Summary
Targeted deletion of focal adhesion kinase (fak) in the developing dorsal forebrain resulted in local disruptions of the cortical basement membrane located between the neuroepithelium and pia-meninges. At disruption sites, clusters of neurons invaded the marginal zone. Retraction of radial glial endfeet, midline fusion of brain hemispheres, and gliosis also occurred, similar to type II cobblestone lissencephaly as seen in congenital muscular dystrophy. Interestingly, targeted deletion of fak in neurons alone did not result in cortical ectopias, indicating that fak deletion from glia is required for neuronal mislocalization. Unexpectedly, fak deletion specifically from meningeal fibroblasts elicited similar cortical ectopias in vivo and altered laminin organization in vitro. These observations provide compelling evidence that FAK plays a key signaling roleincortical basement membrane assembly and/or remodeling. In addition, FAK is required within neurons during development because neuron-specific fak deletion alters dendritic morphology in the absence of lamination defects.
Introduction
During development, cortical neurons migrate along radial glial fibers that create a scaffold traversing the ventricular zone to the pial basement membrane (Marin and Rubenstein, 2003). Radial glia endfeet as well as more mature astrocytic processes contribute to the cortical basement membrane that forms between the endfeet (glia limitans) and the leptomeninges (composedofmeningeal fibroblasts). Meningeal fibroblasts are additional sources of extracellular matrix components such as laminin, collagen, and perlecan, as well as modifiers of matrix organization (Sievers et al., 1994). Defective basement membranes often accompany disorders in cortical development and lamination, although it has been unclear whether or not they are the cause or consequence of aberrantly migrating neurons (Olson and Walsh, 2002).
Deletion of basement membrane components or their receptors often results in abnormal cortical development and cortical dysplasia. Mutations in genes encoding several basal lamina constituents (laminin α5 or γ1, perlecan) as well as their cellular receptors (dystroglycan, β1 or α6 integrin) each disrupt normal deposition/ remodeling of the cortical basement membrane and result in a disorganized cortex (Costell et al., 1999; De Arcangelis et al., 1999; Georges-Labouesse et al., 1998; Graus-Porta et al., 2001; Halfter et al., 2002; Miner et al., 1998; Moore et al., 2002). In many instances, these cortical phenotypes strongly resemble those found in some forms of congenital muscular dystrophy, such as Fukuyama Muscular Dystrophy (FCMD), Walker-Warburg Syndrome (WWS), and Muscle-Eye-Brain Disease (MEB) (Ross and Walsh, 2001). The genes responsible for two of these syndromes have been recently identified as glycosyltransferases (Takeda et al., 2003; Yoshida et al., 2001) that potentially regulate glycosylation of α-dystroglycan, which is essential for its activity as a receptor for laminin (Hayashi et al., 2001; Michele et al., 2002; Takeda et al., 2003).
Since dystroglycan and the α6-containing integrins α6β1 and α6β4 are laminin receptors, it is likely that the signaling pathways triggered by laminin binding are essential for basement membrane integrity and may underlie the pathologies of these disorders. Tyrosine phosphorylation and reorganization of the actin cytoskeleton have been shown to be necessary for higher-order laminin polymerization in vitro (Colognato et al., 1999). However, the intracellular signaling pathways that are responsible for promoting basal lamina assembly and remodeling are poorly understood in vivo.
FAK is a nonreceptor tyrosine kinase named for its prominent localization to focal adhesions and is strongly activated following integrin binding to multiple components of the extracellular matrix (Parsons, 2003). fak−/− fibroblasts have an increased number of immature focal adhesions, resulting in cell rounding and slowed cell migration on fibronectin as well as altered regulation of the actin cytoskeleton (Ilic et al., 1995; Sieg et al., 1998). Interestingly, FAK is strongly expressed in the developing brain in neurons and astrocytes (Contestabile et al., 2003). The presence of neural-specific FAK isoforms in the adult brain indicates that FAK is also important in adult CNS function (Grant et al., 1995; Toutant et al., 2002). Yet analysis of FAK in neuronal signaling pathways in vivo has been precluded by the early embryonic lethality of fak−/− embryos, which die shortly after gastrulation (E8.5) (Furuta et al., 1995; Ilic et al., 1995).
In order to determine the role of FAK in cortical development, we generated a conditional fak knockout mouse (fak-flox). Tissue-specific deletion of fak from neuronal land glial cell precursors of the dorsal telencephalon resulted in severe cortical dysplasia resembling type II lissencephaly. The presence of abnormalities following meningeal fibroblast-specific removal of fak and the absence of gross defects following neuron-specific deletion of fak indicate that the primary phenotype is due to altered basement membrane organization rather than to a cell-autonomous defect in neuronal migration. These findings establish FAK as an essential signaling node in the regulation of basement membrane integrity during cortical development. Furthermore, deficiencies in FAK signaling may underlie aspects of the neurological pathologies observed incongenital muscular dystrophies.
Results
Targeting Strategy for Conditional Inactivation of the FAK Gene
To investigate the role of FAK in nervous system development, a conditional “floxed” allele of fak (fak-flox) was generated using Cre/loxP technology. LoxP sites flanking the second kinase domain exon were introduced into ES cells by homologous recombination (Supplemental Figure S1A available at http://www.neuron.org/cgi/content/full/40/3/501/DC1). In the original fak knockout, disruption of the second kinase exon by insertion of a neo targeting cassette resulted in FAK deficiency and early embryonic lethality (Ilic et al., 1995). Similar disruption of the second kinase exon following Cremediated recombination resulted in a premature translational stop codon resulting in ablation of FAK protein expression (Supplemental Figure S1D–S1F). Homozygous fak-flox mice or heterozygous mice carrying one fak-flox allele and one fak null allele from the original FAK knockout were viable, fertile, and showed no showed no obvious phenotype.
Generation of Dorsal Forebrain-Specific fak Knockout Mice
Specific deletion of FAK in the anlage of the dorsal telencephalon was accomplished by mating mice to mice expressing Cre recombinase under the control of the endogenous emx1 promoter (Gorski et al., 2002). The emx1 promoter is active in neuroepithelial precursors of neurons and glia in the developing cortex and hippocampus beginning at embryonic day 9. FAK protein expression was absent in the mutant cortex in regions where emx1IREScre was expressed, as assayed by Western blot and immunohistochemistry (Supplemental Figures S1D and S1F at http://www.neuron.org/cgi/content/full/40/3/501/DC1). The low levels of FAK protein present in the mutant tissues were due to blood vessels, interneurons, meningeal fibroblasts, and other cells not derived from precursors expressing emx1 (Gorski et al., 2002).
FAK has a naturally expressed C-terminal fragment called FAK-related non-kinase (FRNK), derived from an internal promoter and translational start site after the catalytic domain (Nolan et al., 1999). Our targeting construct was designed not to affect downstream FRNK expression. FRNK (43 kDa) was not detected in wildtype or fak-flox cortical extracts from postnatal brains, although it is likely to be present in other parts of the embryo (Supplemental Figure S1D and data not shown; Nolan et al., 1999). Consequently, these results show that FRNK expression was not detectably upregulated in the absence of FAK protein in the dorsal forebrain.
Abnormal Cortical Laminar Organization and Migration Defects in fak−/− Forebrain
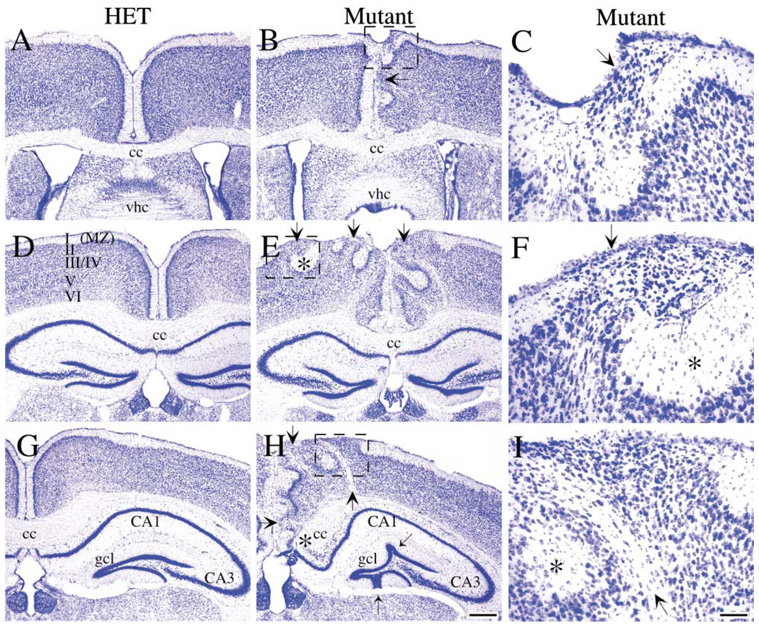
Disruption of FAK expression in the developing dorsal forebrain resulted in mice with frequent neuronal ectopias, manifested by overmigration of cells through the marginal zone and sometimes into the subarachnoid space (Figure 1). Cortical lamination was severely disorganized in areas beneath the ectopic outgrowths (Figures 1E and 1H) and was interrupted by aberrant fiber bundles running from the corpus callosum to the pial surface (Figures 1E and 1H). Defects were most severe at the midline, where there was frequent fusion of the left and right hemispheres (Figure 1H). The frequency of cortical ectopias followed a gradient with dysplasia more often observed in caudal and medial cortical regions (compare Figure 1B to Figures 1E and 1H). Agenesis of the caudal portion of the fak−/− corpus callosum was reproducibly observed, which may be caused by either altered growth cone responsiveness to guidance cues or defects in the glial wedge (asterisk, Figure 1H) (Shu and Richards, 2001).
