Abstract
Aging is associated with an increased incidence and severity of acute renal failure. However, the molecular mechanism underlying the increased susceptibility to injury remains undefined. These experiments were designed to investigate the influence of age on the response of the kidney to ischemic injury and to identify candidate genes that may mediate this response. Renal slices prepared from young (5 mo), aged ad libitum (aged-AL; 24 mo), and aged caloric-restricted (aged-CR; 24 mo) male Fischer 344 rats were subjected to ischemic stress (100% N2) for 0–60 min. As assessed by biochemical and histological evaluation, slices from aged-AL rats were more susceptible to injury than young counterparts. Importantly, caloric restriction attenuated the increased susceptibility to injury. In an attempt to identify the molecular pathway(s) underlying this response, microarray analysis was performed on tissue harvested from the same animals used for the viability experiments. RNA was isolated and the corresponding cDNA was hybridized to CodeLink Rat Whole Genome Bioarray slides. Subsequent gene expression analysis was performed using GeneSpring software. Using two-sample t-tests and a twofold cut-off, the expression of 92 genes was changed during aging and attenuated by caloric restriction, including claudin-7, kidney injury molecule-1 (Kim-1), and matrix metalloproteinase-7 (MMP-7). Claudin-7 gene expression peaked at 18 mo; however, increased protein expression in certain tubular epithelial cells was seen at 24 mo. Kim-1 gene expression was not elevated at 8 or 12 mo but was at 18 and 24 mo. However, changes in Kim-1 protein expression were only seen at 24 mo and corresponded to increased urinary levels. Importantly, these changes were attenuated by caloric restriction. MMP-7 gene expression was decreased at 8 mo, but an age-dependent increase was seen at 24 mo. Increased MMP-7 protein expression in tubular epithelial cells at 24 mo was correlated with the gene expression pattern. In summary, we identified genes changed by aging and changes attenuated by caloric restriction. This will facilitate investigation into the molecular mechanism mediating the age-related increase in susceptibility to injury.
Keywords: ischemia, microarray analysis
Ischemia is a leading cause of acute renal failure (ARF), which develops in ~4–7% of hospitalized patients each year (20). Common conditions leading to ischemia include cardiovascular disease, stroke, dehydration, and surgery, all of which place the elderly population at risk for ischemic ARF. Although the mortality rates for ARF are decreasing, the rates still range from 20 to 35% (45, 47). Importantly, Xue et al. (47) established age as a risk factor for ARF. This is in agreement with previous studies that have associated age with a higher risk for ARF (25, 29, 30). Pascual et al. (26) suggested that the incidence of ARF is 3.5 times higher in patients over 70 and the aged patients had a higher mortality rate. However, little is known about the molecular mechanism(s) that underlie the age-dependent increase in the incidence and severity of ARF.
Animal models have been used extensively to investigate age-related renal dysfunction (2). Structurally, many of the changes observed in the aging human kidney are recapitulated in rats, including thickening of the glomerular basement membrane and degenerative changes in the proximal tubules, while the most notable functional deficits are proteinuria and reduced urine concentrating ability (12, 34). Important findings include that the development of renal disease is more severe in males compared with females (3) and that nutrition influences age-related renal dysfunction (50). Interestingly, male Fischer 344 rats develop severe renal disease similar to end-stage renal disease due to the development of severe glomerulosclerosis and interstitial fibrosis (7); an effect that can be attenuated by lifelong caloric restriction (CR) (39). Therefore, rat models to investigate age-related changes in the kidney, as well as changes secondary to glomerulosclerosis and fibrosis, have been well-characterized.
A number of studies in aging rats indicate that there is a greater susceptibility to both ischemic and toxic injury. Beierschmidt et al. (4) demonstrated an age-related increase in acetaminophen nephrotoxicity in male Fischer 344 rats, comparing rats at 2–4, 12–14, and 22–25 mo of age. Interestingly, baseline blood urea nitrogen (BUN), urine osmolality, and urine volume were similar in all groups, suggesting that a major component of aging was increased sensitivity to insult as opposed to a gradual loss of renal function. Miura et al. (24) demonstrated that old rats (female Fischer-344; 37–38 mo) were more sensitive to ischemia (45 min) followed by reperfusion. Zager and Alpers (49) verified these findings but suggested that the lack of a relationship between the decrease in glomerular filtration rate (GFR) and morphological damage indicated that age-related changes reflected changes in renal hemodynamics, rather than differences in the tubular susceptibility to injury. However, Miura et al. (24) demonstrated that slices of kidney from old rats were more susceptible to in vitro anoxia (100% N2) when compared with slices from young animals as assessed by organic anion transport in the proximal tubules, indicating that a component of the increased sensitivity to injury involves age-dependent alterations in the proximal tubules.
To investigate the influence of age on the response of the kidney to ischemic injury and to provide insight into the pathways that may underlie the increased susceptibility of the aging kidney to injury, the objectives of this study were to 1) characterize the increased susceptibility of the aging kidney to injury, 2) determine whether this response was reversible using aged caloric-restricted rats, and 3) identify genes changed by age and corrected by CR. Our results demonstrate that there is an intrinsic change in the aging kidney that renders it more susceptible to injury and, importantly, this change is preventable by CR. Using the CodeLink Rat Whole Genome Bioarray, we identified 92 changes in gene expression that parallel the functional changes, i.e., altered during aging and attenuated by CR. Several interesting candidates were identified and verified by quantitative PCR including claudin-7, kidney injury molecule-1 (Kim-1), and matrix metalloproteinase-7 (MMP-7). In summary, several genes were identified that may be associated with the increased susceptibility of aging kidney to ischemic insult.
METHODS
Animals
All experimental procedures complied with the Guide for Care and Use of Laboratory Animals and were approved by the Texas A&M University Laboratory Animal Care Committee. Male Fischer 344 rats [young (4–5 mo), aged-ad libitum (AL; 8, 12, 18, and 24 mo), aged-CR (24 mo); CR begins at 10 wk, 10% restriction until 15 wk where it is increased to 25 and 40% restriction beginning at 4 mo] were purchased from NIA colony and housed in the College of Medicine Animal Facilities, Texas A&M Health Science Center. The animal room was temperature controlled and on a 12:12-h light-dark cycle. Following anesthesia (87 mg/kg ketamine and 13 mg/kg body wt xylazine), the abdominal cavity was opened, and the kidneys were removed and weighed.
