Abstract
Purpose
To evaluate, both at initial glaucoma diagnosis and during treatment, the role of demographic and clinical factors on intraocular pressure (IOP).
Design
Cohort study of patients enrolled in a randomized clinical trial.
Participants
607 patients with newly diagnosed, open-angle glaucoma (OAG) were enrolled at 14 U.S. centers.
Methods
After randomization to initial surgery or medications, patients were followed at six-month intervals. IOP was measured by Goldmann applanation tonometry. Predictive factors for IOP at baseline and during follow-up were analyzed using linear mixed models.
Main Outcome Measure
IOP at baseline and during follow-up.
Results
The mean baseline IOP was 27.5 mmHg (standard deviation, 5.6 mmHg). Predictive factors for higher baseline IOP included younger age (0.7 mmHg per 10 years), male sex (2.4 mmHg higher than females), pseudoexfoliative glaucoma (5.4 mmHg higher than primary OAG), and pupillary defect (2.2 mmHg higher than those without a defect). During nine years of follow-up, both surgery and medications dramatically reduced IOP from baseline levels, but the extent of IOP reduction was consistently greater in the surgery group. Over follow-up years 2–9, mean IOP was 15.0 vs. 17.2 mmHg for surgery vs. medicine, respectively. Predictive associations with higher IOP during follow-up included higher baseline IOP (P<0.0001), worse baseline visual field (mean deviation; P<0.0001), and lower level of education (P=0.0019). Treatment effect was modified by smoking status: non-smokers treated surgically had lower IOP than smokers treated surgically (14.6 vs. 16.7 mmHg, respectively; P=0.0013). Clinical center effects were significant (P<0.0001) in both the baseline and follow-up models.
Conclusions
In this large cohort of newly diagnosed glaucoma patients, predictors of pre-treatment IOP and IOP measurements over nine years of follow-up were identified. Our findings lend credence to the postulate that sociodemographic, economic, compliance, or other environmental influences play a role in IOP control during treatment.
Introduction
Controlling intraocular pressure (IOP) has been the primary focus of glaucoma treatment. Recent clinical trials have added sound evidence, consistent with findings from observational studies, that reducing IOP has therapeutic and preventive efficacy.1–3 Studies of factors that influence IOP have been conducted in large populations consisting mostly of people without glaucoma. Increasing age has been associated with increasing IOP in most studies,4–10 although decreasing IOP with increasing age has been reported in two studies of Asian populations.11,12 Schulzer and Drance found that adjustment for systolic blood pressure accounted for any age effect.13 Consistent associations with increasing IOP have been reported for increasing systolic blood pressure,5,7,8,10,12,16,17 darker iris color,9,14,18 and myopia.6,14,18
Once glaucoma is diagnosed and treatment is initiated, the influence on IOP attributable to demographic, systemic, and ocular factors might be overwhelmed by the impact of glaucoma treatment. Our purpose in conducting these analyses was to evaluate, both at the time of initial glaucoma diagnosis and while under prolonged IOP-reducing treatment, the role of all of these factors on IOP.
Patients and Methods
This investigation is based on the 607 patients enrolled in the Collaborative Initial Glaucoma Treatment Study (CIGTS). These newly diagnosed, previously un-treated, phakic, open-angle glaucoma (OAG) patients were enrolled at 14 clinical centers, where Investigational Review Board approval was obtained for the study. The study adhered to the tenets of the Declaration of Helsinki. Upon obtaining written, informed consent, eligible patients were randomized to initial medication (n=307) or initial trabeculectomy (n=300) in the period from October 1993 to April 1997. Because patients presented with either one or both eyes eligible for treatment, before randomization occurred the treating ophthalmologist selected a study eye to receive initial treatment. Baseline data included demographic and clinical variables. Follow-up data were collected through December 2004, when patients had been followed for a median of 7.7 years. At six-month intervals, patients returned to their CIGTS Clinical Center for a comprehensive ophthalmologic examination that included measurement of IOP by Goldmann applanation tonometry. Details of the study’s design and conduct have been previously reported.19
The protocol for measuring IOP followed recommended guidelines.20 An operator and a reader were involved. The operator performed the Goldmann tonometry measurement at the slit lamp according to a specified protocol, and the reader recorded the tonometer dial reading. Two IOP measurements were recorded, and unless they varied by more than 2 mmHg, the average became the final IOP value for data entry. A third measurement was taken if the first two varied by more than 2 mmHg, and the median of the three was then used as the IOP value of record. At the two baseline visits wherein eligibility was assessed, three IOP values were obtained as described above, separated by at least one hour. Thereby, six baseline IOP values (three per visit) were obtained, and one value was obtained at each follow-up visit. Tonometers were calibrated monthly. All staff involved in IOP measurement were certified during site visits.