Figure 1. Emx1IREScre-Induced Deletion of fak Results in Cortical Abnormalities.
Coronal sections (40 µm) from adult control heterozygote (A, D, and G) and emx1IREScre/fak mutant (B, C, E, F, H, and I) forebrain stained with Nissl. In the more anterior sections (A and B), the neuronal dysplasia was less severe and was manifested in small ectopic clusters of neurons migrating into the marginal zone (MZ) at the midline, shown in higher magnification in (C). In the more posterior sections (D and E), the neuronal dysplasia became more severe, with prominent streams of neurons invading the marginal zone and disrupting the lamination of the cortex (arrows, [E and F]). Marginal zone material accumulated in relatively cell-sparse nodules adjacent to ectopias (asterisk, [E and F]). Aberrant fiber bundles (arrow, [H and I]) inappropriately coursed through some cortical regions near the midline, and caudal sections showed partial agenesis of the corpus callosum (asterisk, [H]). The dentate gyrus showed similar ectopic outgrowths of granule cells toward the presumptive pial surface (thin arrows, [H]). I-VI, cortical layers; cc, corpus callosum; gcl, granule cell layer; HET, heterozygote; MUT, mutant; MZ, marginal zone; vhc, ventral hippocampal commissure. Scale bar, 250 µm (H) and 25 µm (I).
The hippocampus was also affected in the fak−/− forebrain (Figure 1H). Notably, the upper and lower branches of the dentate gyrus were undulated, with ectopic migration of granule cells toward the presumptive pial surface.
Cellular Composition of fak−/− Cortical Dysplasia
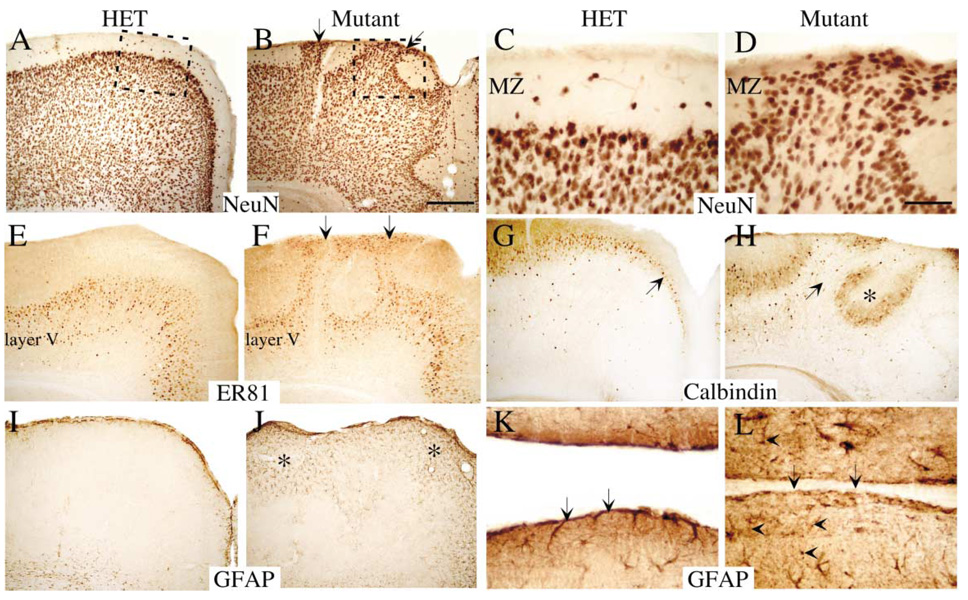
The ectopic cells present in the fak−/− cortex were indeed neurons, as shown by immunostaining with the panneuronal marker NeuN (Figures 2A–2D). Furthermore, neurons from both deep and superficial cortical layers contributed to the ectopias (Figures 2E and 2F; ER81 antibody, layer V; Otx-1 antibody, layers III and V; data not shown).
Figure 2. Cellular Composition of fak−/− Cortical Ectopias.
Coronal sections (40 µm) from P14 heterozygote and fak−/− mice.
(A–D) Panneuronal NeuN mAb immunostaining revealed that the ectopias were composed in part by neurons inappropriately migrating through the marginal zone.
(E and F) Deep layer V cortical neurons that are normally localized to a discrete interior stripe of cells were mislocalized in the fak mutant and streamed to the pial/glial surface as demonstrated by anti-ER81 labeling.
(G and H) Calbindin-positive interneurons that are normally scattered throughout the cortex as well as prominently localized beneath the marginal zone strikingly encircled areas of dysplastic cortex.
(I–L) Numerous GFAP-positive astrocytes characterized areas of fak−/− ectopia, while the control showed normal glial staining within the cortical basement membrane as well as scattered throughout the cortex (I and J). Thinner paraffin sections (7 µm) of the midline revealed the astrocytic contribution to the pial surface in the control more clearly (arrows, [K]). In the mutant, the pial/meningeal surface had clear interruptions (arrows, [L]), and the astrocytes displayed abnormally beaded processes (arrowheads, [L]).
Scale bar, 200 µm (B) and 50 µm (D).
Interneurons and other neurons expressing calbindin were strikingly localized in a ring-like fashion around dysplastic regions (Figures 2G and 2H). Although calbindin-expressing neurons include a subset of pyramidal neurons that are expected to be recombined as a consequence of emx1IREScre expression, inhibitory interneurons are derived from neurons in the pallium that do not express emx1IREScre (Gorski et al., 2002). This indicates that interneurons may be mislocalized either because of abnormally localized guidance cues or through displacement by overmigrating fak−/− cortical neurons.
A striking phenotype of the fak−/− forebrains was the presence of numerous GFAP-positive astrocytes localized in the dysplastic cortical areas (Figures 2I and 2J). These fak−/− astrocytes had punctate beads along their processes (triangles, Figure 2L), very similar to those observed in postnatal cases of Fukuyama Congenital Muscular Dystrophy (Yamamoto et al., 1999), and showed a decreased contribution to the glia limitans (Figures 2K and 2L).
Aberrant Organization and Branching of Cortical Dendrites
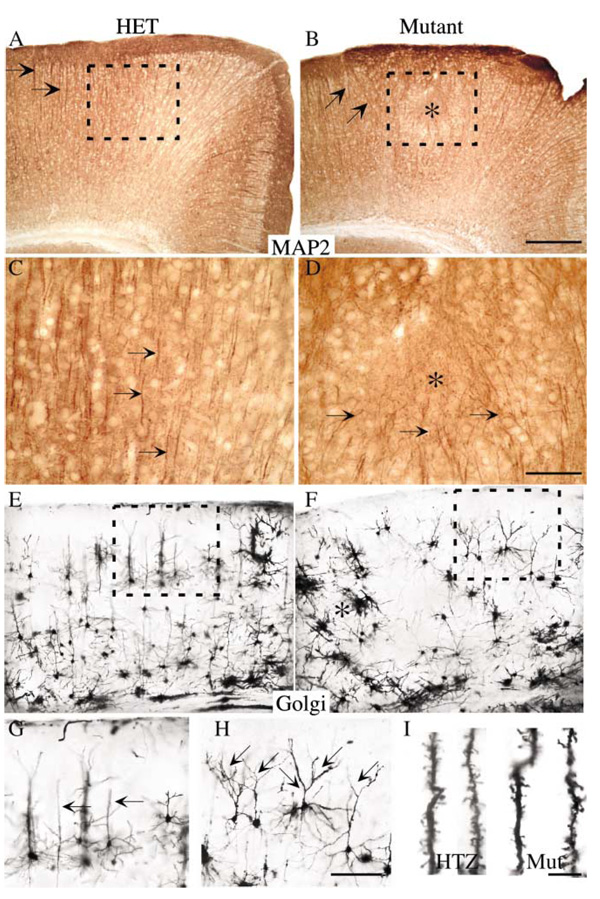
Interestingly, MAP2 staining revealed severe disorientation of fak−/− dendritic processes, which formed rosette-like nodules filled with aberrantly oriented neuronal processes (asterisk, Figures 3A–3D). These ectopic neuronal clusters in the FAK-deficient brain displayed hyperexcitability, consistent with previously described electrophysiological properties of dysplastic cortex (M.E. Calcagnotto and S.C. Baraban, personal communication).
Figure 3. Altered Dendritic Orientation and Branching of fak−/− Neurons.
(A–D) MAP-2-positive dendrites of cortical neurons in control brain had a parallel, striped orientation (arrows, [A and C]), whereas, in the fak−/− cortex, they were frequently abnormally oriented (arrows, [B]) or were drawn into a tangled nodule in areas of cortical dysplasia (arrows, [D]).
(E–H) Golgi impregnation of control (E and G) and fak mutant cortex further revealed lamination defects (asterisk, [F]) but additionally uncovered defects in dendritic branching. Normally, pyramidal neurons extend a single apical dendrite perpendicular to the pial surface. fak−/− cortical neurons displayed a highly branched dendritic morphology. At higher magnification of the boxed areas, the increased branching of fak−/− pyramidal neurons was clearly evident (arrows, [H]), even in areas of less-disturbed dysplasia.
(I) Dendritic spines from cortical neurons of control and fak−/− mice were relatively normal. Scale bar, 200 µm (B and F), 50 µm (D), 100 µm (H), and 20 µm (I).
Aberrant dendritic morphologies of fak−/− neurons were also observed using Golgi stains (Figures 3E–3H). Even in areas where lamination was relatively intact, fak−/− neurons consistently displayed a clear change in dendritic branch complexity, prominently affecting the apical dendrite of layer III and V projection neurons (box, Figure 3F). There was no obvious change in the shape or number of dendritic spines (Figure 3I).
