Abstract
Studies of the biobehavioral actions of psychostimulants commonly focus on locomotion and less commonly on feeding, and only rarely are these measures considered in conjunction within the same animal. The present study compared the impact of (+)-amphetamine and three amphetamine analogs, PAL-287, PAL-313, and PAL-353, on eating and locomotion assessed concurrently using an automated activity/feeding chamber during a daily 45 min session. Each analog is a potent releaser of norepinephrine and of dopamine, but exerts differential serotonin releasing activity (PAL-287 > PAL-313 > amphetamine > PAL-353). Rats were tested with each of five doses of drug (0, 2, 4, 8, or 16 umol/kg, i.p.), given in equimolar concentrations and in random dose order. PAL-353, an analog with minimal serotonin-relasing capacity, markedly stimulated forward locomotion at 2, 4, 8 and 16 umol/kg, as did amphetamine, whereas PAL-287 and PAL-313 did not. In contrast to the locomotor findings, all four amphetamine-like drugs exerted similar effects on the suppression of food intake. These results suggest that the capacity of an amphetamine analog (i.e. amphetamine and PAL-353) to stimulate serotonin release can diminish its psychostimulant action on locomotion, but does not reliably augment drug-induced hypophagia.
Keywords: Body Weight, Cumulative Food Intake, Monoamine, Amphetamine
1. Introduction
Psychostimulants such as cocaine and amphetamine enhance forward locomotion in rats and can induce stereotypic movements (Berthold et al., 1992; Drouin et al., 2002; Drouin et al., 2000; Wellman et al., 2002). The use of cocaine by humans is often explained in terms of the capacity of this drug to alter vigilance/attention, enhance performance, and to reinforce behavior through activation of brain dopamine systems (Clarkson and Thompson, 1997). Another factor that contributes to cocaine use is the capacity of cocaine to suppress caloric intake (hypophagia: (Balopole et al., 1979; Blosser et al., 1987; Cooper and van der Hoek, 1993; Wellman et al., 2002) and to control body weight, especially in women (Cochrane et al., 1998).
Measures of feeding in animals represent a means by which preclinical efficacy data can be obtained to estimate the utility of a drug to act as an appetite supressant (and therefore as an antiobesity agent) in humans. An issue that arises in this regard is the assessment of whether a potential antiobesity drug has significant abuse potential. Multiple behavioral assays can be used to index abuse potential (Ator and Griffiths, 2003; Griffiths et al., 2003) including the capacity of a drug to induce hyperlocomotion (Wise and Bozarth, 1987), the capacity to induce conditioned place preference (Bardo and Bevins, 2000), and the capacity to support intravenous drug self-administration (Brady and Griffiths, 1976). Intravenous self-administration is considered to be the “gold-standard” for abuse liability, whereas the capacity of a drug to stimulate locomotion is an indicator of abuse potential.
Assays of the locomotor and of the hypophagic actions of psychostimulants, however, are commonly assessed using separate paradigms (for exceptions see Baldo et al., 2002; O'Dell et al., 2007). Separate behavioral analyses may miss important differences as to the pattern of actions of a psychostimulant drug on eating and on other psychostimulant measures. We have recently developed a concurrent method in which feeding and locomotion are assayed simultaneously using locomotor/feeding chambers (Wellman et al., 2005). In this method, rats are offered an aliquot of a palatable mash diet that is suspended via a leash from an electronic balance positioned on the ceiling of an activity chamber. The use of a palatable diet ensures that the rats will consume a reliable amount of food during a 45 min test. This method does not require food restriction and attendant weight loss that are known to alter the effects of psychostimulants in behavioral assays (Bell et al., 1997; Carr, 2002).
Amphetamine-like molecules possess varying degrees of releasing activity at the transporters for dopamine (DAT), norepinephrine (NET) and serotonin (SERT). The effects of psychostimulants on locomotion have been related to drug action at DAT (Baldo et al., 2002; Bassareo and Di Chiara, 1999) as well as NET (Drouin et al., 2002; Drouin et al., 2000; Wellman, 2000; Wellman, 2005; Wellman et al., 2002). Amphetamine-like drugs are thought to suppress feeding via actions on DA and/or NE neurotransmission (Burridge and Blundell, 1979; Leibowitz, 1975; Wellman et al., 2003), whereas drugs that act predominantly via serotonin release also inhibit eating (Blundell and Lawton, 1995; Carr, 2002; Carroll and Meisch, 1981; Halford et al., 2005).