IOP summary measures (mean, standard deviation, and range) were described for the first and second baseline visits, and comparisons were made with the paired Student’s t-test. Independent Student’s t-tests were used to compare mean IOP between the medical and surgical treatment groups at specific times. Linear mixed models were used to evaluate factors predictive of IOP both at baseline and over follow-up. The baseline model was also used to assess trends in the three IOP measurements taken within each of the two baseline eligibility visits. Both baseline and longitudinal models included a random subject intercept; the longitudinal model also included a random subject slope over time and a Toeplitz covariance structure for the repeated measures. All interactions with treatment and time were tested. Treatment assignment was “as randomized” in the initial longitudinal analyses, and “as treated” (including changes during follow-up) in a subsequent analysis. All but three patients received initial treatment as randomized; two patients randomized to surgery received initial medicine, and one patient randomized to surgery (excluded from “as treated” model) received initial ALT. SAS® 9.1 software (Cary, NC) was used for all analyses.
Results
Enrolled patients (n=607) had newly diagnosed OAG, and had an average age at baseline of 57.5 years. 55% were male and 38% were blacks. Further details on CIGTS participants are provided in Musch et al.19
Table 1 presents the mean baseline IOP values for study eyes of the 607 patients. A slight but statistically significant (P=0.02) decline in mean IOP was found from the first (27.6 mmHg) to second (27.3 mmHg) baseline visit. Within each visit, a more substantial decline in IOP was observed across the three intra-visit IOP measurements taken, with the third measurement on average being 2.1 mmHg (1st visit) and 1.3 mmHg (2nd visit) lower than the first measurement (P<0.001). Interestingly, the mean values for the third IOP measurement at each baseline visit were equivalent (26.6 mmHg). Measures of variation also differed significantly between the two baseline visits, with greater variation in IOP measurements taken within the first versus within the second visit for both the pooled standard deviation and the range (P<0.001).
Table 1.
Baseline variability in repeated intraocular pressure (IOP) measurements
| Mean (standard deviation) of IOP (in mmHg) |
|||
|---|---|---|---|
| Baseline IOP | Baseline Visit #1 | Baseline Visit #2 | P-Value* |
| Mean of 3 measurements | 27.6 (5.9) | 27.3 (5.8) | 0.0192 |
| 1st measure | 28.7 (6.5) | 27.9 (6.1) | <0.0001 |
| 2nd measure | 27.6 (6.2) | 27.4 (6.1) | 0.3721 |
| 3rd measure | 26.6 (6.1) | 26.6 (5.9) | 0.9127 |
| Range of 3 measurements | 4.3 (2.9) | 3.8 (2.5) | 0.0005 |
| Standard deviation of 3 measurements |
2.3 (1.5) | 2.0 (1.3) | 0.0004 |
P-values from paired t-tests
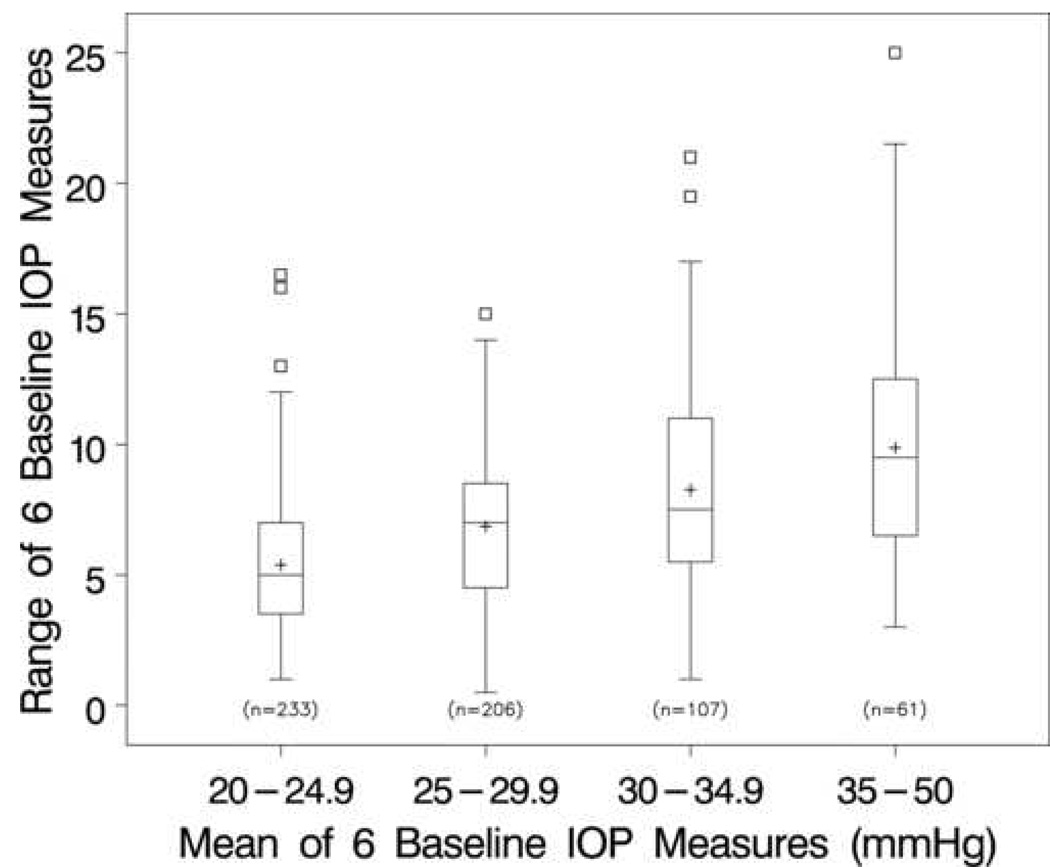
The distributions of ranges (maximum minus minimum) of the six baseline IOP measurements by four categories of the mean IOP are presented using box plots (Figure 1). As the mean IOP increases from <25 mmHg to 35 mmHg or greater, the range of the six IOP values increases. For example, in patients with a baseline mean IOP <25 mmHg, one-half had a range of ≥5 mmHg between their largest and smallest values, whereas in patients with a baseline mean IOP ≥35 mmHg, one-half had a range of ≥10 mmHg.