Kidney slice culture model
Kidneys were isolated from young, aged-AL, and aged-CR rats. Kidney slices were made using a Brendel-Vitron tissue slicer and placed into a roller culture incubator for 1 h before simulated ischemic injury (100% N2 for 30 or 60 min) (24). The rat kidney slices were maintained in 1.7 ml/vial DMEM/F12 medium (Sigma), supplemented with 10% fetal bovine serum. Viability was assessed by intracellular ATP and GSH content or the leakage of lactate dehydrogenase (LDH) and α-glutathione-S-transferase (αGST) into the culture media. The results were compared with control slices (cultured in 95:5 O2-CO2) from each respective group and are presented as percent control.
The slices analyzed for ATP and GSH were weighed, homogenized in 10% trichloroacetic acid with glass homogenizer, snap-frozen in liquid nitrogen, and stored at −70°C. The slice homogenates were thawed and centrifuged (11,000 g, 10 min, 4°C) before analysis. For the ATP determination, an aliquot (4 µl) of slice homogenate was added to a white 96-well flat bottom microliter plate (Dynex Technologies) containing 6.0 µl of 0.5 M Tris-EDTA buffer (pH 8.9). One hundred microliters of luciferin plus luciferase reagent (ATP Determination Kit, Molecular Probes) were added and luminescence was read with a Synergy HT Multi-Detection Microplate Reader (Bio-Tek). For GSH measurements, an aliquot of slice homogenate (50 µl) was transferred to a 96-well microtiter plate, and 200 µl of Ellman’s reagent [39.6 mg dithiobis-nitrobenzoic acid/10 ml EtOH diluted 1:10 with 0.5 M Tris-EDTA buffer (pH 8.9)] were added. The absorbance was determined at 405 nm using a Synergy HT Multi-Detection Microplate Reader and the values were extrapolated from a standard curve of reduced glutathione (0–250 µM). For LDH measurements, culture medium was collected and centrifuged at 1,000 g for 4 min. One hundred microliters of culture medium were assayed using the In Vitro Toxicology Assay Kit (Sigma). For measurement of αGST leakage, 100 µl of culture medium were assayed using the Biotrin Rat αGST EIA assay kit (Biotrin International).
Histological evaluation
The kidney slices were harvested and placed in 4% paraformaldehyde for 24 h. After being rinsed with PBS, the tissue was placed in 70% ethanol for 24 h and then embedded in Paraplast-Plus (Oxford Labware). Five-micrometer sections from the paraffin-embedded tissue slices were used for histological evaluation following hematoxylin/eosin staining.
RNA isolation and purification
Total cellular RNA was isolated from snap-frozen kidney tissue using the RNAqueous-4PCR kit (Ambion). Briefly, the kidney tissue was homogenized in a lysis solution containing guanidinium thiocyanate. The tissue homogenate was then added to a silica-based filter that selectively binds RNA. Following washes, the purified RNA was eluted in nuclease-free H2O before being treated with DNase to remove contaminating genomic DNA. Finally, RNA quantity and purity were assessed via spectrophotometry using the A260 and A260:A280 ratio, respectively.
Identification of candidate genes via microarray analysis
Microarray hybridization and scanning were performed by the Genomics Core Facility of the Center for Environmental and Rural Health at Texas A&M University. RNA that passed the Agilent Technologies 2100 Bioanalyzer quality control test was used to generate biotin-labeled cRNA via a modified Eberwine RNA amplification protocol. Labeled cRNA was applied to the CodeLink Rat Whole Genome Bioarray for 18 h (GE Healthcare); four animals per group were used. After incubation, the slide was washed, stained, and scanned. Array images were processed using CodeLink software. Raw Codelink data output was imported into GeneSpring GX 7.1 (Agilent Technologies) and normalized by setting all measurements <0.01 to 0.01, normalizing each chip to the 50th percentile of all measurements taken for that chip, and normalizing each gene to the median measurement for that gene across all chips. To focus on genes with reliable measurements, the normalized data were filtered for 1) signal intensity greater than background in at least 4 of the 12 samples (based on Codelink data flags), 2) data present in at least 6 of the 12 samples (based on Codelink data flags), and 3) an average GeneSpring Control Signal in at least 3 of 4 samples per treatment group greater than the ratio of the fixed error to proportional error for that treatment group (based on base/proportional value in GeneSpring Cross-Gene Error model). To identify those genes changed by age and/or CR, a series of two-sample Welch t-tests with a Bejamini and Hochberg false discovery rate (FDR) < 0.05 and twofold restriction filters were utilized as indicated (Fig. 1) (5). Gene annotations were acquired using the accession numbers provided with the arrays and the GeneSpider function in GeneSpring. The data discussed in this publication have been deposited in NCBIs Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE6110.
Fig. 1.
Schematic depiction of the process used to filter and analyze the microarray data.
Quantitative real-time PCR
Total RNA samples were reverse transcribed to cDNA using the iScript cDNA Synthesis Kit (Bio-Rad). Quantitative real-time PCR (qPCR) was performed using the iCycler iQ real-time PCR detection system (Version 3.1; Bio-Rad) and iQ SYBR Green Supermix (Bio-Rad). Genes of interest were targeted using specific RT2 Real-Time PCR primer sets to claudin-7, Kim-1, MMP-7, and β-actin (SuperArray). Relative mRNA quantitation was performed using the ΔΔCt method; β-actin being was selected as the internal control gene and Rat Universal Reference RNA (Stratagene) was selected as the calibrator sample (19). Briefly, the quantity of target gene mRNA in each experimental sample (young, aged-AL, or aged-CR) relative to the internal control gene is normalized to the calibrator/reference sample.
Western blot
Whole kidney lysates were quantified by the Bradford method and diluted to 1 µg/µl in 2× sample buffer (250 mM Tris · HCl, pH 6.8, 4% SDS, 10% glycerol, 2% β-mercaptoethanol, 0.006% bromophenol blue). Samples were boiled for 5 min before electrophoresis and 20 µg of protein were separated by 8% SDS-PAGE. Separated proteins were transferred onto a Hybond-ECL nitrocellulose membrane (Amersham) in transfer buffer (25 mM Tris, 200 mM glycine, 20% methanol, and 1% SDS). Nonspecific binding was blocked by incubation with Tris-buffered saline plus Tween 20 (TBST) blocking buffer (0.1% Tween 20, 10 mM Tris, pH 7.5, 100 mM NaCl) supplemented with 5% nonfat dry milk for 1 h at room temperature. A primary antibody against Kim-1 [R9; (14)], MMP-7 (GeneTex), or claudin-7 (Santa Cruz Biotechnology) was diluted in the same buffer and incubated at 4°C overnight. After subsequent washes with TBST, membranes were incubated with secondary antibody (anti-rabbit IgG:horseradish peroxidase, 1:20,000 in TBST:5% nonfat dry milk) for 1 h at room temperature. The blots were washed 3× in TBST and proteins were detected with the Amersham ECL system and exposed to X-ray film.
