Abstract
The antipsychotic agent haloperidol regulates gene transcription in striatal medium spiny neurons (MSNs) by blocking dopamine D2 receptors (D2Rs). We examined the mechanisms by which haloperidol increases the phosphorylation of histone H3, a key step in nucleosomal response. Using BAC-transgenic mice that express EGFP under the control of the promoter of the dopamine D1 receptor or the D2R, we found that haloperidol induced a rapid and sustained increase in the phosphorylation of histone H3 in the striatopallidal MSNs of the dorsal striatum, with no change in its acetylation. This effect was mimicked by raclopride, a selective D2R-antagonist, and prevented by blockade of adenosine A2A receptors (A2ARs) or genetic attenuation of the A2AR-associated G protein, Gαolf. Mutation of the cAMP-dependent phosphorylation site (Thr34) of the 32-kDa dopamine- and cAMP-regulated phosphoprotein (DARPP-32) decreased haloperidol-induced H3 phosphorylation, supporting the role of cAMP in H3 phosphorylation. Haloperidol also induced extracellular signal-regulated kinase (ERK) phosphorylation in striatopallidal MSNs, but this effect was not implicated in H3 phosphorylation. The levels of mitogen- and stress-activated kinase 1 (MSK1), which has been reported to mediate ERK-induced H3 phosphorylation, were lower in striatopallidal than in striatonigral MSNs. Moreover, haloperidol-induced H3 phosphorylation was unaltered in MSK1-knockout mice. These data indicate that, in striatopallidal MSNs, H3 phosphorylation is controlled by the opposing actions of D2Rs and A2ARs. Thus, blockade of D2Rs promotes histone H3 phosphorylation via A2AR-mediated activation of Gαolf and inhibition of protein phosphatase-1 via PKA-dependent phosphorylation of DARPP-32.
Keywords: Basal ganglia, DARPP-32, ERK, Gαolf, haloperidol, MSK1
Introduction
The medium spiny neurons (MSNs) of the striatum are targeted by many psychoactive substances, including drugs used in the treatment of schizophrenia and other psychotic disorders. One major problem associated to the use of conventional antipsychotic drugs, such as haloperidol, is their propensity to generate extrapyramidal side effects, such as parkinsonism and tardive dyskinesia. These complications are most likely related to the ability of these drugs to antagonize dopamine D2 receptor (D2R)-mediated transmission. A better understanding of the molecular mechanisms by which blockade of D2Rs affects striatal function is therefore of high clinical relevance.
In the striatopallidal MSNs, D2Rs activate a Gαi/o protein negatively coupled to adenylyl cyclase (Kebabian and Calne, 1979, Stoof and Kebabian, 1981). Therefore, blockade of D2Rs promotes cAMP signaling. This effect depends on the expression, in striatopallidal MSNs, of adenosine A2A receptors (A2ARs) (Fink et al, 1992, Schiffmann et al, 1991), which are tonically activated and which are coupled to Gαolf-dependent stimulation of adenylyl cyclase (Corvol et al, 2001, Fredholm, 1977). Thus, pharmacological blockade or genetic inactivation of A2ARs prevents the ability of haloperidol and other D2R antagonists to increase cAMP-dependent phosphorylation of downstream targets, such as the dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) and the GluR1 subunit of the glutamate AMPA receptor (Håkansson et al, 2006, Svenningsson et al, 2000). In addition, A2ARs are required for the changes in the expression of several genes, including immediate early genes, caused by blockade of D2Rs (Chen et al, 2001).
Alterations in chromatin structure, through changes in the state of phosphorylation, acetylation, or methylation of specific histones, are critically involved in the control of gene expression. Indeed, phosphorylation of histone H3 at Ser10 by various protein kinases, including mitogen- and stress-activated kinases 1 and 2 (MSK1 and 2), is an important component of the nucleosomal response, which promotes chromatin decondensation, thereby allowing access to DNA by transcription factors and increasing the expression of early response genes, such as c-fos and c-jun (Davie, 2003, Soloaga et al, 2003). In hippocampus and striatum, it has been shown that histone H3 can be phosphorylated by MSK1 following activation of the extracellular signal-regulated kinases (ERK) (Brami-Cherrier et al, 2005, Chwang et al, 2006, Chwang et al, 2007).
A previous study showed that haloperidol induces phosphorylation of the acetylated form of histone H3 in the striatum and that this effect is blocked by inhibition of cAMP-dependent protein kinase (PKA) (Li et al, 2004). In addition, it has been recently reported that haloperidol increases histone H3 phosphorylation selectively in striatopallidal neurons (Bertran-Gonzalez et al, 2008). These observations raise the possibility that combined control of cAMP signaling via D2Rs and A2ARs orchestrates nucleosomal response in striatopallidal MSNs. In the present study, we have employed pharmacological tools and transgenic mice to examine the signaling pathways that link blockade of D2Rs to histone H3 modification in the nucleus. Our results indicate that haloperidol, acting as an antagonist at D2Rs, increases histone H3 phosphorylation in striatopallidal MSNs by promoting A2AR/Gαolf-mediated activation of cAMP/DARPP-32 signaling. We also show that, in contrast to what has been observed in response to psychostimulants in striatonigral neurons, the effects of haloperidol on histone H3 phosphorylation are independent of ERK/MSK1 activation.
Materials and Methods
Animals
Male 7–8-week old mice were maintained in a 12-h light-dark cycle, in stable conditions of temperature (22°C), with food and water ad libitum. All experiments were carried out in accordance with the guidelines of the Swedish Animal Welfare Agency and the French Agriculture and Forestry Ministry (decree 87849, license A75-05–22). Swiss-Webster mice carrying Drd1a-EGFP or Drd2-EGFP bacterial artificial chromosome (BAC) transgenes, were generated by the GENSAT (Gene Expression Nervous System Atlas) program at the Rockefeller University (Gong et al, 2003). DARPP-32 T34A mutant, Gnal+/− (Gαolf) heterozygous and MSK1 knock out mice were generated as described in previous studies (Belluscio et al, 1998, Svenningsson et al, 2003, Wiggin et al, 2002) and were backcrossed for at least 10 generations on a C57Bl/6 background.
Drugs
Haloperidol (0.5 mg/kg, Sigma-Aldrich, Sweden) was dissolved in saline containing 5% (vol/vol) acetic acid and the pH was adjusted to 6.0 with 1 M NaOH. Raclopride (0.3 mg/kg, Sigma-Aldrich, France) was dissolved in 0.9% NaCl. KW6002 (3 mg/kg) and SL327 (50 mg/kg), gifts from Dr. Edilio Borroni (Hoffmann-La Roche, Basel, Switzerland), were suspended by sonication in a solution of 5% (vol/vol) Tween 80 in saline and administered 5 and 30 min before haloperidol, respectively. All drugs were administered intraperitoneally (i.p.). Mice were habituated to handling and saline injection for three consecutive days before the experiment.
