Abstract
Background
Attention has turned to neurorestorative therapies, including erythropoietin (EPO), for experimental ischemic stroke and head injury. Treatments for intracerebral hemorrhage (ICH) need to be developed, as this represents a particularly devastating and common form of neurological injury.
Aim
The aim of this study is to investigate the therapeutic potential of EPO after ICH in rats and to measure its effects on mechanisms of recovery and neurogenesis.
Methods
ICH was induced in 24 Wistar male rats by intrastriatal infusion of autologous blood. Recombinant human EPO (rhEPO) (5000 U/kgBW/day or 10000 U/kgBW/day) or saline was administered starting 1 day after ICH and continued daily for 1 week (n = 8 for each group). To label proliferating cells, bromodeoxyuridine (BrdU) was injected daily for 13 days after ICH. All animals survived for 2 weeks after ICH. Functional outcome, area of tissue loss and immunohistochemical staining were measured at 14 days after ICH. Global test or ANOVA was used to test the EPO dose effect.
Results
Rats receiving rhEPO after ICH exhibited significant improvement in mNSS and corner test at 14 days (P<0.05). Increased expression of phenotypes of synaptogenesis and proliferating immature neurons were shown by immunohistochemical staining. Only the group receiving a lower dose of rhEPO had significantly less tissue loss compared to the control group (P<0.05). In rats treated with rhEPO, double staining for BrdU and TUJ1 revealed a subpopulation of cells that express an immature neuronal marker while still dividing.
Conclusions
EPO improves neurological outcome and increases histochemical parameters of neurogenesis when given after ICH in rats. Intriguingly, only the lower dose of rhEPO was effective in reducing tissue loss in the region of ICH.
Keywords: erythropoietin, neural regeneration, intracerebral hemorrhage
Introduction
Spontaneous intracerebral hemorrhage (ICH) represents 10-20% of all strokes, but the neurological outcome is much worse and mortality rates are higher than that of ischemic stroke (1). Once the hematoma forms there is immediate injury to the brain parenchyma where the clot is located (2). Treatment of patients has been focused on prevention of expansion of the clot with medical management, or possibly consideration of surgical removal of the hematoma if there is progressive neurological deterioration or if the clot is superficially located (1). As with other conditions causing sudden loss of neurological function due to the destruction of cerebral tissue, research in recent years has concentrated on the brain's intrinsic, albeit limited, ability to restore lost cells and lost function (3, 4).
Neurorestorative therapies including statins, marrow stromal cells, and other pharmacologic agents such as erythropoietin (EPO) have been used successfully to treat experimental conditions of ischemic stroke and traumatic brain injury (TBI) with observed increases in neurogenesis and improved functional recovery (5-7). These compounds may act via different mechanistic pathways, but the end result is a cellular and extracellular environment more conducive for neural recovery. Endogenous pluripotent cells from the subventricular zone are part of the neuroregenerative process (8, 9). EPO is a naturally occurring hormone which increases red blood cell production, but also has neuroprotective properties as it can attenuate the insults of hypoxia, excess glutamate, tissue nitric oxide (NO), inflammation and apoptosis (3). In this study we test the hypothesis that EPO, which has shown neurorestorative effects after cerebral ischemia, spinal cord injury, and TBI, will improve functional outcome after ICH and have a measurable positive effect on the histological signs of neurogenesis in the perihematomal and subventricular regions.
Experimental Procedures
Animals and Reagents
Adult male Wistar rats were purchased from the Jackson Laboratory (Bar Harbor, ME). Animal studies were performed according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Health System. Recombinant human erythropoietin (rhEPO) was provided by Amgen, Inc. (Thousand Oaks, CA). 5′-bromo-2′ deoxyuridine (BrdU) was obtained from Sigma (St. Louis, MO). The following primary antibodies were used for immunohistochemistry: monoclonal antibody against BrdU (0.25 μg/ml; Roche, Indianapolis, IN); monoclonal antibody against synaptophysin (1:40; Clone SY 38; Chemicon, Temecula, CA); doublecortin (DCX) (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), and monoclonal antibody against tubulin isotype III (TUJ1) (1:400; BABCO, Berkeley, CA).