Immunohistochemistry
Paraformaldehyde-fixed kidneys were harvested and placed in 4% paraformaldehyde for 24 h. At that time, the sections were rinsed with PBS and placed in 70% ethanol for embedding/sectioning. Immunohistochemical localization of Kim-1 and MMP-7 was performed using peroxidase/DAB staining via a commercially available system (Zymed) as previously used in our laboratory (17). Five-micrometer sections were deparaffinized by xylene incubation for 12 min and rehydrated in a graded series of ethanol (95, 80, 70, 50% ethanol) for 5 min each and then washed with PBS for 10 min. Peroxidase quenching was performed by incubation for 12 min with 9:1 dilution of methanol:30% H2O2 to block endogenous peroxidase activity. After being washed with PBS three times, sections were blocked with solution A for 45 min and blocking solution B for 20 min. The primary antibodies [Kim-1 (MARKE monoclonal-anti-rat Kim-1 ectodomain) (14)] and MMP-7 (rabbit polyclonal, GeneTex) were applied at a dilution of 1:200 at room temperature for 1 h in a humidfied chamber. After being rinsed in PBS, the sections were incubated for 30 min at room temperature with biotinylated secondary antibody. The streptavidin-peroxidase enzyme conjugate was added to each section for 15 min and peroxidase activity was visualized with AEC and DAB for Kim-1 and MMP-7, respectively. Slides were mounted for light microscope study with mounting solution. Negative controls were incubated with blocking solution B in place of the primary antibody.
For localization of claudin-7, 5-µm sections were deparaffinized in a 56°C oven overnight, followed by xylene incubation for 10 min and rehydrated in a graded series of ethanol (100, 95, 70, 50%) for 3 min each, and then washed with TBS for 10 min. Heat-induced epitope retrieval was performed for 4 min at 123.5°C in a Biocare Medical Decloaking Chamber using a reveal antigen retrieval solution. Peroxidase quenching was performed by incubation for 5 min with 9:1 dilution of methanol:30% H2O2 to block endogenous peroxidase activity. After being washed with TBS three times, sections were subjected to a casein background blocking solution for 5 min and a 20-min avidin-biotin blocker. An antibody against claudin-7 (rabbit polyclonal, Santa Cruz Biotechnology) was applied at a dilution of 1:100 at room temperature for 1 h in a humidfied chamber. After being rinsed in TBS, the sections were incubated for 15 min at room temperature with biotinylated secondary antibody. A streptavidin-peroxidase enzyme conjugate was added to each section for 10 min and peroxidase activity was visualized with AEC and DAB for Kim-1 and MMP-7, respectively. Slides were counterstained with hematoxylin and dehydrated through a series of alcohol and xylene solutions before a coverslip was mounted to the slides. Negative controls were incubated with TBS in place of the primary antibody.
Urinary Kim-1 quantiation
Urine samples were coded so that individuals performing the analyses were blinded as to the identity of the samples. Kim-1 protein was measured using Microsphere-based Luminex xMAP technology with monoclonal antibodies (MARKE-Trap and MARKE) raised against rat Kim-1 in the Vaidya/Bonventre Laboratory. This assay has been used to determine urinary levels of Kim-1 in several recent studies in rats (8, 27, 43, 44). For measurements, 30 µl of urine samples were analyzed in duplicate.
Statistics
For all statistics except microarray analysis, an ANOVA followed by post hoc t-tests with the Bonferoni correction was used to assess statistical significance (P < 0.05) via the SPSS program.
RESULTS
Age-related susceptibility to injury
Initial studies were designed to test the hypothesis that aging was associated with an increased susceptibility to injury. An in vitro system that preserves organ architecture and heterogeneity was used to eliminate the variables of renal blood flow and inflammation, which are also affected by aging (6, 11, 16). Precision-cut kidney slices generated from young, aged-AL, and aged-CR rats were challenged by a simulated ischemic insult (100% N2). Slice viability was measured at 30 and 60 min using a number of parameters including intracellular ATP and GSH levels and leakage of LDH and αGST (a marker of proximal tubular damage) into the culture media. There were no significant differences in ATP and GSH content in control slices from young, aged-AL, and age-CR rats at either 30 or 60 min, or for LDH and αGST leakage into the media (Table 1). There was an increased susceptibility to ischemic injury in aged-AL rats compared with young counterparts, as assessed by intracellular ATP and leakage of LDH and αGST following either 30 or 60 min of simulated ischemic injury (Fig. 2A). While there was significant difference between aged-CR and young at 60 min with respect to LDH and αGST, CR significantly attenuated the loss of viability seen in aged-AL slices for all parameters, at both 30 and 60 min. Although intracellular GSH was a less sensitive indicator of the loss of viability, there was still a significant difference between young and aged-AL after 60 min of simulated ischemia (Fig. 2A).
Table 1.
Control slice values
| ATP, nmol/mg Tissue | GSH, nmol/mg Tissue | LDH, U/l/mg Tissue | αGST, µg/ml/mg Tissue | ||||
|---|---|---|---|---|---|---|---|
| 30 min | 60 min | 30 min | 60 min | 30 min | 60 min | 60 min | |
| Young | 2.11±0.19 | 1.67±0.18 | 1.92±0.20 | 1.89±0.13 | 1.31±0.16 | 1.30±0.21 | 1.20±0.07 |
| Aged-CR | 1.90±0.23 | 1.51±0.21 | 1.70±0.23 | 1.67±0.19 | 1.21±0.11 | 1.30±0.14 | 0.97±0.25 |
| Aged-AL | 1.86±0.14 | 1.63±0.09 | 1.95±0.16 | 1.89±0.09 | 1.42±0.25 | 1.34±0.22 | 1.10±0.18 |
Each data point represents means ± SD for 4 animals (4 slices per animal). Control slices from each group were evaluated for intracellular ATP and GSH content as well as leakage of LDH and αGST into the culture media as described in METHODS. Aged-CR, aged calorie-restricted rats; aged-AL, aged ad libitum rats.
Fig. 2.
Impact of ischemia on the viability of renal tissue slices. A: kidney slices were harvested from young, aged ad libitum (aged-AL), or aged caloric-restricted (aged-CR) rats and challenged by simulated ischemia (100% N2) for 30 or 60 min. Viability was assessed by intracellular ATP and GSH content or leakage of LDH and αGST into the culture media. The results were compared with control slices (cultured in 95:5 O2-CO2) from each respective group. Each data point represents means ± SD for 4 animals (4 slices per animal). *Significant difference between young and aged-AL. **Significant difference in aged-AL compared with young and aged-CR. B: following 60 min, slices were harvested and processed for histological evaluation. Normal tubular structure is seen in control slices from young, aged-AL, and aged-CR rats; however, anoxia is associated with significant damage to tubules including areas of flattened tubular epithelium, cell vacuolization, reduced eosin staining, suggesting loss of cytoplasmic proteins and cell sloughing in aged-AL but not young and aged-CR rats. The width of the field is 870 µm.