Tissue preparation and immunofluorescence
Mice were rapidly anaesthetized with pentobarbital (500 mg/kg, i.p., Sanofi-Aventis, France) and transcardially perfused with 4% (weight/vol) paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.5). Brains were post-fixed overnight in the same solution and stored at 4°C. Thirty µm-thick sections were cut with a vibratome (Leica, France) and stored at −20°C in a solution containing 30% (vol/vol) ethylene glycol, 30% (vol/vol) glycerol and 0.1 M sodium phosphate buffer, until they were processed for immunofluorescence. Brain regions were identified using a mouse brain atlas (Franklin and Paxinos, 1997), and sections equivalent to the following bregma coordinates were taken (mm): 1.10 (dorsal striatum). Free-floating sections were rinsed in Tris-buffered saline (TBS; 0.25 M Tris and 0.5 M NaCl, pH 7.5), incubated for 5 min in TBS containing 3% H2O2 and 10% methanol (vol/vol), and then rinsed three times for 10 min each in TBS. After 20 min incubation in 0.2% Triton X-100 in TBS, sections were rinsed three times in TBS again. Finally, they were incubated overnight at 4°C with the different primary antibodies. NaF (0.1 mM) was included in all buffers and incubation solutions. Different histone H3 modifications were identified using rabbit polyclonal antibodies against phospho-Ser10-H3, acetyl-Lys14-H3 and phospho-Ser10-acetyl-Lys14-H3 (1:500, Upstate Ltd., UK). Gαolf protein levels in wild type and Gnal +/- mice were assessed using rabbit polyclonal antibodies (1:500) (Herve et al, 2001). Activated ERK was detected with rabbit polyclonal antibodies (1:400, Cell Signaling Technology, Danvers, MA) and a mouse monoclonal antibody (1:400, Promega, Charbonnière, France) against diphospho-Thr202/Tyr204-ERK1/2. MSK1 was identified using a rabbit polyclonal antibody (1:500, Santa Cruz Biotechnology, Santa Cruz, CA). Following incubation with primary antibodies, sections were rinsed three times for 10 min in TBS and incubated for 45 minutes with goat Cy3-coupled (1:400, Jackson Laboratory, Bar Harbor, ME) and/or goat A488/A633 (1:400, Invitrogen AB, Sweden) secondary antibodies. Sections were rinsed for 10 min twice in TBS and twice in TB (0.25 M Tris) before mounting in Vectashield (Vector Laboratories, Burlingame, CA).
Immunofluorescence Analysis
Single- and double-labeled images from each region of interest were obtained bilaterally using sequential laser scanning confocal microscopy (Leica SP2 and Zeiss LSM). Neuronal quantification was performed in 375 × 375 µm images by counting Cy3-immunofluorescent nuclei (for P-AcH3 and P-ERK immunostaining). Cell counts were done by an observer unaware of the treatment received by the mice. For the analysis of MSK1 expression in striatonigral and striatopallidal neurons, the average fluorescence intensity of each individual MSK1 immunoreactive nucleus was assessed automatically in Drd1a- and Drd2-EGFP mice samples, according to the colocalization of the EGFP signal. A home-written program based on Metamorph software (Molecular Devices, Sunnyvale, CA) was used to compute all parameters.
Statistical Analysis
Data from the quantifications of P-H3, P-AcH3 and AcH3 in Drd2-EGFP mice (means ± SEM, n = 3–4) were analyzed using two-way ANOVA, and post hoc comparisons between groups were made using Bonferroni multiple comparison test. In the other P-AcH3 and P-ERK assessments, data (means ± SEM, n = 2–5) were analyzed using one-way ANOVA and Newman-Keuls post hoc multiple comparison test. Comparisons between MSK1 intensities in striatonigral or striatopallidal neurons (means ± SEM, n = 310–452) were performed using unpaired t test. In all cases, significance threshold was set at p < 0.05
Results
Haloperidol induces a sustained phosphorylation of histone H3 in striatopallidal MSNs
MSNs represent the vast majority of striatal neurons and form two distinct efferent pathways, which exert opposite regulations on thalamo-cortical projection neurons (Gerfen, 1992). The MSNs of the direct pathway project to the substantia nigra pars reticulata and entopeduncular nucleus, whereas the MSNs of the indirect pathway innervate the external globus pallidus, which projects to the subthalamic nucleus (Gerfen, 1992). Striatonigral MSNs predominantly express dopamine D1 receptors (D1Rs), whereas striatopallidal MSNs contain D2Rs and A2ARs (Fink et al, 1992, Gerfen, 1992, Schiffmann et al, 1991).
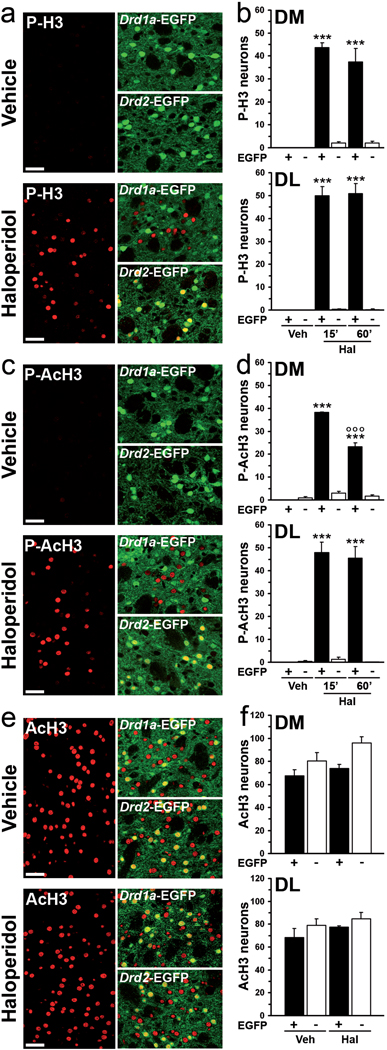
Drd1a-EGFP and Drd2-EGFP mice provide a very efficient way to identify distinct neuronal populations in the striatum (Gong et al, 2003). Using these mice it was shown that haloperidol induced histone H3 phosphorylation selectively in striatopallidal neurons (Bertran-Gonzalez et al, 2008). Here, we further investigated the action of haloperidol on histone modifications by comparing its effects on phosphorylation and acetylation of histone H3 (Fig. 1). Immunofluorescence analysis in Drd1a-EGFP and Drd2-EGFP confirmed that administration of haloperidol (0.5 mg/kg) induced a rapid (15 min) and prolonged (60 min) increase in the levels of Ser10 phosphorylated histone H3 in the D2R, but not in the D1R expressing neurons of dorsomedial and dorsolateral striatum (Fig. 1a, b). Similar results were obtained using an antibody against the Ser10 phosphorylated form of Lys14-acetylated histone H3 (Fig. 1c, d). In contrast, haloperidol did not modify the number of neurons immunoreactive for acetyl-Lys14 histone H3 (Fig. 1e, f), nor did it affect the number of acetyl-Lys12 histone H4-positive neurons (data not shown). Since the phosphoacetylated form of histone H3 is the most functionally significant (Cheung et al, 2000, Salvador et al, 2001), antibodies against this form were used in the rest of the study.
Figure 1.