Normal Localization of Cajal Retzius Neurons and Reelin
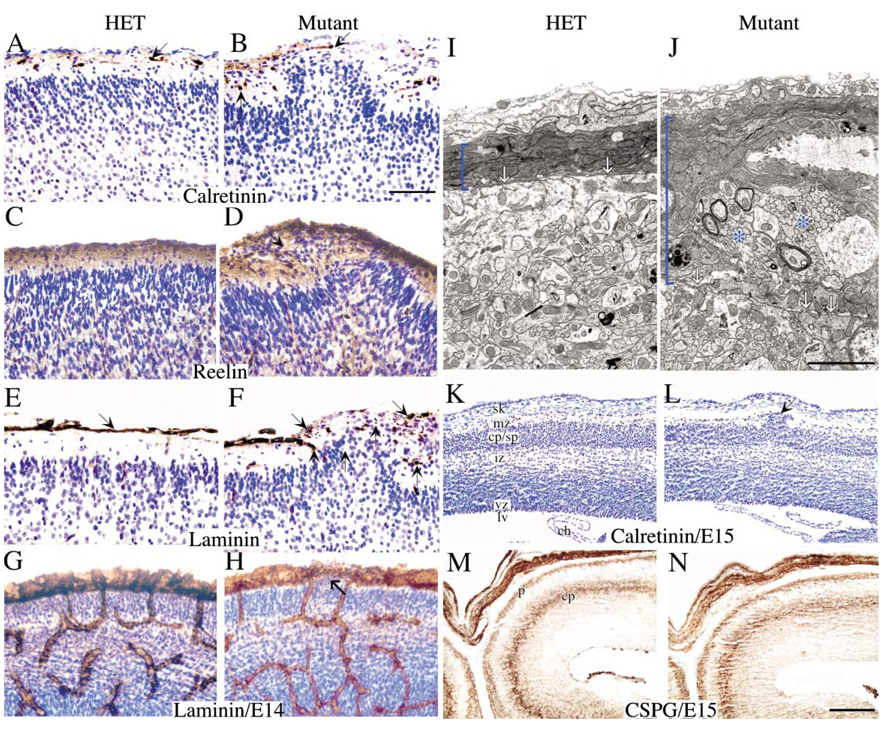
In previous work, absence or mislocalization of Cajal Retzius (CR) neurons has been shown to result in the misplacement of other neurons beneath and around them, in part because they secrete reelin (Graus-Porta et al., 2001; Ligon et al., 2003). To determine if mislocalization of CR neurons contributed to the neuronal dysplasia, fak−/− sections were stained with Calretinin antibodies. In areas without ectopia, CR neurons were normally positioned (Figure 4B). However, in regions surrounding cortical ectopias, the CR neurons were occasionally displaced (along with other constituents of layer 1) to slightly deeper than normal locations. Inward displacement of CR neurons was only observed in regions immediately adjacent to ectopias and was probably an indirect consequence and not a cause of ectopia formation. CR neurons were also located at the peak of ectopic outgrowths, indicating that overmigrating neurons do not appear to be moving through gaps in the CR cell layer (Figure 4B, arrow).
Figure 4. Role of Cajal Retzius Neurons, Reelin, and Basement Membrane Constituents in fak−/− Mice and Developmental Onset of Cortical Abnormalities.
(A–F) Paraffin sections (7 µm) from P1 control and FAK mutant animals counterstained with Nissl. (A and B) Calretinin staining labeled singular Cajal Retzius (CR) neurons localized to the marginal zone proximal to the pial/meningeal membrane (A and B). This localization was largely maintained in fak−/− mutants, except in areas of neuronal ectopia where CR neurons were either present at the peak of ectopias or accumulated within the marginal zone contents to either side. (C and D) Reelin was localized normally to the marginal zone in both control and mutant animals. However, ectopic fak−/− neurons migrated through deposited reelin and did not halt migration at the border of the marginal zone (D). (E and F) Laminin staining with an anti-EHS laminin antibody showed continuous, uninterrupted staining along the cortical basement membrane in control animals (E). In the fak mutants, laminin was degraded over and within areas of ectopia and showed a punctate, fragmented staining suggestive of active degradation. Developmental onset of fak−/− cortical abnormalities noted in 40 µm frozen sections from E14 embryos (G and H) or paraffin (7 µm) sections of E15 embryos (K–N). (G and H) The earliest time point when ectopias were found was in E14 embryos, stained with an anti-laminin α1 subunit. At this early stage, arrows mark the initiation of ectopic outgrowths. These areas coincided with thinned regions of the basement membrane that upon higher magnification showed punctate staining characteristic of laminin degradation. (K and L) Nissl staining revealed ectopias invading the marginal zone of fak−/− cortex at E15 (arrow, [B]). (M and N) The preplate split properly into the cortical plate and marginal zone in the fak−/− mutant cortex as shown by chondroitin sulfate immunoreactivity (CSPG). (I and J) Electron micrographs of the cortex and pial/meningeal interface in adult control and fak−/− brains. Arrows indicate the first layer of the cortical basement membrane, and bracket indicates overall thickness of membranes underneath the skull. The fak−/− basement membrane had allowed cortical material to break through (asterisk, [J]) and was considerably thicker. ch, choroid plexus; cp, cortical plate; iz, intermediate zone; lv, lateral ventricle; mz, marginal zone; p, pial membrane; sk, skull; sp, subplate; vz, ventricular zone. Scale bar, 50 µm (A–H), 2.5 µm (J), and 100 µm (K–N).
The pattern of reelin expression was comparatively normal in the mutant cortex, although high levels were present in neuron-rich regions of the ectopias (Figure 4D). Total levels of reelin appeared to be normal as assessed by Western blot of P1 cortical extracts (data not shown). Since fak−/− neurons inappropriately invaded areas of reelin immunoreactivity in the marginal zone (Figure 4D), it is possible that fak−/− neurons do not exhibit normal responses to reelin. However, because the phenotype observed in the fak−/− forebrain is localized to subregions of cortex in contrast to the inverted lamination observed throughout the cortex in mutants in the reelin signaling pathway (Tissir and Goffinet, 2003), the presence of FAK cannot be required for neuronal responses to reelin in the absence of other contributing factors.
Localized Disruption of Pial-Meningeal Basement Membrane
Disruptions of the basement membrane are thought to contribute to the formation of neuronal dysplasias (Olson and Walsh, 2002). In order to determine if the cortical basement membrane was perturbed by nearby ectopias, sections were stained with a pan-laminin antibody (against Engelbreth-Holm-Swarm laminin). Laminin expression was severely fragmented over ectopic regions of the fak−/− forebrain, although it appeared comparatively normal elsewhere (Figure 4F). Punctate laminin staining was also prominent within ectopias, suggesting active degradation or displacement of the extracellular matrix. Similar disruptions in the expression patterns of collagen IV and perlecan were also observed (data not shown).
Abnormalities in the outer surface of the fak−/− cortex were also revealed by electron microscopy (Figures 4I and 4J). In control brains, a defined basement membrane immediately overlying the glia limitans was observed at the interface of the CNS and the pia mater. However, in ectopic domains of the fak−/− forebrain, these features could not be observed. Instead, there were abnormal clusters of neural cells that had broken through and were resting above a partially disrupted basement membrane. In addition, the layers in the pia and meninges appeared highly abnormal.
Time Course of Ectopia Development in the fak−/− Forebrain
In order to determine at what developmental stage the fak−/− phenotype occurred, sections from E14 and E15 cortex were analyzed. The presence of developing ectopias could be observed as early as E14 and was temporally associated with the onset of basement membrane abnormalities (Figures 4G and 4H). Note that these are much thicker frozen sections, with laminin staining not only the basement membrane but also blood vessels, which are not targeted by emx1IREScre and are not obviously affected in the mutant. At E15, immunostaining with anti-chondroitin sulfate proteoglycan revealed that an early step in cortical development—splitting of the preplate into the subplate and marginal zone—occurred normally in the fak−/− forebrain (Figures 4K and 4L). No major changes in apoptosis or proliferation were observed by TUNEL labeling or immunostaining with Ki67 antibody (data not shown).
Defective Radial Glia Endfeet
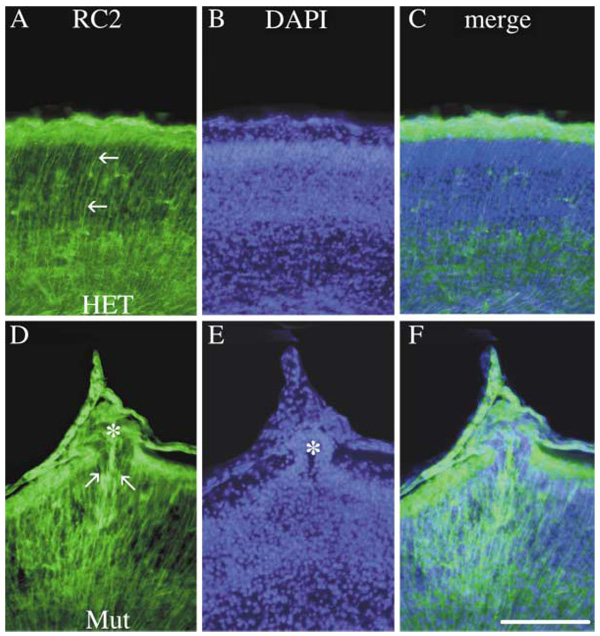
Radial glia processes extend from the ventricular zone to the cortical surface, where their tufted endfeet are the preferred site of basal lamina assembly and organization (Halfter et al., 2000). Therefore, a potential cause of basement membrane defects might be abnormally organized radial glia. In the fak−/− forebrain, RC2-positive glia were disorganized and clearly interrupted in regions of cortical ectopia, where their endfeet were retracted from the cortical surface (Figure 5D, arrow). Occasionally, some of the radial glial processes aberrantly extended into the middle of an ectopic outgrowth (Figure 5D, asterisk).