Animal Surgical Procedures of Intravenous Hemorrhage
Twenty-four male Wistar rats weighing between 270 and 320 g were selected for this study. Briefly, the rat under general anesthesia was placed in a stereotactic frame (Kopf Instruments, Tujunga, CA). A 1-mm craniectomy was performed and a stereotactically guided needle was placed into the right striatum (3.5 mm lateral to midline, 0.5 mm anterior to bregma, depth 5.5 mm below the surface to midline). ICH lesion was induced by injecting 100 μl of autologous blood, taken from the femoral artery at a steady infusion rate of 10 μl per min with an infusion pump (600-910/920, Harvard Apparatus, Holliston, MA) (4).
rhEPO Treatments
At 24-h post ICH, the ICH-induced animals were randomly divided into three experimental groups with each group consisting of 8 rats. Group 1 received an intraperitoneal injection of 5000 U/kg/day rhEPO. Group 2 received an intraperitoneal injection of 10000 U/kg/day rhEPO. Group 3 received the same volume of saline used as a control. All injections of rhEPO were administered 24 h after induction of ICH, and the injections were continued daily for 7 days. In addition, all rats received a daily intraperitoneal injection of 100 mg/kg BrdU starting from Day 1 after ICH to Day 14. The dosage of rhEPO was selected based on earlier studies of TBI and ischemic stroke (5, 10).
Neurological Functional Studies
Functional outcomes were measured using both the modified neurological severity score (mNSS)(11-13) and the corner turn testing score (14, 15), performed by observer blinded to the individual treatment status of the animals. Functional outcomes for each rat were tested at Days 1, 7 and 14 after ICH. The mNSS is a composite score in which motor, sensory, balance, and reflex measures are used to assess the aspects of neurological function in a grade ranging from 1 to 18. One score point is awarded for the inability to perform the test or for the lack of a tested reflex, with the higher score implying greater neurological injury. The corner turn test is performed by placing the rat in a corner and counting the number of times (as a percentage) it turns right or left before exiting (the normal state is a value of 50%). The number of right turns from 10 trials for each test was recorded and used for statistical analysis (e.g., an injured rat preferentially turns toward the unimpaired ipsilateral (left) side).
At the end of 2 weeks, the animals were anesthetized and perfused transcardially with PBS, followed by 4% paraformaldehyde in PBS. Brain tissues were excised, further fixed in 4% paraformaldehyde and sliced into seven 2-mm-thick sections. Each block was processed and embedded in paraffin. Four of the adjacent 6-um-thick sections were cut from each block and stained with hematoxylin & eosin (H & E). The percentage of tissue loss in the ipsilateral compared to the contralateral hemisphere of the brain was measured using an image analysis system as previously described (Data Translation, Marlboro, MA) (4).
Histology and Immunohistochemistry
The brain tissue residing between +0.1-0.86 mm of the bregma on the third block was the most severely injured and therefore the third block was specifically selected for immunostaining. Every 40th coronal section from +0.1-0.86 mm of the bregma was used for immunohistochemical staining with the same antibody. Sections were blocked in a Tris-buffered saline solution containing 5% normal goat serum, 1% BSA and 0.05% Tween-20. Sections were then incubated with the primary antibodies for localization of BrdU (1:100; a marker for proliferation cells), TUJ1 (1:5,000; a marker for a microtubule element of the tubulin family found in developing neurons.), DCX (1:50; a microtubule-associated protein expressed almost exclusively in immature neurons) (16) and synaptophysin (1:1000; a marker for a protein located on synaptic vesicles which contain the neurotransmitters for the labeling of nerve terminals.). After sequential incubation with biotinylated IgG, the sections were treated with an avidin-biotin-peroxidase system (ABC Kit, Vector Laboratories, Burlingame, CA). DAB was then used as a sensitive chromogen for light microscopy. To visualize the cellular co-localization of BrdU and TUJ1, double staining was employed. The sections were incubated for 1 h with monoclonal mouse anti-BrdU, followed by 30 min at room temperature in fluorescein isothiocyanate anti-mouse FITC secondary antibodies. After brief washing, the sections were incubated with polyclonal rabbit anti-TUJ1 overnight at 4°C and subsequently with anti-rabbit Cy5 for 30 min. All immunostainings were performed at the same time with two negative controls (i.e., the omission of primary antibody and the use of pre-immune serum) for quality control of the immunoassaying procedure.