Figure 1.
Box plots of the range (maximum minus minimum) of the six baseline intraocular pressure (IOP) measures by increasing groups of baseline IOP values. [Note: The box extends from the 25th to the 75th percentiles; the bar across the box and the “+” sign indicate the median and mean, respectively. The whiskers extend to the nearest observation within 1.5 times the inter-quartile range, and the square symbols beyond the whiskers represent outlying observations.]
Factors that were influential in predicting baseline IOP are presented in Table 2. These factors included age, sex, diagnosis, pupillary response, and center. On average, baseline IOP decreased 0.7 mmHg with each ten year age increment (P=0.001), and females had lower baseline IOPs than males (2.3 mmHg lower, P<0.0001). The 29 patients enrolled with a diagnosis of pseudoexfoliative OAG had significantly higher baseline IOPs (5.4 mmHg higher; P<0.0001) than those diagnosed with primary OAG (n=550); pigmentary OAG patients (n=28) did not differ in baseline IOP from primary OAG patients (P=0.96). Those with a baseline pupillary defect (afferent, n=68; other, n=2) had a 2.2 mmHg higher IOP than those with a normal pupil response (P=0.0018). Baseline IOP also varied significantly across centers more than would be expected by chance alone (P=0.0002). Using the mean baseline IOP from all patients as a reference, two centers had mean IOPs that were significantly lower (by 2.0 mmHg) and two centers were significantly higher (by 1.9 and 1.6 mmHg) than the overall mean. IOP at baseline did not differ for blacks vs. whites and others (mean IOP of 27.5 mmHg in both groups). Many other factors were evaluated and found to have no significant association with baseline IOP, including education, income, systemic disease (hypertension, diabetes), smoking history, alcohol use, family history of glaucoma, iris color, refractive error, mean deviation (from visual field testing), and corneal thickness.
Table 2.
Mixed model of predictive factors for baseline intraocular pressure (IOP)
| Variables* | Estimate (SE) | P-Value |
|---|---|---|
| Age (10 year increments) | −0.67 (0.20) | 0.0010 |
| Sex (female vs. male) | −2.35 (0.44) | <0.0001 |
| Glaucoma diagnosis (vs. Primary open angle glaucoma, n=550) |
||
| Pigmentary (n=28) | +0.05 (1.05) | 0.9591 |
| Pseudoexfoliative (n=29) | +5.40 (1.03) | <0.0001 |
| Pupillary response (defect vs. normal) | +2.24 (0.72) | 0.0018 |
| Center (vs. overall mean effect) | 0.0002 | |
| Lower than mean IOP: | ||
| Center B | −2.07 (1.25) | |
| Center E | −2.03 (0.69)† | |
| Center M | −2.03 (0.86)† | |
| Center C | −0.86 (1.18) | |
| Center J | −0.73 (0.80) | |
| Center F | −0.47 (0.68) | |
| Center L | −0.39 (1.49) | |
| Higher than mean IOP: | ||
| Center K | +0.17 (0.71) | |
| Center N | +0.90 (0.80) | |
| Center G | +0.96 (0.68) | |
| Center I | +1.25 (0.69) | |
| Center A | +1.65 (0.66)† | |
| Center D | +1.77 (0.82) | |
| Center H | +1.88 (0.73)† |
All variables listed were entered into a single, multivariable model. Day, order, and day × order interaction (P<0.002) were also included in the model. See Table 1 for day and order effects.
