Abstract
The use of visual habituation in the study of infant cognition and learning is reviewed. This article traces the history of the technique, underlying theory, and procedural variation in its measurement. In addition, we review empirical findings with respect to the cognitive processes that presumably contribute to habituation, studies of developmental course and long-term prediction, as well as recent attempts to address or explain the phenomenon of visual habituation through the use of mathematical or quantitative models. The review ends with an appeal for a return to the study of habituation per se as a valid measure of infant learning, rather than relegating the phenomenon to its use as a technique for familiarizing infants in procedures testing for discrimination or recognition.
Introduction
The use of visual responses as a means to study perceptual function in preverbal human subjects dates back to early in the twentieth century. Given the limited response systems available for studying the preverbal human infant, and given that invasive behavioral or physiological techniques are not appropriate for use with this population, developmental scientists learned early on to take advantage of infants’ natural propensity to direct their visual regard to stimuli during the first months of life. Investigators working on the development of color vision reasoned that one could use infants’ eye movements to draw inferences about infants’ perceptions of the external world.
The initial use of such responses was to assess simple visual preferences for particular stimuli. Valentine (1913), Segers (1936), and Stirnimann (1944) all reasoned that if one presented infants with multiple stimuli and could establish systematic visual preferences among them, then one could logically conclude at least that the infant was capable of discriminating the preferred stimuli from those that were significantly less preferred. Furthermore, by carefully controlling the characteristics on which the stimuli differed, one could presumably infer which stimulus property or properties infants used to make the discrimination. Chase (1937) described a somewhat different procedure, in which infants were laid supine and then shown a circle of one color embedded within another color of the same brightness; infants’ ability to discriminate color was inferred from changes in the infants’ attention when the embedded circle was put in motion.
The eventual proliferation of the logic and use of preferential looking for research with human infants may be traced to Robert Fantz who, in the late 1950s, began publishing research based on the tendency for various organisms to examine or inspect some stimuli more than others. His initial publication on the topic (Fantz, 1956) described the general logic and the paradigm, and subsequent publications demonstrated its efficacy with chicks (Fantz, 1957, 1958a), infant chimpanzees (Fantz, 1958b), and then infant humans (Fantz, 1958c). It is noteworthy that Fantz’ graduate mentor at the University of Chicago was Eckhard Hess, who had spent the latter part of his career there looking for clues to covert emotional and cognitive processes by examining the autonomic activity (i.e., the pupillary reflex) of the eye (see Hess, 1975a,b).
Most of Fantz’ early papers sought to demonstrate that young infants responded differentially to various visual patterns (Fantz, 1961a, b; Fantz & Ordy, 1959), thus establishing the existence of both pattern vision and rudimentary discriminative abilities early in life. Contemporaneous with these publications were reports from other laboratories using the preferential-looking method in evaluating newborns’ perceptual capacities (Berlyne, 1958; Hershenson, 1964; Spears, 1966), primarily in the domains of color and form perception.
Early Studies of Visual Habituation
Historically, the seminal demonstration of visual habituation in human infants is often attributed to Fantz (1964). This report, which appeared in Science, extended the use of the preferential looking method by implementing a simple but elegant manipulation. Two stimuli were simultaneously provided to the infant for visual inspection, one each to the left and right of the infant’s midline. One of the two stimuli was different or “novel” on every trial; the other of the two stimuli remained unchanged, and the amount of time the infant looked or attended to each was noted. Over trials, infants progressively distributed their looking away from the unchanging stimulus and toward the novel one; that is, their looking to the familiar stimulus can be said to have “habituated”, although the term itself was not used in the Fantz’ (1964) narrative.
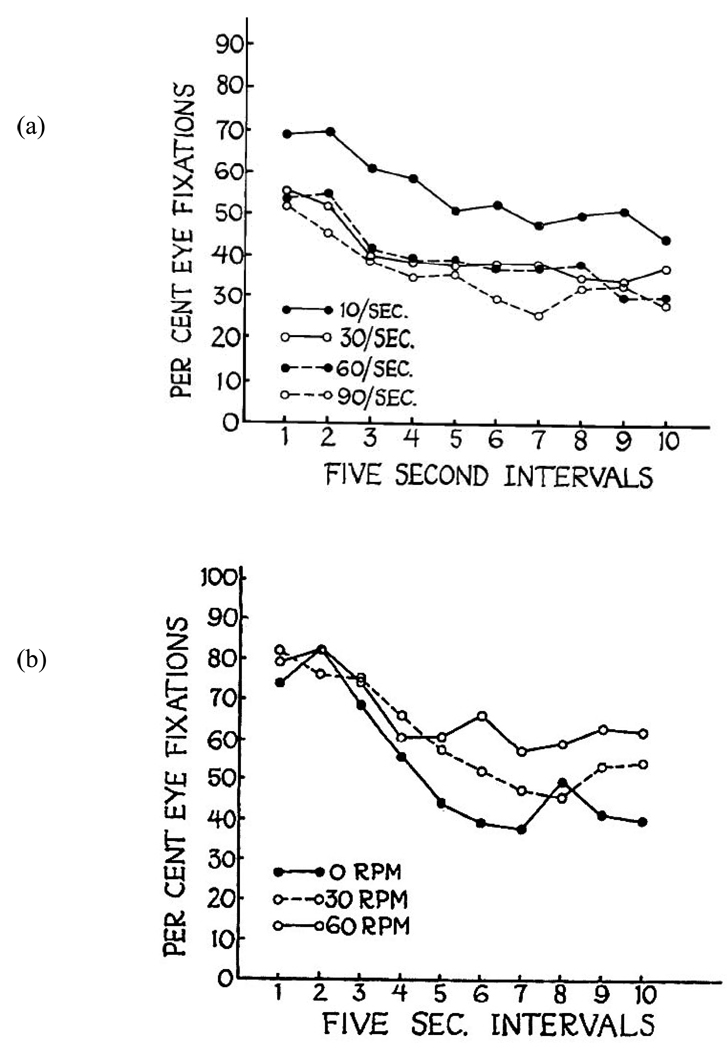
In fact, however, Fantz’ (1964) finding was predated by several others showing decrements in newborns’ responses to auditory stimuli (Bartoshuk, 1962a, b; Bridger, 1961; Hickman, 1963). We find that the first descriptions of visual habituation (i.e., the decline in visual regard or fixation) can be attributed to Gershon Berkson and his colleagues, in a series of papers published in the early 1960s. Berkson had been using visual regard and eye movements as dependent measures in studies of populations with developmental disabilities (O’Connor & Berkson, 1963). Berkson and Fitz-Gerald (1963a,b) demonstrated visual habituation in infant chimpanzees in two reports that preceded Fantz’ (1964) publication. In the first (Berkson & Fitz-Gerald, 1963a), infant chimpanzees were presented with lights that flickered at different rates and stimuli that rotated at different rates. From this came the first demonstrations of the robustness of the decline in looking across repeated redundant presentation, as well as the effects of stimuli on the overall height of the curve and on the rate of the decline of visual regard (See Figure 1). In the same year, these two authors published a paper in Science that replicated the decline in looking with repeated presentation. Following the initial habituation sequence, however, they also showed recovery of fixation and subsequent habituation to a novel stimulus (Berkson & Fitz-Gerald, 1963b), thus providing perhaps the first example of using the habituation technique for testing for visual discrimination. Subsequently, Berkson (1965a) showed the decrement in looking to be more apparent and robust with single-stimulus presentation than with paired stimuli, and that it could also be observed in kittens (Berkson, 1965b).
Figure 1.
The earliest plots of visual habituation. In both panels, decline in looking across time is shown for infant chimpanzees. The height of the habituation curve varies as a function of the relative rate of stimulus flicker (a), and the rate of habituation varies with speed of rotation (b). Both figures reprinted with permission from Berkson, G. & Fitz-Gerald, F. L. (1963a), Perceptual and Motor Skills, 17, 107–117.
Interestingly, despite the fact that habituation was long known in other disciplines (e.g., Coover & Angell, 1907; Harris, 1943; Meyer, 1909; Pepper, 1921), the term does not appear in these early publications. It first appears in Cohen (1966), and then in the title of several publications shortly thereafter (Caron & Caron, 1969; Cohen, 1969; McCall & Melson, 1969). In any case, within a few years, both the term (e.g., Miller, 1972) and the paradigm (Caron, Caron, Caldwell, & Weiss, 1973; Friedman, 1972; Pancratz & Cohen, 1970; Saayman, Ames, & Moffett, 1964) was in frequent use. Interestingly, much of the early work on visual habituation emphasized individual differences (Caron & Caron, 1969; McCall & Melson, 1969; McCall & Kagan, 1970; Messer, McCall, & Kagan, 1970).
That being said, with few exceptions, the literature generated using habituation was not focused on the phenomenon of habituation per se. Rather, in keeping with zeitgeist of the 1970s and early 1980s (Stone, Smith, & Murphy, 1973), emphasis was placed on demonstrating the human infant as a competent cognitive and intellectual being (see Horowitz & Colombo, 1990). As a result, for nearly the next 30 years, the habituation paradigm and many of its later-emerging procedural variants (e.g., high-amplitude sucking, violation of expectancy, familiarization/novelty) were used largely to document the extant cognitive abilities, skills, and products possessed by the infant (Colombo, 2002). Indeed, in preparing for this paper in the winter of 2007, a PsycInfo™ search using the term “habituation” as a keyword and with prenatal, neonatal, infants, and preschoolers as population parameters was found to yield 777 publications from the years 1962–2008. Of those, only about one in four papers included or addressed conclusions concerning the phenomenon of habituation per se; the remaining 75% were content to use habituation merely as a tool for familiarizing infants with a visual stimulus, typically prior to the administration of a test phase in which discrimination or some other differential response was assayed.
Theoretical Models
Although the literature on habituation flourished during the 1960s and 1970s (Groves & Thompson, 1970; Thompson & Spencer, 1966), the theoretical context for addressing infant visual habituation was relatively sparse (Lewis & Baldini, 1979). The next few sections delineate the traditional theoretical approaches to explaining infant habituation, with emphasis on the implications of these approaches for the study of attention.