After staining with BrdU, synaptophysin, TUJ1 and DCX, the series of six slides at various levels from the same block were used for semiquantitative measurements.(6) Quantitative measurements of immunostaining were performed by observer blinded to the individual treatment status of the animals. Regions of interest (ROIs) were defined in the SVZ, at the dorsal end of the lateral ventricle, as a zone with 100 μm width and 800 μm length for counting BrdU+cells, TUJ1+BrdU+ cells, TUJ1and DCX positive signals, in the subcortical striatum for synptophysin staining and with 200 μm width in the perihemorrhagic striatum for counting TUJ1+BrdU+ cells, All slides were digitized under a 20× objective lens (Olympus BX40; Olympus Optical Co, Tokyo, Japan) by using a 3D-CCD color video camera (model DXC-970MD; Sony Corp, Tokyo, Japan) interfaced with the MCID image analysis system (Imaging Research, Inc., St. Catharine's, ON, Canada). For synaptophysin, TUJ1 and DCX, data are presented as a percentage of the immunopositive areas in each field divided by the total areas in the field. The data for BrdU are shown as the number of positive cells in the SVZ. Cells that immunoreacted both to BrdU and TUJ1 were expressed as percent of the total BrdU-positive cells in the ROIs.
Statistical Analysis
the study was interested in rhEPO dose effect on the 14 days functional recovery (primary), and the neurogenesis (secondary). Data were evaluated for normality. Data transformation was performed if data were not normal. As a result, the behaviors tests were not normal and ranked data were used for the analysis. The global test using Generalize Estimating Equation (GEE) was implanted to test the dose effect on functional recovery measured from two behavior tests (corner test and NSS), followed by the pair-wise dose comparisons or treatment effect on individual functional outcome at 0.05, If the overall dose effect was significant at 0.05 level. For each neurogenesis variable, ANOVA was used to test the dose effect, followed by the pairwise group comparisons if the overall dose effect was observed at 0.05 level. Spearman correlation was used to measure the correlation between the functional recovery and histological estimates of tissue loss and neurogenesis at day 14 adjusting for the treatments and p-value less than 0.05 indicated a significant correlation compared to no correlation at all.
Results
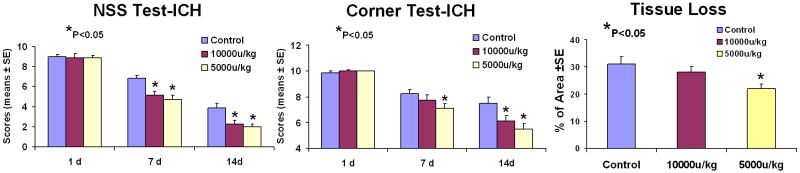
As evaluated by both mNSS and corner turn tests, all ICH-induced rats had similar neurological impairments at Day 1 after ICH (Fig. 1). After treatment with rhEPO for 7 days, rats in the lower dose group showed statistically significant improvement (P<0.05), as assessed using corner testing and the mNSS. The higher dose group also demonstrated significantly improved neurological outcome, as indicated by the mNSS data (P<0.05). The corner turn test showed a trend of improvement for the higher dose group, but with no statistical significance. At Day 7 after termination of the rhEPO injection (Day 14 after ICH), both groups treated with rhEPO showed significant improvements in neurological function over the control group, assessed by both mNSS and corner turn tests (Fig. 1).
Fig. 1.
Neurological functional tests and striatal tissue loss percentage of the injured side. Quantitative bar graph results of NSS (left panel), corner turn test (middle panel) and striatal tissue loss percentage in the ICH region relative to the contralateral normal region (right panel) of control and two rhEPO-treated groups are presented. Statistical significance level is *P<0.05.