Significantly different from the overall mean baseline IOP
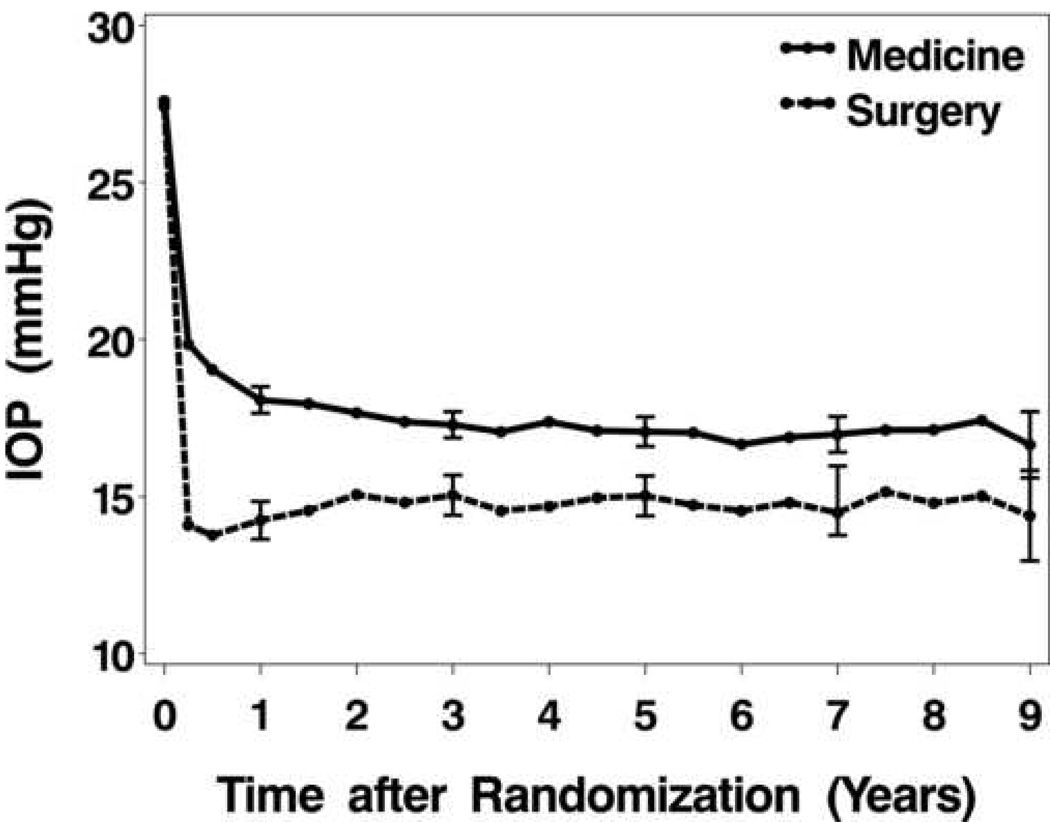
In the first nine years of follow-up after treatment initiation, during which 19 protocol-required follow-up visits were possible, there were an average of 14.1 visits in the medicine group and 13.4 visits in the surgery group. There was no indication of differential dropout between the two treatment groups over time. For example, at five years post treatment initiation, IOP data were available on 247 (80.5%) of the 307 medically-treated patients and on 232 (77.3%) of the 300 surgically-treated patients (P=0.35). Figure 2 shows the pattern of IOP change from baseline through Year 9 by treatment group. Both surgical and medical treatment produced dramatic drops in mean IOP from baseline to Month 3, but the decrease was greater in the surgery group. After this initial drop, the medical treatment group showed slightly declining average IOP and the surgery group slightly increasing IOP to Year 2. From Year 2 on, both groups’ average IOPs remained quite stable, with the surgery group’s mean IOP consistently less than the medicine group’s IOP. Descriptive and comparative statistics on mean IOP at selected follow-up intervals, and summary statistics on mean, maximum, and SD measures for the Year 2 to 9 interval (the period of stable IOP) are shown in Table 3. Highly significant differences between treatment groups are evident for the IOP means in Years 2 through 9 (17.2 mmHg for initial surgery, 15.0 mmHg for initial medicine, P<0.0001), and in the average of the maximum IOP measured in that interval (21.6 mmHg for initial surgery, 18.8 mmHg for initial medicine, P<0.0001). Smaller difference, though, is observed in variation around these means, with mean standard deviation (SD) values of 2.6 and 2.4 mmHg (medicine and surgery, respectively; P=0.0516).
Figure 2.
Intraocular pressure (IOP) mean values across time for the two treatment groups. Error bars indicate 95% confidence intervals.
Table 3.