The Comparator Model
Sokolov’s research program on the neural mechanisms of learning and memory spanned half a century and anticipated many current trends, including neural networks (Sokolov, 1969) and statistical learning (e.g., Arana & Sokolov, 1961). Sokolov’s model is the most popular theoretical explanation for infant habituation, even though the most widely-cited source for this model is a brief passage in the appendix of a small book titled Perception and the conditioned reflex (Sokolov, 1963). The model is developed in other publications (Sokolov, 1960, 1966, 1977), and is derived from a long program of work on the orienting reflex (Novikova & Sokolov, 1957; Roger, Voronin, & Sokolov, 1958; Sokolov & Mikhalevskaya, 1961; Sokolov & Paramanova, 1951; Vinogradova & Sokolov, 1955, 1957; Voronin & Sokolov, 1960).
Sokolov’s model is based on the orienting reflex (OR), a phenomenon first noted by Sechenov (1863/1965), and subsequently by Pavlov (1927) in the context of curiosity and exploration and their effect on conditioning. Behaviorally, the OR is a cluster of responses elicited by the detection of a nonthreatening, moderately intense novel or unexpected stimulus: suppressed heart rate, respiration, skin resistance, pupil dilation, and reduced motor activity, including the fixing of sensory receptors at or toward the source or location of the stimulus. All of these components may be traced ultimately to predominant activation of the parasympathetic nervous system. Stimuli that are more intense or threatening tend to evoke predominant activation of the sympathetic nervous system, which may involve heart rate acceleration, and decreased blood flow to the extremities (Graham & Clifton, 1966).
At face value, the OR would appear to prepare the animal for stimulus intake (e.g., enhance stimulus gain or improve the stimulus-to-noise ratio). Sokolov (1963) reasoned that upon the organism’s encounter with a novel stimulus, the magnitude of the OR would be large. However, repeated exposure to that stimulus would allow the organism to form an internal representation of it, and the magnitude of the OR would be manifest to the degree that the internal representation matched the external stimulus. Thus, in this model, the magnitude of the OR is inversely proportional to the match between that external stimulus and the organism’s internal representation of it. As such, with repeated presentation and increased familiarity with the stimulus, the OR habituates. Because habituation of the OR occurred as a function of the organism’s comparison between the external stimulus and internal representation of it, Sokolov’s (1963) scheme is often called the comparator model (see also Bernstein, 1979, 1981; Siddle, 1979)
Implications of the Comparator Model
This model linked the manifestation of an attentional function directly to the quality of learning and memory, and it had two major implications for the study of infant cognition. First and foremost, it implied that individual differences in the rate of decline in the magnitude of the OR reflected individual differences in infants’ rate of learning or encoding; infants whose OR habituated more rapidly learned faster than infants who habituated more slowly. This was quickly exploited in studies of the concomitant and lagged consequences of “fast-habituating” and “slow-habituating” infants. Second, the magnitude of the OR (relative to its initial value) indicated the quality of the organism’s memory trace for the external stimulus. This meant that it was theoretically possible to equate the level of processing across infants if one adopted a strategy in which infants could be habituated to the same relative criterion. This paved the way for the development of complex procedural variants of the habituation paradigm that allowed for large-scale experimental designs in which individual differences in habituation rate could be strictly controlled. As a result of this, the infant-controlled habituation procedure (Horowitz, Culp, Paden, Bhana, & Self, 1972; Horowitz, Paden, Bhana, & Self, 1972) became a standard technique for research in infant cognition during the 1980s and 1990s. This will be discussed in detail in the sections that follow.
Serial Habituation
The comparator model tied attentional behaviors to the quality of information processing. What was left unspecified, however, was what stimulus aspects were being processed by the infant during habituation. Jeffrey (1968) proposed that, during habituation, infants processed different features of the stimulus during each trial or look, the order of which was determined by the relative salience of the different features.
Implications of the Serial Habituation Model
This hypothesis brought the stimulus into the consideration of the habituation process, and implied that infants processed stimulus information in a bottom-up manner, and encoded individual stimulus features or aspects in discrete steps rather than gradually improving the engram for the entire stimulus over repeated exposures. This model generated some supportive empirical work during the 1970s with infants and children (Miller, 1972; Miller, Ryan, Sinnott, & Wilson, 1976; Yi, 1977, 1979), and subsequent work (Mitchell & Horowitz, 1988) suggested that the type of processing in habituation might be mediated by individual differences. However, critical tests of the model with eye movement tracking data failed to confirm its predictions (e.g., Leahy, 1976) and infant data supportive of the original hypothesis was subsequently shown to be plausibly explained by statistical artifacts (Lasky, 1979). As a result, the serial habituation model did not figure prominently within the study of infant visual habituation beyond that time.
Dual-Process Theory
One theoretical approach to infant visual habituation that gained a fair level of acceptance during the 1980s and early 1990s was drawn directly from theory derived from cellular and synaptic models of habituation and dishabituation (Thompson & Spencer, 1966). An extension of these models by Groves and Thompson (1970) posited that the profile of behavioral responses to a repetitive stimulus series was determined by two independent and dissociable processes.
One of the processes was indeed the decrease in response to the stimulus that characterized habituation, although in Groves and Thompson’s (1970) model, this was presumably mediated at the cellular level by a much simpler and more primitive neural mechanism than that proposed by Sokolov (1963). The second process was sensitization, which was attributable to a transient “spike” in arousal or excitability produced by the stimulus’ onset or intensity. Sensitization was proposed to be manifest as an increase in response strength (in the infant habituation protocol, this translated to an increase in look duration) that occurred during the early part of the habituation sequence, with its strength and presence varying as a function of the relative intensity of the stimulus. In addition to the influence of sensitization on the form of the overall habituation function, the theory made strong predictions about the effect of stimulus parameters on dishabituation (see Connolly & Frith, 1978; Harding & Hagman, 1977; Waters & McDonald, 1975). In addition, the framework of dual-process theory has been extended beyond the cellular level by some authors to refer to dual activation of parasympathetic and sympathetic nervous systems in response to some stimuli or environmental situations (e.g., Hine & Paolino, 1972).
Through the 1970s and 1980s, the predictions of dual-process theory were subjected to various tests with animal preparations (Wester, 1972; Whitlow, 1975), and human psychophysiological variables (Barry & James, 1981; Kelsey, 1993; Megela & Teyler, 1979; Ornitz, Russell, Yuan, & Liu, 1996), with generally positive results. The theory also saw some application to human clinical concerns (Watts, 1979) before the amount of research activity directed toward this approach fell off during the 1980s.
Dual-process theory was revived in the 1980s and applied to infant visual habituation by Bashinski, Werner, and Rudy (1985) and then extended in a series of subsequent reviews and research summaries (Kaplan & Werner, 1991; Kaplan, Werner, & Rudy, 1990). In support of the theory, most of the studies published in this area demonstrated that more intense or complex stimuli did in fact generally produce sensitization (i.e., increases in looking) at early points during repetitive stimulus sequences (Bashinski et al., 1985; Colombo, Frick, & Gorman, 1997; Kaplan & Werner, 1986, 1987; Peterzell, 1993; but see Maikranz, Colombo, Richman, & Frick, 2000). In addition, given that infants of different ages would perceive various stimuli as being more or less complex (Brennan, Ames, & Moore, 1966; Cohen, DeLoache, & Rissman, 1975), it was demonstrated that different stimuli would elicit sensitization at different ages (see Kaplan et al., 1990). Evidence for the effects of arousal and sensitization has been obtained for visual spatial frequencies (Aposhyan, Kaplan, Peterzell, & Werner, 1988), color (Bieber, Kaplan, Rosier, & Werner, 1997), auditory frequency modulation (Kaplan & Owren, 1994), and multimodal combinations (Kaplan & Fox, 1991; Kaplan, Fox, & Huckeby, 1992)
Implications of Dual-Process Theory
For researchers in the area of infant attention, the application of dual-process theory promoted a neglected connection with the traditional animal literature on habituation. In addition, however, the theory had three important implications.
First, the application of this theory suggested that the design of infant habituation experiments in which posthabituation test sequences are constructed by alternating novel and familiar stimuli might be contaminated by the influence of Thompson-Spencer dishabituation (see Kaplan & Owren, 1994; Kaplan & Werner, 1987). In this phenomenon, the neural program of habituation is interrupted and reset by a stimulus presentation that is sufficiently novel or intense, and arousal is reactivated; with this comes the tendency for sensitization to again be manifest in the session. Under conditions where novel stimuli are presented first in the posthabituation sequence, stimuli following such novel presentations might also therefore elicit increased looking. Given that investigators rely on infants to differentiate between novel and familiar stimuli during these sequences by showing more looking to the former and less to the latter, the presence of this phenomenon may serve to obscure evidence for infants’ recognition of stimuli.
Second, the theory holds that the processes presumed to underlie the habituation function and, by extension, infant attention might be far less “cognitive” than comparator theory would predict. That is, the typical indicators used in habituation, such as response decrement and recovery may reflect processes involving sensitization and arousal, in addition to the Sokolovian processes of encoding and comparison. It should be noted that, aside from the demonstrations of the viability of dual process theory in infant attention, there has been little research on arousal and sensitization and its influence on infant cognition. Colombo et al. (1997) did observe that 4-month-olds showing sensitization habituated more slowly than those who did not; however, a subsequent study using both behavioral and psychophysiological measures did not observe any convergent autonomic correlate in infants showing sensitization versus those who did not (Maikranz et al., 2000).
A final implication of dual-process theory is that increases in arousal as a function of stimulus intensity or magnitude should improve attention and thereby facilitate learning and memory. According to the theory, infants showing sensitization or presented with stimuli during the period of increased arousal should show improved recognition or retention of those stimuli. One specific area in which this point has been studied is in the attentional effects elicited by the exaggerated pitch excursions and increased fundamental frequencies that are typically observed in infant-directed speech (e.g., Fernald & Simon, 1984). A number of studies have examined the arousing or sensitizing effects of these stimulus properties (Kaplan, Goldstein, Huckeby, & Cooper, 1995; Kaplan, Goldstein, Huckeby, & Owren, 1995; Kaplan, Jung, Ryther, & Zarlengo-Strouse, 1996; Kaplan & Owren, 1994; Kaplan, Zarlengo-Strouse, Kirk, & Angel, 1997). In addition, a series of follow-up studies have examined the learning characteristics of infants of depressed mothers, whose vocalizations typically lack the acoustic characteristics of infant-directed speech. As predicted by the theory, several studies have shown that such infants may be disadvantaged in certain specific contexts (Kaplan, Bachorowski, & Zarlengo-Strouse, 1999; Kaplan, Bachorowski, Smoski, & Zinser, , 2001; Kaplan, Bachorowski, Smoski, & Hudenko, 2002), and a few studies (Hernandez-Reif, Field, Diego, & Largie, 2002, 2003) have actually suggested that such infants show a broader deficit in their response to environmental stimuli.
