Abstract
Most advanced non–small-cell lung cancers (NSCLCs) with activating epidermal growth factor receptor (EGFR) mutations (exon 19 deletions or L858R) initially respond to the EGFR tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib. However, over time (median of 6−12 months), most tumors develop acquired resistance to EGFR TKIs. Intense research in these NSCLCs has identified two major mechanisms of resistance to gefitinib/erlotinib: secondary resistance mutations and “oncogene kinase switch” systems. The secondary T790M mutation occurs in 50% of EGFR-mutated patients with TKI resistance, and in vitro, this mutation negates the hypersensitivity of activating EGFR mutations. Sensitive detection methods have identified a proportion of TKI-naive tumors that carry T790M, and these resistant clones may be selected after exposure to gefitinib or erlotinib. Other secondary resistance mutations (D761Y, L747S, T854A) seem to be rare. The amplification of the MET oncogene is present in 20% of TKI-resistant tumors; however, in half of the cases with this “oncogene kinase switch” mechanism the T790M is coexistent. It is possible that other kinases (such as insulin-like growth factor-1 receptor [IGF-1R]) might also be selected to bypass EGFR pathways in resistant tumors. The growing preclinical data in EGFR-mutated NSCLCs with acquired resistance to gefitinib or erlotinib has spawned the initiation or conception of clinical trials testing novel EGFR inhibitors that in vitro inhibit T790M (neratinib, XL647, BIBW 2992, and PF-00299804), MET, or IGF-1R inhibitors in combination with EGFR TKIs, and heat shock protein 90 inhibitors. Ongoing preclinical and clinical research in EGFR-mutated NSCLC has the potential to significantly improve the outcomes of patients with these somatic mutations.
Keywords: BIBW 2992, D761Y, Gefitinib, ErbB3, Erlotinib, HKI-272, L747S, MET, PF-00299804, T790M, XL647
Introduction
Lung cancer continues to be the leading cause of cancer deaths in the United States.1 First-line platinum-based chemotherapy, the standard of care for patients with advanced non–small-cell lung cancer (NSCLC), yields a response rate (RR) of 30% or less and a median overall survival (OS) of fewer than 12 months even with the addition of the vascular endothelial growth factor (VEGF) antibody bevacizumab.2,3 Two chemotherapies, docetaxel4 and pemetrexed,5 can be used in second-line therapy for advanced NSCLC. However, the prognosis for patients with advanced NSCLC remains poor.
Two anilinoquinazoline epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TK; TKIs) have gained approval for use in unselected patients with NSCLC in the second- and third-line setting after failure of first-line platinum-based chemotherapy.6,7 The first compound, developed by AstraZeneca, was designated ZD1839 and later renamed gefitinib. Soon after, OSI Pharmaceuticals started its clinical development of the related compound OSI 774, later renamed erlotinib. Erlotinib, in the second- or third-line setting of NSCLC, derived a small, statistically significant improvement in survival compared with placebo, but the RR was only 8.9%, median progression-free survival (PFS) was 2.2 months, and OS was 6.7 months in the treatment arm.7 Gefitinib was not statistically better than placebo in controlling disease progression and OS in the overall cohort of previously treated NSCLC, despite a benefit in the prespecified subgroups of never-smokers and Asian patients.7
Insight into the selective response of a fraction of NSCLC to these agents occurred in 2004, with the identification of oncogenic mutations in the EGFR gene. Two groups from the Dana-Farber/Harvard Cancer Center (DFHCC) and one from Memorial Sloan-Kettering Cancer Center (MSKCC) identified somatic mutations in the TK domain of EGFR in most patients with NSCLC responsive to gefitinib or erlotinib.8-10 EGFR mutations are more common in NSCLC from tumors with adenocarcinoma histology, in women, in Asian patients, and in never-smokers.11-13 EGFR mutations are seldom found in squamous cell carcinomas of the lung, small-cell lung cancer, or other epithelial malignancies. Thus, activating somatic EGFR mutations are a unique feature of a subclass of NSCLC.
The most prevalent EGFR mutations consist of small inframe deletions around the conserved LREA motif of exon 19 (corresponding to amino acid residues 747−750) and a point mutation (L858R) in exon 21,13,14 which account for more than 90% of all EGFR kinase mutations. These EGFR mutations activate the EGFR signaling pathway and promote EGFR-mediated prosurvival and antiapoptotic signals through downstream targets, such as AKT-PI3K, ERK, and STAT.15 Inhibition of the EGFR network leads to upregulation of proapoptotic molecules, such as BIM, that activated the intrinsic mitochondrial apoptotic pathway.16-19 These signaling cascades make these EGFR-mutated cells dependent on a functional EGFR for their survival,14 and hence, they become “oncogene addicted” to EGFR. Therefore, EGFR-mutated NSCLC has an “Achilles’ heel” in the inhibition of EGFR by small-molecule TKIs.9,20,21
Retrospective studies of patients treated with gefitinib and erlotinib demonstrated that close to 80% of NSCLCs with classic EGFR mutant tumors attain radiographic responses to these oral agents.12,22 In some reports, PFS and OS were significantly better for EGFR TKI–treated patients with EGFR mutations than with EGFR wild-type.12 More than 8 prospective trials have evaluated gefitinib or erlotinib monotherapy in EGFR-mutated NSCLC.23-31 These have confirmed that around 75% of patients with L858R or exon 19 deletion mutations achieve responses (Table 1). However, in almost all reports, the PFS did not exceed 12 months32 and acquired resistance developed in most patients.
Table 1.
Prospective Clinical Trials of Gefitinib or Erlotinib for Patients with Advanced Non–Small-Cell Lung Cancer and Activating Epidermal Growth Factor Receptor Mutations
| Study | Drug | RR, n (%) | Median TTP/PFS, Months (95% CI) | 1-Year Survival, % | Median OS, Months | Median Follow-up, Months |
|---|---|---|---|---|---|---|
| Inoue et al23 | Gefitinib | 12 of 16 (75) | 9.7 (7.4−9.9) | NR | Not reached | 7.6 |
| Asahina et al24 | Gefitinib | 12 of 16 (75) | 8.9 (6.7−11.1) | 83 | Not reached | 12.7 |
| Sutani et al25 | Gefitinib | 21 of 27 (78) | 9.4 (NR) | NR | 15.4 | NR |
| Yoshida et al26 | Gefitinib | 19 of 21 (90.5) | 7.7 (NR) | 80 | Not reached | NR |
| Sunaga et al27 | Gefitinib | 16 of 19 (84) | 12.9 (NR) | 85 | Not reached | 12.6 |
| Tamura et al28 | Gefitinib | 21 of 28 (75) | 11.5 (7.3-Not reached) | 79 | Not reached | 18.6 |
| Sequist et al29 | Gefitinib | 17 of 31 (55)* | 9.2 (6.2−11.8) | 73 | 17.5 | 12.3 |
| Paz-Aires et al†30 | Erlotinib | 31 of 38 (82) | 13.3 (NR) | 82 | Not reached | 7 |
| Porta et al†31 | Erlotinib | 111 of 152 (73) | 12 (NR) | NR | 24 | 10 |
This trial included patients with other EGFR mutations such as G719A, L861Q, T790M/L858R, and exon 20 insertion. None of these patients had a response. The RR for L858R was 78% and for exon 19 deletions was 59%.