Descriptive statistics [mean and standard deviation (SD)] for intraocular pressure (IOP) over time, by treatment group
| # of Patients | Mean (SD) IOP (mmHg) | |||
|---|---|---|---|---|
| Time | (Medicine/Surgery) | Medicine | Surgery | P-Value* |
| Baseline | 307/300 | 27.6 (5.5) | 27.4 (5.6) | 0.6979 |
| 2-year | 281/268 | 17.7 (4.0) | 15.1 (5.3) | <0.0001 |
| 3-year | 269/253 | 17.3 (3.5) | 15.0 (5.1) | <0.0001 |
| 5-year | 247/232 | 17.1 (3.8) | 15.0 (4.9) | <0.0001 |
| 7-year | 186/160 | 17.1 (3.7) | 14.5 (4.6) | <0.0001 |
| 9-year | 56/49 | 16.7 (3.9) | 14.4 (5.0) | 0.0122 |
| Summary measures+ | ||||
| Mean of years 2–9 | 293/285 | 17.2 (2.7) | 15.0 (4.1) | <0.0001 |
| Maximum of years 2–9 | 293/285 | 21.6 (4.7) | 18.8 (5.4) | <0.0001 |
| SD of years 2–9 | 290/274 | 2.6 (1.3) | 2.4 (1.4) | 0.0516 |
P-values are based on 2-sample t-tests (for unequal variances) at each point, without correction for multiple testing
Summary measures (mean, maximum, and SD) were calculated within each subject prior to summarizing by treatment group
To characterize factors assessed at baseline that influenced IOP during long-term treatment in the CIGTS, we focused on IOP measurements taken during the two to nine year period after treatment initiation, thereby avoiding the first two years after treatment when treatment influences dominated and post-treatment occurrences, like cataract extraction, were frequent. Strong independent predictors of IOP (all P-values <0.0001) included a treatment effect that was modified by smoking status, baseline IOP, baseline perimetric mean deviation, and center. Other significantly predictive factors included education (p=0.0024), follow-up time (P=0.0070), and hypertension (P=0.0186). Table 4 shows the model estimates. The effect of treatment on IOP interacted significantly with the effect of current smoking. While both non-smokers and smokers treated surgically had lower IOPs over time than medically treated patients [on average, 2.6 mmHg lower for non-smokers (P<0.0001) and 0.8 mmHg lower for smokers (P=0.12)], the extent to which IOP was lower differed significantly by smoking status (P=0.0008). Higher baseline IOPs on average yielded higher follow-up IOPs. The opposite was true for baseline perimetric mean deviation, for which more negative (or worse) baseline perimetric mean deviation was associated with lower IOP during follow-up. Average IOPs during follow-up differed among the 14 centers, resulting in a significant center effect on IOP. The effect of follow-up time on IOP in the model was best characterized by a quadratic function, since on average IOP decreased slightly from year 2 to midway (at about year 5) through the interval and increased slightly thereafter. Education was a significant predictor of IOP during treatment. Mean IOP during follow-up was lower for those with more education, such that among the seven categories of educational attainment, for example, a person who completed college had on average a 0.5 mmHg lower IOP than a person whose formal education ended upon completing high school. Finally, participants who reported being diagnosed with hypertension at baseline had significantly higher IOP (0.5 mmHg higher) during follow-up. Many other factors were evaluated and found to have no significant association with IOP during treatment, including age, sex, race, income, glaucoma diagnosis, diabetes, alcohol use, family history of glaucoma, iris color, and baseline measures of pupillary response and refractive error.
Table 4.
Mixed model of predictive factors for mean intraocular pressure (IOP) from Year 2 to 9
| Variable | Estimates (standard error) |
P-value |
|---|---|---|
| Treatment (vs. medicine) | ||
| ▪ Surgery/current smoker | −0.77 (0.49) | 0.1211 |
| ▪ Surgery/non-smoker | −2.56 (0.27) | <0.0001 |
| Baseline IOP (mmHg) | +0.18 (0.02) | <0.0001 |
| Baseline perimetric mean deviation (dB) | +0.25 (0.02) | <0.0001 |
| Education (7 categories) | −0.24 (0.08) | 0.0024 |
| Hypertension | +0.49 (0.21) | 0.0186 |
| Time on treatment (years) | −0.34 (0.12) | 0.0070 |
| [Time on treatment]2 | +0.03 (0.01) | 0.0225 |
| Center (vs. mean effect) | <0.0001 | |
| Lower than mean IOP: | ||
| Center I | −1.65 (0.33)* | |
| Center K | −1.40 (0.66)* | |
| Center F | −1.29 (0.59)* | |
| Center J | −0.52 (0.36) | |
| Center L | −0.46 (0.32) | |
| Center E | −0.06 (0.31) | |
| Higher than mean IOP: | ||
| Center B | +0.06 (0.32) | |
| Center C | +0.11 (0.34) | |
| Center M | +0.33 (0.44) | |
| Center A | +0.56 (0.31) | |
| Center D | +0.68 (0.38) | |
| Center H | +0.77 (0.34)* | |