Procedural Considerations in Habituation
As with all of the looking-based paradigms, the observation and recording of infant looking is conducted using a protocol that can be traced back to the corneal reflection technique, which has been described at length in many previous reviews (see, for example, Colombo & Mitchell, 1990). However, there is substantial variation in how habituation protocols are conducted, and this variation is relevant to points of theory and developmental function.
Fixed Trial Habituation Protocols
The simplest habituation protocols are fixed trial (FT) procedures, which involve administering a set of discrete, repetitive stimulus presentations to an infant; each trial has a fixed duration, and a fixed intertrial interval. For example, one might present 8 trials of a checkerboard to an infant, each lasting 10 s with a 10 s intertrial interval (e.g., Colombo et al., 1997). In simple FT protocols, the presentations are in no way linked to, or contingent upon, the infant’s behavior or visual attention; the infant is free to look or not look at the stimulus, and the procedure continues. There are a number of distinct advantages to running FT procedures: they are easy to automate, easy to conduct, and they yield data that are simple, uniform for each subject, and easy to analyze. The drawback of FT protocols, however, is that individual infants may habituate at different rates. To the degree that habituation rate reflects stimulus encoding (a central tenet of the comparator model), this implies that at the end of the FT protocol, infants may be at very different levels of processing. For instance, in the example described above (8 trials at 10 seconds each), infants might have looked for as much as 80 seconds and as little as 0 seconds. If one is interested in individual differences in infant processing, this may well be desirable (presuming that the infant has, in fact, looked at the stimulus), as it becomes analogous to a speeded cognitive task, where subjects are given a limited amount of time or exposure to an item before being administered a probe. However, if the aim of the protocol is to demonstrate a generic infant ability or skill, individual and developmental differences in visual behavior may obscure group effects that one is seeking to establish.
Infant-Controlled Habituation Protocols
This problem is addressed by the development of the infant-controlled procedure (ICP; Horowitz et al., 1972). There are two major differences between this procedure and the fixed trial protocol. The first is that the infant is administered repetitive stimulus presentations, but the beginning and end of the trial is contingent upon the infant’s looking. That is, the trial is not considered to start until the infant has looked at the stimulus, and it is terminated when the infant looks away.
Second, the trials continue until the infant has reduced his/her looking to some criterion. This criterion may be absolute (e.g., the sessions may end when the infant looks for a total of, say, no more than 3 seconds), but more often the criterion is relative to the infant’s initial level of looking. For example, the infant may be required to show a decline to a particular percentage (typically 50%) of their initial or longest levels. It should be noted that the use of a relative criterion makes theoretical sense from the point of view of the comparator model; if the initial size of the OR (i.e., the length of the look) represents the response when there is no internal representation of the stimulus, then using a constant percentage of decline as a criterion for habituation theoretically equates all of the subjects for the extent to which the external stimulus is encoded. We note that this reasoning is based on a set of assumptions with regard to looking and the OR, but it is also reasonable to point out that the ICP works extremely well in practice.
There are several other issues to consider with respect to the ICP, however. The first one is that this procedure is more difficult to design and implement than the FT protocol. Because criteria are computed, set, and typically reset several times within a session (based on the looks that the infant makes to the stimulus), it is difficult to conduct this without the aid of a microcomputer and custom programming.
Second, there are many decisions to be made about the parameters of the habituation protocol, for which there are neither consensual standards nor empirical evidence to guide the investigator. These decisions also complicate the programming for the administration of the task. Group habituation curves will mislead many novice investigators into thinking that the first look of a habituation sequence is always the longest one. In fact, however, this occurs only about 60% of the time (Colombo & Mitchell, 1990). Thus, it is common to encounter long looks quite late in the habituation session, and if the habituation criterion is rigidly set by the length of the initial looks, infants will continue on in the session longer than necessary, or may fail to attain the habituation criterion at all. In response to this, many investigators choose to allow the habituation criterion to “float,” or to be reset when longer looks occur later (i.e., beyond the first or second look) in the habituation sequence. In addition, the number of looks on which the criterion is based can vary; it may be based on the longest look, or the average of the two longest looks, or so forth. Similar issues must be faced when choosing how many criterion looks must be elicited to consider the infant to have habituated; here, the standard used by most investigators is two consecutive looks at or below criterion, but there is no empirical justification for this. accepted standard.
Third, the ICP dictates that the coding of looks must be done online, because the criterion for habituation is based on the duration of looks that have been observed earlier in the session. This has some implications for the reliability of online coding, but the coding of looks using the corneal reflection technique (or other less restrictive judgment criteria) tends to be quite easy and reliable. However, because the individual “look” now serves as the primary datum within the session, one must decide what constitutes a “look.” It is possible to place no constraints on the definition of a look; that is, to count any look to the stimulus as valid for purposes of setting or reaching the habituation criterion. However, one finds quickly that this may yield sequences of brief looks that result in very brief stimulus presentations that interfere with encoding; in addition, such looks may make it difficult to attain the habituation criterion, and have lower reliability (Colombo & Horowitz, 1985). In response, most investigators constrain the definition of looks in the ICP; looks must be of some minimum length (usually 1 or 2 sec) to be counted as valid, and be terminated by some minimum (again, 1 or 2 sec) look away from the stimulus. One of the few empirical evaluations of these procedural parameters, Colombo and Horowitz (1985) found 1 sec to be optimal for both the minimum look length and for the interval to characterize look termination, although this evidence has engendered little/no adoption of these standards, and no research has been conducted to determine whether different parameters might be appropriate for different aged infants.
Finally, it should be noted that like all criterion-based learning paradigms, the ICP will yield data sets that are not uniform in length for all participants; one infant may attain the habituation criterion in 5 looks, while another may take 25 looks to attain the criterion. As a result, the data structures for such files are not readily amenable to conventional forms of analysis, and require considerable forethought in arranging.
Cognitive Processes in Habituation
The dependent measure in the infant visual habituation paradigm is look duration, and as the major theoretical position concerning this form of habituation comes from Sokolov’s (1963) consideration of the orienting reflex, it is tempting to interpret infant looking as a reflection of attention. Indeed, the early literature on infant visual habituation was replete with references to many of the constructs that we consider colloquially to be within the realm of attention today, such as arousal and orienting (Berg, 1972; Hunt, 1970; McCall & Melson, 1970; Moreau, Birch, & Turkewitz, 1970; Sameroff, 1970), and many of the early studies made direct reference to the term “attention” itself (Collins, Kessen, & Haith, 1972; Cohen, 1972; Friedman, 1972; McCall & Kagan, 1970; Moffitt, 1973).
Implicit in Sokolov’s (1963) comparator model is the assumption that the infant is encoding or actively attending while looking. However, the notion that looking may not always reflect visual processing in the human infant was noted as far back as 30 years ago, in the use of terms like “obligatory looking” (Stechler, 1964; Stechler & Latz, 1966) or “blank staring” (e.g., Greenberg & Weizmann, 1971; Weizmann, Cohen, & Pratt, 1971). Using respiratory and cardiac measures as convergent validation of infants’ attention while looking, Richards (e.g., 1985, 1987, 1994; Richards & Casey, 1991, 1992) has demonstrated that the degree of infants’ active processing varies systematically within a look, and that active processing may be restricted to periods characterized by sustained heart rate deceleration.
This picture is further complicated by the fact that attention has long been recognized as a heterogeneous construct (e.g., Posner, 1980; Posner, & Cohen, 1984; Posner, Rafal, Choate, & Vaughan, 1985; Posner & Rothbart, 1991); the category of phenomena to which we apply the word “attention” probably reflects at least four independent processes (arousal/alertness, visuospatial orienting, object perception, and endogenous attention) each with its own neural substrate and its own developmental course (see Colombo, 2001 for a detailed exposition and review). There is clear evidence that all four of the listed attentional processes contribute to infants’ performance in habituation. Studies demonstrating the effect of arousal on infant visual behavior and habituation are plentiful, especially in newborns and younger infants (Gardner & Karmel, 1984, 1995; Gardner, Karmel, & Flory, 2003; Karmel, Gardner, & Magnano, 1991). Aspects of visuospatial orienting, especially the component of visual disengagement, have also been implicated (Blaga & Colombo, 2006; Frick, Colombo, & Saxon, 1999; Colombo, Richman, Shaddy, Greenhoot, & Maikranz, 2001). Finally, it is clear that the time that infants spend looking is, in fact, related to the processes involved in encoding the objects or stimuli presented (e.g., Colombo, Frick, Ryther, & Gifford, 1996; Frick & Colombo, 1996; Stoecker, Colombo, Frick, & Ryther, 1998) and that, at least at 6 months and beyond, the more voluntary and integrated forms of endogenous attention (see Colombo & Cheatham, 2006, for a review).
Alternative accounts of visual habituation have stressed the role of underlying components that are separate from cognitive constructs such as attention or encoding. Malcuit, Pomerleau, and Lamarre (1988) have posited an operant-oriented analysis, in suggesting that habituation might be attributable to the waning power of the stimulus to reinforce fixation. Although this attracted some attention (e.g., see Cohen, 1988; Colombo & Mitchell, 1988; Lécuyer, 1988) this position has not recruited broader acceptance in the last two decades.
Developmental Studies of Infant Visual Habituation
The next issue in the consideration of infant visual habituation is its developmental course. Although Colombo and Mitchell (1990) reviewed this literature some time ago, more recent studies have not suggested that the fundamental conclusions drawn there are in need of appreciable change.