Data updated from American Society of Clinical Oncology meeting presentations.
Abbreviations: NR = not reported in the published material; OS = overall survival; PFS = progression-free survival; RR = response rate; TTP = time to progression
This review focuses on the clinically relevant mechanisms of acquired resistance to EGFR TKIs and discusses ongoing phase I/II clinical trials for patients with NSCLC and acquired resistance to gefitinib or erlotinib. Mechanisms of primary resistance to EGFR-targeted therapy, such as K-ras mutations and EGFR exon 20 insertion mutations, have been reviewed elsewhere.13,33,34 Because the mechanisms of sensitivity and resistance to EGFR TKIs have not been clearly established in EGFR wild-type NSCLC, we will address the well-established mechanisms that have been described in EGFR-mutated NSCLC.
Mechanisms of Acquired Resistance to Gefitinib and Erlotinib
Secondary Mutations: EGFR T790M
The first identified mechanism of acquired resistance to EGFR TKIs was the EGFR T790M mutation by investigators at the DFHCC35 and MSKCC36 in 2005. Both groups analyzed patients with NSCLC harboring either exon 19 deletions or the L858R mutation that progressed after a period of response to gefitinib or erlotinib. In postprogression biopsies, the original EGFR mutation and the novel T790M in exon 20 were identified.35,36 There are many similarities among structures of TKs, and T790M (EGFR, NSCLC) is analogous to T315I (ABL1, chronic myeloid leukemia [CML]) and T670I (KIT, gastrointestinal stromal tumors [GIST]).37
When T790M was introduced in vitro to sequences containing wild-type EGFR, exon 19 deletion–EGFR, or L858R-EGFR, the resulting proteins were significantly more resistant to gefitinib in the constructs containing T790M.35,38 The inhibitory concentrations to erlotinib and gefitinib in T790M-containing constructs exceeded 5 μM, which is a concentration more than 100-fold higher than that required to inhibit exon 19 deletions or L858R-EGFR.39 A NSCLC cell line with the L858R-T790M mutation (H1975) was significantly more resistant to gefitinib or erlotinib than lines with L858R (H3255) and an exon 19 deletion (HCC827).36,38,40 The T790M mutation is most often seen in cis; however, it can occur in trans, to L858R or exon 19 deletions.35,40 EGFR-mutated cell lines that have L858R-T790M or exon 19 deletions-T790M continue to be dependent on EGFR, because alternative EGFR inhibitors (which will be described in greater detail below) halt cell proliferation and lead to apoptosis.38,40,41 Some EGFR-mutated NSCLC cell lines (H3255 and PC-9) that are exposed to incremental concentrations of gefitinib in culture end up acquiring T790M,40,42 and mouse lung cancer models of L858R-T790M confirmed that these tumors are resistant to gefitinib and erlotinib.43 These preclinical and clinical data support T790M as a main mechanism of resistance to EGFR TKIs.
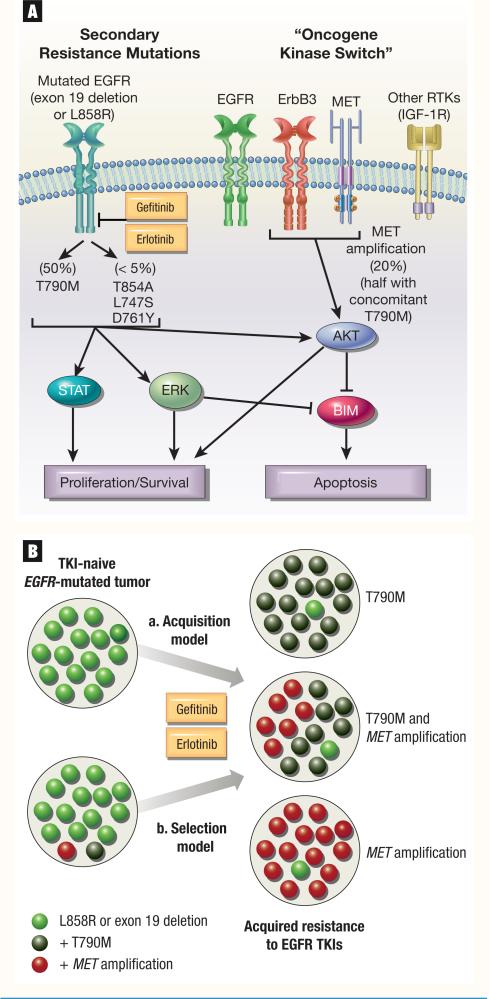
Consistently, T790M has been identified in half of NSCLC patients with acquired resistance to gefitinib and erlotinib (Figure 1A) in North American and Asian cohorts of EGFR-mutated patients who underwent re-biopsy after progression.40,44-46
Figure 1. Mechanisms of Acquired Resistance to Gefitinib/Erlotinib in Epidermal Growth Factor Receptor–Mutated Non–Small-Cell Lung Cancer.
A. Secondary resistance mutations and “oncogene kinase switch” models that account for resistance. B. A model of “acquisition” (a) versus “selection” (b) of T790M and MET amplification during the course of treatment of EGFR-mutated tumors with gefitinib/erlotinib.
Abbreviations: EGFR = epidermal growth factor receptor; IGF-1R = insulin-like growth factor-1 receptor; RTK = receptor tyrosine kinase; TKI = tyrosine kinase inhibitor
How T790M affects the hypersensitivity of activating EGFR mutations is still not completely clear. Initially, it was speculated, based on the crystallographic structure of the kinase domain of EGFR, that the bulkier methionine residue of the “gatekeeper” T790M changed the ATP-binding pocket of the kinase, therefore blocking the engagement of erlotinib or gefitinib.35 However, more recently, it was demonstrated that T790M affected minimally the binding of gefitinib to L858R-EGFR.47 Instead, L858R-T790M-EGFR had increased affinity to ATP when compared with L858R alone, which is predicted to decrease binding of gefitinib and erlotinib because these drugs are ATP-competitive kinase inhibitors.47 These findings will certainly affect the development of the next generation of EGFR inhibitors with the ability to overcome T790M.