| Center N | +0.84 (0.54) | |
| Center G | +2.04 (0.40)* |
Significantly different from the overall mean IOP
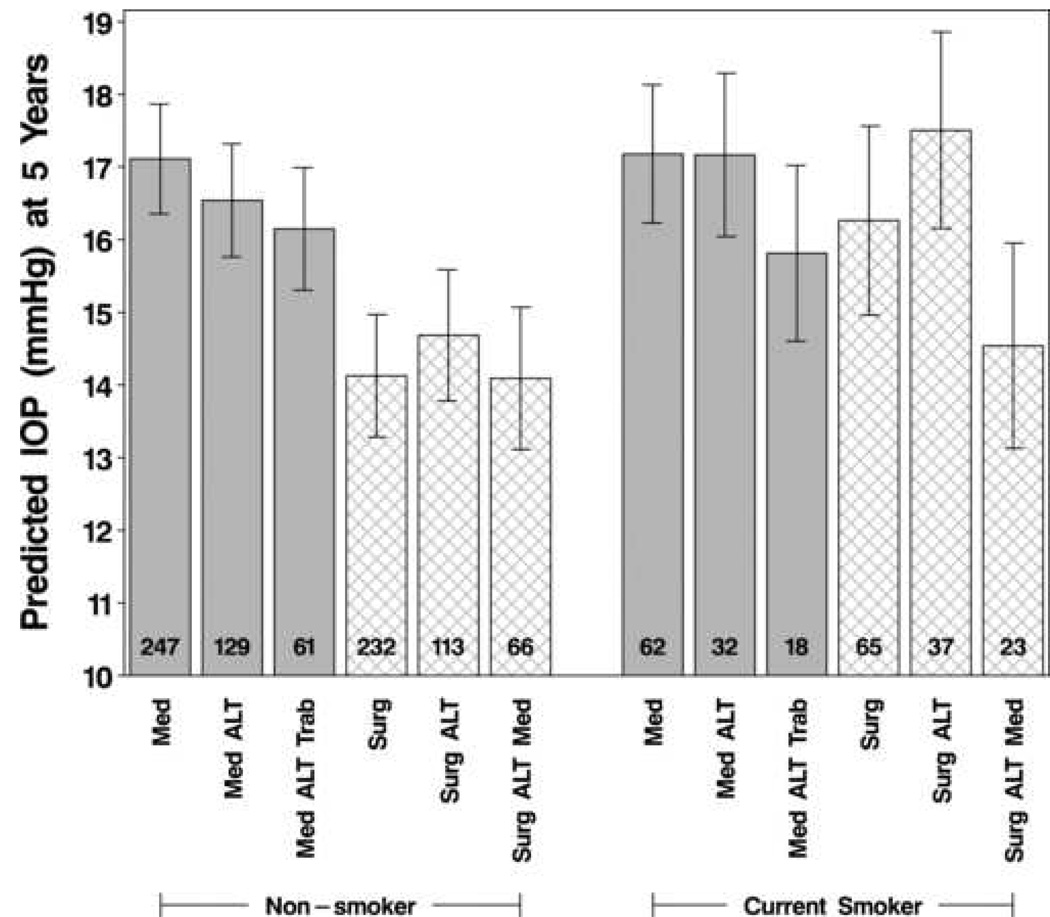
To assess IOP levels with changes in treatment during follow-up (e.g., ALT after initial medicine or initial surgery), we fit a mixed model with the same covariates listed in Table 4, but with time-varying covariates for treatment and treatment by smoking interaction. Other than treatment, all of the other covariates had effects similar to those shown in Table 4. Figure 3 displays the predicted IOP at 5 years, separately for current smokers and non-smokers, for patients in each step of the CIGTS treatment protocol (medicine→ALT→trabeculectomy or trabeculectomy→ALT→medicine). The most striking effect shown in Figure 3 is the impact of initial surgery on the IOP of non-smokers relative to smokers, which is consistent with the “as-randomized” analysis (Table 4). Regardless of smoking status, treatment crossover (e.g., medicine→trabeculectomy or trabeculectomy →medicine) resulted in the same or lower predicted IOP at 5 years as the initial treatment.
Figure 3.
Predicted intraocular pressure (IOP) values at five years, by smoking status, for each step of the treatment sequence (medicine→ALT→trabeculectomy or trabeculectomy→ALT→ medicine) in an “as treated” analysis. Error bars indicate 95% confidence intervals, and the number at the base of each bar is the sample size.
Discussion
During the two eligibility examinations, our evaluation of IOP in 607 subjects newly diagnosed with glaucoma revealed some expected results. Repeated measurements of IOP taken with an applanation tonometer resulted in a significant lessening of IOP, even with the protocol requiring at least a one-hour separation of the three measurements taken within each visit. The IOP-reducing effect of repeated applanation has been reported,21–23 although most studies of this effect involved repeated measurements over several minutes rather than the several hour interval in this study. We did not control for diurnal variation in IOP, which has been reported to result in a mean range of 5.8 mmHg in patients with open-angle glaucoma.24 Although our three IOP measurements taken at each baseline visit were from a 2 to 3 hour period of time, the time of day of these visits may have contributed to variation between visits..
A number of factors were associated with the mean IOP obtained in these newly diagnosed glaucoma patients prior to the onset of treatment. Older age was associated with lower IOP in our patients, which differs from that found in many population studies. Females in CIGTS presented with significantly lower mean IOP than males. In population studies, the reported effect of a person’s sex on IOP has not been consistent.4,7,8,11,12,17 The small number of patients with pseudoexfoliative OAG (n=29) presented with substantially higher IOP than those with primary or pigmentary forms of OAG. Mitchell et al. noted that the presence of pseudoexfoliation in non-glaucoma participants of the Blue Mountains Eye Study was significantly associated with higher IOP.17 A defect in pupillary response at baseline, almost all of which were afferent defects, was associated with a higher baseline IOP. Since an afferent pupillary defect (APD) in a glaucoma patient is typically due to optic nerve damage, this finding is most likely indicative of more severe glaucoma damage in the eye with an APD. A significant center effect remained after adjustment for demographic and clinical factors. Unmeasured factors, such as sociodemographic variables, may be operative in producing significant IOP differences among the 14 centers.
Our investigation of glaucoma patients under treatment, either by medications or by trabeculectomy surgery, found a definite impact of the treatment approach on IOP. Initial surgery reduced IOP more than initial medications, so that on average over the 2 to 9 year follow-up interval, surgically-treated patients had a 2.2 mmHg lower IOP than medically-treated patients. While the impact of this treatment difference in IOP reduction on controlling visual field loss was not evident in interim analyses involving shorter follow-up,25 its longer term influence will be the subject of a separate manuscript. The pattern of mean IOP over the 2 to 9 year period was quite stable in both treatment groups. While subjects who were older or had more substantial initial visual field loss may have been more likely to dropout, there was no evidence of differential dropout between initial treatment groups over time.