Effect of haloperidol on histone H3 phosphorylation and acetylation in striatal MSNs. Mice expressing EGFP in striatonigral (Drd1a-EGFP) or striatopallidal (Drd2-EGFP) MSNs were treated with vehicle or haloperidol and perfused 15 min (15’; a–f), or 60 min later (60’; b, d). (a, c, e). Confocal sections of the dorsal striatum, showing immunofluorescence (red) for phospho-Ser10-histone H3 (P-H3, a), phospho-Ser10-acetyl-Lys14-histone H3 (P-AcH3, c) and acetyl-Lys14-histone H3 (AcH3, e) alone (left panels), or in combination with EGFP fluorescence (green; right panels). (b, d, f) Quantification of P-H3- (b), P-AcH3- (d), andAcH3- (f) immunoreactive neurons among EGFP-positive (EGFP+) or EGFP-negative (EGFP-) neurons in the dorsomedial (DM) and dorsolateral (DL) striata of vehicle (Veh) or haloperidol (Hal) treated Drd2-EGFP mice (*** p < 0.001 vs. Veh; °°°p < 0.001 vs. Hal 15’). Scale bars: 40 µm.
Although haloperidol is an excellent D2R antagonist, it also binds to other receptors, including D1Rs (Missale et al, 1998). To confirm that the effects of haloperidol actually resulted from blockade of D2R, we compared its action with that of raclopride, a highly selective D2 antagonist (Missale et al, 1998). Raclopride induced a robust phosphorylation of Lys14-acetylated histone H3 in D2R expressing neurons of the dorsal striatum (Suppl. Fig. 1). The effect of raclopride was less persistent than that of haloperidol (Suppl. Fig. 1b), a difference most likely related to its shorter half-life (Farde et al, 1988, Kudo and Ishizaki, 1999).
Haloperidol-induced phosphorylation of histone H3 depends on A2ARs
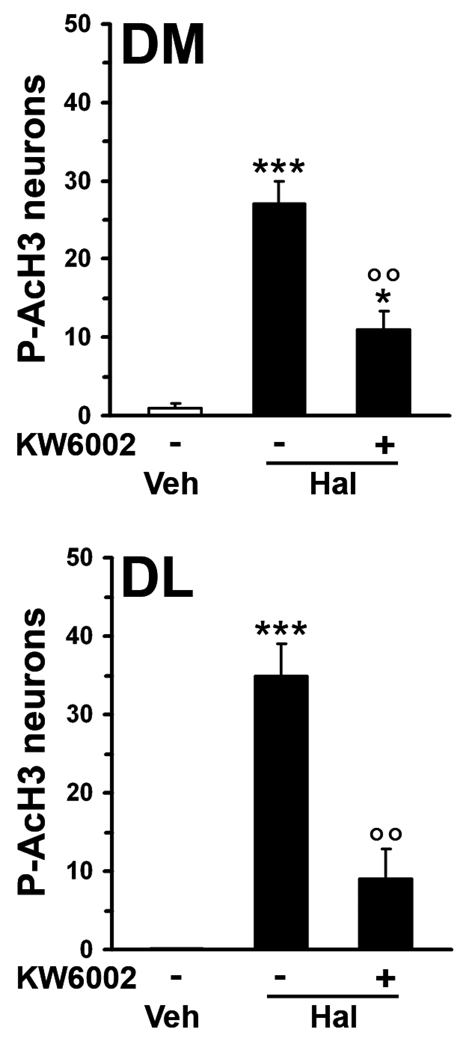
In the striatum, haloperidol promotes cAMP/PKA signaling by removing the inhibition exerted by D2Rs on adenylyl cyclase (Kebabian and Calne, 1979, Stoof and Kebabian, 1981). This effect depends on basal activation of A2ARs, which are selectively expressed in striatopallidal MSNs (Fink et al, 1992, Schiffmann et al, 1991) and are primarily responsible for the synthesis of cAMP in these neurons through activation of Gαolf (Corvol et al, 2001, Zhuang et al, 2000). As shown in Fig. 2, blockade of A2ARs, achieved with the selective antagonist KW6002 (3 mg/kg), dramatically reduced the increase in the number of P-AcH3-immunoreactive neurons produced by haloperidol. These results strongly support the hypothesis that blockade of D2Rs promotes the phosphorylation of Lys14-acetylated histone H3 through disinhibition of an A2AR-triggered signaling cascade in striatopallidal MSNs. They also indicate that, in these neurons, the state of phosphorylation of histone H3 is regulated in an opposite way by dopamine, acting on D2Rs, and adenosine, acting on A2ARs.
Figure 2.
Haloperidol-induced histone H3 hosphorylation is prevented by blockade of A2ARs. Wild type mice were treated with haloperidol alone, or in combination with the A2AR antagonist KW6002 (injected 5 min prior to haloperidol) and perfused 15 min later. Quantification of phospho-Ser10-acetyl-Lys14-histone H3 (P-AcH3) immunoreactive neurons in the dorsomedial (DM) and dorsolateral (DL) striata of mice treated with vehicle (Veh), haloperidol (Hal), or haloperidol plus KW6002 (* p < 0.05, *** p < 0.001 vs. vehicle; °°p < 0.01 vs. Hal).
Haloperidol increases the phosphorylation of histone H3 through Gαolf
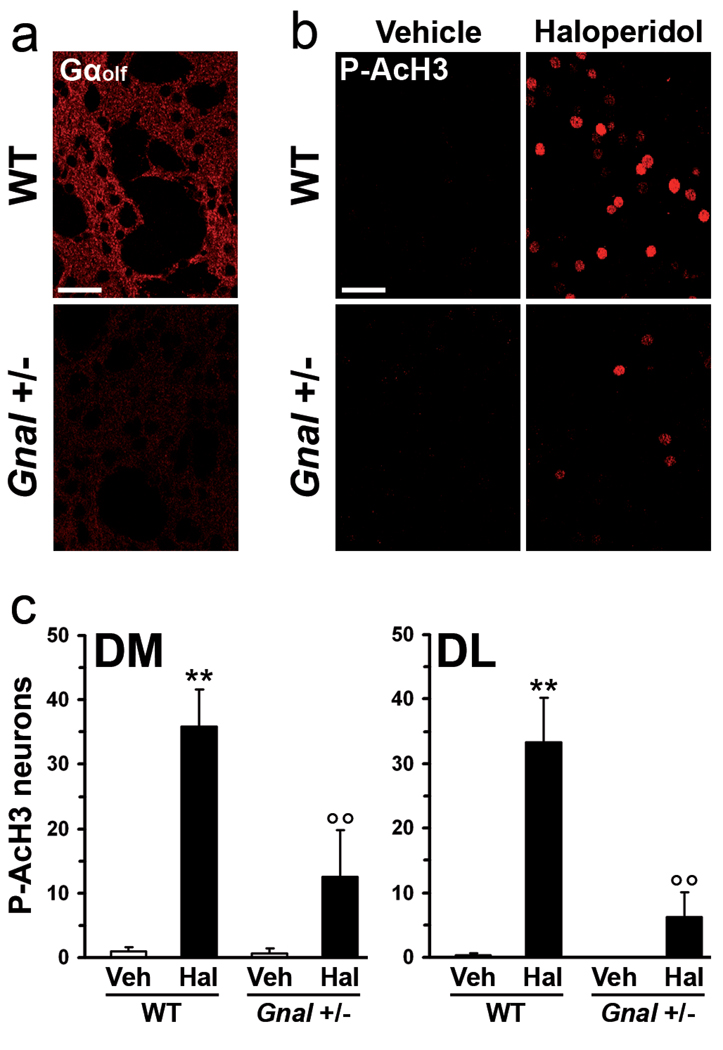
In striatal MSNs, receptor-mediated activation of adenylyl cyclase depends on stimulation of the GTP-binding protein, Gαolf (Corvol et al, 2001, Zhuang et al, 2000). We therefore analyzed whether phosphorylation of histone H3 induced by haloperidol was altered in Gnal+/− mice carrying a heterozygous mutation of the gene encoding for Gαolf. In these animals, Gαolf expression is reduced by about 50% (cf. Fig. 3a), leading to impaired A2AR-mediated activation of adenylyl cyclase (Corvol et al, 2001, Zhuang et al, 2000). We found that, following administration of haloperidol (0.5 mg/kg), the number of P-AcH3-positive neurons was strongly reduced in the dorsal striatum of Gnal+/− mice, as compared to wild type mice (Fig. 3b, c). These results indicate that the increase in the phosphorylation of Lys14-acetylated histone H3 produced by blockade of D2Rs depends on a signaling pathway in which Gαolf is a limiting factor.