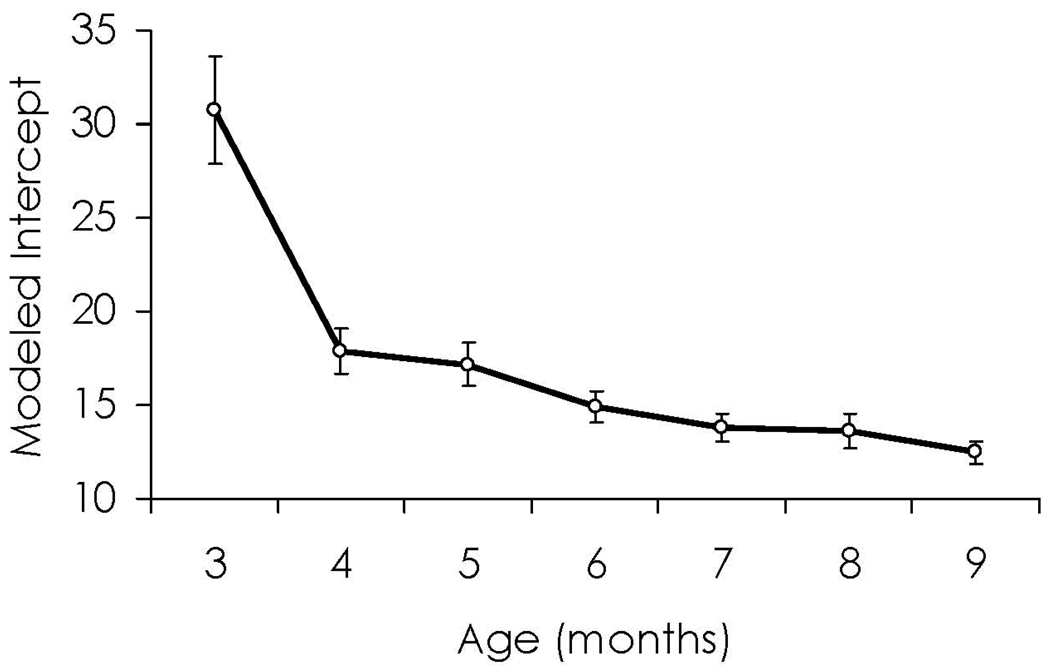
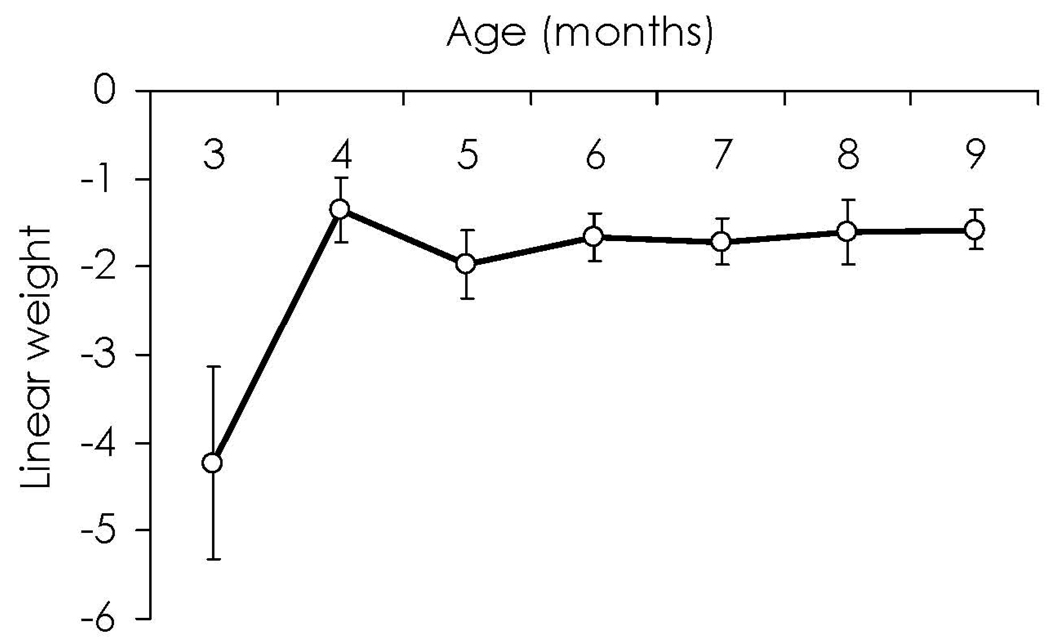
Procedural differences represent a critical parameter in interpreting the developmental functions for habituation. In FT procedures, the amount of fixation that infants devote to a stimulus decreases with age (Barten & Ronch, 1971; Horowitz et al., 1972; Lewis, 1969; Schaffer, Greenwood, & Parry, 1972; Wetherford & Cohen, 1973) and rate of visual habituation appears to increase with age (Cohen, 1969; Fantz, 1964; Lewis, 1969; Martin, 1975). In the ICP, the trend toward shorter durations with age holds true (Bornstein, Pecheaux, & Lecuyer, 1988; Colombo & Mitchell, 1990; Colombo, Shaddy, Richman, Maikranz, & Blaga, 2004; Mayes & Kessen, 1989; Rose, Slater, & Perry, 1986) but the rate of habituation appears to be shallower with age. Figure 2 and Figure 3 present a secondary analysis of habituation data from the longitudinal study reported in Colombo et al. (2004). Here, we have used regression modeling to derive a “best fitting” habituation curve (i.e., plotting duration of look against the each successive stimulus presentation) for each age of testing. From those best-fit curves, we have generated the intercept of the curve (i.e., the height of the habituation curve at the y-intercept; see Figure 2) and the unstandardized linear weight for the curve (i.e., the slope of the best-fitting straight line; see Figure 3) for each of the ages tested. As expected, in the ICP, the initial height of the habituation curve (i.e., initial length of looking) declines drastically across the first year of life, and the slope of curve (i.e., the degree of decrement) becomes more shallow.
Figure 2.
Data on infant-controlled habituation modeled from the Colombo et al. (2004) database. This graph shows the modeled intercept as a function of age, showing clearly that the intercept (height of curve) drops across the first year, with significant drops from 3 to 4 months and from 6 months to beyond.
Figure 3.
Data on infant-controlled habituation modeled from the Colombo et al. (2004) database. This graph shows the linear component of the modeled curves as a function of age. The linear term increases from a strong negative toward zero, thus showing that the curve flattens out with age.
Although the relationship between look duration taken from habituation protocols and age was once posited to be more or less a linear one (Colombo & Mitchell, 1990), recent work suggests a more complex pattern (Colombo, 2002). Newborns clearly habituate (e.g., Slater, Morison, & Rose, 1983, 1984), but there is evidence of an increase in look duration from birth to two months (Hood, Murray, King, & Hooper, 1996; Slater, Brown, Mattock, & Bornstein, 1996) prior to the decline described above. The decline in look duration culled from habituation protocols appears to plateau after 6 months (Colombo & Mitchell, 1990; Colombo et al., 2004). It has been suggested that looking, as derived from visual habituation protocols, may reflect different components of attention and cognitive function at different points during infancy and early childhood (Colombo, 2001), but elaboration on this issue is beyond the scope of this article.
Infant Habituation and Long-Term Outcome
Given that the variables culled from infant visual habituation likely represent important aspects of early cognition, one might posit that individual differences in visual habituation might be related to later cognitive or intellectual outcomes. This issue was explored in smaller studies through the 1970s and 1980s, and the reader would be best directed to any number of literature reviews that have collated and appraised the work (Bornstein & Sigman, 1986; Colombo, 1993; McCall & Carriger, 1993). However, the findings can be summarized as follows. Early studies using FT paradigms suggested that the rate or decrement of habituation (presumably, a measure of the rapidity or efficiency of learning) was correlated positively with standardized test outcomes later in childhood, while studies using ICP methodology focused on raw look duration, showing that this measure was correlated negatively with outcomes. An important caveat in these studies is that the magnitude of prediction was relatively modest. Although a few studies (e.g., Rose, Slater, & Perry, 1986) suggest that almost half of the variance in later IQ measures might be predicted by habituation, it has been far more common to see much smaller percentages of variance (e.g., 4% to 10%) accounted for by habituation indices in more recent reports.
More sophisticated analyses of the habituation construct have been attempted using structural equation modeling, and there have been reports of successful prediction using a latent-trait or latent-factor statistical approach to quantifying habituation performance (Tamis-LeMonda & Bornstein, 1989; Bornstein et al., 2006). Recent studies of the link between habituation measures and later cognitive and language outcomes (Bornstein et al., 2006; Colombo et al., 2004) suggest that the paths from habituation performance and cognitive development later in life are complex. That is, habituation does not appear to represent a fundamental or primary component of intelligence, but rather represents a building block for learning and cognition upon which higher-order functions are constructed across early childhood.
Statistical/Quantitative and Dynamical Systems Models of Infant Habituation
The more recent attempts to advance the study of infant visual habituation have attempted to provide sophisticated mathematical or quantitative approaches to explain or characterize the habituation process more precisely. This endeavor has two implications, the first being that we might understand the underlying phenomena that give rise to the habituation curve better, and the second involving the simple refinement of the measurement of habituation for its use both as a tool and a predictive measure. These quantitative approaches can be characterized as falling into three distinct categories.
Mathematical Models
Mathematical models in explaining infant cognitive behavior are not new; over three decades ago, Thomas (1973) attempted to use two different mathematical approaches to explain infants’ visual preferences. Over the years, pure mathematical modeling has been used to try to derive the “true” functions for visual habituation.
The first of these attempts involved the explication of different phases of visual processing that presumably took place during habituation. The seminal paper here (Cohen & Gelber, 1975) took infant visual habituation curves and plotted them “backward” from the attainment of the criterion. Surprisingly, this method yielded an apparent increase in looking to the habituation stimulus prior to the decline; this suggested that infants passed through a period of allocating more visual attention or visual regard to the familiar stimulus before finally distributing attention preferentially to a novel stimulus. This had important implications for testing infant recognition and memory, as most tests for such cognitive components involved the assessment of novelty preferences. This finding suggested that if infants had not fully processed a visual stimulus, the discriminative response could take very different forms. Indeed, this issue is fundamental to current debates about data that purport to show advanced cognitive capacities in very young infants (e.g., see Cohen & Marks, 2002; Wynn, 1992, 1995). The presence of the shift in the nature of the discriminative response has been theoretically formalized (Wagner & Sakovits, 1986) and empirically confirmed in many studies (e.g., Frick, Colombo, & Allen, 2000; Lasky, 1980; Roder, Bushnell, Sasseville, 2000; Wetherford & Cohen, 1973). However, it has also been shown that backward habituation curves are likely artifactual in nature (Cohen & Menten, 1981) and are not the product of this shift.