Histological evaluation of the tissue following 60 min of simulated ischemia demonstrated significant tubular damage in slices harvested from aged-AL rat (i.e., flattened tubular epithelium, cell vacuolization, and sloughing and loss of eosin staining) while relatively little tubular damage was seen in young and aged-CR samples (Fig. 2B). Taken together, these results demonstrate that 1) susceptibility to injury increases with age, and can be attenuated by CR, and 2) the increased injury response is, in part, intrinsic to the kidney.
Microarray analysis and validation
In an effort to identify the molecular mechanisms mediating the increased susceptibility to injury in the aging kidney, microarray analysis was performed. The data were analyzed as described in methods and filtered to identify genes changed by aging, CR, or aging and attenuated by CR (Fig. 1). Complete lists of genes changed by aging (1,325 genes), CR (790 genes), age-induced changes potentiated by CR (2 genes), and age-induced changes attenuated by CR (92 genes) are shown in the online version of this article as it contains the supplemental data.
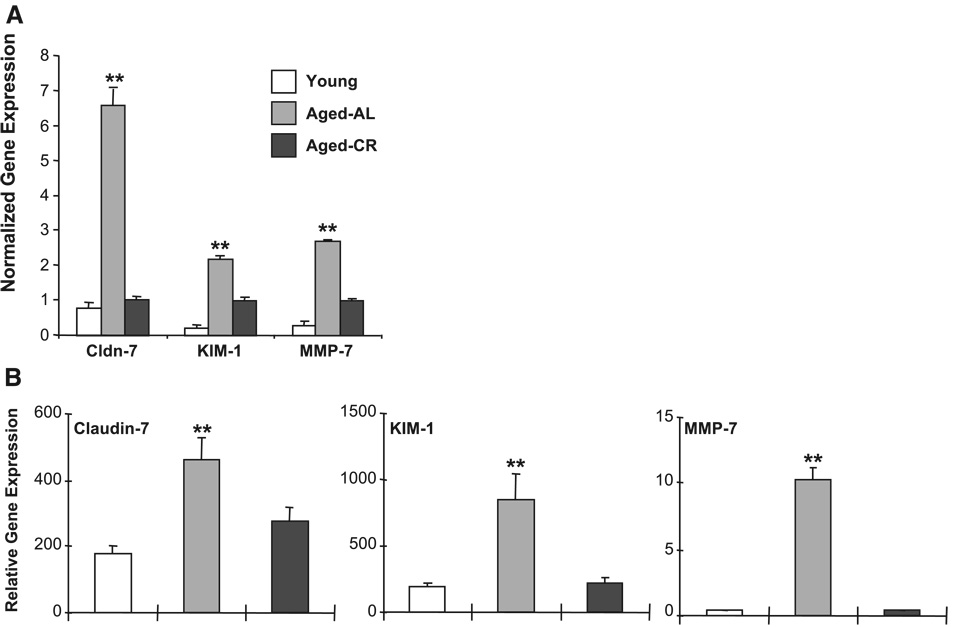
Based on the injury data, we focused on the group of genes that was changed by aging, but attenuated by CR. The well-annotated genes (named genes) downregulated by age but corrected by CR and upregulated by age but corrected by CR are shown in Table 2 and Table 3, respectively. Several upregulated genes were also further pursued, including claudin-7, an integral membrane protein of tight junctions, Kim-1, a putative epithelial adhesion molecule that is upregulated following injury (14) and thought to be a promising biomarker for renal injury (13, 15), and MMP-7 (matrilysin) (Fig. 3A). The expression changes in these genes of interest were confirmed by quantitative PCR. Similar to the microarray data, the expression of claudin-7, Kim-1, and MMP-7 was increased during aging, but attenuated by CR (Fig. 3B).
Table 2.
Genes decreased by age, attenuated by caloric restriction
| Description | Aged-AL Fold Decrease | Aged-CR Fold Decrease |
|---|---|---|
| Solute carrier family 21, member 1 | 555.56 | 9.71 |
| Similar to E130103I17Rik protein (predicted) | 7.57 | 1.88 |
| Similar to RCK | 6.89 | 1.83 |
| Adenylate cyclase 1 (predicted) | 4.69 | 1.79 |
| Sema domain, transmembrane domain (TM), and cytoplasmic domain, (semaphorin) 6A (predicted) | 3.95 | 1.46 |
| Phosphatase and actin regulator 1 | 3.93 | 1.84 |
| Immunoglobulin superfamily, member 11 (predicted) | 3.09 | 1.43 |
| Phosphatase and actin regulator 1 | 2.95 | 1.18 |
| Similar to HS1 binding protein 3 (predicted) | 2.89 | 1.08 |
| Phosphatase and actin regulator 1 | 2.85 | 1.35 |
| V-maf musculoaponeurotic fibrosarcoma (avian) oncogene homolog (c-maf) | 2.81 | 0.99 |
| ATP-binding cassette, subfamily C (CFTR/MRP), member 2 | 2.73 | 0.98 |
| PreB-cell leukemia transcription factor 1 (predicted) | 2.5 | 1.06 |
| START domain containing 7 (predicted) | 2.48 | 1.07 |
| Prickle-like 2 (Drosophila) (predicted) | 2.42 | 1.12 |
| Serine/threonine kinase | 2.16 | 0.93 |
| Cytochrome P-450, family 4, subfamily v, polypeptide 3 (predicted) | 2.13 | 0.99 |
| Solute carrier family 8 (sodium/calcium exchanger), member 1 | 2.14 | 0.7 |
| Phosphatase and actin regulator 1 | 2.04 | 0.63 |
Data are presented as the fold-decrease as compared with young. Well-annotated genes downregulated by age but corrected by caloric restriction in the kidney as identified by microarray analysis. For these genes, the magnitude of expression was significantly depressed by at least 2-fold in aged-AL vs. young and significantly elevated at least 2-fold in aged-CR vs. aged-AL.
Table 3.