Figure 5. Defective Radial Glia in fak−/− Cortex.
Fluorescent micrographs of frozen sections (20 µm) from E16 heterozygous (A, B, and C) and fak mutant (D, E, and F) cortex stained with RC2 antibodies to label radial glia processes and DAPI to visualize individual cell nuclei. The tufted endfeet of radial glial cells were prominently stained at this stage, where they contribute to the cortical basement membrane (A). Cortical ectopias within the marginal zone of the fak mutant are marked by asterisks (D and E) and coincided with interrupted RC2 staining due to retracted and disorganized radial glial processes. Occasionally, radial glial processes were seen extending through the ectopic clusters. mz, marginal zone. Scale bar, 100 µm.
Neuron-Specific fak Deletion Is Not Sufficient to Induce Ectopias but Results in Altered Dendritic Branching
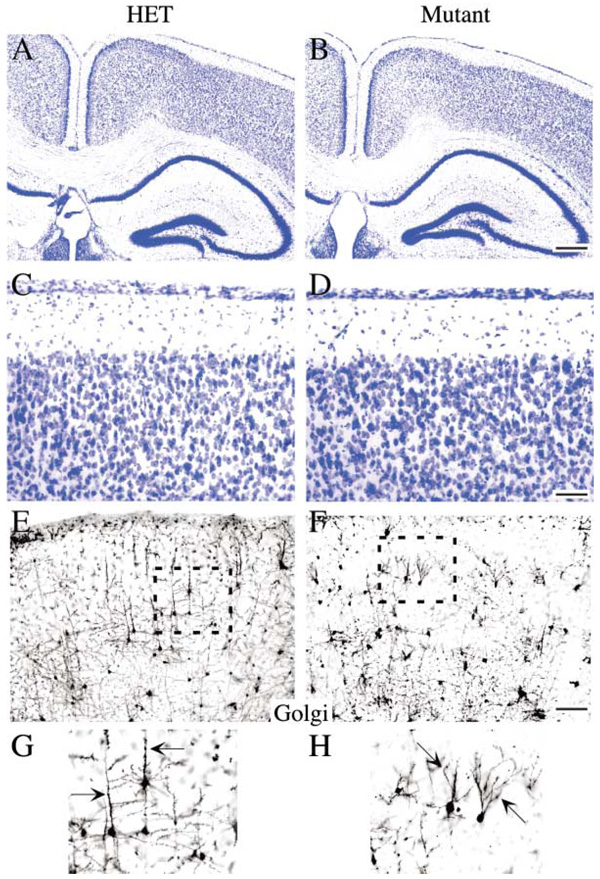
In order to determine if the deletion of FAK from neurons (as opposed to glia) was necessary for development of the fak−/− phenotype, the conditional fak-flox mouse was crossed to the neuronal-specific Cre line nex-Cre. Nex-Cre drives recombination in migrating neurons as early as E11, with robust expression occurring throughout the forebrain by E12.5 (S.G. and K.-A.N., unpublished data). When fak-flox mice were crossed to the nex-Cre line, their forebrains looked remarkably normal (Figures 6A–6D). The fak−/− cortex was nicely laminated with no ectopic clusters of cells in the marginal zone or in the hippocampal dentate gyrus (Figures 6B and 6D). Therefore, it is likely that defective radial glia endfeet as opposed to neurons are the primary contributors to the cortical lamination defects found in the emx1IREScre dorsal forebrain-specific fak−/− mutant.
Figure 6. fak Deletion in Neurons Is Not Sufficient to Induce Cortical Dysplasia but Does Induce Altered Dendritic Morphology.
Coronal sections (40 µm) from adult control and nex-Cre/fak mutant forebrain stained with Nissl are indistinguishable at the gross anatomical level (A–D). The marginal zone was intact with no ectopic clusters of cells, and the hippocampus exhibited a normal morphology. However, Golgi staining of coronal sections (100 µm) did reveal abnormalities in dendritic branching in nex-Cre/fak mutants. Pyramidal neurons from layer III and V have a prominent apical dendrite extending perpendicular to the brain surface in control cells (arrow, [G]) that is notably perturbed in the fak−/− mutant (arrow, [H]). Scale bar, 500 µm (B) and 50 µm (D and F).
Intriguingly, Golgi staining of individual neurons form nex-Cre forebrains revealed that the observed change in dendritic complexity was a phenotype intrinsic to the fak−/− neurons and not a result of other cortical abnormalities (Figures 6E–6H). This defect was particularly obvious in the apical dendrites of pyramidal neurons, which were perturbed in the nex-Cre/fak mutant even though cortical lamination was intact (Figures 6G and 6H, arrow).
Targeting Meningeal Fibroblasts Is Sufficient to Generate Cortical Ectopia
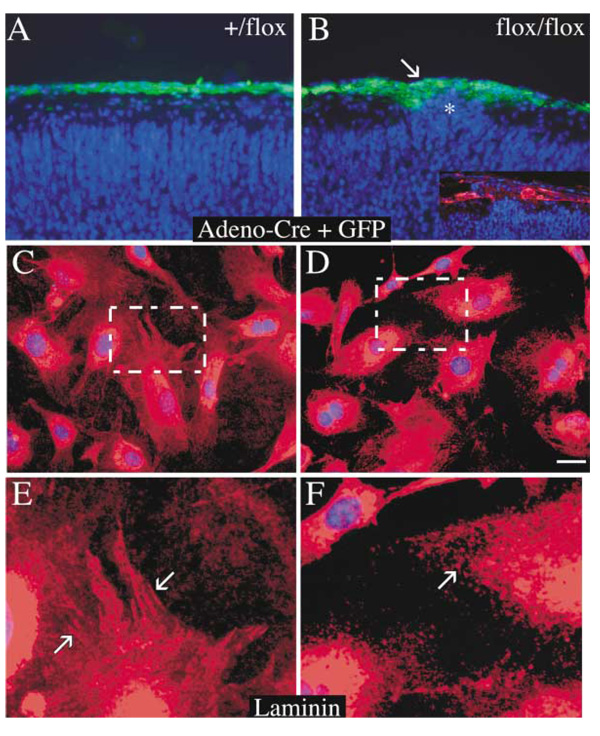
If basement membrane fragility resulting from fak deficiency in radial glial endfeet is indeed the primary cause of ectopia formation, one would predict that manipulation of other cells that contribute to the cortical basement membrane would also lead to generation of ectopic cell clusters. To test this prediction, we deleted fak from meningeal fibroblasts, which also play a role in synthesizing and organizing the cortical basement membrane (Figure 7). Homozygous (fak-flox/fak-flox) or heterozygous (+/fak-flox) embryos at E12.5–13 were injected in utero with an adenovirus expressing Cre recombinase and GFP under a dual CMV promoter. E12.5–13 was the earliest time one could easily resolve the interhemispheric fissure injection site, thereby applying virus only to the outer brain surface underneath the skull. After 5.5 days, control (+/fak-flox) embryos displayed prominent GFP staining in the meninges, indicating that the virus had robustly infected the cells of this layer without infecting underlying cortical cells or disturbing lamination (Figure 7A). Surprisingly, the infected homozygous (fak-flox/fak-flox) embryos showed evidence of heterotopic clusters of cells invading the marginal zone (Figure 7B, asterisk). Furthermore, disrupted laminin staining was associated with the ectopic outgrowths (Figure 7B, inset). These aberrantly positioned neurons did not express GFP, were not targeted by the adenovirus-Cre, and consequently expressed normal levels of FAK protein. Therefore, the presence of these cells in the marginal zone can only be a non-cell-autonomous defect, secondary to a primary defect in the cortical basement membrane resulting from fak deficiency in the pia and meninges.
Figure 7. Adenoviral Recombination of Meningeal Fibroblasts Is Sufficient to Induce Cortical Dysplasia.
(A and B) Paraffin sections (7 µm) from E18.5 embryo heterozygous (+/flox, [A]) or homozygous (flox/flox, [B]) cortex following in utero injection of an adenovirus bearing Cre recombinase and GFP at E12.5/13 and stained with DAPI (blue) and anti-GFP (green). Cortical ectopias were present in the marginal zone of the flox/flox animal and coincided with interrupted laminin staining of the cortical basement membrane (inset, [B]).
(E–H) Laminin staining of primary meningeal fibroblasts isolated from E15 (flox/flox) and infected with either an adenovirus expressing GFP alone (E and G) or GFP plus Cre recombinase (F and H). fak−/− fibroblasts were unable to organize laminin into fibrillar structures as in control. Scale bar, 50 µm (B) and 25 µm (A–D).
fak-Deficient Meningeal Fibroblasts Exhibit Defective Basal Lamina Organization In Vitro
Fibroblasts do not generate basement membranes in vitro, but classical studies have indicated that they are essential for basal lamina organization and secretion of matrix components (Sanderson et al., 1986; Sievers et al., 1994). In order to determine if laminin organization was defective on the surface of fak−/− meningeal fibroblasts, primary meningeal cells were isolated from fak-flox/fak-flox animals and infected with an adenovirus-Cre or control adenovirus GFP for 3 days in vitro. Cells infected with control virus organized laminin into both fibrillar and punctate structures (Figures 7C and 7E), whereas fibroblasts infected with adenovirus Cre showed only punctate laminin staining, with a loss of the fibrillar-like structures (Figures 7D and 7F). This impairment in laminin organization may contribute to the basement membrane deficiencies observed in FAK-deficient meningeal fibroblasts in vivo. Interestingly, the fak−/− meningeal fibroblasts survived for the 3 days required to perform this experiment and did not exclusively exhibit the rounded morphology described in immortalized mouse embryo fibroblast cell lines derived from germline fak deficiency (Ilic et al., 1995).