Much debate also exists in regard to the “selection” or “acquisition” of T790M in EGFR-mutated systems (Figure 1B). In the original reports, preprogression samples lacked T790M, and it was thought that this abnormality was acquired only after exposure to gefitinib or erlotinib.35,36 However, in rare instances, T790M has been identified in biopsy specimens of treatment-naive NSCLCs using standard sequencing techniques.29,48,49 A group from the DFHCC used a circulating tumor cell chip to capture circulating tumor cells in the blood of EGFR-mutated NSCLC patients, and identified the T790M mutation in 10 of 26 (38%) pretreatment samples using a sensitive polymerase chain reaction method.50 Interestingly, they found that a relatively high number of amplification cycles were required to detect T790M, suggesting that the mutation was present in only a fraction of the tumor cells.50 Serial analyses of EGFR-mutated patients treated with gefitinib and erlotinib revealed the increased prevalence of the resistant T790M allele within circulating tumor cells over time.50 The presence of pretreatment T790M did not preclude the response to gefitinib or erlotinib but was prognostic of a shorter time to progression on EGFR TKIs in these EGFR-mutated patients.50 These findings might be indicative that T790M is not uncommon in some tumor clones of TKI-naive patients with EGFR mutations, and clones with this alteration are selected for after treatment with EGFR TKIs (Figure 1B). The clinical use of noninvasive methods to detect T790M is ongoing evaluation, but assays that test for tumor-derived DNA in plasma or circulating tumors cells might one day supplement the need for a repeat biopsy to identify this mechanism of resistance.51
Other Secondary Mutations
To date, few secondary mutations other than T790M have been described in patients with acquired resistance to gefitinib or erlotinib (Figure 1A). Interestingly, in the case of CML and GIST, many distinct mutations have been described in the BCR-ABL and KIT kinase domains from patients with resistance to imatinib.52,53 The predominance of T790M as a secondary mutation in NSCLC could be because of the binding of gefitinib/erlotinib to the active conformation of EGFR, while imatinib binds to the inactive conformation of ABL and KIT.44
The first non-T790M secondary mutation described was D761Y. The patient that acquired this change had a background of L858R-EGFR, and the compound L858R-D761Y mutation was identified in a growing central nervous system lesion.44 In vitro, L858R-D761Y was more resistant to the inhibitory effects of gefitinib when compared with L858R but many fold less resistant than L858R-T790M.44 We described the L747S secondary mutation in the pleural effusion of a L858R-EGFR–mutated patient who had a prolonged response to single-agent gefitinib before detection of L747S.16 L858R-L747S is more resistant in vitro to gefitinib/erlotinib than L858R alone; however, it demonstrated a pattern of resistance that was less pronounced than that observed with L858R-T790M. In TKI-naive patients, both D761Y54,55 and L747S56 have been described, and the few patients treated with gefitinib with these mutations did not have responses. The mechanism of resistance of these non-T790M mutations is unclear, but they might affect the conformation of the active and inactive states of L858R-EGFR and consequently the binding of gefitinib.16 One interesting clinical aspect of the in vitro studies with L858R-L747S and L858R-D761Y is that doses of gefitinib or erlotinib that reach 1 μM are able to inhibit these EGFR mutants, which indicates that in these rare patients, a dose escalation of a reversible TKI (either by increasing the dose of gefitinib or switching to erlotinib) could potentially overcome their initial resistance to gefitinib as will be described herein.57 More recently, the T854A mutation was identified in a patient with L858R-EGFR NSCLC after long-term treatment with gefitinib and erlotinib.58 In vitro L858R-T854A was resistant to erlotinib, and the crystal structure of EGFR suggests the T854 side chain is within contact distance of gefitinib and erlotinib.58 A recent in vitro erlotinib-resistance screen also disclosed L858R-T854A as an erlotinib-resistant mutation with 3-fold increase in inhibitory concentration when compared with L858R-EGFR alone, while L858R-T790M was more than 300-fold more resistant than L858R.39
Overall, based on the largest patient series of EGFR-mutated TKI-resistant patients,44,45,58 the frequency of non-T790M mutations is below 5%.
MET Amplification and Other “Kinase Switch” Mechanisms
In other “oncogene-addicted” cancers, acquired resistance to TKIs can develop through a “kinase switch” mechanism. In GIST, cells dependent on mutated KIT have been able to overcome imatinib by switching their dependency to AXL (another TK) that sustained common downstream signaling pathways.59
A similar mechanism occurs in EGFR-mutated tumors with acquired resistance to gefitinib or erlotinib. In 2007, 2 groups identified the acquired amplification of MET as a cause of TKI resistance.60,61 The amplification of MET, a transmembrane TK receptor, is seen in gastric and esophageal cancers62,63 but is less common in untreated NSCLC.64-67 However, both MET expression and mutations are present in NSCLC.66
Acquired amplification of the wild-type MET oncogene was identified in approximately 20% of EGFR-mutated patients with acquired resistance to gefitinib or erlotinib.60,61 In most cases, MET was not amplified in pretreatment specimens. The methods of identification of MET (either fluorescence in situ hybridization or quantitative DNA analysis) have not been compared head-to-head in patient's samples, and it is unclear if the method used could affect the percentage of cases with this abnormality. MET couples with other ErbB members, specifically ErbB3, and activates downstream signals mediated by AKT that bypass the inhibited EGFR.60 The in vitro resistance to erlotinib and gefitinib in this model was in the range of 5−10 μM. Inhibition of MET signaling by RNA interference restored sensitivity to gefitinib, and dual inhibition of EGFR and MET with TKIs was able to overcome MET-amplified EGFR TKI–resistant tumors.60 In almost half of the patients with MET amplification, T790M was identified either in the same biopsy specimen or in other sites of the same patient.60,61 In some instances, patients with EGFR-activating mutations and primary resistance to gefitinib had concomitant MET amplification,29 and it is possible that MET-amplified clones are selected in the presence of gefitinib or erlotinib (Figure 1B).
Other kinases might also participate in similar “kinase switch” alterations in EGFR-dependent NSCLCs that become resistant to gefitinib or erlotinib (Figure 1A). Although no patient data support this assumption, recent in vitro models of resistance to EGFR TKIs have shown that the insulin-like growth factor (IGF)-1 receptor (IGF-1R) can activate downstream targets that bypass dependency in EGFR.68,69 In an EGFR wild-type–amplified cell line, loss of IGF binding proteins with subsequent activation of IGF-1R was seen when the cells were made resistant to gefitinib in culture.68 In the H1975 NSCLC cell line (L858R-T790M-EGFR) chronic exposure to increasing concentrations of the EGFR inhibitor CL-387,785 resulted in an EGFR TKI–resistant line with activated IGF-1R.69 Because the identification of IGF-1R activation could be technically challenging in human samples, it might be difficult to replicate these results in NSCLC samples from TKI-resistant patients.