Other, more recent attempts in mathematical modeling of habituation have attempted to derive some truer evaluation of the parameters of habituation. Dannemiller (1984) conducted a Monte-Carlo simulation to determine whether or not the calculations for determining if habituation has taken place in the ICP were accurate; he concluded that the use of a trials-to-criterion variable was not warranted. Ashmead and Davis (1996) and Thomas and Gilmore (2004) have sought to improve calculations for determining the criteria for habituation in individual infants; although they espouse different models, both found that criteria imposed by a nonlinear regression model-fitting approach are an improvement over common algorithms for calculating percent decrement. It has been claimed that the standard 50% decrement routine commonly used in ICP may guarantee that only a bare majority of infants truly habituate (Gilmore & Thomas, 2002), or that it raises the probability of misclassifying infants who are not truly habituated to unacceptably high levels (Thomas & Gilmore, 2004). These issues have important implications, given the fact that the nature of the infant’s discriminative response will vary as a function of the degree to which the stimulus in question has been processed (i.e., the degree to which the infant has habituated).
Connectionist Approaches
In the mid-1990s, connectionist modeling and methodology was applied to developmental psychology (see Elman et al., 1996, for an excellent introduction). Essentially, connectionist models are designed to represent either simple circuits based on fundamental neural structures, or on the function of one or more hypothetical systems thought to underlie a process. The systems are then run to determine whether they can simulate data that match extant reports in the empirical literature, or if they can generate nonobvious predictions that can be empirically verified in subsequent examinations.
In two papers, Sirois and Mareschal (2002, 2004) have sought to use a connectionist approach to address basic issues in infant visual habituation. In the first, Sirois and Mareschal (2002) identified key characteristics of habituation (temporal unfolding, exponential decrease, familiarity-to-novelty shift, habituation to repeated testing, discriminability of habituated items, selective inhibition, and cortical-subcortical interactions) and attempted to determine whether any of the fundamental model types (function estimators, symbolic models, simple recurrent networks, auto-associator models, novelty filters, and reproductive auto-associator models) might be sufficient to account for all characteristics. Indeed, no model accounted for the familiarity-novelty shift in an adequate manner. No one model generated simulations that met all of Sirois and Mareschal’s (2002) criteria for habituation, although it might be noted that perhaps not all the critical characteristics chosen as criteria for habituation would have a high degree of consensus among habituation researchers.
A subsequent model (Sirois & Mareschal, 2004) was designed based on the interaction among functions localized in entorhinal cortex, hippocampus, and thalamic relays. This model reproduced important aspects of Fantz’ (1964) data, thereby suggesting the feasibility of circuits that involve only these neural structures.
Such modeling has engendered progress in other areas of early cognition (Cohen, Chaput, & Cashon, 2002). These initial attempts in explaining habituation show similar promise, and the ability of models to generate new predictions about the phenomenon will be welcome.
Dynamical Systems
More recently, in an expansive paper, Schöner and Thelen (2006) have argued for the value of applying some of the principles inherent in dynamical systems models to analyzing and explaining infant habituation. This approach seeks to explain the behavioral output of habituation by marrying the operation of fairly simple fundamental processes (e.g., activation of looking, inhibition of looking away) with characteristics of stimulus input and procedural parameters in formal models and simulations. A challenge for this approach will be to provide a meaningful prospective and explanatory account of habituation that goes beyond the generation of data that reproduce the quantitative aspects of extant phenomena.
Summary
Over the past 50 years, the study of infant visual habituation has yielded rich and vibrant insights into the nature of early cognition. Despite the obvious utility and importance of studying visual habituation per se, the vast proportion of its use comes as a tool for familiarizing infants with stimuli prior to a test for discrimination or recognition. A better understanding of infant visual habituation will make it a more precise tool for this use, but it is also likely to contribute greatly to our understanding of the nature of early cognitive function and to the neural processes that give rise to those functions. It is our hope that, within the context of this special issue, investigators will see the importance of habituation and devote more attention to it as a phenomenon, rather than as a technique. Clear advances toward this goal await us, particularly through the use of studies of individual differences, and connectionist and mathematical modeling.
Acknowledgments
We are grateful to Lori Curtindale and Leah Kapa for readings of an earlier draft of the manuscript.
Preparation of this article was supported by grants P30 DC005803, R01 HD41184, and R01 HD047315. Research cited in the article from the senior author’s laboratory was supported by grants R01 HD29960 and R01 HD35903.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aposhyan HM, Kaplan PS, Peterzell DH, Werner JS. Spatial frequency analysis of infant habituation. Infant Behavior and Development. 1988;11:145–157. [Google Scholar]
- Arana L, Sokolov EN. The stochastic theory of perception: Communication II Perception of elementary figures characterized by different probability of occurrence. Doklady Akademii Pedagogicheskikh Nauk RSFSR. 1961;4:65–69. [Google Scholar]
- Ashmead DH, Davis DL. Measuring habituation in infants: An approach using regression analysis. Child Development. 1996;67:2677–2690. [PubMed] [Google Scholar]
- Barry RJ, James AL. Fractionation of respiratory and vascular responses with simple visual stimulation. Physiological Psychology. 1981;9:96–101. [Google Scholar]
- Barten S, Ronch J. Continuity in the development of visual behavior in young infants. Child Development. 1971;42:1566–1571. [PubMed] [Google Scholar]
- Bartoshuk AK. Response decrement with repeated elicitation of human neonatal cardiac acceleration to sound. Journal of Comparative and Physiological Psychology. 1962a;55:9–13. doi: 10.1037/h0041490. [DOI] [PubMed] [Google Scholar]
- Bartoshuk AK. Human neonatal cardiac acceleration to sound: Habituation and dishabituation. Perceptual and Motor Skills. 1962b;15:15–27. doi: 10.2466/pms.1962.15.1.15. [DOI] [PubMed] [Google Scholar]
- Bashinski HS, Werner JS, Rudy JW. Determinants of infant visual fixation: Evidence for a two-process theory. Journal of Experimental Child Psychology. 1985;39:580–598. doi: 10.1016/0022-0965(85)90058-x. [DOI] [PubMed] [Google Scholar]
- Berg WK. Habituation and dishabituation of cardiac responses in 4-month-old, alert infants. Journal of Experimental Child Psychology. 1972;14:92–107. doi: 10.1016/0022-0965(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Berkson G. Eye fixation responses to one or two stimuli. Journal of Comparative and Physiological Psychology. 1965a;59:422–426. doi: 10.1037/h0022035. [DOI] [PubMed] [Google Scholar]
- Berkson G. Eye fixation responses of kittens and infant chimpanzees to onset and offset of a stimulus. Perceptual and Motor Skills. 1965b;21:619–624. doi: 10.2466/pms.1965.21.2.619. [DOI] [PubMed] [Google Scholar]
- Berkson G, Fitz-Gerald FL. Temporal sequence of eye fixations in the orienting response of infant chimpanzees. Perceptual and Motor Skills. 1963a;17:107–117. doi: 10.2466/pms.1963.17.1.107. [DOI] [PubMed] [Google Scholar]
- Berkson G, Fitz-Gerald FL. Eye fixation aspect of attention to visual stimuli in infant chimpanzees. Science. 1963b;139:586–587. doi: 10.1126/science.139.3555.586. [DOI] [PubMed] [Google Scholar]
- Berlyne DE. The influence of the albedo and complexity of stimuli on visual fixation in the human infant. British Journal of Psychology. 1958;49:315–318. doi: 10.1111/j.2044-8295.1958.tb00669.x. [DOI] [PubMed] [Google Scholar]
- Bernstein AS. The orienting response as novelty and significance detector: Reply to O’Gorman. Psychophysiology. 1979;16:263–273. doi: 10.1111/j.1469-8986.1979.tb02989.x. [DOI] [PubMed] [Google Scholar]
- Bernstein AS. The orienting response and stimulus significance: Further comments. Biological Psychology. 1981;12:171–185. doi: 10.1016/0301-0511(81)90010-7. [DOI] [PubMed] [Google Scholar]
- Bieber ML, Kaplan PS, Rosier E, Werner JS. Sensitizing properties of spectral lights in 4-month-old human infants. Developmental Psychobiology. 1997;30:275–281. doi: 10.1002/(sici)1098-2302(199705)30:4<275::aid-dev1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Blaga OM, Colombo J. Visual processing and infant ocular latencies in the overlap paradigm. Developmental Psychology. 2006;42:1069–1076. doi: 10.1037/0012-1649.42.6.1069. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Hahn C-S, Bell C, Haynes OM, Slater A, Golding J, Wolke D. Stability in cognition across early childhood: A developmental cascade. Psychological Science. 2006;17:151–158. doi: 10.1111/j.1467-9280.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Pecheux M-G, Lecuyer R. Visual habituation in human infants: Development and rearing circumstances. Psychological Research. 1988;50:130–133. doi: 10.1007/BF00309213. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Sigman MD. Continuity in mental development from infancy. Child Development. 1986;57:251–274. doi: 10.1111/j.1467-8624.1986.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Brennan W, Ames EW, Moore RW. Age differences in infants’ attention to patterns of different complexities. Science. 1966;151:354–356. doi: 10.1126/science.151.3708.354. [DOI] [PubMed] [Google Scholar]