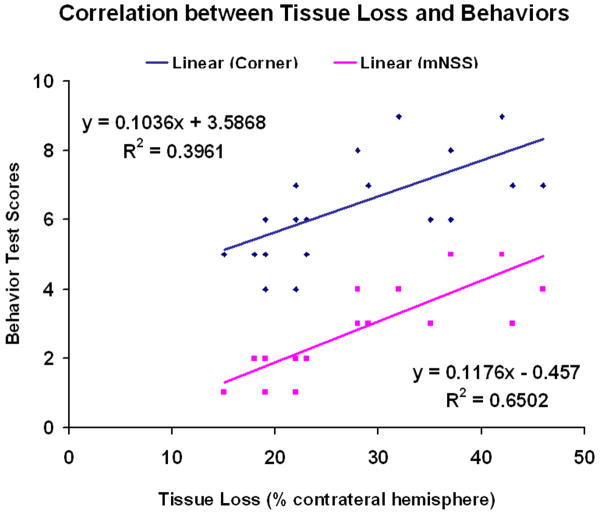
The data for the percentage of striatal tissue loss on the side of hemorrhage is shown in Figure 1. The areas of tissue loss as a percentage of the normal contralateral side are as follows: 5000 U rhEPO, 22 ± 1.5% (P<0.05) and 10000 U rhEPO, 28±4% and control 30±2.7%. Consequently, the lower dose group showed significantly reduced tissue loss compared to control at 2 weeks after ICH. The higher dose group also showed the same trend, but with no significant difference compared to the control group and the lower dose group. Finally, it is observed that there is a negative correlation between the tissue loss and the neurological functional recovery (Fig. 2). The percentage of tissue loss is significantly correlated with either mNSS or the corner turn testing score (P<0.05).
Fig. 2.
Linear regression and correlation analyses. Analyses were done to assess the relationship between tissue loss and NSS (pink) or corner turn test (blue) (P<0.05).
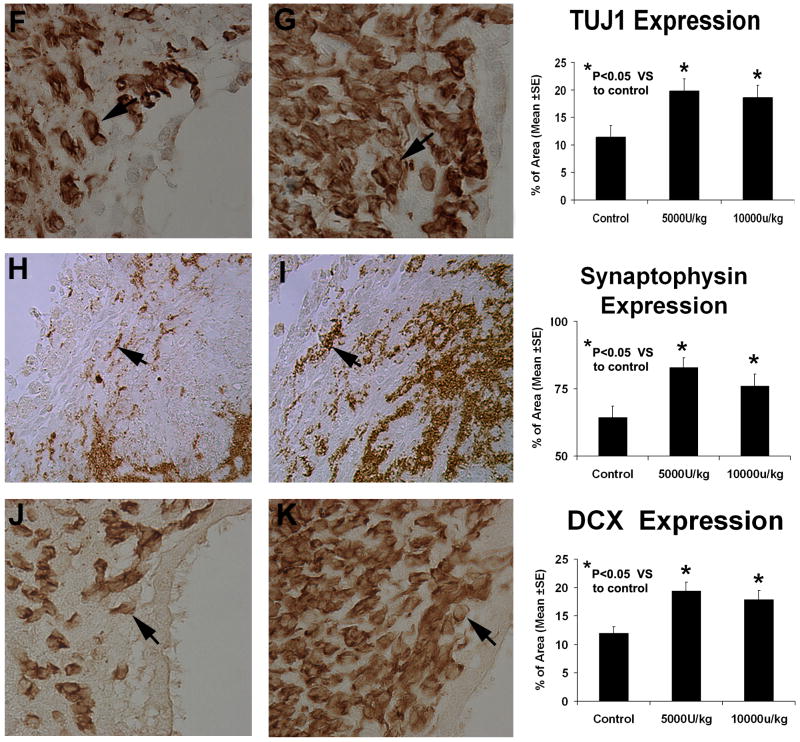
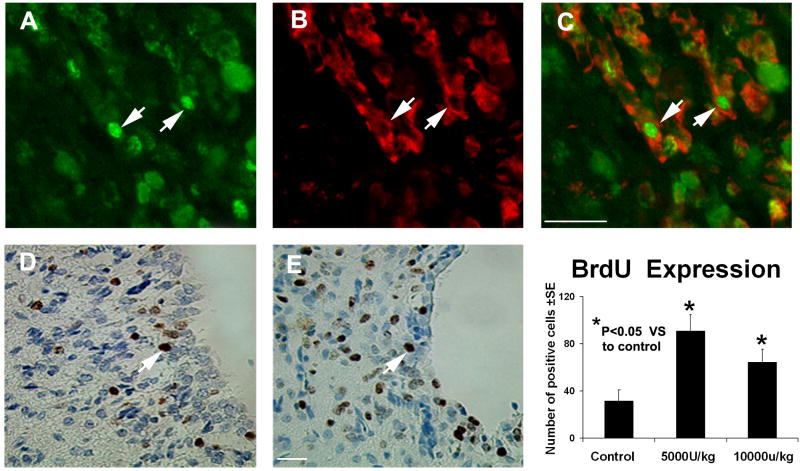
To further understand how rhEPO administration promotes neurological recovery, histology and immunohistochemistry were performed. Typical immunostainings for synaptophysin (panel H and I), DCX (pane J and K) and TUJ1 (panel F and G) in the control group and the lower dose group were detected (Fig 3). Statistical analysis indicated that staining for all three neuron markers in both treated groups had been significantly increased, suggesting that rhEPO can enhance synapse and neuronal formation, respectively. Consistent with the neurological functional studies, the lower dose group exhibits, observationally, stronger staining for all three markers than that of the higher dose group. However, we did not observe significant difference for all three markers between the lo-w and high-dose groups. To determine if rhEPO can enhance cell proliferation around the injured site, immunostaining for BrdU was also performed (Fig. 4). Both groups treated with rhEPO demonstrated significantly increased BrdU-positive cells in the SVZ when compared with the ICH rats injected with placebo, suggesting that rhEPO promotes the proliferation of local cells. Further double staining for BrdU and TUJ1 identified a sub-population of cells co-stained with both BrdU and TUJ1, indicating newly formed immature cells with neural phenotype (Fig. 4). The percent of cell expressing BrdU and TUJ1 positive double immunoreactivity is significantly higher in rhEPO treated groups than that of the control group in the region of the subventricular zone and the boundary of hematoma. (Table 1) However, more than 90% of these BrdU positive cells do not colabeled with TUJ1, indicating that only limited newly formed cells represent neurogensis.
