS
Nineteen subjects completed both laboratory assessments and are included in the analysis. Average age of subjects was 26.1 ± 6.9 (SD), average BMI was 23.9 ± 2.8 and 53% of subjects were female.
For plasma epinephrine concentrations a significant treatment x period effect was found [F (2, 16) = 12.7, p < 0.001). In those receiving placebo, plasma epinephrine concentration remained largely unchanged during the one hour period after placebo administration. However, in those receiving naltrexone, plasma epinephrine concentrations increased by 10.4 pg/ml during this period (p< 0.001). Although plasma epinephrine concentrations increased in both conditions during the CPT, the increase was similar across the two conditions. Due to the increase in epinephrine concentrations prior to the CPT when receiving naltrexone (but not placebo), epinephrine concentrations after the CPT were higher in those receiving naltrexone compared to placebo 32.2 versus 28.9 pg/ml, although this difference was not statistically significant (figure 3a).
Figure 3.
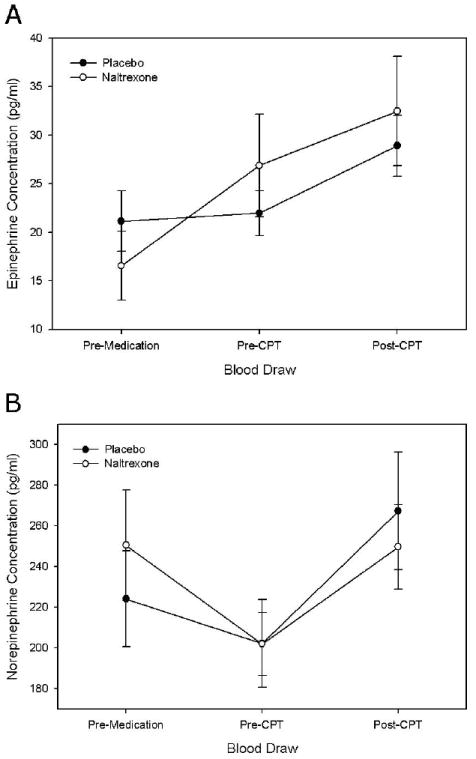
Plasma epinephrine (panel A) and norepinephrine (panel B) concentrations (mean ± SE) in subjects while receiving placebo or naltrexone. Pre-CPT = Immediately prior to cold pressor test; Post-CPT = immediately following cold pressor test.
A significant treatment × period effect was also found for plasma norepinephrine concentrations [F(2, 14) = 5.4, P < 0.02], however the pattern was that there was a greater decrease in plasma norepinephrine concentrations during the one hour period after medication and a smaller increase in response to the CPT (figure 3b).
No differences were observed between treatments in measures of SBP, DBP, HR, McGill Pain Questionnaire score or pain ratings. Although cortisol concentrations increased after the administration of the cold presor test as indicated by a main effect of period (F (7, 10) = 9.32; p < 0.001), no significant differences were observed between treatments (Figure 2). Distress increased [F(7, 11) = 3.44, p = 0.03)] and positive affect decreased with time [F(7, 11) = 3.2, p = 0.04] during the CPT as indicated by significant main period effects, although no significant treatment by period effects were found.
Figure 2.