Genes increased by age, attenuated by caloric restriction
| Description | Aged-AL Fold Increase | Aged-CR Fold Increase |
|---|---|---|
| Similar to Immunoglobulin kappa-chain VJ precursor | 47.9 | 17.2 |
| Similar to immunoglobulin kappa-chain | 39.4 | 8.84 |
| Similar to Ig kappa chain | 30.2 | 9.8 |
| Similar to Myb proto-oncogene protein (C-myb) | 21.3 | 8.84 |
| Clone 126.42 immunoglobulin kappa light chain variable region | 15.9 | 4 |
| CD163 antigen (predicted) | 11.3 | 2.31 |
| Kidney injury molecule 1 | 10.57 | 4.83 |
| Similar to immunoglobulin heavy chain 6 (Igh-6) | 10.53 | 4.77 |
| Matrix metalloproteinase 7 | 9.08 | 3.38 |
| Gamma-2a immunoglobulin heavy chain | 8.68 | 2.76 |
| Immunoglobulin delta heavy chain constant region | 8.45 | 2.27 |
| Claudin 7 | 8.26 | 1.26 |
| Pancreatic lipase-related protein 2 | 7.12 | 2.12 |
| Cell-line YFC 511.1 immunoglobulin light chain mRNA, partial cds; and CDR1, CDR2, and CDR3 genes, complete sequence | 6.92 | 2.51 |
| Immunoglobulin joining chain (predicted) | 6.79 | 3.38 |
| Immunoglobulin heavy chain 1a (serum IgG2a) (predicted) | 6.53 | 1.7 |
| Killer cell lectin-like receptor subfamily G, member 1 | 6.23 | 2.41 |
| Chemokine (C-C motif) ligand 5 | 5.62 | 2.67 |
| FK506 binding protein 5 | 5.54 | 1.16 |
| S100 calcium binding protein A8 (calgranulin A) | 5.07 | 2.05 |
| Serine dehydratase | 4.43 | 0.98 |
| S100 calcium binding protein A9 (calgranulin B) | 4.34 | 1.64 |
| Transglutaminase 1; transglutaminase 1 | 4.31 | 2.13 |
| RasGEF domain family, member 1A (predicted) | 4.29 | 1.77 |
| Core promoter element binding protein | 4 | 1.94 |
| Killer cell lectin-like receptor, family E, member 1 | 3.98 | 1.81 |
| Echinoderm microtubule associated protein like 2 | 3.64 | 1.53 |
| Similar to two pore domain K channel subunit | 3.62 | 1.68 |
| Similar to T cell receptor V delta 6 (predicted) | 3.54 | 1.51 |
| Myosin, heavy polypeptide 4 | 3.36 | 1.22 |
| Similar to immunoglobulin light chain | 3.3 | 1.59 |
| Alcohol dehydrogenase 1 | 2.98 | 1.14 |
| Synaptotagmin 1 | 2.89 | 1.03 |
| A kinase (PRKA) anchor protein (gravin) 12 | 2.76 | 1.09 |
| Similar to Golgi autoantigen golgin subtype a4; tGolgin-1 | 2.66 | 1.24 |
| Testis expressed gene 2 (predicted) | 2.56 | 1.24 |
| Sulfotransferase family 2A, dehydroepiandrosterone (DHEA)-preferring, member 2 (predicted) | 2.44 | 1.01 |
| Ring finger protein 14 | 2.36 | 1.18 |
| Similar to lipoma HMGIC fusion partner-like 3 | 2.21 | 0.45 |
| Transmembrane protein with EGF-like and two follistatin-like domains 2 (predicted) | 2.21 | 1.1 |
| Rap guanine nucleotide exchange factor (GEF) 5 | 2.07 | 0.98 |
| Potassium voltage gated channel, Shab-related subfamily, member 2 | 2.06 | 0.58 |
Data are presented as the fold-increase relative to the young values. Well-annotated genes upregulated by age but corrected by caloric restriction in the kidney as identified by microarray analysis. For these genes, the magnitude of expression was significantly elevated by at least 2-fold in aged-AL vs. young and significantly depressed at least 2-fold in aged-CR vs. aged-AL.
Fig. 3.
Impact of aging on renal gene expression. A: normalized Claudin-7 (Cldn-7), kidney injury molecule-1 (Kim-1), and matrix metalloproteinase-7 (MMP-7) gene expression in young, aged-AL, and aged-CR rats as assessed by microarray analysis using the CodeLink Rat Whole Genome Bioarray. Each data point represents the normalized mean intensity ± SD for that gene across all arrays (4 animals). **Significant difference in aged-AL compared with young and aged-CR. B: age-related changes in the above target genes were verified by quantitative PCR. The β-actin normalized Cldn-7, Kim-1, and MMP-7 gene expression in young, aged-AL, and aged-CR rats is presented relative to the gene expression in an arbitrary reference sample (Stratagene Rat Universal Reference RNA). The values represent means ± SD of relative gene expression of 8 animals per group. **Significant difference in aged-AL compared with young and aged-CR.
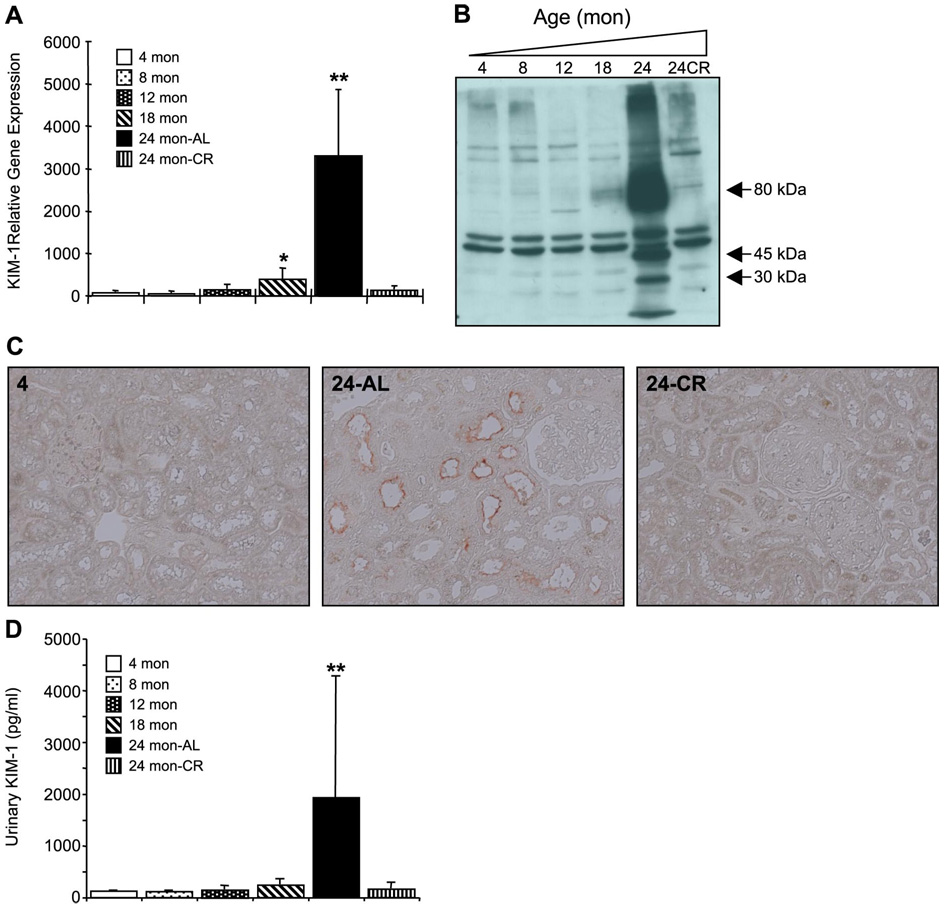
Investigation into the time course of changes in claudin-7 revealed that gene expression was increased significantly at 18 mo, but not at 24 mo (Fig. 4A). Interestingly, protein expression was increased at 24 mo as assessed by Western blot (Fig. 4B), as well as increased staining in certain tubular epithelial cells (Fig. 4C). We further examined the impact of aging on the expression of Kim-1 and MMP-7. Kim-1 gene expression increased with age and was significantly different from control at 18 and 24 mo (Fig. 5A). Western blot analysis showed a marked elevation of Kim-1 at 24 mo (Fig. 5B); full-length Kim-1 is seen at ~80 kDa, while fragments at 30 and 45 kDa were also seen, similar to previous reports of Kim-1 protein expression in the kidney (44). Kim-1 was localized to the proximal tubules (Fig. 5C) and ELISA demonstrated significant increases in Kim-1 urine levels (Fig. 5D). Importantly, similar to the gene expression data, CR attenuated this increase in protein expression.