Ongoing research is attempting to identify other mechanisms that account for the 30%-40% of EGFR TKI–resistant EGFR-mutated tumors that do not carry secondary resistance mutations or MET amplification.
Clinical Management of Patients with Acquired Resistance to Gefitinib or Erlotinib
Continued Use of Gefitinib/Erlotinib
In EGFR-mutated patients with acquired resistance to either gefitinib or erlotinib continued EGFR inhibition might be clinically beneficial, akin to the continued blockage of ErbB2 (HER2) with trastuzumab in ErbB2-amplified breast cancers.70,71 The re-administration of gefitinib or erlotinib in 2 cohorts of previously TKI-responsive NSCLC patients upon radiographic progression decreased the rate of clinical deterioration and stabilized some of the growing lesions72,73; however, most tumors continued to display progressive disease. Ongoing randomized phase II trials such as erlotinib plus pemetrexed versus pemetrexed alone in patients with a previous response to erlotinib (ClinicalTrials.gov identifier: NCT00660816) are attempting to address if maintaining some form of the original EGFR TKI (be it gefitinib or erlotinib) in addition to other lines of therapy is better than removing the TKI altogether in a clinically enriched population of NSCLC with resistance to gefitinib or erlotinib.
Another intriguing clinical question that has emerged asks if patients initially responsive to gefitinib should receive erlotinib after tumor progression. Gefitinib's clinical dose of 250 mg/day is less than its maximum tolerated dose (MTD) of 1000 mg/day, and the mean steady state serum concentration of gefitinib at 250 mg/day does not exceed 0.5 μM.74 Erlotinib is used clinically at a dose of 150 mg/day,6 which is its MTD, and the median steady state trough concentrations at this dose exceeds 1.5 μM.75 In preclinical models, the in vitro concentrations of gefitinib/erlotinib that inhibit EGFR-mutated NSCLC cell lines that harbor T790M and/or MET amplification exceed 5 μM.35-38 Because gefitinib-resistant EGFR-mutated tumors predominantly have T790M and/or MET amplification, the prevailing hypothesis is that these tumors would be cross-resistant to erlotinib. Two recent Korean phase II trials of erlotinib 150 mg/day in patients with either primary or acquired resistance to gefitinib have shown that erlotinib has a very limited activity profile with a short time of disease control rate in these patients.76,77 Over 80% of the patients (36 of 44) had rapid tumor growth qualified as progressive disease (PD) with this gefitinib-to-erlotinib switch.76,77 In a cohort of 18 EGFR-mutated NSCLCs with resistance to gefitinib that subsequently received erlotinib, 78% of the patients had PD on erlotinib, and the median PFS was a dismal 2 months.78 Most patients with acquired resistance in that series carried the T790M mutation, and the only responding patient had the acquired L858R-L747S mutation, which in vitro can be inhibited by gefitinib/erlotinib concentrations of 1−2 μM that could be achieved by erlotinib 150 mg/day but not gefitinib 250 mg/day.78
Based on the data above, it seems that continued EGFR inhibition with gefitinib or erlotinib might have a role in the management of patients with EGFR mutations after resistance ensues; however, it is also clear that meaningful and sustained clinical and radiographic responses will seldom be obtained with such an approach, and alternative treatment options need to be explored.
Novel Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors
Initial steps have begun to use preclinical data for rationale design of clinical trials of patients with acquired resistance to gefitinib or erlotinib. In the original publication of EGFR-T790M, the authors had demonstrated that an irreversible EGFR inhibitor (CL-387,785, an ATP mimetic that covalently bind to Cys-797 of EGFR) was able to in vitro inhibit T790M in cis to activating exon 19 deletion mutation at lower concentrations than gefitinib or erlotinib.35,38 Following that original observation, many groups have shown that other irreversible and second-generation EGFR inhibitors in vitro can partially overcome L858R-T790M and exon 19 deletions-T790M EGFR mutations.37,41,79-82 In some cases, the drugs tested in vitro have already entered the clinical arena.83
The above-mentioned findings have spawned phase II trials to address if these novel EGFR inhibitors will be useful in patients with EGFR T790M and/or acquired resistance to gefitinib or erlotinib. Table 2 describes 4 compounds (neratinib [HKI-272], XL647, BIBW 2992, and PF-00299804) that are being tested in this setting.
Table 2.
Completed or Planned Phase II Clinical Trials of Novel Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for Patients with Acquired Resistance to Gefitinib/Erlotinib or the T790M Mutation
| Compound (Type of Tyrosine Kinase Inhibitor) |
Manufacturer |
ClinicalTrials.gov Identifier |
Population of Interest |
Preclinical Rationale | Status | Comments* |
|---|---|---|---|---|---|---|
| Neratinib (HKI-272; Irreversible Pan ErbB–EGFR/ErbB2/ErbB3) | Wyeth | NCT00266877 | Failed gefitinib/erlotinib after previous benefit and EGFR mutation or EGFR wild-type | Active against cell lines and mice carrying EGFR mutations with/without T790M; however, inhibitory dose higher in EGFR T790M than L858R or exon 19 deletion alone; active in EGFR exon 20 insertion mutations | Completed accrual (number estimated: 138) | No radiographic response in phase I trial that included patients with EGFR mutations and resistance to gefitinib/erlotinib85; lack of TKI-induced rash in phase I trial |
| XL647 (EGFR/ErbB2/VEGFR-2/EphB4) | Exelixis Inc. | NCT00522145 | Failed gefitinib/erlotinib after previous benefit and/or EGFR T790M mutation | Active against cell line carrying EGFR L858R mutation with T790M (H1975) | Completed accrual (number estimated: 34) | No radiographic responses seen in patients with T790M mutation; XL647 has activity against exon 19 deletions and L858R EGFR mutations in a phase II trial for EGFR TKI-naive patients87 |
| BIBW 2992 (Irreversible Dual EGFR/ErbB) | Boehringer Ingelheim GmbH | NCT00711594 | Failed chemotherapy and gefitinib/erlotinib; requires previous benefit from gefitinib/erlotinib | Active against cell lines and mice carrying EGFR mutations with/without T790M; however, inhibitory dose higher in EGFR T790M than EGFR-L858R alone; active in EGFR exon 20 insertion mutations | Ongoing (number estimated: 72) | Phase II trial in EGFR-mutated patients showed responses in patients with EGFR exon 19 deletion, L858R, L861Q, and G719S/S768I mutations89; a phase IIb/III randomized trial of BIBW 2992 vs. placebo in third-line setting of NSCLC after failure of gefitinib/erlotinib is planned (NCT00656136; name: LUX-Lung 1) |
| PF-00299804 (Irreversible Pan ErbB–EGFR/ErbB2/ErbB3) | Pfizer Inc. | NCT00548093 | Failed chemotherapy and erlotinib; K-ras wild-type | Active against cell lines carrying EGFR mutations with/without T790M; however, inhibitory dose higher in EGFR T790M; active in EGFR exon 20 insertion mutations | Ongoing (number estimated: 74) | Phase I trial showed response in a patient with an exon 20 insertion mutation90 |
See text for further details.