- Bridger WH. Sensory habituation and discrimination in the human neonate. American Journal of Psychiatry. 1961;117:991–996. [Google Scholar]
- Caron RF, Caron AJ. Degree of stimulus complexity and habituation of visual fixation in infants. Psychonomic Science. 1969;14:78–79. [Google Scholar]
- Caron AJ, Caron RF, Caldwell RC, Weiss SJ. Infant perception of the structural properties of the face. Developmental Psychology. 1973;9:385–399. [Google Scholar]
- Chase WP. Color vision in infants. Journal of Experimental Psychology. 1937;20:203–222. [Google Scholar]
- Cohen LB. Observing responses, visual preferences and habituation to visual stimuli in infants. Dissertation Abstracts. 1966;27(1B):310. doi: 10.1016/0022-0965(69)90004-6. [DOI] [PubMed] [Google Scholar]
- Cohen LB. Observing responses, visual preferences, and habituation to visual stimuli in infants. Journal of Experimental Child Psychology. 1969;7:419–433. doi: 10.1016/0022-0965(69)90004-6. [DOI] [PubMed] [Google Scholar]
- Cohen LB. Attention-getting and attention-holding processes of infant visual preferences. Child Development. 1972;43:869–879. [PubMed] [Google Scholar]
- Cohen LB. A two-process model of infant attention. Merrill-Palmer Quarterly. 1973;19:157–180. [Google Scholar]
- Cohen LB. The relationship between infant habituation and infant information processing. Cahiers de Psychologie Cognitive/Current Psychology of Cognition. 1988;8:445–454. [Google Scholar]
- Cohen LB, Chaput HH, Cashon CH. A constructivist model of infant cognition. Journal of Cognitive Development. 2002;17:1323–1343. [Google Scholar]
- Cohen LB, DeLoache J, Rissman M. The effect of stimulus complexity on infant visual attention and habituation. Child Development. 1975;46:611–617. [PubMed] [Google Scholar]
- Cohen LB, Gelber E. Infant visual memory. In: Cohen L, Salapatek P, editors. Infant perception: From sensation to cognition. Vol. 1. New York: Academic Press; 1975. pp. 347–403. [Google Scholar]
- Cohen LB, Marks KS. How infants process addition and subtraction events. Developmental Science. 2002;5:186–201. [Google Scholar]
- Cohen LB, Menten TG. The rise and fall of infant habituation. Infant Behavior and Development. 1981;4:269–280. [Google Scholar]
- Collins D, Kessen W, Haith M. Note on an attempt to replicate a relation between stimulus unpredictability and infant attention. Journal of Experimental Child Psychology. 1972;13:1–8. doi: 10.1016/0022-0965(72)90002-1. [DOI] [PubMed] [Google Scholar]
- Colombo J. Infant cognition: Predicting later intellectual functioning. Newbury Park, CA: Sage Publications; 1993. [Google Scholar]
- Colombo J. The development of visual attention in infancy. Annual Review of Psychology. 2001;52:337–367. doi: 10.1146/annurev.psych.52.1.337. [DOI] [PubMed] [Google Scholar]
- Colombo J. Infant attention grows up: The emergence of a developmental cognitive neuroscience perspective. Current Directions in Psychological Science. 2002;11:196–199. [Google Scholar]
- Colombo J, Cheatham CL. The emergence and basis of endogenous attention in infancy and early childhood. In: Kail R, editor. Advances in Child Development and Behavior. New York: Academic Press; 2006. pp. 283–322. [DOI] [PubMed] [Google Scholar]
- Colombo J, Frick JE, Gorman SA. Sensitization during visual habituation sequences: Procedural effects and individual differences. Journal of Experimental Child Psychology. 1997;67:223–235. doi: 10.1006/jecp.1997.2406. [DOI] [PubMed] [Google Scholar]
- Colombo J, Frick JE, Ryther JS, Gifford JJ. Individual differences in infant visual attention: Four-month-olds’ recognition of forms connoted by complementary contour. Infant Behavior and Development. 1996;19:113–119. [Google Scholar]
- Colombo J, Horowitz FD. A parametric study of the infant control procedure. Infant Behavior and Development. 1985;8:117–121. [Google Scholar]
- Colombo J, Mitchell DW. Infant visual habituation: In defense of an information-processing analysis. Cahiers de Psychologie Cognitive/Current Psychology of Cognition. 1988;8:455–461. [Google Scholar]
- Colombo J, Mitchell DW. Individual and developmental differences in infant visual attention: Fixation time and information processing. In: Colombo J, Fagen JW, editors. Individual differences in infancy: Reliability, stability, and prediction. Hillsdale, NJ: Lawrence Erlbaum; 1990. pp. 193–227. [Google Scholar]
- Colombo J, Richman WA, Shaddy DJ, Greenhoot AF, Maikranz JM. Heart rate-defined phases of attention, look duration, and infant performance in the paired-comparison paradigm. Child Development. 2001;72:1605–1616. doi: 10.1111/1467-8624.00368. [DOI] [PubMed] [Google Scholar]
- Colombo J, Shaddy DJ, Richman WA, Maikranz JM, Blaga OM. The developmental course of habituation in infancy and preschool outcome. Infancy. 2004;5:1–38. [Google Scholar]
- Connolly JF, Frith CD. Effects of a varying stimulus context on habituation and sensitization of the OR. Physiology and Behavior. 1978;21:511–514. doi: 10.1016/0031-9384(78)90121-x. [DOI] [PubMed] [Google Scholar]
- Coover JE, Angell F. General practice effect of special exercise. American Journal of Psychology. 1907;18:328–340. [Google Scholar]
- Dannemiller JL. Infant habituation criteria. I. A Monte Carlo study of the 50% decrement criterion. Infant Behavior and Development. 1984;7:147–166. [Google Scholar]
- Elman JL, Bates EA, Johnson MH, Karmiloff-Smith A, Parisi D, Plunkett K. Rethinking innateness: A connectionist perspective on development. Cambridge, MA, US: The MIT Press; 1996. [Google Scholar]
- Fantz RL. A method for studying early visual development. Perceptual and Motor Skills. 1956;6:13–15. [Google Scholar]
- Fantz RL. Form preferences in newly hatched chicks. Journal of Comparative and Physiological Psychology. 1957;50:422–430. doi: 10.1037/h0044973. [DOI] [PubMed] [Google Scholar]
- Fantz RL. Depth discrimination in dark-hatched chicks. Perceptual and Motor Skills. 1958a;8:47–50. [Google Scholar]
- Fantz RL. Visual discrimination in a neonate chimpanzee. Perceptual and Motor Skills. 1958b;8:59–66. [Google Scholar]
- Fantz RL. Pattern vision in young infants. Psychological Record. 1958c;8:43–47. [Google Scholar]
- Fantz RL. A method for studying depth perception in infants under six months of age. Psychological Record. 1961a;11:27–32. [Google Scholar]
- Fantz RL. The origin of form perception. Scientific American. 1961b;204:66–72. doi: 10.1038/scientificamerican0561-66. [DOI] [PubMed] [Google Scholar]
- Fantz RL. Visual experience in infants: Decreased attention familar patterns relative to novel ones. Science. 1964;146:668–670. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- Fantz RL, Ordy JM. A visual acuity test for infants under six months of age. Psychological Record. 1959;9:159–164. [Google Scholar]
- Fernald A, Simon T. Expanded intonation contours in mothers’ speech to newborns. Developmental Psychology. 1984;20:104–113. [Google Scholar]
- Frick JE, Colombo J. Individual differences in infant visual attention: Recognition of degraded visual forms by 4-month-olds. Child Development. 1996;67:188–204. [PubMed] [Google Scholar]
- Frick JE, Colombo J, Allen JR. Temporal sequence of global-local processing in 3-month-old infants. Infancy. 2000;1:375–386. doi: 10.1207/S15327078IN0103_6. [DOI] [PubMed] [Google Scholar]
- Frick JE, Colombo J, Saxon TF. Individual and developmental differences in disengagement of fixation in early infancy. Child Development. 1999;70:537–548. doi: 10.1111/1467-8624.00039. [DOI] [PubMed] [Google Scholar]
- Friedman S. Habituation and recovery of visual response in the alert human newborn. Journal of Experimental Child Psychology. 1972;13:339–349. doi: 10.1016/0022-0965(72)90095-1. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Karmel BZ. Arousal effects on visual preferences in neonates. Developmental Psychology. 1984;20:374–377. doi: 10.1037//0012-1649.35.3.640. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Karmel BZ. Development of arousal-modulated visual preferences in early infancy. Developmental Psychology. 1995;31:473–482. [Google Scholar]
- Gardner JM, Karmel BZ, Flory MJ. Arousal modulation of neonatal visual attention: Implications for development. In: Soraci S, Murata-Soraci K, editors. Visual information processing. Westport, CT, US: Praeger Publishers/Greenwood Publishing Group; 2003. pp. 125–153. [Google Scholar]
- Gilmore RO, Thomas H. Examining individual differences in infants’ habituation patterns using objectives quantitative techniques. Infant Behavior and Development. 2002;25:399–412. [Google Scholar]
- Graham FK, Clifton RK. Heart-rate change as a component of the orienting response. Psychological Bulletin. 1966;65:305–320. doi: 10.1037/h0023258. [DOI] [PubMed] [Google Scholar]
- Greenberg DJ, Weizmann F. The measurement of visual attention in infants: A comparison of two methodologies. Journal of Experimental Child Psychology. 1971;11:234–243. doi: 10.1016/0022-0965(71)90079-8. [DOI] [PubMed] [Google Scholar]
- Groves PB, Thompson RF. Habituation: A dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Harding GB, Hagman JD. Stimulus intensity effects in electrodermal habituation. Journal of Experimental Psychology: Human Learning and Memory. 1977;3:600–607. [PubMed] [Google Scholar]
- Harris JD. Habituatory response decrement in the intact organism. Psychological Bulletin. 1943;40:385–422. [Google Scholar]
- Hernandez-Reif M, Field T, Diego M, Largie S. Depressed mothers’ newborns show longer habituation and fail to show face/voice preference. Infant Mental Health Journal. 2002;23:643–653. [Google Scholar]