Fig. 4.
Impact of aging on claudin-7 expression. A: claudin-7 gene expression was assessed using quantitative PCR; the β-actin normalized claudin-7 gene expression is presented relative to the gene expression in an arbitrary reference sample (Stratagene Rat Universal Reference RNA). The values represent means ± SD of relative gene expression of 4 animals per group. **Significant difference in aged-AL compared with young and aged-CR. B: Western blot analysis of claudin-7 protein levels in kidney lysates. Full-length claudin-7 is seen at ~25 kDa; membranes were stripped and reprobed with an antibody against β-actin to demonstrate equal loading. C: paraffin-embedded sections were processed for immunohistochemical localization of claudin-7 with a commercially available system. The arrows point to tubules with increased intensity of claudin-7 staining; similar results were seen in duplicate experiments.
Fig. 5.
Impact of aging on Kim-1 expression. A: quantiative PCR analysis of Kim-1 gene expression; the β-actin normalized Kim-1 gene expression is presented relative to the gene expression in an arbitrary reference sample (Stratagene Rat Universal Reference RNA). The values represent means ± SD of relative gene expression of 4 animals per group. *Significant difference in 24-mo AL compared with young. **Significant difference in aged-AL compared with young and aged-CR. B: Western blot analysis of Kim-1 protein levels in kidney lysates. Full-length Kim-1 is seen at ~80 kDa, while fragments at 30 and 45 kDa are also seen; the bands at 50 kDa are nonspecific but demonstrate equal protein loading. C: paraffin-embedded sections were processed for immunohistochemical localization of Kim-1 with a commercially available system (Zymed); similar results were seen in duplicate experiments. D: Kim-1 levels in rat urine as assessed by ELISA. Each data point represents means ± SD of 4 animals per group. **Significant difference in 24-mo AL compared with young and 24-mo CR.
The time course of changes in MMP-7 gene expression was also examined. Interestingly, expression was decreased at 8 mo and did not significantly increase compared with young animals until 24 mo (Fig. 6A). The increased MMP-7 gene expression correlated with increased protein expression as assessed by Western blot (Fig. 6B), as well as elevated staining of MMP-7 in tubular epithelial cells at 24 mo (Fig. 6C), suggesting that the changes in gene expression result in elevated MMP-7 protein expression.
Fig. 6.
Impact of aging on MMP-7 expression. A: MMP-7 gene expression was assessed using quantitative PCR; the β-actin-normalized MMP-7 gene expression is presented relative to the gene expression in an arbitrary reference sample (Stratagene Rat Universal Reference RNA). The values represent means ± SD of relative gene expression of 4 animals per group. *Significant difference in 24-mo AL compared with young. **Significant difference in aged-AL compared with young and aged-CR. B: Western blot analysis of MMP-7 protein levels in kidney lysates. Full-length MMP-7 is seen at ~27 kDa; membranes were stripped and reprobed with an antibody against β-actin to demonstrate equal loading. C: paraffin-embedded sections were processed for immunohistochemical localization of MMP-7 with a commercially available system; similar results were seen in duplicate experiments.
DISCUSSION
Aging causes structural and functional changes in human systems, sometimes leading to organ failure (36, 37). As such, susceptibility to ARF in the elderly may be due to an underlying compromise of renal function. Several factors may account for this including reductions in renal blood flow and altered glomerular structure and function (decreased number, increased size, and sclerosis) (10). In addition, the cellular antioxidant defense in the tubular cells declines with age (1, 9, 35). Twenty-years ago, scientists found that aged animal kidney is more susceptible to ischemic injury than young animals (24, 49), suggesting that animal models may parallel the clinical situation.
In the present study, we evaluated susceptibility of kidney slices from young, aged-AL, and aged-CR rats to ischemic and nephrotoxic injury and examined gene expression profiles using microarray analysis with Codelink Rat Whole Genome Bioarray. An in vitro kidney slice model was used to exclude the influences of reduced renal blood flow and increased inflammatory mediators associated with aging. An increased susceptibility to renal injury was seen in aged-AL rats. This effect is due, in part, to an inherent susceptibility of the proximal tubular epithelial cells as assessed by the response of renal tissue slices to ischemic challenge. Importantly, CR attenuated the increased susceptibility of aged rats to renal injury in vitro, suggesting that the increased susceptibility is preventable. The effect of CR on aging nephropathy may be related to decreased protein intake, anti-oxidative action (48), suppression of renal tubular apoptosis (18), and decreased inflammatory infiltration (38) in aged animals. However, other data suggest that the development of glomerulosclerosis, which is attenuated by CR, does not influence the increased susceptibility to ischemia in the rat kidney (33).