Abbreviations: EGFR = epidermal growth factor receptor; NSCLC = non–small-cell lung cancer; TKI = tyrosine kinase inhibitor; VEGFR = vascular endothelial growth factor receptor
Neratinib was one of first compounds to be tested in this specific population. This is an irreversible pan-ErbB TKI (EGFR, ErbB2, ErbB3) that in vitro and in vivo has activity against classic EGFR mutations (L858R, exon 19 deletions), exon 20 EGFR insertions (which are more resistant to gefitinib/erlotinib), amplified or mutated ErbB2, and compound EGFR mutations with T790M.41,84,85 In a phase I trial of neratinib for patients with solid tumors, the MTD was determined as 320 mg/day, and 12 patients with NSCLC were enrolled.85 Despite no responses noted in the NSCLC cohort, 5 patients with acquired resistance to gefitinib/erlotinib had stable disease for more than 24 weeks.85 These findings led to a 3-arm randomized trial of neratinib at 240 mg/day in patients with NSCLC who had progressed after clinical benefit of gefitinib/erlotinib or were never- or light smokers (ClinicalTrials.gov identifier: NCT00266877). The accrual for this trial is complete, and the arms were divided into patients with or without EGFR mutations. Results are anticipated and should help determine if neratinib is active or inactive in this select patient population that is enriched for patients with acquired resistance to TKIs because of T790M or MET amplification in the EGFR-mutated arm. However, since the initiation of the trial, additional preclinical data have become available for neratinib. In vitro, an EGFR exon 19 deletion–mutated cell line (PC-9) exposed to neratinib at doses achieved in the phase I clinical trial85 acquired EGFR T790M.86 In a mouse model of EGFR L858R-T790M, neratinib alone was ineffective in generating a radiographic response in established tumors.43 These data, and the fact that neratinib did not lead to development of rash (a surrogate marker of EGFR TKI dose) in patients,85 raise the possibility that, at the achievable clinical concentrations of this and other novel EGFR inhibitors, T790M will still not be inhibited.
XL647 is a reversible TKI of EGFR, HER2, VEGF receptor (VEGFR)-2, and EphB4, among other kinases. In an EGFR L858R-T790M–mutated model (H1975), XL647 was able to inhibit the growth of tumors with a lower concentration than that achieved by gefitinib or erlotinib.81 In a phase II trial of an enriched population of NSCLC with chemotherapy-naive EGFR-mutated tumors, XL647 had activity against classic (L858R, exon 19 deletions) EGFR mutations.87 These findings led to a phase II trial of XL647 at 300 mg/day in patients with NSCLC who had clinical benefit with erlotinib/gefitinib for over 3 months before progression or the T790M mutation (ClinicalTrials.gov identifier: NCT00522145). The trial tested for T790M in the plasma of all patients. Accrual is complete, and preliminary results were presented in 2008.88 Of the estimated 34 patients enrolled, only 1 achieved a partial response. This never-smoker had an exon 19 deletion in her original tumor, had no T790M detected in her plasma and had been off of erlotinib for over 6 months. None of the patients with T790M reported achieved a radiographic response, and most of the patients had PD within the first 2 months of study drug use.88 Final results of the study are awaited to determine the median time to progression, rate of stable or progressive disease, and extent of toxicities.
BIBW 2992 is another irreversible TKI of EGFR and ErbB2. In vitro and in vivo models of EGFR-mutated NSCLC have shown that BIBW 2992 might inhibit EGFR-mutated tumors with lower concentrations than neratinib.82 Specifically, BIBW 2992 was able to shrink tumors in transgenic mice with the L858R-T790M mutation and was effective in exon 20 insertion EGFR mutations.82 In a phase II trial of patients with EGFR mutations (ClinicalTrials.gov identifier: NCT00525148), BIBW 2992 led to responses in patients with EGFR exon 19 deletion, L858R, L861Q, and G719S/S768I mutations.89 These data have led to the launch of a clinical trial of BIBW 2992 at 50 mg/day as third-line therapy for NSCLC patients who have failed chemotherapy and had previous clinical benefit from gefitinib/erlotinib before progression (ClinicalTrials.gov identifier: NCT00711594). A phase IIb/III randomized trial of BIBW 2992 versus placebo in the third-line setting of NSCLC after failure of gefitinib/erlotinib is also planned (ClinicalTrials.gov identifier: NCT00656136; LUX-Lung 1). Results of the above-mentioned trials will help determine if BIBW 2992 will have clinical activity against EGFR-mutated tumors with acquired resistance to gefitinib/erlotinib.
PF-00299804 is a pan-ErbB TKI with a better in vitro and in vivo inhibition profile against EGFR-T790M than gefitinib or erlotinib.79 The drug also has activity against exon 20 EGFR insertion mutations.79 Furthermore, PF-00299804 showed adequate distribution in human tumor xenografts with optimal pharmacokinetic properties in preclinical studies.80 In a phase I trial of PF-00299804 for patients with advanced solid tumors, patients with EGFR mutations and resistance to gefitinib/erlotinib showed responses.90 A patient with an exon 20 insertion had a partial radiographic response.90 A phase II trial of third-line PF-00299804 at 45 mg/day for K-ras wild-type NSCLC patients who experienced failure with chemotherapy and erlotinib is ongoing (ClinicalTrials.gov identifier: NCT00548093) and will help determine the activity of this compound in EGFR-mutated patients with acquired resistance to gefitinib/erlotinib.
If neratinib, XL647, BIBW 2992, and PF-00299804 are not effective in the setting of acquired resistance to gefitinib/erlotinib in NSCLC, they still might have a role in the first-line treatment of EGFR TKI–naive patients with classic EGFR mutations (L858R or exon 19 deletions), atypical mutations less sensitive to gefitinib/erlotinib (exon 20 insertions), or in ErbB2-amplified breast cancer.85,87,89,90 A novel EGFR inhibitor specifically selected to target EGFR mutations with T790M could eventually have clinical activity in patients with NSCLC and acquired resistance to gefitinib/erlotinib.