Analysis of Signaling Pathways Regulated by FAK
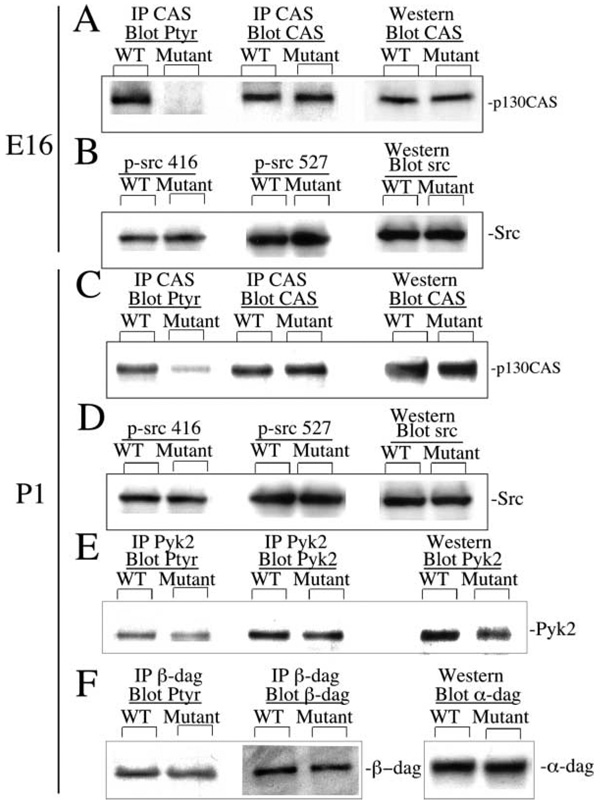
Integrin-mediated binding to laminin triggers the tyrosine phosphorylation of intracellular signaling proteins, including FAK and the adaptor protein p130CAS, and results in the reorganization of the actin cytoskeleton. P130CAS binds to the FAK C terminus through its SH3 domain and is phosphorylated by both FAK and FAK bound Src family kinase members (reviewed in Bouton et al., 2001; Hanks et al., 2003). Tyrosine phosphorylation of p130CAS was significantly reduced in both E16 and P1 fak−/− cortical extracts (Figures 8A and 8C) and was not restricted to regions of cortical abnormality. This decrease in p130CAS phosphorylation may identify p130CAS as a direct substrate of FAK catalytic activity or may be attributed to the loss of Src family recruitment and binding to the FAK autophosphorylation site Y397 (Hanks et al., 2003). In contrast, phosphorylation of the Src autophosphorylation site (Y416) or negative regulatory c-terminal phosphotyrosine (Y527) was not reduced in the absence of FAK at these time points (Figures 8B and 8D).
Figure 8. P130CAS Is Altered, While Src, Pyk2, and Dystroglycan Are Unchanged in fak−/− Cortical Extracts.
Cortical extracts from E16 and P1 wild-type and mutant brains were immunoprecipitated or probed with antibodies to p130CAS (A and C), phospho-Src (B and D), Pyk2 (E), or β-dystroglycan (F) and probed with anti-phosphotyrosine (Ptyr) or their corresponding antibodies. In lanes marked Western, 20 µg of cell extracts were directly fractionated and probed with antibodies to p130 CAS, Src, Pyk2, or β- and α-dystroglycan to compare total cellular expression levels. p130CAS phosphorylation was significantly decreased in the FAK mutant brain at both E16 and P1, whereas its protein expression levels were unchanged (A). The level and phosphorylation of the non-receptor tyrosine kinase Src or of FAK family member Pyk2 was unchanged (B and C). Similarly, the level and phosphorylation of β-dystroglycan was unchanged in the mutant, as was the expression level of α-dystroglycan (D).
Significantly, it has been previously shown that the FAK family member Pyk2 is upregulated in immortalized fak−/−; p53−/− embryonic fibroblasts derived from the original fak knockout mouse (Sieg et al., 1998). In order to determine if Pyk2 was also upregulated in the conditional fak−/− line and possibly compensating for fak deficiency, cortical extracts were immunoprecipitated with antibodies to Pyk2 and blots were probed with anti-phosphotyrosine and anti-Pyk2 (Figure 8E). Pyk2 phosphorylation was slightly reduced; however, its protein levels were unchanged, indicating that it is not upregulated in the dorsal forebrains of fak−/− animals.
Recently, the dystroglycan complex was shown to provide a link between the muscle pathology and neurological deficits found in several congenital muscular dystrophies (Michele et al., 2002; Moore et al., 2002). Since β1 integrin has been shown to regulate dystroglycan expression levels in embryonic stem cells (Li et al., 2002b), it seemed possible that the presence of FAK is essential for a signaling cascade that promotes the expression and/or glycosylation of α-dystroglycan or the phosphorylation of β-dystroglycan. However, the level of α-DAG expression was unaffected in the fak−/− forebrain as was the phosphorylation of β-DAG (Figure 8F).
Discussion
In the present study, generation of a floxed allele of fak has made it possible to examine essential FAK functions during development of the nervous system. Such studies were not possible before, because FAK-deficient embryos die during early embryogenesis prior to extensive development of the nervous system. The major conclusion of these studies is that FAK is required for the formation of a normal basal lamina at the interface between radial glial endfeet and meningeal fibroblasts. FAK deficiency in either cell type results in local disruptions of the cortical basement membrane that have profound effects on dorsal forebrain development.
Non-Cell-Autonomous Effect on Ectopic Neurons
Our results show that when fak is deleted from neurons alone, it does not result in aberrant neuronal migration. Yet, clusters of displaced neurons are found in the marginal zone when fak is deleted from meningeal fibroblasts or neuroepithelial/glial precursors. Since the mislocalization of these neurons is not cell-autonomous, what is provoking them into leaving their normal position? Several possibilities stem from disruption of localized environmental cues triggered by basal lamina breakage. Proteolytically degraded laminin fragments released from a locally fragmented basement membrane could stimulate motility, as has been described in other cell types (Giannelli et al., 1997; Pirila et al., 2003). Indeed, increased matrix metalloprotease (MMP) activity has been demonstrated in glia following brain injury and could arise from gliotic areas in the fak−/− brains (Leppert et al., 2001; Pagenstecher et al., 1998; Rosenberg et al., 2001). Alternatively, matrix remodeling by meningeal cells following basal lamina breakage or injury could result in neuritic growth into the area, as has been shown in the spinal cord (Duchossoy et al., 2001). Other possibilities that could locally influence neuronal migration include release of a chemoattractant present in the meninges or marginal zone (such as SDF-1 or semaphorin III) or loss of a potential repellent or instructive signal, such as reelin. Meningeal cell secretion of SDF-1 controls granule cell migration in the cerebellum and hippocampus, and SemaIII has been shown to attract cortical dendritic growth toward the pial surface (Bagri et al., 2002; Polleux et al., 2000; Zhu et al., 2002). Although reelin was originally thought to act as a “stop” signal to cortical neurons in the marginal zone, it is currently viewed as more of a permissive signal acting in concert with additional positional cues (Dulabon et al., 2000; Magdaleno et al., 2002; Tissir and Goffinet, 2003). However, reelin deposition does not seem to be affected in the fak mutant, and neuron-specific fak deletion does not result in altered cell positioning.
Although the primary defect responsible for abnormal migration of cortical neurons appears to reside in the basement membrane, fak−/− neurons are not completely unaffected. More subtle defects were revealed by Golgi staining of individual cells within the cortex. Unexpectedly, fak−/− cortical neurons displayed an abnormal morphology with a notable alteration in dendritic branch complexity. This morphological defect represents a cell-autonomous phenotype intrinsic to the fak−/− neurons, since it is present in the neuron-specific (nex-Cre) fak-deficient forebrain. Recently, overexpression of a FAK point mutant (S732A) was shown to impair cortical neuron migration (Xie et al., 2003). However, we see no evidence of altered neuronal positioning when FAK is deleted from migrating neurons.
Cellular Functions of FAK in Basement Membrane Remodeling: Radial Glial Endfeet and Meningeal Fibroblasts
Our results indicate that aberrant neuronal migration occurs following FAK deletion in either radial glia or meningeal fibroblasts but not in neurons alone. Importantly, both types of cells involved are required for normal organization of the cortical basement membrane which is formed at the interface between radial glia endfeet (glia limitans) and the pia/meninges (Shearer and Fawcett, 2001; Sievers et al., 1994).
Since FAK loss from radial glia or meningeal fibroblasts results in only localized disruptions of the basal lamina, FAK is not essential for the formation of the cortical basement membrane. However, FAK deficiency may contribute to increased membrane fragility, especially in regions that endure prolonged and consistent pressure from the expanding brain vesicle. Constant membrane remodeling may further patch other areas of disruption before contributing to a developmental defect, resulting in a more localized phenotype. Mutations in genes encoding constituents of the basal lamina, such as perlecan, result in basement membrane deterioration in areas of high mechanical stress, such as the heart and the expanding brain vesicle, but not in the skin or gut (Costell et al., 1999). The timing and completeness of emx1IREScre expression at early stages may also play a role in the regional severity of the ectopias. Emx1 is initially expressed in a low rostrolateral to high caudalmedial gradient (Gulisano et al., 1996), which corresponds to more severe cortical dysplasia at the midline and in the caudal regions of the cortex. However, a much more severe and less localized phenotype may be expected if FAK were deleted from both radial glial and the meningeal fibroblasts.