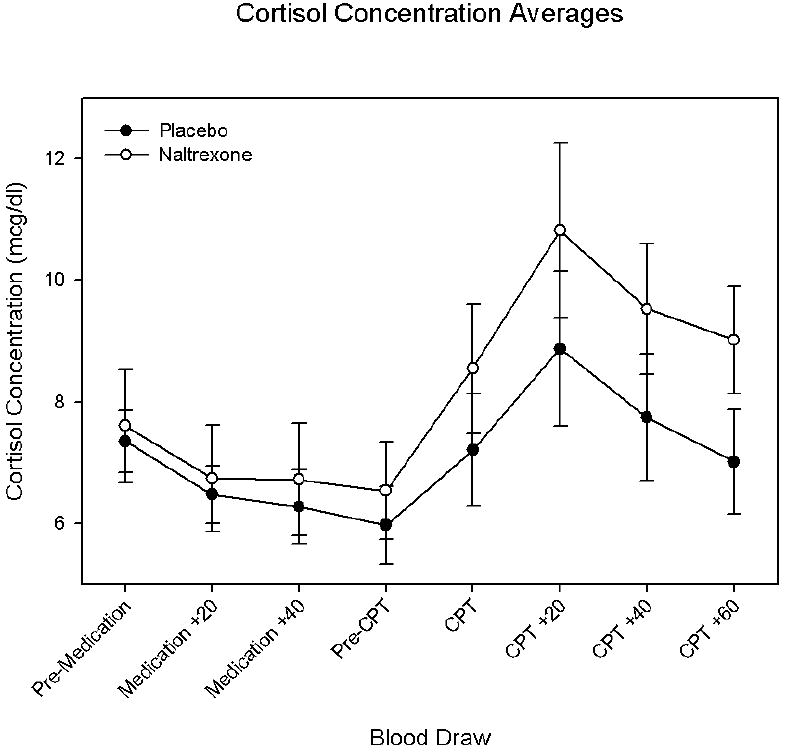
Plasma cortisol concentrations (mean ± SE) in subjects while receiving placebo or naltrexone. Pre-CPT = Immediately prior to cold pressor test; CPT = immediately following cold pressor test; CPT + 20 = 20 minutes following cold pressor test; CPT + 40 = 40 minutes following cold pressor test; CPT + 60 = 60 minutes following cold pressor test.
Discussion
The main finding is that naltrexone administration prior to a CPT has significant effects on plasma catecholamine (i.e. epinephrine, norepinephrine) concentrations, particularly epinephrine concentrations which increased after naltrexone administration (but prior to the CPT).
The data regarding opioid antagonist (i.e. naloxone, naltrexone) effects on HR and BP have been somewhat inconsistent; however most data suggest that opioid blockade increases epinephrine and cortisol concentrations (al’Absi et al 2004; Bouloux et al 1985; Bouloux et al 1989; Hughes et al 1991; Litschauer et al 2005; Morris et al 1990). Inconsistent results may perhaps be due to differences related to endogenous opioid levels of those studied. For example, one study found that naloxone only potentiated the BP response to a mental stressor in those with low baseline BP (McCubbin et al 1988). Another that naltrexone increased the HR response to a math task only in aerobically fit individuals (McCubbin et al 1992). Additionally, gender may impact naltrexone’s effect on blood pressure response to a CPT (al’Absi et al 2004). The data as a whole therefore suggest that in certain subpopulations of subjects, opioid blockade increases sympathetic response to either a mental or a cold presor stressor and may also have effects on resting values. Effects on epinephrine and cortisol appear to be most consistent.
Several limitations are present in this study. The small sample size does not permit sub-analyses to be performed to determine predictors of naltrexone response and likely explains the lack of significant cortisol effects despite relatively consistent reports of opioid blockers increasing cortisol concentrations (although the trends observed were in the predicted duration). A limitation related to the catecholamine data is the timing of the post cold-pressor blood draw. Because catecholamine concentrations change rapidly, a better measure of catecholamine response would have been to draw blood while their hand was submerged. Additionally, the time between removal of the hand from ice water and drawing of the blood varied slightly between lab sessions due to factors such as how long it took the nurse to start the blood draw and how long it took for blood to be collected. Nonetheless, the data are generally consistent with previously published reports and add to the totality of information regarding the interactions between the endogenous opioid system and the hypothalamic-pituitary-adrenal axis and the hypothalamo-sympathetic axis. Free cortisol concentrations (i.e. the biologically active component) were not measured in this study; however it is unlikely that the unbound fraction would have changed substantially in healthy volunteers during the course of this relatively short study. Other studies have similarly measured total cortisol concentrations and have found it is affected by the cold pressor task and by opioid blockade.
In summary, this study found that naltrexone administration alters baseline and cold-pressor induced plasma catecholamine concentrations. Further research is necessary to determine the subpopulations in which these effects are of greatest magnitude and the long term safety implications of these effects.