Targeting Other Oncogenes in Addition to Epidermal Growth Factor Receptor
A new area of intense clinical investigation resides in the combination of different TKIs. Based on the novel findings that “kinase switch” mechanisms might play a role in the resistance to gefitinib/erlotinib in NSCLC,60,68 new trials are being developed based on these preclinical observations.
Because MET amplification was identified in a proportion of patients with acquired resistance to gefitinib/erlotinib,60,61 novel MET TKIs could be a viable treatment strategy for a small fraction of patients with MET amplification as the main mechanism of resistance. In preclinical models, EGFR-mutated cells with MET amplification responded to a combination of EGFR and MET TKIs but were resistant to each agent given alone.60 One small caveat of this approach is that almost half of the cases with MET amplification also displayed concomitant T790M,60,61 indicating that combination therapies with MET TKIs might need to be accompanied by an EGFR TKI capable of inhibiting T790M.91 XL184 is a novel TKI with activity against MET, VEGFR-2, and RET. A phase II clinical trial of XL184 with or without erlotinib for patients with NSCLC who have progressed after responding to erlotinib is planned if the combination is safe in the anteceding phase I trial (ClinicalTrials.gov identifier: NCT00266877). Trials with other MET inhibitors (such as ARQ 197, PF-2341066, SGX523) will follow.
Despite no patient data supporting that IGF-1R is responsible for acquired resistance to gefitinib/erlotinib, preclinical models indicate that this is a potential mechanism of resistance in different tumor types.68,69 Clinical trials of IGF-1R inhibitors with activity in unselected NSCLC patients, such as figitumumab (CP-751,871),92 in association with EGFR inhibitors will likely be planned for NSCLC with acquired resistance to gefitinib/erlotinib in the near future. It has also been shown that mutant EGFR can activate the gp130/JAK/STAT3 pathway by means of interleukin-6 upregulation in lung adenocarcinomas, which indicates that JAK inhibition in combination with EGFR TKIs might be effective in the treatment of mutant EGFR-driven tumors that are less sensitive or resistant to TKIs.93
Another area of interest is the use of inhibitors of multiple TKIs by a nonkinase-dependent mechanism. The heat shock protein (HSP) 90 chaperone system plays a role in the conformational maturation of kinases, including EGFR, ErbB2, and MET, among others.94 HSP90 inhibitors are active in preclinical models of NSCLCs bearing EGFR mutations with or without T790M.69 Phase I/II clinical trials of some of the HSP90 inhibitors, such as retaspimycin (IPI-504; ClinicalTrials.gov identifier: NCT00431015), for patients who have failed gefitinib/erlotinib, will help determine if these agents will have a role in the management of these “oncogene-addicted” tumors in the clinical arena.
Another attractive approach would be to target a common key effector pathway of receptor tyrosine kinases including EGFR. The proapoptotic BH3-only protein BIM was shown to be the most important molecule that determines the apoptotic signal of EGFR inhibition.16-19 BH3-mimetics, such as ABT-737 or ABT-263, have enhanced the effects of erlotinib and gefitinib in EGFR-mutated cells.17,18 If BH3-mimetics move forward in clinical development, it may be interesting to test them in NSCLCs with TKI resistance.
Conclusion
The rapid pace of preclinical work in the field of EGFR mutations and mechanisms of resistance to gefitinib and erlotinib continues to shed light on potential clinical approaches for this NSCLC subtype. The main mechanisms of resistance identified to date include secondary mutations and an “oncogene kinase switch.” The EGFR T790M mutation accounts for 50% of cases, other secondary resistance mutations are rare, and MET amplification is detected in 20% (however, half have concomitant T790M) of patients with EGFR-mutated TKI-resistant NSCLC. As always, clinical investigation lags behind the exciting laboratory findings of the field. However, a multitude of clinical trials evaluating novel EGFR, MET, IGF-1R, and HSP90 inhibitors in NSCLC are under way or planned for this growing cohort of patients with acquired resistance to gefitinib/erlotinib and/or EGFR mutations.
Acknowledgements
This work was supported by the National Institution of Health (NIH) grants K99CA126026-02 (to S.K.) and Specialized Program of Research Excellence (SPORE) in Lung Cancer 2PA50-CA090578-06 (to Daniel B. Costa); a Young Investigator Award (YIA) by the American Society of Clinical Oncology (to Daniel B. Costa), an American Association for Cancer Research (AACR)-Astra-Zeneca-Cancer Research and Prevention Foundation Fellowship in Translational Lung Cancer Research (to Daniel B. Costa), a Clinical Investigator Training Program from Beth Israel Deaconess Medical Center - Harvard/MIT Health Sciences and Technology in collaboration with Pfizer Inc. and Merck & Co. (to Daniel B. Costa).