Fig. 3.
Representative immunostaining and quantitative immunoreactivities of TUJ1, synaptophysin and DCX. TUJ1 (panels F and G) and DCX (panels J and K) examples are from the subventricular zone. The synaptophysin example (panels H and I) is from the ICH border zone. Results on the left panels are shown only for the control (panels F, H and J) and low-dose treated groups (panels G, I and K). A higher percentage of per area positively reacting cells to TUJ1, synaptophysin and DCX is seen in the sections derived from rhEPO-treated rats vs control ones. Quantitative immunoreactivities for all three groups are presented as bar graphs on the right side of each panel.
Fig. 4.
Representative immunostaining and quantitative immunoreactivities of BrdU and colocalization of BrdU and TUJ1 immunostaining in the subventricular zone. BrdU results on panels D and E are shown only for the control and low-dose treatment groups, respectively. Quantitative immunoreactivities for all three groups are presented as bar graphs on the right side of panels D and E. Colocalization of BrdU (panel A) and TUJ1 (panel B) in a subpopulation of cells near the injured site of the low-dose treatment group is presented on panel C. Arrows indicate cells that stained positively for both BrdU and TUJ1.
Table 1. TUJ1/BrdU Expression.
| % total BrdU labeled cells in | ||
|---|---|---|
| Groups | Ipsilateral Boundary Zone | Ipsilateral SVZ |
| Control | 5.63±1.11 | 5.13±1.60 |
| rhEPO 5000U | 8.77± 0.73* | 9.92±1.32* |
| rhEPO 10000U | 8.38± 0.42* | 9.15±0.91* |
Values are mean ± SE
P<0.05 VS Control group
Discussion
The present study has demonstrated that treatment with rhEPO promotes neurological recovery in an animal model of ICH, as evidenced by improved neurological functional outcome in both treated groups (low- and high-dose) and significantly diminished tissue loss in the low-dose group. Immunostaining for BrdU and neuronal markers also illustrated significantly more newly generated cells expressing immature neuronal markers in the perilesional region of all rhEPO treated rats.
rhEPO has been shown to improve a number of neurological conditions, including ischemic stroke, spinal cord injury, TBI and neonatal brain injury (5, 10, 17, 18), Several potential mechanisms may be relevant to the neuroprotective and neurorestorative effects of rhEPO. Anti-apoptotic, anti-inflammatory, antioxidative, angiogenetic and neurotrophic effects reduce the ischemic infarct, the loss of cells and tissues, and promote cell survival in these animal models.