Moreover, pattern of action of drugs at the different biogenic amine transporters may modulate the behavioral actions of psychostimulants. Drugs that exert a nonselective action at DAT, NET, and SERT may induce less behavioral activation and are less likely to be abused than are drugs that are selective for DAT/NET relative to SERT (Negus et al., 2007). Of interest here was the evaluation of whether amphetamine-like drugs that exert similar actions at DAT and at NET, but differential actions at SERT, would induce similar profiles of stimulation of locomotion as well as induction of hypophagia. It has been argued that an amphetamine-induced hypophagia may result, in part, because eating is incompatible with drug-induced hyperlocomotion (Carlton, 1963; Cole, 1973). A series of amphetamine-like compounds have been characterized as DA, NE, and 5-HT releasers using an in vitro assay (Negus et al., 2007; Rothman and Baumann, 2003; Rothman et al., 2001). In the present study, we compare the impact on feeding and on locomotion of (+)-amphetamine with that of three amphetamine-like molecules (PAL-287, PAL-313, and PAL-353). Each of these PAL drugs exert similar actions at NET and at DAT, but exhibit a range of release activity at SERT. To the extent that locomotion plays a role in psychostimulant-induced hypophagia, our expectation is that drugs with low SERT activity (amphetamine, PAL 353) would induce greater degrees of hypophagia relative to drugs with high SERT activity (PAL-313, PAL-287).
2. Materials and Methods
This study was approved by the Texas A&M University Laboratory Animal Care Committee and all procedures were in accordance with National Institutes of Health guidelines.
2.1 Animals
Adult male Sprague-Dawley rats (Harlan Industries: Houston, TX) weighing approximately 250–275 g at the beginning of the study were housed in plastic hanging cages in colony room maintained at 23.0 ± 1 ° C under a 12 h light/dark cycle (lights off at 1100 h). The rats were maintained on standard chow pellets (Teklad 8604, Madison, WI) and tap water in the home cage for 23 hours per day. A mash diet (see below) was offered for an hour per day in a separate test chamber.
2.2 Drugs
A vehicle saline solution was prepared as 0.9% sodium chloride in distilled water. (+)-amphetamine sulfate was obtained from Sigma Chemical (St. Louis, MO). Three amphetamine-like drugs were used in the present study (drugs were synthesized by Dr. Bruce E. Blough). PAL-287 is 1-napthyl-aminopropane; PAL-313 is para-methylamphetamine; PAL-353 is meta-fluoramphetamine (Wee et al., 2005). The profile of activity of these drugs at DAT, NET, and SERT is given in Table 1. Each molecule exerts a similar releasing effect at DAT and at NET. Of these, PAL-287 exerts the greatest SERT releasing activity, whereas PAL-313 and PAL-353 exert substantially less SERT releasing activity. The releasing profile of (+)-amphetamine is very similar to that of PAL-353. Drug doses (2, 4, 8, and 16 umol/kg) were calculated as the weight of the base molecule and all injections were administered i.p. in a volume of 1 ml/kg.
Table 1.
In vitro potency values of (+)-amphetamine and PAL compounds for monoamine release. Values are means for three experiments (the data and methods are described in Rothman et al., 2001; Wee et al., 2005).
| Releasing Activity (IC50, in nMol) | |||
|---|---|---|---|
| DRUG: | Dopamine | Serotonin | Norepinephrine |
| (+)-AMPHETAMINE | 24.8 | 1,765.0 | 7.2 |
| PAL-287 | 12.6 | 3.4 | 11.1 |
| PAL-313 | 44.1 | 53.4 | 22.2 |
| PAL-353 | 24.2 | 1937.0 | 16.1 |
2.3 Diet
A palatable mash diet (4.03 kcal/g) consisted of 470 g of Teklad ground rat chow, 10 g of maltodextrin (Product # 31410: Fluka BioChemica, St. Louis, MO, USA), 440 g of sweetened condensed milk (Hill Country Fare: San Antonio TX, USA), and 80 g of corn oil (Albertson’s; Boise, ID, USA). The mash diet contained 13.2% fat, 14.6% protein, and 49.8% carbohydrate. The mash diet was prepared fresh every 3–4 days, stored in a sealed plastic container in a refrigerator and allowed to reach room temperature prior to use in the activity-feeding cages. The texture of this diet is such that it is possible to press a ball of the diet into a suspended plastic cylinder for presentation within a locomotor chamber. The mash diet is palatable, easily consumed, and is not readily spilled during the act of eating by a rat (Farley et al., 2003; Wellman et al., 2005).