Our findings of other factors associated with IOP show intriguing correlates with and differences from findings in non-glaucoma subjects. Demographic variables such as age, sex, and race were not predictive of IOP in patients being treated over time. However, four patient-level factors – education, smoking, center, and hypertension – and two eye-level factors – baseline IOP and perimetric mean deviation – were significantly associated with mean IOP over follow-up. The association of IOP with smoking status in the surgery group is perhaps the most intriguing association, since smoking is a behavior that can be changed. The effect of smoking on IOP must be viewed in the presence of an interaction with treatment. While both smokers and non-smokers treated surgically had lower IOP over time than patients treated medically, smokers treated surgically had much higher mean IOP over time than non-smokers treated surgically. Smoking had no effect on the mean IOPs of medically treated patients. Several studies have reported that smoking was associated with higher IOP,8,17,18 but this would affect both those treated medically and surgically. The literature does not address if smoking affects trabeculectomy outcomes adversely. Even so, this finding provides another reason to advise glaucoma patients, particularly those needing surgery, to cease smoking.
A patient’s education level was inversely related to mean IOP over time. Patients who were more highly educated had lower mean IOP, which is similar to that reported by Yip et al.10 The distribution of educational levels within the two treatment groups showed no significant differences, so this effect is not due to confounding between treatment and education. We hypothesize that the association of education with IOP during treatment was influenced by unmeasured factors like compliance, socioeconomic factors, or dietary influences on IOP.
These effects may also be operative in producing the significant center effect on mean IOP over time. All centers treated patients according to a standard protocol with certified personnel using regularly calibrated tonometers, and so one would not expect one center to differ from another in aggressiveness of applying IOP reducing therapy, differing measurement technique, or instrument variation. Even so, some centers may have been more aggressive in their application of the treatment protocol. Surgeon skill may have varied as well. There is some supportive evidence for consistency of differences among the centers, as five centers (A, D, G, H, & N) with relatively high average IOP during follow-up also had higher average IOP at baseline.
The positive association we found between reported hypertension and IOP during follow-up is consistent with findings from many studies of a positive association between systolic blood pressure and IOP.5,7,8,10,15
Higher baseline IOP was directly related to higher IOP under treatment, with no significant interaction between baseline IOP and initial treatment. This would be an expected finding if one assumes that IOP reduction from a higher baseline level usually results in a higher IOP under treatment than those who began with a lower IOP level. However, it may be counterintuitive to the view that when a surgical opening is made in an eye via trabeculectomy, IOP should decrease to a similar level among operated eyes. The effect of higher baseline IOP on subsequent IOP was common to both treatment groups, which does not validate the common reduction view of surgical treatment.
The finding that patients who had more visual field loss at baseline (as measured by the perimetric mean deviation) had lower mean IOP while under treatment is difficult to explain. It may be that patients with more visual field loss at baseline were treated more aggressively within the protocol guidelines, or were more compliant in medication use. However, this finding was observed not only in medically treated patients, but also in surgically treated patients. Within the medically-treated patients, no differences were seen in number of glaucoma medications used over time between patients with greater (−10 dB or worse) versus patients with lesser (−2 dB or better) perimetric mean deviation at baseline (results not shown).
The associations found with baseline IOP – age, sex, center, diagnosis, and pupillary defect – are unique in the sense that they were uncovered in a large group of patients with newly-diagnosed OAG. This uniqueness limits our ability to make head-to-head comparisons with IOP associations reported from large population or community studies of healthy subjects. Likewise, the IOP associations found while these patients were under treatment are unique in that even after controlling for treatment effects, factors like a patient’s smoking status, education, and center of origin impact on mean IOP level. The long-term influence of smoking on a surgical patient’s IOP gives added support to advising glaucoma patients to quit smoking for not only their systemic but also their ocular health. The fact that person-level factors like education and center impact as well implies that sociodemographic, economic, compliance, or other environmental influences play a role in IOP control during treatment.