Intriguingly, when fak is deleted exclusively in meningeal cells, it is sufficient to elicit abnormal neuronal migration into the marginal zone. Meningeal fibroblasts contribute to the basement membrane by secreting and organizing the majority of basal lamina constituents at the surface of the brain. This includes laminin, collagen IV, nidogen, the heparin sulfate proteoglycan perlecan, plus additional fibrillar collagens and other ECM constituents (Shearer and Fawcett, 2001; Sievers et al., 1994). In contrast, although glial processes contribute to the basement membrane, they do not normally secrete matrix proteins during development. Chemical ablation of meningeal cells at birth in rodents results in reduced expression of matrix proteins at the brain surface followed by development of gaps in the basement membrane, disorganization of the glial limitans, and gliosis as assessed by elevated expression of GFAP (Abnet et al., 1991; Sievers and Pehlemann, 1986). Proliferation of meningeal cells is followed in a few days by restoration of the basement membrane and glial limitans. Our experiments indicate that the presence of FAK in meningeal cells promotes the organization and maintenance of this basal lamina, which is in turn essential for the organization of the glial limitans in vivo.
In cell culture, fak-deficient meningeal fibroblasts have a clear deficit in the organization of laminin into fibrils, similar to the deficit observed in skeletal myocytes in the presence of a general tyrosine kinase inhibitor (Colognato et al., 1999). However, unlike skeletal myocytes, meningeal fibroblasts do not generate a basement membrane in culture. To distinguish whether FAK is primarily affecting basement membrane assembly, maintenance, or remodeling will be an interesting topic for future investigation. Since we observe only localized disruption and not a total loss of the basal lamina overlying fak−/− brains, disruptions appear to require additional factors, such as mechanical stress or proteolytic activity. The analysis of these defects may be difficult to analyze in vitro.
Interestingly, deletion of FAK from primary meningeal fibroblasts does not result in immediate apoptosis nor does it cause the striking rounded phenotype observed in p53−/− immortalized cell lines derived from FAK null embryos (Ilic et al., 1995; Sieg et al., 1998). Similarly, we observed very little apoptosis when FAK was deleted in vivo from cells in the developing dorsal forebrain. Differences between FAK deletion in meningeal fibroblasts and the FAK null embryo fibroblast cell lines may be a consequence of cell type, age of isolation, or substrate. Furthermore, any phenotypic variation could be explained by differences between primary cells and an immortalized clonal population or compensatory events that are avoided by conditional deletion of fak. Subsequent studies have found morphological variability in the FAK null embryo fibroblasts and have isolated FAK-deficient lines that have a normal spread phenotype while maintaining defects in cell migration (Owen et al., 1999; Wang et al., 2001).
Macromolecular Signaling Complexes and Laminin Polymerization
FAK deficiency produces a phenotype similar to mutations in CNS basal lamina constituents or their cellular receptors, resulting in disrupted basement membranes and localized neuronal ectopias. Examples include the matrix proteins laminin α5, γ1, and perlecan and the integrins α3, α6, and β1 and dystroglycan (Costell et al., 1999; De Arcangelis et al., 1999; Georges-Labouesse et al., 1998; Graus-Porta et al., 2001; Halfter et al., 2002; Miner et al., 1998). Our results indicate that FAK modulates the function of one or more of these cellular receptors, some of which have been shown to activate FAK upon ligand binding (Georges-Labouesse et al., 1998; Giancotti and Ruoslahti, 1999). Notably, laminin binding and organization seems to be a key factor in many of these mutations. Consistent with this, genes that are responsible for the human hereditary diseases Fukuyama Congenital Muscular Dystrophy (FCMD) and Muscle-Eye-Brain disease have recently been identified as glycosyltransferases that appear to regulate receptor binding to laminin by glycosylation (Takeda et al., 2003; Yoshida et al., 2001). In addition to defects in brain development, these diseases are also characterized by dystrophic muscle pathology and ocular defects. Interestingly, we have observed pathologies similar to those seen in these dystrophies when FAK is targeted in the developing muscle and eye (H.E.B., unpublished data.
Phenotypic Variation in Mouse Lines with Laminin Receptor Deficiencies
Utilization of different Cre lines may be sufficient to account for phenotypic variations between FAK deficiency and mutations in laminin receptors, such as α6β1 integrin or dystroglycan. However, there are additional possibilities to account for such differences. For example, brain-specific β1-integrin-deficient mice have interiorly displaced Cajal Retzius neurons, resulting in a more scalloped cortical lamination pattern than seen in FAK-deficient mice. Yet, β1-integrin binds to other α subunits that do not signal exclusively through FAK, and these heterodimers may be what affects CR cell positioning. Similarly, FAK signals downstream of multiple other (non-β1) integrin receptors, adhesion molecules (Beggs et al., 1997), and growth factor systems (Ivankovic-Dikic et al., 2000; Sieg et al., 2000). Inhibition of these pathways may therefore result in distinctive phenotypes, such as the agenesis of the caudal portion of the fak−/− corpus callosum or the increase in dendritic branching of cortical neurons.
Brain-specific dystroglycan (DG) deficiency results in a more disorganized cortex than observed following either β1 integrin or FAK deficiency alone (Graus-Porta et al., 2001; Moore et al., 2002). It has been proposed that DG is responsible for the initial binding of laminin on cell surfaces, followed by recruitment of integrins into a large complex (Henry et al., 2001; Olson and Walsh, 2002). This hierarchy in laminin binding may therefore account for disparity in phenotypic severity. In support of this theory, DG null ES cells do not detectably bind laminin, whereas β1-integrin null ES cells are capable of binding laminin but fail to organize it into morphologically complex structures (Henry et al., 2001; Lohikangas et al., 2001). Additionally, lack of DG glycosylation can prevent efficient integrin binding to laminin (Michele et al., 2002). Since FAK is activated following integrin engagement to extracellular matrix components, it is likely that FAK deficiency impairs signaling downstream of integrin engagement. Additionally, one report found that FAK is present in β-dystroglycan complexes immunoprecipitated from brain synaptosomes (Cavaldesi et al., 1999). Subsequent studies have shown that FAK does not directly interact with β-dystroglycan (Sotgia et al., 2001), suggesting that FAK coimmunoprecipitation with dystroglycan was a result of gentle detergent extraction, resulting in the preservation of large protein complexes. Nonetheless, we cannot rule out the possibility that FAK is involved in intracellular communication between the integrin and dystroglycan pathways at this time. β-dystroglycan can be phosphorylated by Src (Sotgia et al., 2003), so it is possible that loss of Src recruitment to FAK may impair DG phosphorylation. However, we did not see a reduction in β-dystroglycan phosphorylation in fak−/− brain extracts.
Model of Signaling Complex Regulating Basement Membrane Integrity
FAK exerts its biological effects not only through its tyrosine kinase activity but also by acting as a scaffolding protein that connects cell surface proteins to the actin cytoskeleton in a large macromolecular complex. Disruption of FAK signaling may prevent the cytoskeletal reorganization and signal transduction cascades that are necessary to convey the bidirectional signals required for laminin organization and overall basement membrane stability (Model, Figure 9). The dramatic reduction in p130CAS phosphorylation throughout fak−/− forebrains suggests that perturbations in localization or activities of this adaptor protein may play a significant role in the FAK phenotype. Although FAK can directly phosphorylate p130CAS, this reduction in phosphorylation may alternatively be due to the loss of Src recruitment into FAK/p130CAS signaling complexes (Ruest et al., 2001). FAK and p130CAS have been shown to cooperate in the promotion of cell migration in many cell types (Cary et al., 1998; Hsia et al., 2003; Panetti, 2002). However, the mechanistic details of how FAK and p130CAS modulate the actin cytoskeleton are not fully known. Given that FAK signaling has been shown to both suppress and activate rho activity, FAK may serve as a regulatable switch dynamically controlling rho-mediated effects on the actin cytoskeleton (Ren et al., 2000; Zhai et al., 2003). An intriguing further possibility is that FAK can act as a “mechanosensor,” altering its link to the cytoskeleton in response to environmental tension or rigidity of the extracellular matrix (Geiger et al., 2001; Li et al., 2002a; Wang et al., 2001). FAK deletion may disrupt this mechanosensory communication enough to alter the dynamic force necessary to assemble or remodel the matrix. This will be an interesting source of future study.
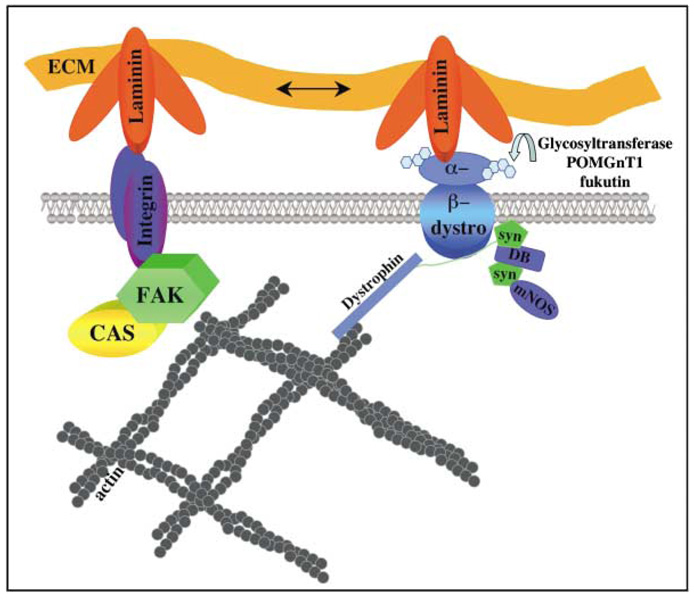
Figure 9. Model.