2.4 Apparatus
The concurrent assessments of eating, drinking, and locomotion were made in a set of 8 automated optical beam activity monitors (Model RXYZCM-16; Accuscan Instruments, Columbus, OH, USA). Each monitor is housed within a 40 X 40 X 30.5 cm acrylic cage. The locomotion monitors were modified to include an electronic balance affixed to the ceiling of each chamber. A food ring, packed with the mash diet, was suspended from the weigh-below hook of each balance. Software programs were used to record forward locomotion and food intake for each minute of a 45 minute test session. A calibrated drinking tube (Wahmann) with a metal sipper spout is inserted through a hole drilled above the back left corner of the chamber and the end of the spout is positioned just above the vertical infrared beams. A metal ball bearing placed within each sipper tube serves to minimize water spillage. Details on the concurrent eating/locomotion monitoring system are given in (Wellman et al., 2005)
2.5 Procedures
In the present studies, rats were provided with continuous access to a chow pellet diet and tap water in the home cage for 23 h per day. Each rat was adapted to the mash test diet for a 3 day period. In this phase, a leash filled with the mash diet was suspended from the side of the home cage wall for an hour per day.
The rats were then adapted daily to activity-monitoring procedures for an additional 8 baseline days. Each rat was weighed at 1000 h and separately transported to the locomotor testing room in a small plastic container. At 1015 h, under red light (15 W) and white noise, the rats were placed in their respective activity chambers for 15 min without food or water. This period served to adapt the rats to the chambers and to reduce initial locomotor activity (Wellman et al., 2002). After 15 min, each rat was injected (i.p.: 1 ml/kg) with saline and replaced in the chamber. The balance lid was placed on the chamber, the balance was zeroed, and the food leash was checked for clearance from the left and front walls of the chamber. The drinking tube start volume was then recorded to the nearest ml. The activity monitor and the food monitor programs were then initiated for 45 min. At the end of the 45 min period, the final volumes of the water tubes were recorded, and the leash assembly was weighed to verify the actual food consumption. The chambers were checked for food spillage (which was negligible). Rats were then transported back to the colony room and again allowed food and water ad libitum in the home cage until the next test day. Testing chambers were thoroughly cleaned with a mild disinfectant and dried with paper towels after each test session.
After baseline testing, the rats were assigned to one of four drug conditions: (+)-amphetamine, PAL-287, PAL-313, or PAL-353. Drug was administered in place of saline on days 1, 4, 7, 10, and 13. Drug doses were given in random order and included 0, 2, 4, 8 and 16 umol/kg. Each rat received each drug dose once. On the two days in between each drug dose, no injections were given and tests were conducted as above. The rats were run in three squads to yield a group size of n=6 per drug condition. Drug conditions were counterbalanced across the three squads.
2.6 Data Analyses
Separate t-tests were computed for baseline measures of total distance travelled (cm/45 min) and total food intake (g/45 min). Separate analyses of variance (ANOVA) were computed for each dependent measure using a split-plot (mixed) model with drug (amphetamine, PAL-287, PAL-313, or PAL-353) as a between-group factor and dose (0, 2, 4, 8 and 16 umol/kg) and time after injection (three 15 min blocks) as within-group factors. Additional contrasts between drug groups were computed using the Bonferroni procedure. Difference probabilities that were less than 0.05 were deemed statistically significant.
3. Results
After injection of vehicle, there were no significant differences between the groups in total distance traveled scores (see figure 1A and Figure 2B). ANOVA of the total distance traveled scores during the three 15 min time periods indicated significant effects of drug [F(3,20) = 238.1, P < .0001], of dose [F(4,80) = 13.3, P < .0001] and of time [F(2,40) = 42.1, P < .0001]. Additionally, there was a significant three way interaction between the factors of drug, dose, and time [F(24,160) = 2.655, P < .0001]. This interaction reflected the fact that the total distance traveled scores increased with time for rats receiving amphetamine and PAL-353, whereas rats treated with PAL-287 or PAL-313 exhibited minimal changes in total distance traveled scores (see Figs 1A-1E and Fig. 3A). Overall contrasts between drugs indicated no significance difference between (+)-amphetamine and PAL-353 or between PAL-287 and PAL-313. Whereas there was no signficant difference betweeen the groups at 0.0 mg/kg, rats in groups amphetamine and PAL-353 exhibited significantly greater locomotion relative to rats treated with PAL-313 and PAL-287 at doses of 2, 4, 8 and 16 umol/kg.
Fig.1.