Acknowledgments
Grant support: Supported by NIH/NEI grants EY015860 and EY09148, and an unrestricted grant from Allergan, Inc. (Irvine, CA)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior presentation: Presented in part at the Annual Meeting of the Association for Research in Vision and Ophthalmology, Ft. Lauderdale, FL, May 2006 and at the Fourth U.S. Symposium on Ocular Epidemiology, Sarasota, FL, January 2007
Commercial relationships: DC Musch: Allergan, Inc. (F); Bausch & Lomb, Inc. (C, R); Glaukos, Inc. (C, R), Iridex, Inc. (C, R). BW Gillespie: Allergan, Inc. (F). LF Cashwell: Allergan, Inc. (F). PR Lichter: Allergan, Inc. (F). LM Niziol: None (N)
References
- 1.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 2.Leske MC, Heijl A, Hussein M, et al. Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 3.Gordon MO, Beiser JA, Brandt JD, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 4.Armaly MF. On the distribution of applanation pressure. I. Statistical features and the effect of age, sex, and family history of glaucoma. Arch Ophthalmol. 1965;73:11–18. doi: 10.1001/archopht.1965.00970030013005. [DOI] [PubMed] [Google Scholar]
- 5.Klein BE, Klein R. Intraocular pressure and cardiovascular risk variables. Arch Ophthalmol. 1981;99:837–839. doi: 10.1001/archopht.1981.03930010837009. [DOI] [PubMed] [Google Scholar]
- 6.David R, Zangwill L, Stone D, Yassur Y. Epidemiology of intraocular pressure in a population screened for glaucoma. Br J Ophthalmol. 1987;71:766–771. doi: 10.1136/bjo.71.10.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein BE, Klein R, Linton KL. Intraocular pressure in an American community: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992;33:2224–2228. [PubMed] [Google Scholar]
- 8.Wu SY, Leske MC. Associations with intraocular pressure in the Barbados Eye Study. Arch Ophthalmol. 1997;115:1572–1576. doi: 10.1001/archopht.1997.01100160742012. [DOI] [PubMed] [Google Scholar]
- 9.Hashemi H, Kashi AH, Fotouhi A, Mohammad K. Distribution of intraocular pressure in healthy Iranian individuals: the Tehran Eye Study. Br J Ophthalmol. 2005;89:652–657. doi: 10.1136/bjo.2004.058057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yip JL, Aung T, Wong TY, et al. Socioeconomic status, systolic blood pressure and intraocular pressure: the Tanjong Pagar Study. Br J Ophthalmol. 2007;91:56–61. doi: 10.1136/bjo.2006.099549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori K, Ando F, Nomura H, et al. Relationship between intraocular pressure and obesity in Japan. Int J Epidemiol. 2000;29:661–666. doi: 10.1093/ije/29.4.661. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Choi YR, Lee JE, et al. Relationship between intraocular pressure and systemic health parameters in the Korean population. Korean J Ophthalmol. 2002;16:13–19. doi: 10.3341/kjo.2002.16.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Schulzer M, Drance SM. Intraocular pressure, systemic blood pressure, and age: a correlational study. Br J Ophthalmol. 1987;71:245–249. doi: 10.1136/bjo.71.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weih LM, Mukesh BN, McCarty CA, Taylor HR. Association of demographic, familial, medical, and ocular factors with intraocular pressure. Arch Ophthalmol. 2001;119:875–880. doi: 10.1001/archopht.119.6.875. [DOI] [PubMed] [Google Scholar]
- 15.Bulpitt CJ, Hodes C, Everitt MG. Intraocular pressure and systemic blood pressure in the elderly. Br J Ophthalmol. 1975;59:717–729. doi: 10.1136/bjo.59.12.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dielemans I, Vingerling JR, Algra D, et al. Primary open-angle glaucoma, intraocular pressure, and systemic blood pressure in the general elderly population: the Rotterdam Study. Ophthalmology. 1995;102:54–60. doi: 10.1016/s0161-6420(95)31054-8. [DOI] [PubMed] [Google Scholar]
- 17.Carel RS, Korczyn AD, Rock M, Goya I. Association between ocular pressure and certain health parameters. Ophthalmology. 1984;91:311–314. doi: 10.1016/s0161-6420(84)34282-8. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell R, Rochtchina E, Lee A, et al. Iris color and intraocular pressure: the Blue Mountains Eye Study. Am J Ophthalmol. 2003;135:384–386. doi: 10.1016/s0002-9394(02)01967-0. [DOI] [PubMed] [Google Scholar]
- 19.Musch DC, Lichter PR, Guire KE, Standardi CL. The Collaborative Initial Glaucoma Treatment Study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106:653–662. doi: 10.1016/s0161-6420(99)90147-1. [DOI] [PubMed] [Google Scholar]
- 20.Kass MA. Standardizing the measurement of intraocular pressure for clinical research: guidelines from the Eye Care Technology Forum. Ophthalmology. 1996;103:183–185. doi: 10.1016/s0161-6420(96)30741-0. [DOI] [PubMed] [Google Scholar]
- 21.Goldmann H. Some basic problems of simple glaucoma. Am J Ophthalmol. 1959;48:213–220. doi: 10.1016/0002-9394(59)90260-0. [DOI] [PubMed] [Google Scholar]
- 22.Ytteborg J. The role of intraocular blood volume in rigidity measurements in human eyes. Acta Ophthalmol (Copenh) 1960;38:410–436. doi: 10.1111/j.1755-3768.1960.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 23.Moses RA, Liu CD. Repeated applanation tonometry. Am J Ophthalmol. 1968;66:89–91. doi: 10.1016/0002-9394(68)91794-7. [DOI] [PubMed] [Google Scholar]
- 24.David R, Zangwill L, Briscoe D, et al. Diurnal intraocular pressure variations: an analysis of 690 diurnal curves. Br J Ophthalmol. 1992;76:280–283. doi: 10.1136/bjo.76.5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichter PR, Musch DC, Gillespie BW, et al. CIGTS Study Group. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]