FAK is involved in bidirectional signaling from the ECM to the cytoskeleton, regulating laminin polymerization and organization of the cortical basement membrane. Integrin binding to laminin activates FAK and associated molecules, such as p130CAS, that in turn regulate the actin cytoskeleton and feed back on receptor/matrix organization within the basal lamina. Other signaling pathways are involved in basement membrane organization, such as the dystroglycan complex, which is thought to initially bind to laminin and then further recruit integrins into a large macromolecular signaling complex. Modulation of α-dystroglycan glycosylation by glycosyl-transferases (POMGnT1, fukutin) affects its ability to bind laminin. Genetic deletion of fak or other components of this signaling complex in the brain results in local basement membrane disruption and cortical dysplasia as seen in cobblestone lissencephaly.
Experimental Procedures
Generation of Floxed FAK Mice
Please see the Supplemental Data at http://www.neuron.org/cgi/content/full/40/3/501/DC1.
Mouse Lines Expressing Cre Recombinase
The emx1IREScre mice were obtained by collaboration with Jessica Gorski and Kevin Jones (University of Colorado), and the nex-Cre mice were obtained by collaboration with Sandra Goebbels and Klaus Armin Nave before publication (Max-Planck-Institute). The pattern of recombination promoted by the emx1IREScre allele has been described (Gorski et al., 2002). All animals were handled in accordance with protocols approved by the UCSF Committee on Animal Research.
Antibodies
FAK C-20 and A-20 pAb (Santa Cruz, 1:1000), FAK pAb (Upstate Biotechnology, 1:500), FAK clone 77 pAb (BD Transduction Laboratories, 1:1000), NeuN mAb (Chemicon, 1:500), ER81 (Tom Jessell, Columbia University, 1:5000), OTX-1 (Sue McConnell, Stanford University, 1:100), Calbindin mAb (Swant, 1:1000), MAP2 mAb (Sigma, 1:500), GFAP pAb (Dako, 1:250), p130 CAS (C-20) mAb (Santa Cruz Biotechnology, 1:1000), Pyk2 (BD Transduction Laboratories, 1:1000), Src (pan, pY418, pY527) pAb (Biosource, 1:1000), α-dystroglycan (IIH6) and c-terminal β-dystroglycan pAb (Kevin Campbell, University of Iowa, 1:5000 and 1:1000), phosphotyrosine 4G10 mAb (Upstate Biotechnology, 1:1000), Calretinin pAb (Swant, 1:3000), Reelin mAb G10 (Andre Goffinet, University Catholique de Louvain, Belgium, 1:2000), EHS laminin pAb (Sigma, 1:3000), CSPG (CS-56) mAb (Sigma, 1:200), RC2 mAb (University of Iowa Hybridoma Bank), GFP pAb (Novus Biologicals, 1:500).
Immunoprecipitation and Western Blotting
Cortices from postnatal day 1 mice were dissected and lysed in modified RIPA buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris [pH7.4], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1.5 mM MgCl2, 10% glycerol, 1 mM NaVO4, 10 µg/ml leupeptin, 0.11 TIU aprotinin, 1 mM PMSF, 100 mM NaF). For immunoprecipitation, antibodies were incubated in precleared lysates (250 µg) for 2 hr, collected on Protein A/G plus agarose beads (Santa Cruz Biotechnology), and washed three times prior to resolution on 4%–15% gradient SDS-PAGE gels (Biorad). Lysates (15 µg) were directly run on gels. For immunoblotting, gels were transferred overnight onto nitrocellulose membranes, blocked with 5% BSA, and incubated with primary antibodies overnight at 4°C. Membranes were incubated with HRP-conjugated secondary antibodies (Jackson Laboratories, 1:5000) followed by ECL reagent (Amersham).
Histological Analysis
Mice were deeply anesthetized and perfused with (or embryos submerged in) 4% paraformaldehyde/PBS. Tissue for frozen sliding microtome sections (40 µm) or cryostat sections (15 µm) was submerged in 30% sucrose overnight at 4°C and then either directly cut or embedded in OCT. Tissue for paraffin sectioning (7 µm) was dehydrated in ascending ethanol series and xylene prior to paraffin embedding. Nissl-stained sections were dehydrated overnight in 70% EtOH prior to staining with 0.1% cresyl violet/0.5% acetic acid. Sections were then rinsed in dH2O, 70%, 95% ethanol, and chloroform, and differentiated with 1.7% acetic acid in 95% EtOH.
Immunocytochemistry
Paraffin-embedded sections were subjected to heat-based antigen retrieval in 10 mM citrate buffer. Frozen and paraffin sections were quenched with 10% methanol/3% H202, followed by blocking in 10% goat serum, 3% BSA, and 0.3% Triton X-100. Primary antibodies were incubated overnight at 4°C, and sections were incubated with biotinylated mouse or rabbit secondary antibodies (Vector Laboratories, 1:200), ABC solution (Vector), and 0.05% diaminobenzidine/0.0003% H202. For fluorescent labeling of sections, either mouse or rabbit Alexa 488 and 594 goat antibodies (Molecular Probes, 1:500) were used as secondary reagents.
Golgi Staining
Modified Golgi-Cox impregnation of neurons was performed using methods described in the FD Rapid GolgiStain kit (FD NeuroTechnologies). In brief, 3-month-old nonperfused mouse brains were immersed in impregnation solution for 2 weeks, transferred to “Solution C” for 2 days, and cut at 100 µm on the cryostat. Sections were mounted on 3% gelatin-coated slides and allowed to dry for 2 weeks before staining with silver nitrate solution “Solution D and E” and dehydrated through descending alcohol series before mounting with Permount.
Electron Microscopy
Mice were perfused with 0.9% NaCl, followed by 2.5% glutaraldehyde and 1% PFA in 0.1 M sodium cacodylate buffer (pH 7.4). Following overnight fixation, 100 µm vibratome sections were cut to aid dissection of dysplastic regions, dehydrated, and embedded in Epon-Araldite. Ultra thin sections were cut and stained with uranyl acetate and lead citrate and photographed at the San Francisco VA Hospital EM facility.
Adenovirus Generation and Embryo Infection
Recombinant adenovirus stocks expressing GFP and Cre recombinase under a dual CMV promoter were produced using the method described in detail in He et al., 1998. In brief, pML78 was digested with EcoR1 and the ends blunted followed by digestion with Sal1 to release a 1 kb fragment containing Cre modified with a nuclear localization sequence and a Kozak sequence. This fragment was cloned into the pAdTrack vector digested with Sal1 and EcoRV and electroporated along with the pAdEasy1 plasmid into BJ5183 cells for recombination. For in utero infection of E12.5-E13 mouse embryos, timed pregnant mice were anesthetized using intraperitoneal injections of Ketamine (90 mg/kg) and Xylazine (6 mg/kg). Cesarean sections were performed, and embryos were selected for injections. A small incision was made in the uterine wall overlying the embryo head region to expose the skull and the interhemispheric fissure visible underneath. In each embryo, approximately 1 µl containing 106 viruses was pressure injected into the interhemispheric space, using a glass micropipette. A maximum of four embryos were injected per litter, and all uterine sacs were sutured. The maternal abdominal wall was repaired, and the animal was allowed to recover. Five and a half days after surgery and virus infection, embryos were harvested, sacrificed, and processed for histological examination in paraffin sections.
Preparation and Infection of Primary Meningeal Fibroblasts
The meninges were removed from E14 mouse (flox/flox) embryos, gently minced, and plated in culture medium consisting of MEM plus 10% FCS, glutamine, and penicillin/streptomycin. Meningeal fibroblasts migrated out of the clumps onto the tissue culture plastic and eventually formed monolayers. Fibroblasts were then passaged and replated onto 8-well culture slides (Lab Tek, 3 × 103 cells/cm2) treated with 0.5% poly-D-lysine, and allowed to attach for 2 hr. Cells were then infected with adenovirus expressing GFP or expressing both GFP and Cre recombinase adenovirus (10 MOI). After 3 days, cells were fixed in 4% PFA and processed for laminin.
Supplementary Material
Acknowledgments
This research was supported by NIH NRSA grant NS11033 to H.E.B; R01 NS19090toLF.R; R01 NEI 10688 to D.S.; BWF, ACS, and CRCW grants to K.R.J; and the Howard Hughes Medical Institute. We thank Kevin Campbell, Andre Goffinet, Sue McConnell, and Tom Jessell for antibodies; Susan Dymecki for FlpE deletor mice; Gail Martin for the PGK-neo cassette plasmid K11 and Cre plasmid pML78; Dusko Ilic for fak-null mice; Erikki Ruoslahti for the map of the fak 1st and 2nd kinase domain exons; members of the Reichardt lab for helpful discussions; Carey Backus and Miya Yamamoto for technical support; and Ivy Hsieh for EM processing.