Figure 3.
Gαolf-mediated signaling is required for haloperidol-induced histone H3 phosphorylation. (a) Gαolf immunoreactivity in single confocal sections of the dorsal striatum from a wild type (WT) or a Gnal heterozygous (Gnal+/−) mouse. Note the decrease in Gαolf immunoreactivity in the striatum of the Gnal+/− mouse. (b, c) WT and Gnal+/− mice were treated with haloperidol and perfused 15 min later. (b) Phospho-Ser10-acetyl-Lys14-histone H3 (P-AcH3) immunoreactivity in single confocal sections of the dorsal striatum of WT or Gnal+/− mice. (c) Quantification of P-AcH3 immunoreactive neurons in the dorsomedial (DM) and dorsolateral (DL) striata of WT and Gnal+/ - mice 15 min after administration of vehicle (Veh) or haloperidol (Hal) (** p < 0.01 vs. WT Veh; °°p < 0.01 vs. WT Hal). Scale bars: 40 µm.
Involvement of DARPP-32 in haloperidol-induced phosphorylation of histone H3
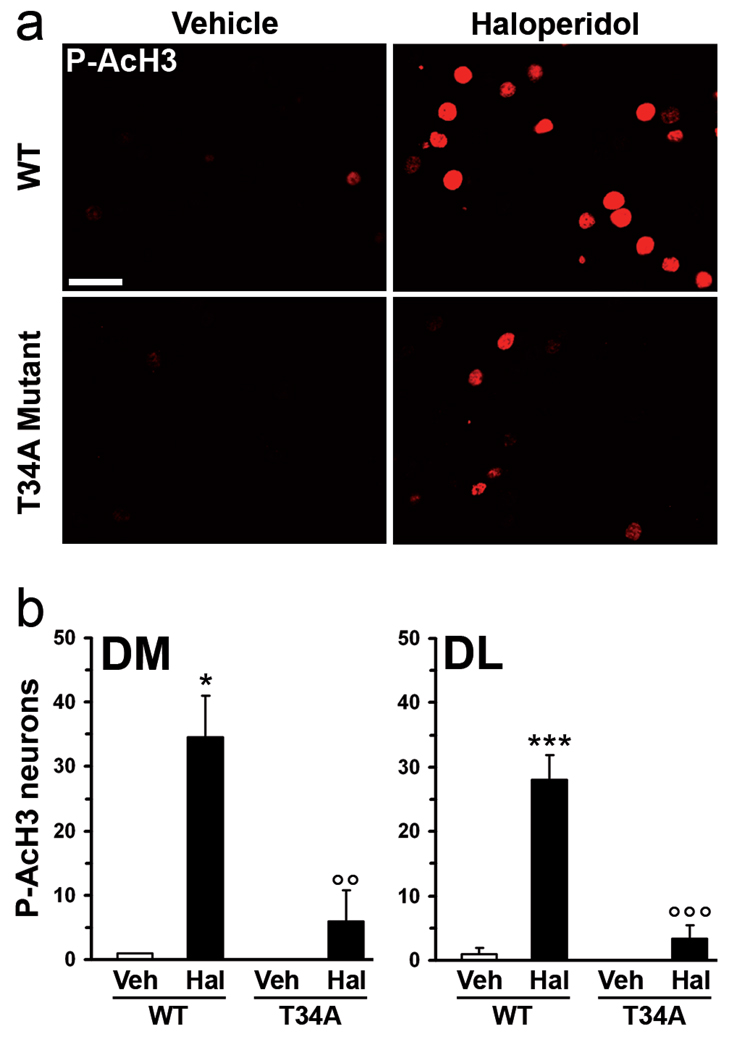
Administration of D2R antagonists, including haloperidol, results in PKA-dependent phosphorylation of DARPP-32 on Thr34 (Svenningsson et al, 2000), which is thus converted into a potent inhibitor of protein phosphatase-1 (PP-1) (Hemmings et al, 1984). DARPP-32-mediated suppression of PP-1 activity plays a key role in the regulation of the state of phosphorylation of many proteins targeted by the cAMP/PKA cascade (Greengard, 2001). Moreover, DARPP-32 is critical for the regulation of histone H3 phosphorylation produced by cocaine (Stipanovich et al, 2008). To examine the involvement of DARPP-32 in the control of the phosphorylation of Lys14-acetylated histone H3 exerted by haloperidol, we employed knock-in mice expressing a mutant form of DARPP-32, in which the PKA phosphorylation site, Thr34, is inactivated by substitution with an Ala (T34A mutant mice). In these mice, the ability of haloperidol to stimulate the phosphorylation of acetylated histone H3 in the dorsal striatum was strongly reduced (Fig. 4a, b). Therefore, we concluded that, in striatopallidal MSNs, blockade of D2Rs reduces the dephosphorylation of histone H3 through DARPP-32-mediated inhibition of PP-1.
Figure 4.
Mutation of Thr34 to Ala in DARPP-32 prevents haloperidol-induced histone H3 phosphorylation. Wild type (WT) or T34A DARPP-32 mutant mice were treated with vehicle or haloperidol and perfused 15 min later. (a) Phospho-Ser10-acetyl-Lys14-histone H3 (P-AcH3) immunoreactivity in single confocal sections of the dorsal striatum from WT or T34A mutant mice. (b) Quantification of P-AcH3 immunoreactive neurons in the dorsomedial (DM) and dorsolateral (DL) striatum, 15 min after administration of vehicle (Veh) or haloperidol (Hal) to WT or T34A DARPP-32 mutant mice (T34A) (* p < 0.05, *** p < 0.001 vs. WT Veh; °°p < 0.01, °°°p < 0.001 vs. WT Hal). Scale bar: 40 µm.