Footnotes
Disclosures
The authors report no relevant conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 5.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 7.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005;366:1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 8.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 9.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 10.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–46. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 12.Sequist LV, Bell DW, Lynch TJ, et al. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–95. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 13.Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–41. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 14.Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 15.Sequist LV, Joshi VA, Janne PA, et al. Epidermal growth factor receptor mutation testing in the care of lung cancer patients. Clin Cancer Res. 2006;12:4403s–8s. doi: 10.1158/1078-0432.CCR-06-0099. [DOI] [PubMed] [Google Scholar]
- 16.Costa DB, Halmos B, Kumar A, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4:1669–79. doi: 10.1371/journal.pmed.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cragg MS, Kuroda J, Puthalakath H, et al. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4:1681–9. doi: 10.1371/journal.pmed.0040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong Y, Somwar R, Politi K, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng J, Shimamura T, Perera S, et al. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 2007;67:11867–75. doi: 10.1158/0008-5472.CAN-07-1961. [DOI] [PubMed] [Google Scholar]
- 20.Politi K, Zakowski MF, Fan PD, et al. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 2006;20:1496–510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji H, Li D, Chen L, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–95. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23:2513–20. doi: 10.1200/JCO.2005.00.992. [DOI] [PubMed] [Google Scholar]
- 23.Inoue A, Suzuki T, Fukuhara T, et al. Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol. 2006;24:3340–6. doi: 10.1200/JCO.2005.05.4692. [DOI] [PubMed] [Google Scholar]
- 24.Asahina H, Yamazaki K, Kinoshita I, et al. A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. Br J Cancer. 2006;95:998–1004. doi: 10.1038/sj.bjc.6603393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutani A, Nagai Y, Udagawa K, et al. Gefitinib for non-small-cell lung cancer patients with epidermal growth factor receptor gene mutations screened by peptide nucleic acid-locked nucleic acid PCR clamp. Br J Cancer. 2006;95:1483–9. doi: 10.1038/sj.bjc.6603466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida K, Yatabe Y, Park JY, et al. Prospective validation for prediction of gefitinib sensitivity by epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer. J Thorac Oncol. 2007;2:22–8. [PubMed] [Google Scholar]
- 27.Sunaga N, Tomizawa Y, Yanagitani N, et al. Phase II prospective study of the efficacy of gefitinib for the treatment of stage III/IV non-small cell lung cancer with EGFR mutations, irrespective of previous chemotherapy. Lung Cancer. 2007;56:383–9. doi: 10.1016/j.lungcan.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Tamura K, Okamoto I, Kashii T, et al. Multicentre prospective phase II trial of gefitinib for advanced non-small cell lung cancer with epidermal growth factor receptor mutations: results of the West Japan Thoracic Oncology Group trial (WJTOG0403). Br J Cancer. 2008;98:907–14. doi: 10.1038/sj.bjc.6604249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–9. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 30.Paz-Ares L, Sanchez JM, Garcia-Velasco A, et al. A prospective phase II trial of erlotinib in advanced non-small-cell lung cancer (NSCLC) patients (p) with mutations in the tyrosine kinase (TK) domain of the epidermal growth factor receptor (EGFR). J Clin Oncol. 2006;24(18 suppl):369s. (Abstract 7020) [Google Scholar]
- 31.Porta R, Queralt C, Cardenal F, et al. Erlotinib customization based on epidermal growth factor receptor (EGFR) mutations in stage IV non-small-cell lung cancer (NSCLC) patients (p). J Clin Oncol. 2008;26(15 suppl):430s. (Abstract 8028) [Google Scholar]
- 32.Costa DB, Kobayashi S, Tenen DG, et al. Pooled analysis of the prospective trials of gefitinib monotherapy for EGFR-mutant non-small cell lung cancers. Lung Cancer. 2007;58:95–103. doi: 10.1016/j.lungcan.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adjei AA. K-ras as a target for lung cancer therapy. J Thorac Oncol. 2008;3:S160–3. doi: 10.1097/JTO.0b013e318174dbf9. [DOI] [PubMed] [Google Scholar]
- 34.Wu JY, Wu SG, Yang CH, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res. 2008;14:4877–82. doi: 10.1158/1078-0432.CCR-07-5123. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 36.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter TA, Wodicka LM, Shah NP, et al. Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc Natl Acad Sci USA. 2005;102:11011–6. doi: 10.1073/pnas.0504952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi S, Ji H, Yuza Y, et al. An alternative inhibitor overcomes resistance caused by a mutation of the epidermal growth factor receptor. Cancer Res. 2005;65:7096–101. doi: 10.1158/0008-5472.CAN-05-1346. [DOI] [PubMed] [Google Scholar]
- 39.Avizienyte E, Ward RA, Garner AP. Comparison of the EGFR resistance mutation profiles generated by EGFR targeted tyrosine kinase inhibitors and the impact of drug combinations. Biochem J. 2008;415:197–206. doi: 10.1042/BJ20080728. [DOI] [PubMed] [Google Scholar]
- 40.Engelman JA, Mukohara T, Zejnullahu K, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clin Invest. 2006;116:2695–706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci USA. 2005;102:7665–70. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogino A, Kitao H, Hirano S, et al. Emergence of epidermal growth factor receptor T790M mutation during chronic exposure to gefitinib in a non small cell lung cancer cell line. Cancer Res. 2007;67:7807–14. doi: 10.1158/0008-5472.CAN-07-0681. [DOI] [PubMed] [Google Scholar]
- 43.Li D, Shimamura T, Ji H, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 45.Kosaka T, Yatabe Y, Endoh H, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res. 2006;12:5764–9. doi: 10.1158/1078-0432.CCR-06-0714. [DOI] [PubMed] [Google Scholar]
- 46.Fukui T, Mitsudomi T. Mutations in the epidermal growth factor receptor gene and effects of EGFR-tyrosine kinase inhibitors on lung cancers. Gen Thorac Cardiovasc Surg. 2008;56:97–103. doi: 10.1007/s11748-007-0193-8. [DOI] [PubMed] [Google Scholar]
- 47.Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA. 2008;105:2070–5. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toyooka S, Kiura K, Mitsudomi T. EGFR mutation and response of lung cancer to gefitinib. N Engl J Med. 2005;352:2136. doi: 10.1056/NEJM200505193522019. [DOI] [PubMed] [Google Scholar]
- 49.Bell DW, Gore I, Okimoto RA, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37:1315–6. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 50.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–77. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janne PA. Challenges of detecting EGFR T790M in gefitinib/erlotinib-resistant tumours. Lung Cancer. 2008;60(suppl 2):S3–9. doi: 10.1016/S0169-5002(08)70099-0. [DOI] [PubMed] [Google Scholar]
- 52.Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370:342–50. doi: 10.1016/S0140-6736(07)61165-9. [DOI] [PubMed] [Google Scholar]
- 53.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–41. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- 54.Toyooka S, Date H, Uchida A, et al. The epidermal growth factor receptor D761Y mutation and effect of tyrosine kinase inhibitor. Clin Cancer Res. 2007;13:3431–2. doi: 10.1158/1078-0432.CCR-07-0070. [DOI] [PubMed] [Google Scholar]