Currently, few studies have focused specifically on the effect of rhEPO on ICH. EPO treatment in the collagenase blood injected ICH animal model showed reduced TUNEL staining in the perihematoma region, suggesting EPO enhances neuronal tolerance to insults sustaining neurons from both physical damage and the subsequent toxic blood breakdown products of the hematoma (19). In the same study, perihematomal inflammation was also shown to be reduced by exogenous EPO, which may subsequently contributes to neuroprotection (19). However, to our knowledge, no study has focusd on observing the neurorestorative effect of rhEPO treatment after ICH. Our study measures the nerogenic parameters, showing that ehEPO may improve neurological functional outcome through neurogenesis.
The immunochemical stainings presented in this study are consistent with improved functional neuronal survival and neurogenesis. Increases of immature cells and neuroblasts, detected by immunostainings for BrdU and DCX, respectively, indicate that many more newly generated cells exist in the SVZ. The SVZ is important source for neurogenesis after injury in rodent adult brain. According, it has been reported that rhEPO improved the neural precursor cells migration toward ischemic boundary from the SVZ after ischemic stroke.(20) Significantly increased staining for TUJ1 and a subpopulation of cells co-stained for TUJ1 and BrdU suggest more cells with immature neural phenotype are induced by the exogenous EPO, supporting the neurotrophic effect. Enhanced expression of synaptophysin in the striatum of treatment group, suggesting EPO may improve the formation of the new nerve terminals or protect the terminals from degeneration.
The present study shows the neurological function is correlated with the tissue loss. In addition, rhEPO significantly improves neurological functional recovery and reduces the tissue loss. These data imply that the increase of new formed neurons or reduction of neuron loss around the hematoma contributes to the neurological recovery.
Although a dosage of 500 U/kg/day rhEPO had been used in the collagenase ICH model (19) to achieve a neuroprotective effect, higher doses of rhEPO (2500-5000 U/kg/day) have been administered in animal models of brain injury than those traditionally used to clinically correct for anemia (< 500 U/kg/day) (21). In the current study, intraperitoneal injection of 5000 U/kg/day rhEPO induced significant neurogenesis and synpatogenesis in rats after ICH. It is interesting to note that when injecting 10000 U/kgBW/day rhEPO after ICH, the indicators of neurological outcome are no better than those of the lower-dose rhEPO group. The histological markers of neurorestorative effect may be more sensitive for recovery than tissue loss and functional scores at 7 days, since the latter two measures showed a trend but no statistical improvement with high-dose rhEPO. It is likely that high-dose rhEPO is less effective than low-dose rhEPO in this experiment. Treatment with rhEPO at a higher dose may cause adverse effects (e.g., hyperviscosity, hypertension and vascular thrombosis) and the endothelial cell receptors may have reached saturation, counteracting the neuroprotective and neurorestorative effects (18, 22).
The complications of the hematopoietic effect have precluded the use of rhEPO in the clinical treatment of chronic neurogenerative diseases (23, 24). The erythropoietic and tissue-protective properties of EPO have been found recently to be conferred via two distinct receptor systems (24, 25). Carbamylated EPO can selectively target the nonerythropoietic receptor and may achieve therapeutic potential, while avoiding the polycythemic effects (25). The use of carbamylated EPO has been recently reported to provide benefit after TBI in rats, and application to the ICH experimental model is indicated (25).
Several limitations in the presented study should be considered. First, the nature of biological variation in disease development in individual animals and the limited numbers of animals used makes temporal comparison sub-optimal. While the sacrifice of rats at designated time points (14 day after ICH) was performed to ensure histochemistrial evaluation, this limited the study of pathophysiological events preceding a poor outcome. Furthermore, the results of histochemistry do not yet demonstrate the hypoghesis that newly formed cells migrate and integrate into the injured area. Futher studies should be performed involving the time course study and the fate of new formed cells.
Conclusion
Exogenous rhEPO treatment improves neurological outcome in an animal model of ICH. The improved outcome is associated with the increased immunohistochemical staining parameters for neuronal markers. These results suggest that rhEPO contributes to functional recovery after ICH by promoting, at least partially, neurogenesis and synaptogenesis.
Acknowledgments
Special thanks to Susan MacPhee-Gray for editorial assistance. This work was supported by Internal (Departmental) Funds and National Institutes of Health grant NSO58581.