Haloperidol-induced phosphorylation of histone H3 is independent of ERK activation
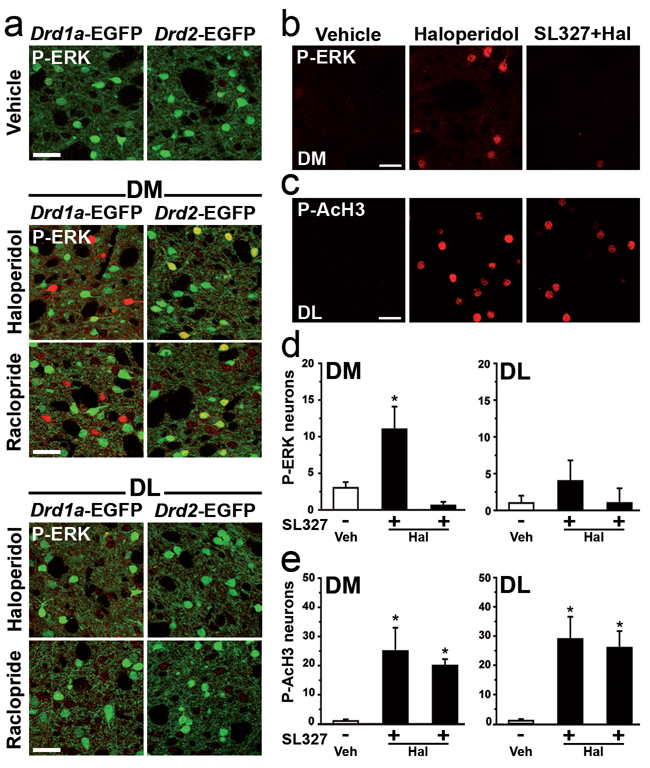
It is known that ERK, acting through its downstream target MSK1, elicits in vivo phosphorylation of histone H3 (Brami-Cherrier et al, 2005, Chwang et al, 2007). It has also been shown that administration of haloperidol induces ERK phosphorylation in the striatum (Gerfen et al, 2002, Pozzi et al, 2003, Valjent et al, 2004) and that this effect occurs selectively in striatopallidal MSNs (Bertran-Gonzalez et al, 2008). In the striatum, activation of ERK is mediated in part through activation of the cAMP pathway (Santini et al, 2007, Valjent et al, 2005). Therefore, it was logical to hypothesize that the haloperidol-induced phosphorylation of Lys14-acetylated histone H3 might result from A2AR/cAMP-mediated activation of ERK. Examination of haloperidol-induced ERK phosphorylation in Drd1a- and Drd2-EGFP mice confirmed, as previously reported (Bertran-Gonzalez et al, 2008), that ERK activation occurred selectively in striatopallidal MSNs (Fig. 5a). Similar results were obtained with raclopride, indicating that, as in the case of histone H3 modification, the effect of haloperidol resulted from the blockade of D2R (Fig. 5a). However, the effects produced by the D2R antagonists were limited to a small number of neurons and were restricted to the dorsomedial part of the striatum (Fig. 5a). Next, we examined the effect of haloperidol in the presence or absence of SL327, a drug that blocks ERK by inhibiting the mitogen-activated protein kinase/ERK kinase (MEK). We found that administration of 50 mg/kg of SL327 abolished haloperidol-induced ERK phosphorylation (Fig. 5b, d), without affecting the concomitant increase in histone H3 phosphorylation observed in the dorsal striatum (Fig. 5c, e). These results demonstrated that the effects of haloperidol on H3 phosphorylation, in contrast with those of cocaine (Brami-Cherrier et al, 2005), were ERK-independent.
Figure 5.
Histone H3 phosphorylation induced by blockade of D2Rs is independent of ERK activation. (a) Mice expressing EGFP in striatonigral (Drd1a-EGFP) or striatopallidal (Drd2-EGFP) MSNs were treated with vehicle, haloperidol, or raclopride, and perfused 15 min later. EGFP fluorescence (green) in single confocal sections of the dorsomedial (DM) or dorsolateral (DL) striatum is shown in combination with fluorescence (red) for diphospho-Thr202/Tyr204-ERK1/2 (P-ERK). Note, in haloperidol and raclopride treated mice, the relatively low number and prevalent localization in the DM of P-ERK-positive MSNs (single-labeled Drd1a-EGFP mice and double-labeled in Drd2-EGFP mice). (b–e) Wild type mice were treated with haloperidol alone or in combination with the MEK inhibitor SL327 (injected 45 min prior to haloperidol) and perfused 15 min later. P-ERK (b, d) and phospho-Ser10-acetyl-Lys14-histone H3 (P-AcH3) (c, e) immunoreactivity was determined in single confocal sections of the DM striatum. (d, e) Quantification of striatal P-ERK (d) and P-AcH3 (e) immunoreactive neurons in the DM and DL striata of mice treated with vehicle (Veh) or haloperidol (Hal) alone or in combination with SL327 (* p < 0.05 vs. vehicle; °p < 0.05 vs. Hal). Scale bars: 40 µm.
MSK1 expression is lower in D2R-expressing than in D1R-expressing neurons
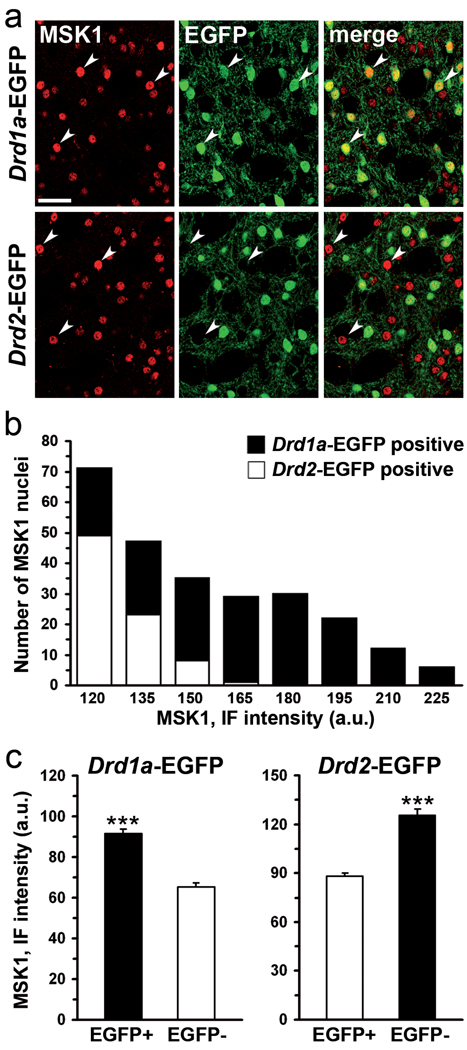
MSK1 plays a critical role in the phosphorylation of histone H3 in several brain regions including the striatum (Brami-Cherrier et al, 2005) and the hippocampus (Chwang et al, 2007). However, MSK1-immunoreactivity has been previously detected in only about 60% of striatal neurons (Heffron and Mandell, 2005), prompting the question of the implication of MSK1 in haloperidol-induced H3 phosphorylation. To address this question, we studied the distribution of MSK1 immunoreactivity in Drd1a- and Drd2-EGFP mice (Fig. 6). As previously reported (Heffron and Mandell, 2005), MSK1 immunoreactivity was restricted to the nuclei (Fig. 6a). Interestingly, there was a clear heterogeneity in labeling intensity (Fig. 6a). Comparison of MSK1 immunoreactivity and EGFP fluorescence in Drd1- and Drd2-EGFP mice showed that intensely labeled nuclei belonged almost invariably to D1R-expressing neurons (Fig. 6b). Quantitative study of immunolabeling in the two neuronal populations showed that the mean level of MSK1 immunoreactivity was lower in D2R-neurons than in D1R-neurons (Fig. 6c). However, it is important to note that at least some MSK1 immunoreactivity was detected in all EGFP-expressing neurons in both transgenic lines. In line with these results, a small but significant increase in phospho-MSK1-positive striatopallidal neurons occurs in response to haloperidol (Bertran-Gonzalez et al, 2008). Thus, our observations revealed that, although MSK1 was present in virtually all striatal MSNs, its expression levels were lower in D2R- than in D1R-containing neurons, raising the question of the contribution of MSK1 to the effects of haloperidol on H3 phosphorylation in striatonigral neurons.