Although gene expression profiling of the aged rat kidney has been reported (28, 40), the impact of CR on age-related changes has not been extensively examined. As such, we identified several genes that are upregulated during aging and attenuated by CR. Claudin-7 is associated with the tight junctions of epithelial cells and in the tubule it is often expressed at the basolateral membrane. Interestingly, overexpression of claudin-7 has been associated with ischemia in the kidney (41). Kim-1 is upregulated following ischemia-reperfusion in rat kidney tubules, and it has been suggested as a new biomarker for ARF (13, 43). The literature also suggests that Kim-1 is also increased in chronic kidney disease, although questions were raised as to the impact of age on Kim-1 levels (32). Our data clearly demonstrate an association between increased Kim-1 and the development of chronic renal dysfunction and suggest that chronological age may not be an important variable in the clinical use of Kim-1. Importantly, increased urinary Kim-1 may be a valuable biomarker to identify aging patients at risk for ARF.
Unlike most MMPs, MMP-7 is constitutively expressed in many epithelial cell types (46). The expression of MMP-7 is low in the normal kidney but dramatically increases during several renal disease states (42). MMP-7 is linked to acute lung injury (19, 22); however, a causative role in kidney injury is less clear. Our results suggest that MMP-7 is overexpressed in the aging kidney; this conclusion is supported by data from the aging human kidney. The expression of MMP-7 was increased in aging human kidneys as assessed by microarray analysis from a total of 74 patients ranging in age from 27 to 92 yr (31). Interestingly, the fold-change (2.9) in MMP-7 expression was the second largest seen in the study. Increased MMP-7 expression during aging in the human kidney has also been confirmed in another study (23).
In conclusion, an increased susceptibility to renal injury was seen in aged rats. Importantly, CR attenuated the increased susceptibility of aged rats to renal injury in vitro, suggesting that the underlying mechanism(s) may be reversible. Microarray analysis identified 92 genes with a twofold or greater change in aged rats (as compared with young rats) whose change in expression was attenuated by CR. Based on the in vitro injury data, these genes are candidates for future investigations into the mechanism underlying the increased susceptibility of the aging kidney to injury. Importantly, both a biomarker (Kim-1) of age-related susceptibility to injury as well as a protein previously identified as having a role in acute organ injury (MMP-7) have been identified and verified as candidate genes for future studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. L. Davidson for assisting with the microarray analysis and GE/Amersham Biosciences for providing 6 CodeLink Rat Whole Genome Bioarray slides through the Center for Environmental and Rural Health (CERH) at Texas A&M University.
GRANTS
This work was supported by the CERH (P30-ES09106), a grant provided by the Texas A&M Health Science Center Vice President for Research and Graduate Studies, National Institutes of Health Grants AG-024179 (A. R. Parrish), DK-39773 (J. V. Bonventre), DK-72831 (J. V. Bonventre), and DK-74099 (J. V. Bonventre), and Scientist Development Grant 0535492T from the American Heart Association (V. S. Vaidya).
REFERENCES
- 1.Akcetin Z, Erdemli G, Bromme HJ. Experimental study showing a diminished cytosolic antioxidative capacity in kidneys of aged rats. Urol Int. 2000;64:70–73. doi: 10.1159/000030494. [DOI] [PubMed] [Google Scholar]
- 2.Baylis C, Corman B. The aging kidney: insights from experimental studies. J Am Soc Nephrol. 1998;9:699–709. doi: 10.1681/ASN.V94699. [DOI] [PubMed] [Google Scholar]
- 3.Baylis C. Age-dependent glomerular damage in the rat: dissociation between glomerular injury and both glomerular hypertension and hypertrophy. Male gender as a primary risk factor. J Clin Invest. 1994;94:1823–1829. doi: 10.1172/JCI117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beierschmidt W, Keenan K, Weiner M. Age-related susceptibility of male Fischer 344 rats to acetaminophen nephrotoxicity. Life Sci. 1986;39:2335–2342. doi: 10.1016/0024-3205(86)90664-8. [DOI] [PubMed] [Google Scholar]
- 5.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Statist Soc. 1995;57:289–300. [Google Scholar]
- 6.Corman B, Michel JB. Glomerular filtration, renal blood flow, and solute excretion in conscious aging rats. Am J Physiol Regul Integr Comp Physiol. 1987;253:R255–R260. doi: 10.1152/ajpregu.1987.253.4.R555. [DOI] [PubMed] [Google Scholar]
- 7.Corman B, Owen R. Normal development, growth, and aging of the kidney. In: Mohr U, Dungworth DL, Capen CC, editors. Pathobiology of Aging Rats. Washington, DC: ILSI Press; 1992. pp. 195–209. [Google Scholar]
- 8.De Borst MH, van Timmeren MM, Vaidya VS, de Boer RA, van Dalen MB, Kramer AB, Schuurs TA, Bonventre JV, Navis G, van Goor H. Induction of kidney injury molecule-1 in homozygous Ren2 rats is attenuated by blockade of the renin-angiotensin system or p38 MAP kinase. Am J Physiol Renal Physiol. 2007;292:F313–F320. doi: 10.1152/ajprenal.00180.2006. [DOI] [PubMed] [Google Scholar]
- 9.De Cavanagh EM, Piotrkowski B, Fraga CG. Concerted action of the renin-angiotensin system mitochondria, and antioxidant defenses in aging. Mol Aspects Med. 2004;25:27–36. doi: 10.1016/j.mam.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Fillit H, Rowe JW. The aging kidney. In: Brocklehurst JC, Tallis RC, Fillit HM, editors. Textbook of Geriatric Medicine and Gerontology. UK: Churchill Livingstone; 1992. pp. 612–628. [Google Scholar]
- 11.Go EK, Jung KJ, Kim JY, Yu BP, Chung HY. Betaine suppresses proinflammatory signaling during aging: the involvement of nuclear factor-kappaB via nuclear factor-inducing kinase/IkappaB kinase and mitogen-activated protein kinases. J Gerontol A Biol Sci Med Sci. 2005;60:1252–1264. doi: 10.1093/gerona/60.10.1252. [DOI] [PubMed] [Google Scholar]
- 12.Haley DP, Bulger RE. The aging male rat: structure and function of the kidney. Am J Anat. 1983;167:1–13. doi: 10.1002/aja.1001670102. [DOI] [PubMed] [Google Scholar]