References
- 1.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116(16):e391–413. doi: 10.1161/CIRCULATIONAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes HM, Siddique S, Banister K, et al. Continuous monitoring of ICP and CPP following ICH and its relationship to clinical, radiological and surgical parameters. Acta Neurochir Suppl. 2000;76:463–6. doi: 10.1007/978-3-7091-6346-7_96. [DOI] [PubMed] [Google Scholar]
- 3.Hasselblatt M, Ehrenreich H, Siren AL. The brain erythropoietin system and its potential for therapeutic exploitation in brain disease. J Neurosurg Anesthesiol. 2006;18(2):132–8. doi: 10.1097/00008506-200604000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Seyfried D, Ding J, Han Y, Li Y, Chen J, Chopp M. Effects of intravenous administration of human bone marrow stromal cells after intracerebral hemorrhage in rats. J Neurosurg. 2006;104(2):313–8. doi: 10.3171/jns.2006.104.2.313. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35(7):1732–7. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Zhang ZG, Li Y, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53(6):743–51. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 7.Chopp M, Zhang XH, Li Y, et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11(13):3001–5. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Zhang ZG, Zhang RL, et al. Neurogenin 1 mediates erythropoietin enhanced differentiation of adult neural progenitor cells. J Cereb Blood Flow Metab. 2006;26(4):556–64. doi: 10.1038/sj.jcbfm.9600215. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Zhang ZG, Zhang RL, et al. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26(22):5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22(9):1011–7. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 11.Schallert T, Kozlowski DA, Humm JL, Cocke RR. Use-dependent structural events in recovery of function. Adv Neurol. 1997;73:229–38. [PubMed] [Google Scholar]
- 12.Shohami E, Novikov M, Bass R. Long-term effect of HU-211, a novel non-competitive NMDA antagonist, on motor and memory functions after closed head injury in the rat. Brain Res. 1995;674(1):55–62. doi: 10.1016/0006-8993(94)01433-i. [DOI] [PubMed] [Google Scholar]
- 13.Borlongan CV, Randall TS, Cahill DW, Sanberg PR. Asymmetrical motor behavior in rats with unilateral striatal excitotoxic lesions as revealed by the elevated body swing test. Brain Res. 1995;676(1):231–4. doi: 10.1016/0006-8993(95)00150-o. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Schallert T, Zhang ZG, et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117(2):207–14. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–11. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Walsh CA. Protein-protein interactions, cytoskeletal regulation and neuronal migration. Nat Rev Neurosci. 2001;2(6):408–16. doi: 10.1038/35077559. [DOI] [PubMed] [Google Scholar]
- 17.Bartesaghi S, Marinovich M, Corsini E, Galli CL, Viviani B. Erythropoietin: a novel neuroprotective cytokine. Neurotoxicology. 2005;26(5):923–8. doi: 10.1016/j.neuro.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Li Y, Cui Y, et al. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005;1034(12):34–9. doi: 10.1016/j.brainres.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Lee ST, Chu K, Sinn DI, et al. Erythropoietin reduces perihematomal inflammation and cell death with eNOS and STAT3 activations in experimental intracerebral hemorrhage. J Neurochem. 2006;96(6):1728–39. doi: 10.1111/j.1471-4159.2006.03697.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang RL, LeTourneau Y, Gregg SR, et al. Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27(12):3157–62. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellert BA, McPherson RJ, Juul SE. A comparison of high-dose recombinant erythropoietin treatment regimens in brain-injured neonatal rats. Pediatr Res. 2007;61(4):451–5. doi: 10.1203/pdr.0b013e3180332cec. [DOI] [PubMed] [Google Scholar]
- 22.Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97(19):10526–31. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torup L. Neuroprotection with or without erythropoiesis; sometimes less is more. Br J Pharmacol. 2007;151(8):1141–2. doi: 10.1038/sj.bjp.0707287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman TR, Westenfelder C, Togel FE, et al. Cytoprotective doses of erythropoietin or carbamylated erythropoietin have markedly different procoagulant and vasoactive activities. Proc Natl Acad Sci U S A. 2006;103(15):5965–70. doi: 10.1073/pnas.0601377103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmood A, Lu D, Qu C, et al. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. 2007;107(2):392–7. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]