- Hernandez-Reif M, Field T, Diego M, Largie S. Haptic habituation to temperature is slower in newborns of depressed mothers. Infancy. 2003;4:47–63. [Google Scholar]
- Hershenson M. Visual discrimination in the human newborn. Journal of Comparative and Physiological Psychology. 1964;58:270–276. doi: 10.1037/h0047340. [DOI] [PubMed] [Google Scholar]
- Hess EH. The role of pupil size in communication. Scientific American. 1975a;233:110–119. doi: 10.1038/scientificamerican1175-110. [DOI] [PubMed] [Google Scholar]
- Hess EH. The tell-tale eye: How your eyes reveal hidden thoughts and emotions. Oxford, England: Van Nostrand Reinhold; 1975b. [Google Scholar]
- Hickman RK. Auditory habituation and discrimination in the human neonate. Dissertation Abstracts. 1963;24:2137. [Google Scholar]
- Hine B, Paolino RM. Increases in heart rate accompanying decreases in activity and defecation: Support for a dual-process theory of habituation? Behavioral Biology. 1972;7:427–433. doi: 10.1016/s0091-6773(72)80115-9. [DOI] [PubMed] [Google Scholar]
- Hood BM, Murray L, King F, Hooper R. Habituation changes in early infancy: Longitudinal measures from birth to 6 months. Journal of Reproductive & Infant Psychology. 1996;14:177–185. [Google Scholar]
- Horowitz FD, Colombo J. Infancy research: A summative evaluation and a look to the future. Detroit, MI, US: Wayne State University Press; 1990. [Google Scholar]
- Horowitz FD, Culp R, Paden L, Bhana K, Self P. Developmental changes in infant visual fixation to differing complexity levels among cross-sectionally and longitudinally studied infants. Developmental Psychology. 1972;7:88–89. [Google Scholar]
- Horowitz FD, Paden L, Bhana K, Self P. An infant-control procedure for studying infant visual fixations. Developmental Psychology. 1972;7:90. [Google Scholar]
- Hunt JM. Attentional preference and experience: I. Introduction. Journal of Genetic Psychology. 1970;117:99–107. [Google Scholar]
- Jeffrey WE. The orienting reflex and attention in cognitive development. Psychological Review. 1968;75:323–334. doi: 10.1037/h0025898. [DOI] [PubMed] [Google Scholar]
- Kaplan PS, Bachorowski JA, Smoski MJ, Hudenko WJ. Infants of depressed mothers, although competent learners, fail to learn in response to their own mothers’ infant-directed speech. Psychological Science. 2002;13:268–271. doi: 10.1111/1467-9280.00449. [DOI] [PubMed] [Google Scholar]
- Kaplan PS, Bachorowski JA, Smoski MJ, Zinser M. Role of clinical diagnosis and medication use in effects of maternal depression on infant-directed speech. Infancy. 2001;2:537–548. doi: 10.1207/S15327078IN0204_08. [DOI] [PubMed] [Google Scholar]
- Kaplan PS, Bachorowski JA, Zarlengo-Strouse P. Child-directed speech produced by mothers with symptoms of depression fails to promote associative learning in 4-month-old infants. Child Development. 1999;70:560–570. doi: 10.1111/1467-8624.00041. [DOI] [PubMed] [Google Scholar]
- Kaplan PS, Fox KB. Cross-modal associative transfer of response sensitization in infants. Developmental Psychobiology. 1991;24:265–276. [Google Scholar]
- Kaplan PS, Fox KB, Huckeby ER. Faces as reinforcers: Effects of pairing condition and facial expression. Developmental Psychobiology. 1992;25:299–312. doi: 10.1002/dev.420250407. [DOI] [PubMed] [Google Scholar]
- Kaplan PS, Goldstein MH, Huckeby ER, Cooper RP. Habituation, sensitization, and infants’ responses to motherese speech. Developmental Psychobiology. 1995;28:45–57. doi: 10.1002/dev.420280105. [DOI] [PubMed] [Google Scholar]
- Kaplan PS, Goldstein MH, Huckeby ER, Owren MJ. Dishabituation of visual attention by infant- versus adult-directed speech: Effects of frequency modulation and spectral composition. Infant Behavior and Development. 1995;18:209–223. [Google Scholar]
- Kaplan PS, Jung PC, Ryther JS, Zarlengo-Strouse P. Infant-directed versus adult-directed speech as signals for faces. Developmental Psychology. 1996;32:880–891. [Google Scholar]
- Kaplan PS, Owren MJ. Dishabituation of visual attention in 4-month-olds by infant-directed frequency sweeps. Infant Behavior and Development. 1994;17:347–358. [Google Scholar]
- Kaplan PS, Werner JS. Habituation, response to novelty, and dishabituation in human infants: Tests of a dual-process theory of visual attention. Journal of Experimental Child Psychology. 1986;42:199–217. doi: 10.1016/0022-0965(86)90023-8. [DOI] [PubMed] [Google Scholar]
- Kaplan PS, Werner JS. Sensitization and dishabituation of infant visual fixation. Infant Behavior and Development. 1987;10:183–197. [Google Scholar]
- Kaplan PS, Werner JS. In: Newborn attention: Biological constraints and the influence of experience. Weiss MJS, Zelazo PR, editors. Westport, CT: Ablex Publishing; 1991. pp. 278–307. [Google Scholar]
- Kaplan PS, Werner JS, Rudy JW. Habituation, sensitization, and infant visual attention. Advances in Infancy Research. 1990;6:61–109. [Google Scholar]
- Kaplan PS, Zarlengo-Strouse P, Kirk LS, Angel CL. Selective and nonselective associations between speech segments and faces in human infants. Developmental Psychology. 1997;33:990–999. doi: 10.1037//0012-1649.33.6.990. [DOI] [PubMed] [Google Scholar]
- Karmel BA, Gardner JM, Magnano CL. Attention and arousal in early infancy. In: Weiss MJ, Zelazo PR, editors. Newborn attention: Biological constraints and the influence of experience. Westport, CT US: Ablex; 1991. pp. 339–376. [Google Scholar]
- Kelsey RM. Habituation of cardiovascular reactivity to psychological stress: Evidence and implications. In: Blascovich JJ, Katkin ES, editors. Cardiovascular reactivity to psychological stress and disease. Washington, DC: American Psychological Association; 1993. pp. 135–153. [Google Scholar]
- Lasky RE. Serial habituation or regression to the mean? Child Development. 1979;50:568–570. [PubMed] [Google Scholar]
- Lasky RE. Length of familiarization and preference for novel and familiar stimuli. Infant Behavior and Development. 1980;3:15–28. [Google Scholar]
- Leahy RL. Development of preferences and processes of visual scanning in the human infant during the first 3 months of life. Developmental Psychology. 1976;12:250–254. [Google Scholar]
- Lécuyer R. Please infant, can you tell me exactly what you are doing during a habituation experiment? Cahiers de Psychologie Cognitive/Current Psychology of Cognition. 1988;8:476–480. [Google Scholar]
- Lewis M. Infants’ responses to facial stimuli during the first year of life. Developmental Psychology. 1969;1:75–86. [Google Scholar]
- Lewis M, Baldini N. Attentional processes and individual differences. In: Hale GA, Lewis M, editors. Attention and cognitive development. New York: Plenum Press; 1979. pp. 135–172. [Google Scholar]
- Maikranz JM, Colombo J, Richman WA, Frick JF. Autonomic correlates of individual differences in sensitization and look duration during infancy. Infant Behavior and Development. 2000;23:137–151. [Google Scholar]
- Malcuit G, Pomerleau A, Lamarre G. Habituation, visual fixation and cognitive activity in infants: A critical analysis and attempt at a new formulation. Cahiers de Psychologie Cognitive/Current Psychology of Cognition. 1988;8:415–440. [Google Scholar]
- Martin RM. Effects of familiar and complex stimuli on infant attention. Developmental Psychology. 1975;11:178–185. [Google Scholar]
- Mayes LC, Kessen W. Maturational changes in measures of habituation. Infant Behavior and Development. 1989;12:437–450. [Google Scholar]
- McCall RB, Carriger MS. A meta-analysis of infant habituation and recognition memory performance as predictors of later IQ. Child Development. 1993;64:57–79. [PubMed] [Google Scholar]
- McCall RB, Kagan J. Individual differences in the infant’s distribution of attention to stimulus discrepancy. Developmental Psychology. 1970;2:90–98. [Google Scholar]
- McCall RB, Melson WH. Attention in infants as a function of magnitude of discrepancy and habituation rate. Psychonomic Science. 1969;17:317–319. [Google Scholar]
- McCall RB, Melson WH. Complexity, contour, and area as determinants of attention in infants. Developmental Psychology. 1970;3:343–349. [Google Scholar]
- Megela AL, Teyler TJ. Habituation and the human evoked potential. Journal of Comparative and Physiological Psychology. 1979;93:1154–1170. doi: 10.1037/h0077630. [DOI] [PubMed] [Google Scholar]
- Messer SB, Kagan J, McCall RB. Fixation time and tempo of play in infants. Developmental Psychology. 1970;3:406. [Google Scholar]
- Meyer M. The nervous correlate of attention: II. Psychological Review. 1909;16:36–47. [Google Scholar]
- Miller DJ. Visual habituation in the human infant. Child Development. 1972;43:481–493. [PubMed] [Google Scholar]
- Miller DJ, Ryan EB, Sinnott JP, Wilson MA. Serial habituation in two-, three-, and four-month-old infants. Child Development. 1976;47:341–349. [PubMed] [Google Scholar]
- Mitchell DW, Horowitz F. Three- and four-month-olds’ processing of high-and low-salience stimulus components. Washington, DC: Paper presented at the International Conference on Infant Studies; 1988. Apr, [Google Scholar]
- Moffitt AR. Intensity discrimination and cardiac reaction in young infants. Developmental Psychology. 1973;8:357–359. [Google Scholar]
- Moreau T, Birch HG, Turkewitz G. Ease of habituation to repeated auditory and somesthetic stimulation in the human newborn. Journal of Experimental Child Psychology. 1970;9:193–207. doi: 10.1016/0022-0965(70)90085-8. [DOI] [PubMed] [Google Scholar]
- Novikova LA, Sokolov EN. Investigation of the electroencephalogram and the motor and skin-galvanic reactions in the orienting and conditioned reflexes in man. Zhurnal Vysshei Nervnoi Deyatel’nosti. 1957;7:363–373. [PubMed] [Google Scholar]
- O’Connor N, Berkson G. Eye movement in normals and defectives. American Journal of Mental Deficiency. 1963;68:85–90. [PubMed] [Google Scholar]