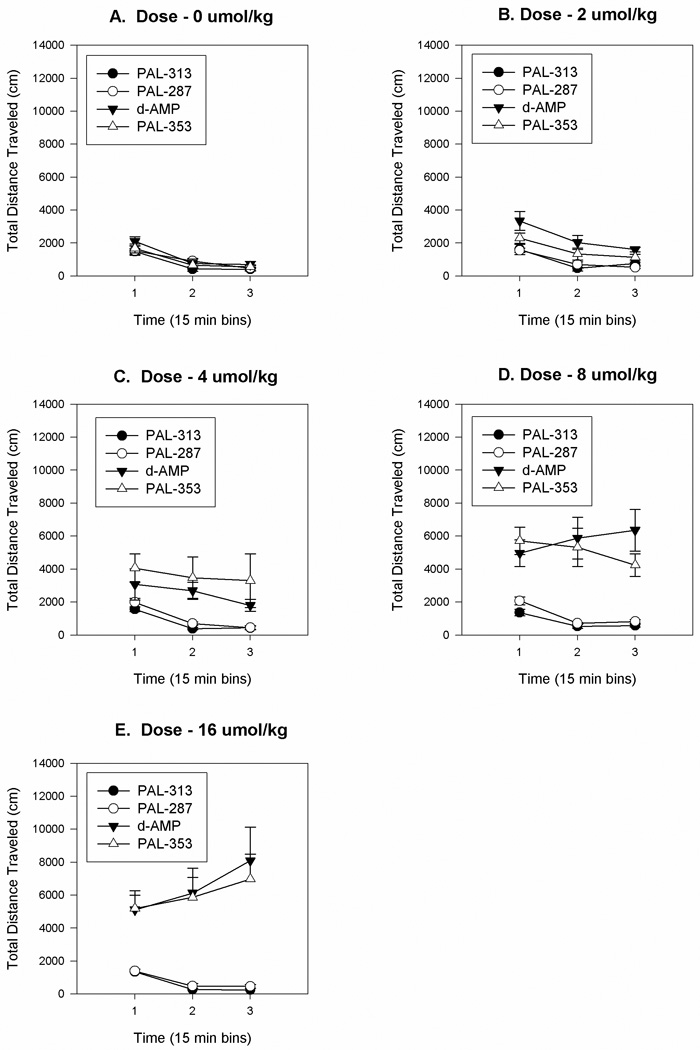
Mean group changes in total distance traveled scores (cm) during successive 15 min periods for rats treated with either amphetamine (AMP) or with PAL-287, PAL-313, or PAL-353. Panel A: 0 umol/kg. Panel B: 2 umol/kg. Panel C: 4 umol/kg. Panel D: 8 umol/kg. Panel E: 16 umol/kg. The line above each symbol represents the SEM for that value.
Fig.2.
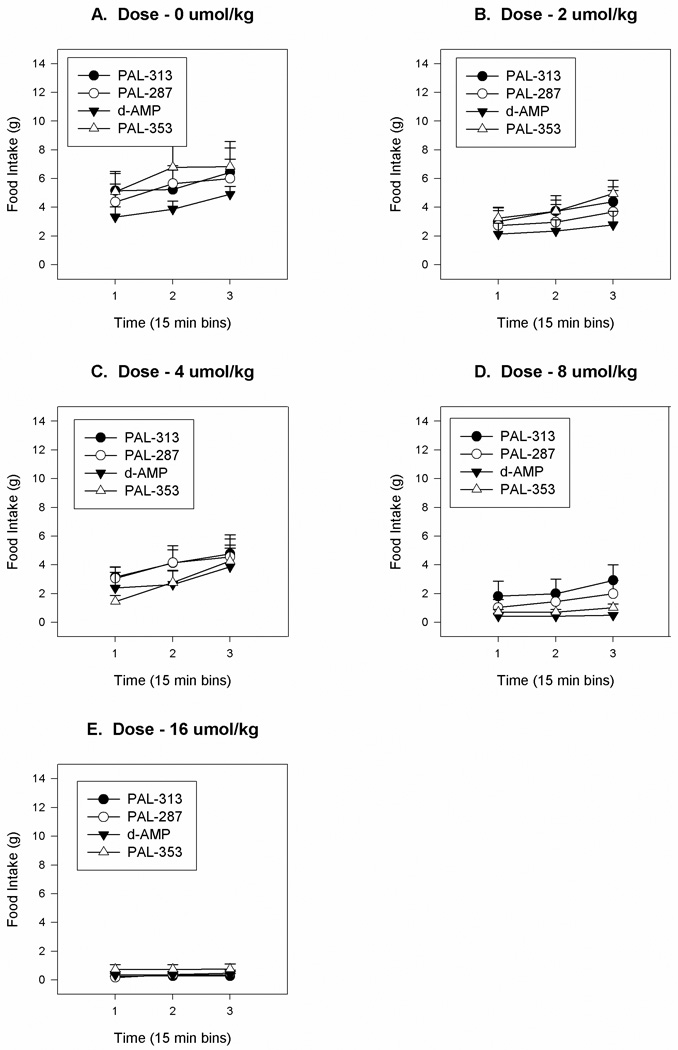
Mean group cumulative food intake values during successive 15 min periods for rats treated with either amphetamine (AMP) or with PAL-287, PAL-313, or PAL-353. Panel A: 0 umol/kg. Panel B: 2 umol/kg. Panel C: 4 umol/kg. Panel D: 8 umol/kg. Panel E: 16 umol/kg. The line above each symbol represents the SEM for that value.