References
- Abnet K, Fawcett JW, Dunnett SB. Interactions between meningeal cells and astrocytes in vivo and in vitro. Brain Res. Dev. Brain Res. 1991;59:187–196. doi: 10.1016/0165-3806(91)90099-5. [DOI] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Beggs HE, Baragona SC, Hemperly JJ, Maness PF. NCAM140 interacts with the focal adhesion kinase p125(fak) and the SRC-related tyrosine kinase p59(fyn) J. Biol. Chem. 1997;272:8310–8319. doi: 10.1074/jbc.272.13.8310. [DOI] [PubMed] [Google Scholar]
- Bouton AH, Riggins RB, Bruce-Staskal PJ. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene. 2001;20:6448–6458. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaldesi M, Macchia G, Barca S, Defilippi P, Tarone G, Petrucci TC. Association of the dystroglycan complex isolated from bovine brain synaptosomes with proteins involved in signal transduction. J. Neurochem. 1999;72:1648–1655. doi: 10.1046/j.1471-4159.1999.721648.x. [DOI] [PubMed] [Google Scholar]
- Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J. Cell Biol. 1999;145:619–631. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A, Bonanomi D, Burgaya F, Girault JA, Valtorta F. Localization of focal adhesion kinase isoforms in cells of the central nervous system. Int. J. Dev. Neurosci. 2003;21:83–93. doi: 10.1016/s0736-5748(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fassler R. Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Arcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development. 1999;126:3957–3968. doi: 10.1242/dev.126.17.3957. [DOI] [PubMed] [Google Scholar]
- Duchossoy Y, Horvat JC, Stettler O. MMP-related gelatinase activity is strongly induced in scar tissue of injured adult spinal cord and forms pathways for ingrowing neurites. Mol. Cell. Neurosci. 2001;17:945–956. doi: 10.1006/mcne.2001.0986. [DOI] [PubMed] [Google Scholar]
- Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, Kreidberg JA, Anton ES. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr. Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SG, Karl KA, Kiebler MA, Kandel ER. Focal adhesion kinase in the brain: novel subcellular localization and specific regulation by Fyn tyrosine kinase in mutant mice. Genes Dev. 1995;9:1909–1921. doi: 10.1101/gad.9.15.1909. [DOI] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Gulisano M, Broccoli V, Pardini C, Boncinelli E. Emx1 and Emx2 show different patterns of expression during proliferation and differentiation of the developing cerebral cortex in the mouse. Eur. J. Neurosci. 1996;8:1037–1050. doi: 10.1111/j.1460-9568.1996.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Schurer B, Osanger A, Schneider W, Ruegg M, Cole GJ. Composition, synthesis, and assembly of the embryonic chick retinal basal lamina. Dev. Biol. 2000;220:111–128. doi: 10.1006/dbio.2000.9649. [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Yip YP, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J. Neurosci. 2002;22:6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Ryzhova L, Shin NY, Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front. Biosci. 2003;8:D982–D996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- Hayashi YK, Ogawa M, Tagawa K, Noguchi S, Ishihara T, Nonaka I, Arahata K. Selective deficiency of alpha-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology. 2001;57:115–121. doi: 10.1212/wnl.57.1.115. [DOI] [PubMed] [Google Scholar]
- Henry MD, Satz JS, Brakebusch C, Costell M, Gustafsson E, Fassler R, Campbell KP. Distinct roles for dystroglycan, beta1 integrin and perlecan in cell surface laminin organization. J. Cell Sci. 2001;114:1137–1144. doi: 10.1242/jcs.114.6.1137. [DOI] [PubMed] [Google Scholar]
- Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, Huang S, Li E, Nemerow GR, Leng J, et al. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 2003;160:753–767. [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Ivankovic-Dikic I, Gronroos E, Blaukat A, Barth BU, Dikic I. Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nat. Cell Biol. 2000;2:574–581. doi: 10.1038/35023515. [DOI] [PubMed] [Google Scholar]
- Leppert D, Lindberg RL, Kappos L, Leib SL. Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res. Brain Res. Rev. 2001;36:249–257. doi: 10.1016/s0165-0173(01)00101-1. [DOI] [PubMed] [Google Scholar]
- Li S, Butler P, Wang Y, Hu Y, Han DC, Usami S, Guan JL, Chien S. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc. Natl. Acad. Sci. USA. 2002a;99:3546–3551. doi: 10.1073/pnas.052018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, Yurchenco PD. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 2002b;157:1279–1290. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon KL, Echelard Y, Assimacopoulos S, Danielian PS, Kaing S, Grove EA, McMahon AP, Rowitch DH. Loss of Emx2 function leadsto ectopic expression ofWnt1 in the developing telencephalon and cortical dysplasia. Development. 2003;130:2275–2287. doi: 10.1242/dev.00421. [DOI] [PubMed] [Google Scholar]
- Lohikangas L, Gullberg D, Johansson S. Assembly of laminin polymers is dependent on beta1-integrins. Exp. Cell Res. 2001;265:135–144. doi: 10.1006/excr.2001.5170. [DOI] [PubMed] [Google Scholar]
- Magdaleno S, Keshvara L, Curran T. Rescue of ataxia and preplate splitting by ectopic expression of Reelin in reeler mice. Neuron. 2002;33:573–586. doi: 10.1016/s0896-6273(02)00582-2. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell Migration in the Fore-brain. Annu. Rev. Neurosci. 2003 doi: 10.1146/annurev.neuro.26.041002.131058. Published online February 26, 2003. [DOI] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J. Cell Biol. 1998;143:1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- Nolan K, Lacoste J, Parsons JT. Regulated expression of focal adhesion kinase-related nonkinase, the autonomously expressed C-terminal domain of focal adhesion kinase. Mol. Cell. Biol. 1999;19:6120–6129. doi: 10.1128/mcb.19.9.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EC, Walsh CA. Smooth, rough and upside-down neocortical development. Curr. Opin. Genet. Dev. 2002;12:320–327. doi: 10.1016/s0959-437x(02)00305-2. [DOI] [PubMed] [Google Scholar]
- Owen JD, Ruest PJ, Fry DW, Hanks SK. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto-and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am. J. Pathol. 1998;152:729–741. [PMC free article] [PubMed] [Google Scholar]
- Panetti TS. Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration. Front. Biosci. 2002;7:d143–d150. doi: 10.2741/A771. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J. Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pirila E, Sharabi A, Salo T, Quaranta V, Tu H, Heljasvaara R, Koshikawa N, Sorsa T, Maisi P. Matrix metalloproteinases process the laminin-5 gamma 2-chain and regulate epithelial cell migration. Biochem. Biophys. Res. Commun. 2003;303:1012–1017. doi: 10.1016/s0006-291x(03)00452-2. [DOI] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 2000;113:3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- Ross ME, Walsh CA. Human brain malformations and their lessons for neuronal migration. Annu. Rev. Neurosci. 2001;24:1041–1070. doi: 10.1146/annurev.neuro.24.1.1041. [DOI] [PubMed] [Google Scholar]
- Ruest PJ, Shin NY, Polte TR, Zhang X, Hanks SK. Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol. Cell. Biol. 2001;21:7641–7652. doi: 10.1128/MCB.21.22.7641-7652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson RD, Fitch JM, Linsenmayer TR, Mayne R. Fibroblasts promote the formation of a continuous basal lamina during myogenesis in vitro. J. Cell Biol. 1986;102:740–747. doi: 10.1083/jcb.102.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer MC, Fawcett JW. The astrocyte/meningeal cell interface–a barrier to successful nerve regeneration? Cell Tissue Res. 2001;305:267–273. doi: 10.1007/s004410100384. [DOI] [PubMed] [Google Scholar]
- Shu T, Richards LJ. Cortical axon guidance by the glial wedge during the development of the corpus callosum. J. Neurosci. 2001;21:2749–2758. doi: 10.1523/JNEUROSCI.21-08-02749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK-cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Sievers J, Pehlemann FW. Influences of meningeal cells on brain development. Findings and hypothesis. Naturwissenschaften. 1986;73:188–194. doi: 10.1007/BF00417722. [DOI] [PubMed] [Google Scholar]
- Sievers J, Pehlemann FW, Gude S, Berry M. Meningeal cells organize the superficial glia limitans of the cerebellum and produce components of both the interstitial matrix and the basement membrane. J. Neurocytol. 1994;23:135–149. doi: 10.1007/BF01183867. [DOI] [PubMed] [Google Scholar]
- Sotgia F, Lee H, Bedford MT, Petrucci T, Sudol M, Lisanti MP. Tyrosine phosphorylation of beta-dystroglycan at its WW domain binding motif, PPxY, recruits SH2 domain containing proteins. Biochemistry. 2001;40:14585–14592. doi: 10.1021/bi011247r. [DOI] [PubMed] [Google Scholar]
- Sotgia F, Bonuccelli G, Bedford M, Brancaccio A, Mayer U, Wilson MT, Campos-Gonzalez R, Brooks JW, Sudol M, Lisanti MP. Localization of phospho-beta-dystroglycan (pY892) to an intracellular vesicular compartment in cultured cells and skeletal muscle fibers in vivo. Biochemistry. 2003;42:7110–7123. doi: 10.1021/bi0271289. [DOI] [PubMed] [Google Scholar]
- Takeda S, Kondo M, Sasaki J, Kurahashi H, Kano H, Arai K, Misaki K, Fukui T, Kobayashi K, Tachikawa M, et al. Fukutin is required for maintenance of muscle integrity, cortical histiogenesis and normal eye development. Hum. Mol. Genet. 2003;12:1449–1459. doi: 10.1093/hmg/ddg153. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat. Rev. Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Toutant M, Costa A, Studler JM, Kadare G, Carnaud M, Girault JA. Alternative splicing controls the mechanisms of FAK autophosphorylation. Mol. Cell. Biol. 2002;22:7731–7743. doi: 10.1128/MCB.22.22.7731-7743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl. Acad. Sci. USA. 2001;98:11295–11300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Sanada K, Samuels BA, Shih H, Tsai LH. Serine 732 phosphorylation of FAK by Cdk5 isimportant for microtubule organization, nuclear movement, and neuronal migration. Cell. 2003;114:469–482. doi: 10.1016/s0092-8674(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Armstrong D, Shibata N, Kanazawa M, Kobayashi M. Immature astrocytes in Fukuyama congenital muscular dystrophy: an immunohistochemical study. Pediatr. Neurol. 1999;20:31–37. doi: 10.1016/s0887-8994(98)00089-7. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, et al. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev. Cell. 2001;1:717–724. doi: 10.1016/s1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- Zhai J, Lin H, Nie Z, Wu J, Canete-Soler R, Schlaepfer WW, Schlaepfer DD. Direct interaction of focal adhesion kinase with p190RhoGEF. J. Biol. Chem. 2003;278:24865–24873. doi: 10.1074/jbc.M302381200. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat. Neurosci. 2002;5:719–720. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.