- 13.Han WK, Bailly V, Abichandi R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (Kim-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 14.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (Kim-1), a putative epithelial cell adhesion molecule containing a novel immunoglobin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 15.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–F563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 16.Jerkic M, Vojvodic S, Lopez-Novoa JM. The mechanism of increased susceptibility to toxic substances in the elderly. I. The role of increased vasoconstriction. Int Urol Nephrol. 2001;32:539–547. doi: 10.1023/a:1014484101427. [DOI] [PubMed] [Google Scholar]
- 17.Jiang J, Dean D, Burghardt RC, Parrish AR. Disruption of cadherin/catenin expression, localization, and interactions during HgCl2-induced nephrotoxicity. Toxicol Sci. 2004;80:170–182. doi: 10.1093/toxsci/kfh143. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Jung KJ, Kim JW. Suppression of apoptosis by calorie restriction in aged kidney. Exp Gerontol. 2004;39:1361–1368. doi: 10.1016/j.exger.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 20.Liano F, Pascual J Madrid Acute Renal Failure Study Group. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol. 2003;162:1831–1843. doi: 10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melk A, Mansfield ES, Hsieh SC, Hernandez-Boussard T, Grimm P, Rayner DC, Halloran PF, Sarwal MM. Transcriptional analysis of the molecular basis of human kidney aging using cDNA microarray profiling. Kidney Int. 2005;68:2667–2679. doi: 10.1111/j.1523-1755.2005.00738.x. [DOI] [PubMed] [Google Scholar]
- 24.Miura K, Goldstein RS, Morgan DG, Pasino DA, Hewitt WR, Hook JB. Age-related differences in susceptibilty to renal ischemia in rats. Toxicol Appl Pharmacol. 1987;87:284–296. doi: 10.1016/0041-008x(87)90290-0. [DOI] [PubMed] [Google Scholar]
- 25.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 26.Pascual J, Orofino L, Uano F. Incidence and prognosis of acute renal failure in older patients. J Am Geriatr Soc. 1990;38:25–30. doi: 10.1111/j.1532-5415.1990.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Rojas J, Blanco JA, Cruz C, Trujillo J, Vaidya VS, Uribe N, Bonventre JV, Gamba G, Bobadilla NA. Mineralocorticoid receptor blockade confers renoprotection in preexisting chronic cyclosporine nephrotoxicity. Am J Physiol Renal Physiol. 2007;292:F131–F139. doi: 10.1152/ajprenal.00147.2006. [DOI] [PubMed] [Google Scholar]
- 28.Preisser L, Houot L, Teillet L, Kortulewski T, Morel A, Tronik-Le Roux D, Corman B. Gene expression in aging kidney and pituitary. Biogerontology. 2004;5:39–47. doi: 10.1023/b:bgen.0000017685.91381.40. [DOI] [PubMed] [Google Scholar]
- 29.Rich WW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age and older: a prospective study. Arch Intern Med. 1990;150:1237–1242. [PubMed] [Google Scholar]
- 30.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR., Jr Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 31.Rodwell GE, Sonu R, Zahn JM, Lund J, Wilhelmy J. A transcriptional profile of aging in the human kidney. PLoS Biol. 2004;2:e427–e428. doi: 10.1371/journal.pbio.0020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen S, Heyman S. Concerns about Kim-1 as a urinary biomarker for acute tubular necrosis. Kidney Int. 2003;63:1955. doi: 10.1046/j.1523-1755.2003.00944.x. [DOI] [PubMed] [Google Scholar]
- 33.Sabbatini M, Sansone G, Uccello F, de Nicola L, Giliberti A, Sepe V, Magri P, Conte G, Andreucci VE. Functional versus structural changes in the pathophysiology of acute ischemic renal failure in aging rats. Kidney Int. 1994;45:1355–1361. doi: 10.1038/ki.1994.177. [DOI] [PubMed] [Google Scholar]
- 34.Sands JM. Urine-concentrating ability in the aging kidney. Sci Aging Knowledge Environ. 2003;24:PE15. doi: 10.1126/sageke.2003.24.pe15. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu MHM, Araujo M, Borges SMM, de Tolosa EMC, Seguro AC. Influence of age and vitamin E on postischemic acute renal failure. Exp Gerontol. 2004;39:825–830. doi: 10.1016/j.exger.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Silva FG. The aging kidney: a review. I. Int Urol Nephrol. 2005;37:185–205. doi: 10.1007/s11255-004-0873-6. [DOI] [PubMed] [Google Scholar]
- 37.Silva FG. The aging kidney: a review. II. Int Urol Nephrol. 2005;37:419–432. doi: 10.1007/s11255-004-0874-5. [DOI] [PubMed] [Google Scholar]
- 38.Son TG, Zou Y, Yu BP, Lee J, Chung HY. Aging effect on myeloperoxidase in rat kidney and its modulation by calorie restriction. Free Radic Res. 2005;39:283–289. doi: 10.1080/10715760500053461. [DOI] [PubMed] [Google Scholar]
- 39.Stern JS, Gades MD, Wheeldon CM, Borchers AT. Calorie restriction in obesity: prevention of kidney disease in rodents. J Nutr. 2001;131:913S–917S. doi: 10.1093/jn/131.3.913S. [DOI] [PubMed] [Google Scholar]
- 40.Sung B, Jung KJ, Song HS, Son MJ, Yu BP, Chung HY. cDNA representational difference analysis used in the identification of genes related to the aging process in rat kidney. Mech Ageing Dev. 2005;126:882–891. doi: 10.1016/j.mad.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 42.Surendran K, Simon TC, Liapis H, McGuire JK. Matrilysin (MMP-7) expression in renal tubular damage: association with Wnt4. Kidney Int. 2004;65:2212–2222. doi: 10.1111/j.1523-1755.2004.00641.x. [DOI] [PubMed] [Google Scholar]
- 43.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitiative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 44.Van Timmeren MM, Bakker SJ, Vaidya VS, Bailly V, Schuurs TA, Damman J, Stegeman CA, Bonventre JV, van Goor H. Tubular kidney injury molecule-1 in protein-overload nephropathy. Am J Physiol Renal Physiol. 2006;291:F456–F464. doi: 10.1152/ajprenal.00403.2005. [DOI] [PubMed] [Google Scholar]
- 45.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988–2002. J Am Soc Nephrol. 2006;17:1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 46.Wilson CL, Heppner KJ, Rudolph LA, Matrisian LM. The metallo-proteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Mol Biol Cell. 1995;6:851–859. doi: 10.1091/mbc.6.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 48.Yu BP. Aging and oxidative stress; modulation by dietary restriction. Free Radic Biol Med. 1996;21:97–105. doi: 10.1016/0891-5849(96)00162-1. [DOI] [PubMed] [Google Scholar]
- 49.Zager RA, Alpers CE. Effects of aging on expression of ischemia acute renal failure in rats. Lab Invest. 1989;61:290–294. [PubMed] [Google Scholar]
- 50.Zawada ET, Alvai FK, Santella RN, Maddox DA. Influence of dietary macronutrients on glomerular senescence. Curr Nephrol. 1997;20:1–47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.