- Ornitz EM, Russell AT, Yuan H, Liu M. Autonomic, electroencephalographic, and myogenic activity accompanying startle and its habituation during mid-childhood. Psychophysiology. 1996;33:507–513. doi: 10.1111/j.1469-8986.1996.tb02426.x. [DOI] [PubMed] [Google Scholar]
- Pancratz CN, Cohen LB. Recovery of habituation in infants. Journal of Experimental Child Psychology. 1970;9:208–216. doi: 10.1016/0022-0965(70)90086-x. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. New York: Dover Press; 1927. [Google Scholar]
- Pepper SC. The law of habituation. Psychological Review. 1921;28:61–71. [Google Scholar]
- Peterzell DH. Individual differences in the visual attention of human infants: Further evidence for separate sensitization and habituation processes. Developmental Psychobiology. 1993;26:207–218. doi: 10.1002/dev.420260404. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bowhuis DG, editors. Attention and Performance X. Hillsdale, NJ: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: Neural basis and function. Cognitive Neuropsychology. 1985;2:211–228. [Google Scholar]
- Posner MI, Rothbart MK. Attentional mechanisms in conscious experience. In: Milner AD, Rugg MD, editors. The Neuropsychology of Consciousness. London, UK: Academic Press; 1991. pp. 91–112. [Google Scholar]
- Richards JE. Respiratory sinus arrhythmia predicts heart rate and visual responses during visual attention in 14 and 20 week old infants. Psychophysiology. 1985;22:101–109. doi: 10.1111/j.1469-8986.1985.tb01567.x. [DOI] [PubMed] [Google Scholar]
- Richards JE. Infant visual sustained attention and respiratory sinus arrhythmia. Child Development. 1987;58:488–496. [PubMed] [Google Scholar]
- Richards JE. Baseline respiratory sinus arrhythmia and heart-rate responses during sustained visual attention in preterm infants from 3 to 6 months of age. Psychophysiology. 1994;30:235–243. doi: 10.1111/j.1469-8986.1994.tb02212.x. [DOI] [PubMed] [Google Scholar]
- Richards JE, Casey BJ. Heart-rate variability during attention phases in young infants. Psychophysiology. 1991;28:43–53. doi: 10.1111/j.1469-8986.1991.tb03385.x. [DOI] [PubMed] [Google Scholar]
- Richards JE, Casey BJ. Development of sustained visual attention in the human infant. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Hillsdale, NJ: Erlbaum; 1992. pp. 30–60. [Google Scholar]
- Roder BJ, Bushnell EW, Sasseville AM. Infants’ preferences for familiarity and novelty during the course of visual processing. Infancy. 2000;1:491–507. doi: 10.1207/S15327078IN0104_9. [DOI] [PubMed] [Google Scholar]
- Roger A, Voronin LG, Sokolov EN. Electroencephalographic study of the conditioned connection with extinction of the orienting reflex in man. Zhurnal Vysshei Nervnoi Deyatel’nosti. 1958;8:3–16. [PubMed] [Google Scholar]
- Rose DH, Slater A, Perry H. Prediction of childhood intelligence from habituation in early infancy. Intelligence. 1986;10:251–263. [Google Scholar]
- Saayman G, Ames EW, Moffett A. Response to novelty as an indicator of visual discrimination in the human infant. Journal of Experimental Child Psychology. 1964;1:189–198. [Google Scholar]
- Sameroff AJ. Respiration and sucking as components of the orienting reaction in newborns. Psychophysiology. 1970;7:213–222. doi: 10.1111/j.1469-8986.1970.tb02227.x. [DOI] [PubMed] [Google Scholar]
- Schaffer HR, Greenwood A, Parry MH. The onset of wariness. Child Development. 1972;43:165–175. [Google Scholar]
- Schöner G, Thelen E. Using dynamic field theory to rethink infant habituation. Psychological Review. 2006;113:273–299. doi: 10.1037/0033-295X.113.2.273. [DOI] [PubMed] [Google Scholar]
- Sechenov IM. Reflexes of the brain. Cambridge, MA: MIT Press; 18631965. [Google Scholar]
- Segers JE. Recent observations relative to the perception of color in babies. Archives Belges de Sciences et Education. 1936;2:52–56. [Google Scholar]
- Siddle DA. The orienting response and stimulus significance: Some comments. Biological Psychology. 1979;8:303–309. doi: 10.1016/0301-0511(79)90012-7. [DOI] [PubMed] [Google Scholar]
- Sirois S, Mareschal D. Models of habituation in infancy. Trends in Cognitive Sciences. 2002;6:293–298. doi: 10.1016/s1364-6613(02)01926-5. [DOI] [PubMed] [Google Scholar]
- Sirois S, Mareschal D. An interacting systems model of infant habituation. Journal of Cognitive Neuroscience. 2004;16:1352–1362. doi: 10.1162/0898929042304778. [DOI] [PubMed] [Google Scholar]
- Slater A, Brown E, Mattock A, Bornstein MH. Continuity and change in habituation in the first 4 months from birth. Journal of Reproductive and Infant Psychology. 1996;14:207–215. [Google Scholar]
- Slater A, Morison V, Rose D. Locus of habituation in the human newborn. Perception. 1983;12:593–598. doi: 10.1068/p120593. [DOI] [PubMed] [Google Scholar]
- Slater A, Morison V, Rose D. Habituation in the newborn. Infant Behavior and Development. 1984;7:183–200. [Google Scholar]
- Sokolov EN. Neural model of the stimulus and the orienting reflex. Voprosy Psychologii. 1960;4:61–72. [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. New York: MacMillan; 1963. [Google Scholar]
- Sokolov EN. Orienting reflex as information regulator. In: Leontyev A, Luria A, Smirnov A, editors. Psychological research in U.S.S.R. Moscow: Progress Publishers; 1966. pp. 334–360. [Google Scholar]
- Sokolov EN. Mechanisms of memory. Oxford, England: Moscow University; 1969. [Google Scholar]
- Sokolov EN. Brain functions: Neuronal mechanisms of learning and memory. Annual Review of Psychology. 1977;28:85–112. [Google Scholar]
- Sokolov EN, Mikhalevskaya MB. Measurement of the effectiveness of a repeatedly present stimulus. Voprosy Psychologii. 1961;5:183–188. [Google Scholar]
- Sokolov EN, Paramonova NP. On extinction of the orienting reactions. Zhurnal Vysshei Nervnoi Deyatel’nosti. 1951;11:3–11. [PubMed] [Google Scholar]
- Spears WC. Visual preference in the four-month-old infant. Psychonomic Science. 1966;4:237–238. [Google Scholar]
- Stechler G. Newborn attention as affected by medication during labor. Science. 1964;144:315–317. doi: 10.1126/science.144.3616.315. [DOI] [PubMed] [Google Scholar]
- Stechler G, Latz E. Some observations on attention and arousal in the human infant. Journal of the American Academy of Child Psychiatry. 1966;5:517–525. [Google Scholar]
- Stirnimann F. On the color perception of the newborn. Annales Paediatrici. 1944;163:1–25. [Google Scholar]
- Stoecker JJ, Colombo J, Frick JE, Ryther JS. Long- and short-looking infants’ recognition of symmetrical and asymmetrical visual forms. Journal of Experimental Child Psychology. 1998;71:63–78. doi: 10.1006/jecp.1998.2460. [DOI] [PubMed] [Google Scholar]
- Stone LJ, Smith HT, Murphy LB. The competent infant: Research and commentary. New York: Basic Books; 1973. [Google Scholar]
- Tamis-LeMonda CS, Bornstein MH. Habituation and maternal encouragement of attention in infancy as predictors of toddler language, play, and representational competence. Child Development. 1989;60:738–751. doi: 10.1111/j.1467-8624.1989.tb02754.x. [DOI] [PubMed] [Google Scholar]
- Thomas H. Unfolding the baby’s mind: The infant’s selection of visual stimuli. Psychological Review. 1973;80:468–488. doi: 10.1037/h0035551. [DOI] [PubMed] [Google Scholar]
- Thomas H, Gilmore RO. Habituation assessment in infancy. Psychological Methods. 2004;9:70–92. doi: 10.1037/1082-989X.9.1.70. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Valentine CW. Colour perception and preferences of an infant at three months. Section on Ophthalmology. 1913;18:689–690. [Google Scholar]
- Vinogradova OS, Sokolov EN. On the extinction of the vascular component of the orientive reaction. Zhurnal Vysshei Nervnoi Deyatel’nosti. 1955;5:344–350. [PubMed] [Google Scholar]
- Vinogradova OS, Sokolov EN. The relationship between reactions of blood vessels of hand and head in some unconditioned responses in man. Fiziologicheskii Zhurnal SSSR. 1957;43:54–59. [PubMed] [Google Scholar]
- Voronin LG, Sokolov EN. Skin galvanic reactions with pairing of two auditory stimuli. Zhurnal Vysshei Nervnoi Deyatel’nosti. 1960;10:3–9. [Google Scholar]
- Wagner SH, Sakovits LJ. A process analysis of infant visual and cross-modal recognition memory: Implications for an amodal code. Advances in Infancy Research. 1986;4:195–217. 240–245. [Google Scholar]
- Waters WF, McDonald DG. Stimulus and temporal variables in the “below-zero” habituation of the orienting response. Psychophysiology. 1975;12:461–464. [PubMed] [Google Scholar]
- Watts FN. Habituation model of systematic desensitization. Psychological Bulletin. 1979;86:627–637. [PubMed] [Google Scholar]
- Weizmann F, Cohen LB, Pratt RJ. Novelty, familiarity, and the development of infant attention. Developmental Psychology. 1971;4:149–154. [Google Scholar]
- Wester K. Sensitization and habituation of the intracerebrally evoked orienting response. Physiology and Behavior. 1972;9:643–647. doi: 10.1016/0031-9384(72)90024-8. [DOI] [PubMed] [Google Scholar]
- Wetherford MJ, Cohen LB. Developmental changes in infant visual preferences for novelty and familiarity. Child Development. 1973;44:416–424. [PubMed] [Google Scholar]
- Whitlow JW. Short-term memory in habituation and dishabituation. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1:189–206. [Google Scholar]
- Wynn K. Addition and subtraction by human infants. Nature. 1992;358:749–750. doi: 10.1038/358749a0. [DOI] [PubMed] [Google Scholar]
- Wynn K. Infants possess a system of numerical knowledge. Current Directions in Psychological Science. 1995;4:172–177. [Google Scholar]
- Yi S. Some implications of Jeffrey’s serial habituation hypothesis: A theoretical basis of resolving one-look versus multiple-look attentional account of discrimination learning. Journal of General Psychology. 1977;97:89–99. doi: 10.1080/00221309.1977.9918505. [DOI] [PubMed] [Google Scholar]
- Yi S. Infant visual habituation as a function of exposure time and stimulus similarity. Genetic Psychology Monographs. 1979;100:139–156. [PubMed] [Google Scholar]