Figure 6.
Expression of MSK1 in striatonigral and striatopallidal MSNs. (a) MSK1 immunoreactive nuclei (red) are co-labeled with EGFP (green) in the striatum of Drd1a- or Drd2-EGFP mice in a double fluorescence analysis. Nuclei intensely labeled with MSK1 antibodies colocalized with EGFP in Drd1a-EGFP but not in Drd2-EGFP mice (arrowheads). Single confocal sections, scale bar: 40 µm. (b) Quantitative analysis of MSK1 fluorescence intensity in Drd1a- and Drd2-EGFP mice. Distribution histograms of intensely labeled MSK1 nuclei (average normalized immunofluorescence intensity > 120 a.u.) colocalized with EGFP in Drd1a-EGFP mice (black bars) and in Drd2-EGFP mice (white bars). (c) Mean values of MSK1 immunofluorescence (arbitrary units, a.u.) of neurons containing (EGFP+) or not (EGFP-) EGFP in Drd1a- and Drd2-EGFP mice (*** p < 0.0001).
Haloperidol-induced phosphorylation of histone H3 in striatopallidal MSNs is independent of MSK1
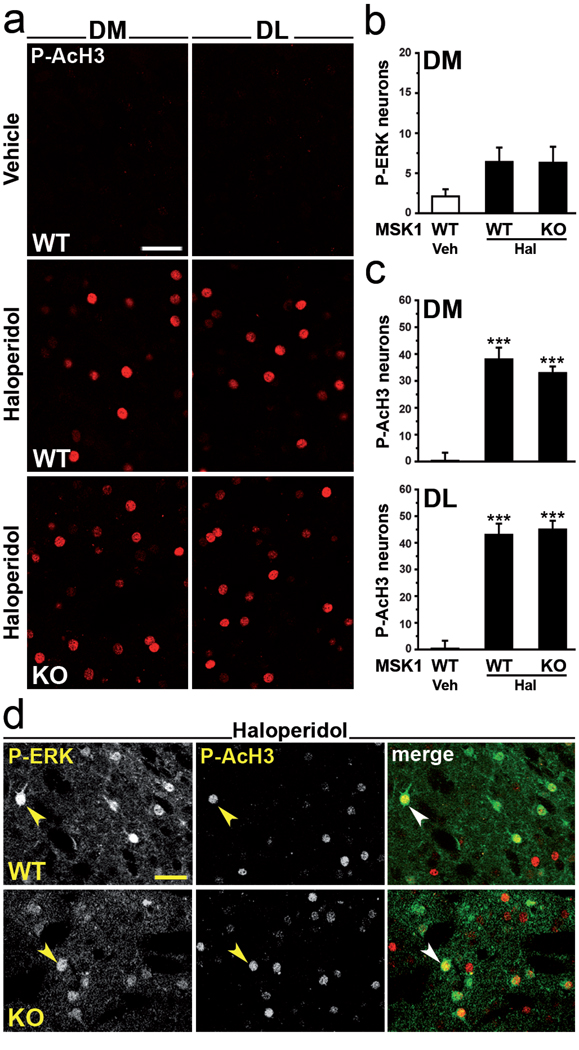
To examine the role of MSK1 in the effects of haloperidol, we used MSK1 knock out mice, in which phosphorylation of histone H3 in response to cocaine is abolished (Brami-Cherrier et al, 2005). In these mice, the haloperidol-induced phosphorylation of histone H3 was virtually identical to that observed in wild type mice (Fig. 7a, c). As expected, the effect of haloperidol on the number of phospho-ERK-positive neurons in the dorsomedial striatum was also unchanged (Fig. 7b).
Figure 7.
Haloperidol-induced histone H3 phosphorylation in striatopallidal MSNs is independent of MSK1. Wild type (WT) or MSK1 knock out (KO) mice were treated with vehicle or haloperidol and perfused 15 min later. (a) Phospho-Ser10-acetyl-Lys14-histone H3 (P-AcH3) immunoreactivity in single confocal sections of the dorsomedial (DM) or dorsolateral (DL) striata of WT and MSK1 KO mice. (b, c) Quantification of (b) diphospho-Thr202/Tyr204-ERK1/2 (P-ERK) and (c) P-AcH3 immunoreactive neurons in the DM (b, c) and DL (c) striata of WT and MSK1 KO mice 15 min after administration of vehicle (Veh) or haloperidol (Hal). No difference in P-AcH3 immunoreactivity was found between vehicle treated WT mice and vehicle treated MSK1 KO mice (the latter are not shown in the figure) (*** p < 0.001 vs. Veh WT). (d) Double immunofluorescence showing P-ERK (green) and P-AcH3 (red) in the DM of WT and MSK1 KO mice after haloperidol treatment. Arrowheads indicate colocalization of P-ERK and P-AcH3 immunoreactivity in the same MSNs. Scale bars: 40 µm.
Our results showed that, following administration of haloperidol, phospho-acetylated-histone H3-positive MSNs largely outnumbered phospho-ERK-positive MSNs (see Fig. 5). However, double-labeling experiments indicated that haloperidol-induced phosphorylation of ERK and histone H3 were both detected in a modest number of neurons (Fig. 7d). Therefore, we tested the role of MSK1 in the phosphorylation of histone H3 in this subset of striatopallidal MSNs. We found that concomitant phosphorylation of ERK and histone H3 was still present in the dorsomedial striata of MSK1 knock out mice (Fig. 7d). In combination with the lack of effect of SL327 on H3 phosphorylation, these results clearly showed that haloperidol-induced histone H3 phosphorylation in striatopallidal MSNs occurred independently of activation of the ERK/MSK1 cascade.
Discussion
In this study, we have characterized the regulation of histone H3 exerted by blockade of D2Rs in striatopallidal MSNs. Our data show that the increase in histone H3 phosphorylation produced by haloperidol is mimicked by the highly selective D2R antagonist raclopride, involves A2AR/αolf -mediated transmission and requires PKA-dependent phosphorylation of DARPP-32, which leads to inhibition of PP-1. In contrast, the effect of haloperidol on histone H3 phosphorylation is independent of ERK and MSK1 activation, which occurs only in a limited subset of striatopallidal MSNs.
Contribution of adenosine A2ARs, Gαolf and DARPP-32 to histone H3 phosphorylation
In striatopallidal MSNs, cAMP signaling is controlled by the opposite actions of A2ARs, which increase cAMP production via Gαolf, and D2Rs, which reduce cAMP production via Gαi/o (Corvol et al, 2001, Kebabian and Calne, 1979, Stoof and Kebabian, 1981). Thus, haloperidol and other D2R antagonists promote cAMP signaling by removing the inhibition exerted by D2Rs on adenylyl cyclase. This action depends on A2AR transmission, which maintains basal cAMP synthesis, provided by endogenous adenosine (Håkansson et al, 2006, Svenningsson et al, 2000).