Acknowledgments
We thank Angie Harju, Deanna Ellestad, and Jennifer Harris for help with data collection and management. This study was supported in part by a pilot grant from the Minnesota TTURC grant DA013333 to the second author and Grant # M01-RR00400 from the General Clinical Research Centers program of the National Center for Research Resources. During this research Dr. Kotlyar was also supported by a grant from the National Institute on Drug Abuse (K23DA017307) and Dr. al’Absi was also supported by grants from the National Institute on Drug Abuse (DA013435; DA016351) and the National Cancer Institute (CA 88272).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Petersen KL. Blood pressure but not cortisol mediates stress effects on subsequent pain perception in healthy men and women. Pain. 2003;106:285–295. doi: 10.1016/S0304-3959(03)00300-2. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Petersen KL, Wittmers LE. Blood pressure but not parental history for hypertension predicts pain perception in women. Pain. 2000;88:61–68. doi: 10.1016/S0304-3959(00)00306-7. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, et al. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med. 2004;66:198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- Bouloux PM, Grossman A, Al-Damluji S, Bailey T, Besser M. Enhancement of the sympathoadrenal response to the cold-pressor test by naloxone in man. Clin Sci (Lond) 1985;69:365–368. doi: 10.1042/cs0690365. [DOI] [PubMed] [Google Scholar]
- Bouloux PM, Newbould E, Causon R, Perry L, Rees LH, Besser GM, et al. Differential effect of high-dose naloxone on the plasma adrenaline response to the cold-pressor test. Clin Sci (Lond) 1989;76:625–630. doi: 10.1042/cs0760625. [DOI] [PubMed] [Google Scholar]
- Gonzalez JP, Brogden RN. Naltrexone. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs. 1988;35:192–213. doi: 10.2165/00003495-198835030-00002. [DOI] [PubMed] [Google Scholar]
- Hughes GS, Jr, Ringer TV, Francom SF, Caswell KC, DeLoof MJ, Spillers CR. Effects of fish oil and endorphins on the cold pressor test in hypertension. Clin Pharmacol Ther. 1991;50:538–546. doi: 10.1038/clpt.1991.179. [DOI] [PubMed] [Google Scholar]
- Kim SW, Grant JE, Adson DE, Shin YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49:914–921. doi: 10.1016/s0006-3223(01)01079-4. [DOI] [PubMed] [Google Scholar]
- Litschauer B, Schaller G, Wolzt M. Naloxone does not influence cardiovascular responses to mild mental stress in postmenopausal women. Am J Physiol Heart Circ Physiol. 2005;289:H2120–2125. doi: 10.1152/ajpheart.01113.2004. [DOI] [PubMed] [Google Scholar]
- McCubbin JA, Cheung R, Montgomery TB, Bulbulian R, Wilson JF. Aerobic fitness and opioidergic inhibition of cardiovascular stress reactivity. Psychophysiology. 1992;29:687–697. doi: 10.1111/j.1469-8986.1992.tb02047.x. [DOI] [PubMed] [Google Scholar]
- McCubbin JA, Surwit RS, Williams RB., Jr Opioid dysfunction and risk for hypertension: naloxone and blood pressure responses during different types of stress. Psychosom Med. 1988;50:8–14. doi: 10.1097/00006842-198801000-00002. [DOI] [PubMed] [Google Scholar]
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- Morris M, Salmon P, Steinberg H, Sykes EA, Bouloux P, Newbould E, et al. Endogenous opioids modulate the cardiovascular response to mental stress. Psychoneuroendocrinology. 1990;15:185–192. doi: 10.1016/0306-4530(90)90029-9. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Cooney JL, Krishnan-Sarin S, Dubin JA, McKee SA, Cooney NL, et al. A controlled trial of naltrexone augmentation of nicotine replacement therapy for smoking cessation. Arch Intern Med. 2006;166:667–674. doi: 10.1001/archinte.166.6.667. [DOI] [PubMed] [Google Scholar]
- Roozen HG, de Waart R, van der Windt DA, van den Brink W, de Jong CA, Kerkhof AJ. A systematic review of the effectiveness of naltrexone in the maintenance treatment of opioid and alcohol dependence. Eur Neuropsychopharmacol. 2006;16:311–323. doi: 10.1016/j.euroneuro.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. European heart journal. 2003;24:690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Velasco M, Gomez J, Blanco M, Rodriguez I. The cold pressor test: pharmacological and therapeutic aspects. Am J Ther. 1997;4:34–38. doi: 10.1097/00045391-199701000-00008. [DOI] [PubMed] [Google Scholar]