- 55.Tokumo M, Toyooka S, Ichihara S, et al. Double mutation and gene copy number of EGFR in gefitinib refractory non-small-cell lung cancer. Lung Cancer. 2006;53:117–21. doi: 10.1016/j.lungcan.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Pallis AG, Voutsina A, Kalikaki A, et al. ‘Classical’ but not ‘other’ mutations of EGFR kinase domain are associated with clinical outcome in gefitinib-treated patients with non-small cell lung cancer. Br J Cancer. 2007;97:1560–6. doi: 10.1038/sj.bjc.6604068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Costa DB, Schumer ST, Tenen DG, et al. Differential responses to erlotinib in epidermal growth factor receptor (EGFR)-mutated lung cancers with acquired resistance to gefitinib carrying the L747S or T790M secondary mutations. J Clin Oncol. 2008;26:1182–4. doi: 10.1200/JCO.2007.14.9039. [DOI] [PubMed] [Google Scholar]
- 58.Bean J, Riely GJ, Balak MN, et al. Acquired resistance to epidermal growth factor receptor kinase inhibitors associated with a novel T854A mutation in a patient with EGFR-mutant lung adenocarcinoma. Clin Cancer Res. 2008;14:7519–25. doi: 10.1158/1078-0432.CCR-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahadevan D, Cooke L, Riley C, et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007;26:3909–19. doi: 10.1038/sj.onc.1210173. [DOI] [PubMed] [Google Scholar]
- 60.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 61.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104:20932–7. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci USA. 2006;103:2316–21. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller CT, Lin L, Casper AM, et al. Genomic amplification of MET with boundaries within fragile site FRA7G and upregulation of MET pathways in esophageal adenocarcinoma. Oncogene. 2006;25:409–18. doi: 10.1038/sj.onc.1209057. [DOI] [PubMed] [Google Scholar]
- 64.Beau-Faller M, Ruppert AM, Voegeli AC, et al. MET gene copy number in non-small cell lung cancer: molecular analysis in a targeted tyrosine kinase inhibitor naive cohort. J Thorac Oncol. 2008;3:331–9. doi: 10.1097/JTO.0b013e318168d9d4. [DOI] [PubMed] [Google Scholar]
- 65.Guo A, Villen J, Kornhauser J, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci USA. 2008;105:692–7. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65:1479–88. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 67.Cipriani NA, Abidoye OO, Vokes E, et al. MET as a target for treatment of chest tumors. Lung Cancer. 2009;63:169–79. doi: 10.1016/j.lungcan.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guix M, Faber AC, Wang SE, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–19. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimamura T, Li D, Ji H, et al. Hsp90 inhibition suppresses mutant EGFR-T790M signaling and overcomes kinase inhibitor resistance. Cancer Res. 2008;68:5827–38. doi: 10.1158/0008-5472.CAN-07-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tripathy D, Slamon DJ, Cobleigh M, et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol. 2004;22:1063–70. doi: 10.1200/JCO.2004.06.557. [DOI] [PubMed] [Google Scholar]
- 71.Moasser MM. Targeting the function of the HER2 oncogene in human cancer therapeutics. Oncogene. 2007;26:6577–92. doi: 10.1038/sj.onc.1210478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150–5. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 73.Yokouchi H, Yamazaki K, Kinoshita I, et al. Clinical benefit of readministration of gefitinib for initial gefitinib-responders with non-small cell lung cancer. BMC Cancer. 2007;7:51. doi: 10.1186/1471-2407-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baselga J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002;20:4292–302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- 75.Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–79. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 76.Cho BC, Im CK, Park MS, et al. Phase II study of erlotinib in advanced non-small-cell lung cancer after failure of gefitinib. J Clin Oncol. 2007;25:2528–33. doi: 10.1200/JCO.2006.10.4166. [DOI] [PubMed] [Google Scholar]
- 77.Lee DH, Kim SW, Suh C, et al. Phase II study of erlotinib as a salvage treatment for non-small-cell lung cancer patients after failure of gefitinib treatment. Ann Oncol. 2008;19:2039–42. doi: 10.1093/annonc/mdn423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costa DB, Nguyen KS, Cho BC, et al. Effects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinib. Clin Cancer Res. 2008;14:7060–7. doi: 10.1158/1078-0432.CCR-08-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Engelman JA, Zejnullahu K, Gale CM, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–32. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- 80.Gonzales AJ, Hook KE, Althaus IW, et al. Antitumor activity and pharmaco-kinetic properties of PF-00299804, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2008;7:1880–9. doi: 10.1158/1535-7163.MCT-07-2232. [DOI] [PubMed] [Google Scholar]
- 81.Gendreau SB, Ventura R, Keast P, et al. Inhibition of the T790M gatekeeper mutant of the epidermal growth factor receptor by EXEL-7647. Clin Cancer Res. 2007;13:3713–23. doi: 10.1158/1078-0432.CCR-06-2590. [DOI] [PubMed] [Google Scholar]
- 82.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–11. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Riely GJ. Second-generation epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. J Thorac Oncol. 2008;3:S146–9. doi: 10.1097/JTO.0b013e318174e96e. [DOI] [PubMed] [Google Scholar]
- 84.Rabindran SK, Discafani CM, Rosfjord EC, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–65. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 85.Wong KK. HKI-272 in non small cell lung cancer. Clin Cancer Res. 2007;13:s4593–6. doi: 10.1158/1078-0432.CCR-07-0369. [DOI] [PubMed] [Google Scholar]
- 86.Godin-Heymann N, Ulkus L, Brannigan BW, et al. The T790M “gatekeeper” mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol Cancer Ther. 2008;7:874–9. doi: 10.1158/1535-7163.MCT-07-2387. [DOI] [PubMed] [Google Scholar]
- 87.Rizvi NA, Kris MG, Miller VA, et al. Activity of XL647 in clinically selected NSCLC patients (pts) enriched for the presence of EGFR mutations: Results from Phase 2. J Clin Oncol. 2008;26(18 suppl):437s. (Abstract 8053) [Google Scholar]
- 88.Miller VA, Wakelee HA, Lara PN, et al. Activity and tolerance of XL647 in NSCLC patients with acquired resistance to EGFR-TKIs: Preliminary results of a phase II trial. J Clin Oncol. 2008;26(18 suppl):430s. (Abstract 8028) [Google Scholar]
- 89.Yang C, Shih J, Chao T, et al. Use of BIBW 2992, a novel irreversible EGFR/HER2 TKI, to induce regression in patients with adenocarcinoma of the lung and activating EGFR mutations: Preliminary results of a single-arm phase II clinical trial. J Clin Oncol. 2008;26(18 suppl):430s. (Abstract 8026) [Google Scholar]
- 90.Janne PA, Schellens JH, Engelman JA, et al. Preliminary activity and safety results from a phase I clinical trial of PF-00299804, an irreversible pan-HER inhibitor, in patients (pts) with NSCLC. J Clin Oncol. 2008;26(18 suppl):430s. (Abstract 8027) [Google Scholar]
- 91.Tang Z, Du R, Jiang S, et al. Dual MET-EGFR combinatorial inhibition against T790M-EGFR-mediated erlotinib-resistant lung cancer. Br J Cancer. 2008;99:911–22. doi: 10.1038/sj.bjc.6604559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karp DD, Paz-Ares LG. Phase II study of anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27:2516–22. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]
- 93.Gao SP, Mark KG, Leslie K, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–56. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shimamura T, Shapiro GI. Heat shock protein 90 inhibition in lung cancer. J Thorac Oncol. 2008;3:S152–9. doi: 10.1097/JTO.0b013e318174ea3a. [DOI] [PMC free article] [PubMed] [Google Scholar]