We show that the increase in histone H3 phosphorylation produced by administration of haloperidol is prevented by pharmacological blockade of A2ARs. This finding indicates that, in the absence of D2R transmission, the tonic activation of A2ARs is able to increase the state of phosphorylation of histone H3. It also suggests that, in striatopallidal MSNs, dopamine and adenosine exert opposing effects on nucleosomal response, an observation that is in line with their opposing actions on gene expression (Dragunow et al, 1990, Svenningsson et al, 1997).
The requirement of A2AR-mediated transmission in the regulation of histone H3 exerted by haloperidol is further supported by the experiments performed in Gnal+/−mice. We show that a reduction in the expression of Gαolf, which dramatically decreases the ability of A2ARs to activate adenylyl cyclase (Corvol et al, 2007), prevents haloperidol from promoting histone H3 phosphorylation. This observation demonstrates the crucial role played by Gαolf in striatopallidal neurotransmission and identifies this protein as a critical mediator for the actions of D2Rs antagonists, including antipsychotic drugs.
The observation that A2ARs and Gαolf are involved in the regulation of histone H3 phosphorylation indicates the importance of the cAMP/PKA signaling cascade in the control of this protein. In support of this view, the present study also shows that PKA-dependent phosphorylation of DARPP-32 at Thr34 is an obligatory step in the phosphorylation of histone H3. This observation is in line with recent results demonstrating the critical role played by nuclear accumulation of DARPP-32 in the control of histone H3 phosphorylation exerted by cocaine (Stipanovich et al, 2008). Thus, it appears that haloperidol enhances H3 phosphorylation through disinhibition of A2ARs, which leads to stimulation of PKA and concomitant suppression of PP-1 activity via phosphoThr34-DARPP-32. The idea that this mechanism occurs at the level of striatopallidal neurons is supported by recent data demonstrating that haloperidol increases DARPP-32 phosphorylation at Thr34 specifically in this group of MSNs (Bateup et al, 2008).
Studies performed in striatal slices, have shown that D2Rs and A2ARs exert an opposite regulation on the state of phosphorylation of DARPP-32 (Lindskog et al, 1999). This observation is in agreement with the contrasting regulation exerted by haloperidol and KW6002 on histone H3 phosphorylation and indicates that, even in intact animals, the actions of these drugs are most likely exerted in the striatum, where D2Rs and A2ARs are abundantly expressed on striatopallidal MSNs (Fink et al, 1992, Gerfen, 1992, Schiffmann et al, 1991).
ERK- and MSK1-independent regulation of histone H3 phosphorylation in striatopallidal MSNs
Drugs able to promote cAMP-dependent signaling in the striatum, such as cocaine and L-DOPA, activate ERK and MSK1 through phosphorylation of DARPP-32 at Thr34 (Santini et al, 2007, Valjent et al, 2000). This activation of ERK and MSK1, which depends on D1Rs, mediates the concomitant increase in the state of phosphorylation of histone H3 (Brami-Cherrier et al, 2005, Santini et al, 2007). Based on this evidence it has been proposed that regulation of histone H3 in striatonigral MSNs involves the coordinated activation of cAMP/PKA/DARPP-32 and ERK/MSK1 signaling (Girault et al, 2007). The idea of a critical role for ERK and MSK1 in the regulation of histone H3 is further supported by recent studies on histone phosphorylation in the hippocampus (Chwang et al, 2006, Chwang et al, 2007).
In contrast to the work mentioned above, several lines of evidence presented in this study show that ERK signaling is not involved in the phosphorylation of histone H3 produced by blockade of D2Rs. First, the modest and regionally restricted stimulation of ERK phosphorylation observed in response to haloperidol contrasts with the large and more widespread increase in histone H3 phosphorylation, suggesting that these events are functionally uncoupled. Second, and most important, neither pharmacological inhibition of ERK, nor genetic inactivation of MSK1, affect the increase in histone H3 phosphorylation produced by blockade of D2Rs. These observations, together with the finding of lower expression of MSK1 in D2R- as compared to D1R-containing MSNs, suggest that ERK/MSK1 signaling is not necessary for histone H3 phosphorylation in striatopallidal neurons. Therefore it is likely that, in these MSNs, A2AR-activated signaling pathways increase histone H3 phosphorylation independently of ERK/MSK1, possibly directly through PKA or through other protein kinases. In support of this interpretation, PKA has been shown to phosphorylate chromatin in vitro (Taylor, 1982) and has been proposed to represent the physiological histone H3 kinase in non-neuronal cells (DeManno et al, 1999, Salvador et al, 2001). The involvement of phosphoThr34-DARPP-32 in the haloperidol-mediated regulation of histone H3 further suggests that PP-1 may be involved in histone dephosphorylation at Ser10.
Recent evidence showed the existence of important differences between striatonigral and striatopallidal MSNs with respect to several forms of synaptic plasticity (Cepeda et al, 2008, Day et al, 2006, Kreitzer and Malenka, 2007, Shen et al, 2007). The divergence in the role of ERK signaling and the mechanisms of regulation of chromatin remodeling, as reflected by histone H3 phosphorylation, further demonstrates the profound differences between these two populations of MSNs. Moreover it sheds light on the molecular basis of the actions of antipsychotic drugs and on mechanisms potentially implicated in their extrapyramidal side effects.
Supplementary Material
Effect of raclopride on phospho-acetyl-histone H3. Mice expressing EGFP in striatonigral (Drd1a-EGFP) or striatopallidal (Drd2-EGFP) MSNs were treated with vehicle or with the D2R antagonist raclopride and perfused 15 min (15’; a, b), 30 min (30’; b) and 60 min (60’; b) later. (a) Immunofluorescence (red) for phospho-Ser10-acetyl-Lys14-histone H3 (P-AcH3) in single confocal sections of the dorsal striatum is shown alone or in combination with EGFP fluorescence (green). (b) Quantification of striatal P-AcH3 immunoreactive neurons among EGFP + or EGFP - neurons in the DM and DL striata of vehicle (Veh) or raclopride (Rac) treated Drd2-EGFP mice (** p < 0.01, *** p < 0.001 vs. vehicle; °°° p < 0.001 vs. Rac 15’). Scale bars: 40 µm.
Acknowledgements
This work was supported by Swedish Research Council grants 13482 and 14862 and the Björn Oscarssons donation (G.F.), The Peter Jay Sharp Foundation, The Picower Foundation and NIH grants MH40899 and DA10044 (P.G.), INSERM, Fondation pour la Recherche Médicale (E.V., D.H. and J.A.G.), grant ANR-05-NEUR-020-03 from Agence Nationale de la Recherche (J.A.G.), ARC (K.C.B.) and Neuropôle de Recherche Francilien-NeRF, Région Ile de France (J.B.G, T.I., K.B.C., D.H., E.V. and J.A.G.). A.U. was a recipient of a fellowship from the Wenner-Gren Foundations.
Footnotes
Disclosure/Conflicts of interest
The authors declare that over the past three years P.G. has received compensation from IntraCellular Therapies, Inc., PsychoGenics, Neurologix, Pfizer, Sanofi-Aventis.
References
- Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, et al. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci. 2008;11:932–939. doi: 10.1038/nn.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Herve D, Darragh J, Corvol JC, Pages C, et al. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur J Neurosci. 2008;27:671–682. doi: 10.1111/j.1460-9568.2008.06038.x. [DOI] [PubMed] [Google Scholar]
- Chen J-F, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, et al. The role of the D2 dopamine receptor (D2R) in A2A adenosine receptor (A2A R)-mediated behavioral and cellular responses as revealed by A2A and D2 receptor knockout mice. Proc. Natl. Acad. Sci. USA. 2001;98:1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell. 2000;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwang WB, Arthur JS, Schumacher A, Sweatt JD. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci. 2007;27:12732–12742. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol JC, Studler JM, Schonn JS, Girault JA, Herve D. Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J Neurochem. 2001;76:1585–1588. doi: 10.1046/j.1471-4159.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- Corvol JC, Valjent E, Pascoli V, Robin A, Stipanovich A, Luedtke RR, et al. Quantitative changes in G αolf protein levels, but not D1 receptor, alter specifically acute responses to psychostimulants. Neuropsychopharmacology. 2007;32:1109–1121. doi: 10.1038/sj.npp.1301230. [DOI] [PubMed] [Google Scholar]
- Davie JR. MSK1 and MSK2 mediate mitogen- and stress-induced phosphorylation of histone H3: a controversy resolved. Sci STKE. 2006;2003:PE33. doi: 10.1126/stke.2003.195.pe33. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- DeManno DA, Cottom JE, Kline MP, Peters CA, Maizels ET, Hunzicker-Dunn M. Follicle-stimulating hormone promotes histone H3 phosphorylation on serine-10. Mol Endocrinol. 1999;13:91–105. doi: 10.1210/mend.13.1.0222. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Robertson GS, Faull RLM, Robertson HA, Jansen K. D2 dopamine receptor antagonists induce c-fos and related proteins in rat striatal neurons. Neuroscience. 1990;37:287–294. doi: 10.1016/0306-4522(90)90399-o. [DOI] [PubMed] [Google Scholar]
- Farde L, Wiesel FA, Jansson P, Uppfeldt G, Wahlen A, Sedvall G. An open label trial of raclopride in acute schizophrenia. Confirmation of D2-dopamine receptor occupancy by PET. Psychopharmacology (Berl) 1988;94:1–7. doi: 10.1007/BF00735871. [DOI] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, et al. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptor in rat striatum. Mol. Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Activation of adenylate cyclase from rat striatum and tuberculum olfactorium by adenosine. Med. Biol. 1977;55:262–267. [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Ann. Rev. Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Håkansson K, Galdi S, Hendrick J, Snyder G, Greengard P, Fisone G. Regulation of phosphorylation of the GluR1 AMPA receptor by dopamine D2 receptors. J Neurochem. 2006;96:482–488. doi: 10.1111/j.1471-4159.2005.03558.x. [DOI] [PubMed] [Google Scholar]
- Heffron D, Mandell JW. Differential localization of MAPK-activated protein kinases RSK1 and MSK1 in mouse brain. Brain Res Mol Brain Res. 2005;136:134–141. doi: 10.1016/j.molbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P, Tung HY, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Herve D, Le Moine C, Corvol JC, Belluscio L, Ledent C, Fienberg AA, et al. Galpha(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J Neurosci. 2001;21:4390–4399. doi: 10.1523/JNEUROSCI.21-12-04390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kudo S, Ishizaki T. Pharmacokinetics of haloperidol: an update. Clin Pharmacokinet. 1999;37:435–456. doi: 10.2165/00003088-199937060-00001. [DOI] [PubMed] [Google Scholar]
- Li J, Guo Y, Schroeder FA, Youngs RM, Schmidt TW, Ferris C, et al. Dopamine D2-like antagonists induce chromatin remodeling in striatal neurons through cyclic AMP-protein kinase A and NMDA receptor signaling. J Neurochem. 2004;90:1117–1131. doi: 10.1111/j.1471-4159.2004.02569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindskog M, Svenningsson P, Fredholm BB, Greengard P, Fisone G. Activation of dopamine D2 receptors decreases DARPP-32 phosphorylation in striatonigral and striatopallidal projection neurons via different mechanisms. Neuroscience. 1999;88:1005–1008. doi: 10.1016/s0306-4522(98)00411-4. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Pozzi L, Håkansson K, Usiello A, Borgkvist A, Lindskog M, Greengard P, et al. Opposite regulation by typical and atypical anti-psychotics of ERK1/2, CREB and Elk-1 phosphorylation in mouse dorsal striatum. J Neurochem. 2003;86:451–459. doi: 10.1046/j.1471-4159.2003.01851.x. [DOI] [PubMed] [Google Scholar]
- Salvador LM, Park Y, Cottom J, Maizels ET, Jones JC, Schillace RV, et al. Follicle-stimulating hormone stimulates protein kinase A-mediated histone H3 phosphorylation and acetylation leading to select gene activation in ovarian granulosa cells. J Biol Chem. 2001;276:40146–40155. doi: 10.1074/jbc.M106710200. [DOI] [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaegen JJ. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J. Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, et al. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci. 2007;10:1458–1466. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, et al. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. Embo J. 2003;22:2788–2797. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanovich A, Valjent E, Matamales M, Nishi A, Ahn JH, Maroteaux M, et al. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 2008;453:879–884. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294:366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, Ongini E, Fredholm BB. Antagonism of adenosine A2A receptors underlies the behavioural activating effect of caffeine and is associated with reduced expression of messenger RNA for NGFI-A and NGFI-B in caudate-putamen and nucleus accumbens. Neuroscience. 1997;79:753–764. doi: 10.1016/s0306-4522(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Ledent C, Parmentier M, Greengard P, Fredholm BB, et al. Regulation of the phosphorylation of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa in vivo by dopamine D1, dopamine D2, and adenosine A2A receptors. Proc Natl Acad Sci U S A. 2000;97:1856–1860. doi: 10.1073/pnas.97.4.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, et al. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302:1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- Taylor SS. The in vitro phosphorylation of chromatin by the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1982;257:6056–6063. [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggin GR, Soloaga A, Foster JM, Murray-Tait V, Cohen P, Arthur JS. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol Cell Biol. 2002;22:2871–2881. doi: 10.1128/MCB.22.8.2871-2881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Belluscio L, Hen R. G(olf)alpha mediates dopamine D1 receptor signaling. J Neurosci. 2000;20:RC91. doi: 10.1523/JNEUROSCI.20-16-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of raclopride on phospho-acetyl-histone H3. Mice expressing EGFP in striatonigral (Drd1a-EGFP) or striatopallidal (Drd2-EGFP) MSNs were treated with vehicle or with the D2R antagonist raclopride and perfused 15 min (15’; a, b), 30 min (30’; b) and 60 min (60’; b) later. (a) Immunofluorescence (red) for phospho-Ser10-acetyl-Lys14-histone H3 (P-AcH3) in single confocal sections of the dorsal striatum is shown alone or in combination with EGFP fluorescence (green). (b) Quantification of striatal P-AcH3 immunoreactive neurons among EGFP + or EGFP - neurons in the DM and DL striata of vehicle (Veh) or raclopride (Rac) treated Drd2-EGFP mice (** p < 0.01, *** p < 0.001 vs. vehicle; °°° p < 0.001 vs. Rac 15’). Scale bars: 40 µm.