Fig.3.
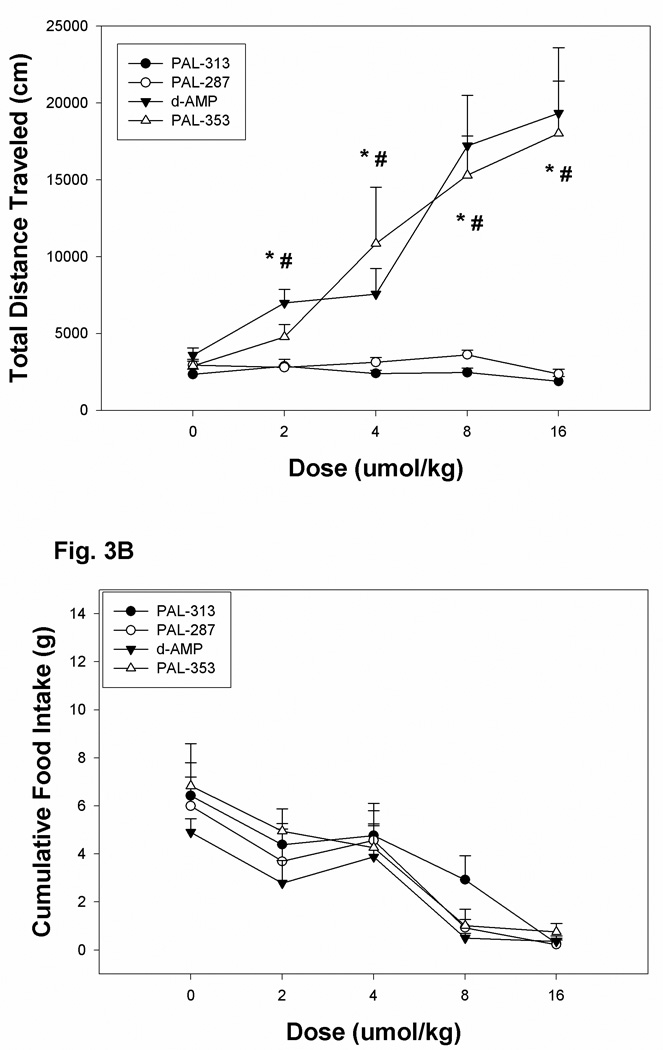
Panel A: Mean group changes in total distance traveled scores (cm) for rats injected with a random order dose series of 0, 2, 4, 8 or 16 umol/kg (i.p.) (+)-amphetamine (AMP), PAL-287, PAL-313, or PAL-353. The * symbol indicates a significant difference (P < 0.05) between the AMP group and either PAL-287 or PAL-313 groups at a particular dose, whereas the # symbol indicates a significant difference (P < 0.05) between the PAL-353 group and either PAL-287 or PAL-313 groups at a particular dose. Panel B. Mean group changes in food intake (0.1g) for rats injected with an random order dose series of 0, 2, 4, 8 or 16 umol/kg (i.p.) (+)-amphetamine (AMP), PAL-287, PAL-313, or PAL-353. The line above each symbol represents the SEM for that value.
As expected, the rats avidly consumed the mash diet during the daily 45 min test period (see Fig. 2A and Fig 3B). After injection of saline, each group consumed between 6.1 and 7.5 g of the mash diet over the 45 min period; these values were not significantly different from each other. ANOVA of the changes in food intake over the three 15 min periods failed to detect a significant effect of drug [F(3,20) = 0.515, P < .677]. Although there was a significant effect of drug dose [F(4,80) = 29.0, P < .0001], there was no significant interaction between drug and drug dose [F(12,80) = 0.7, P < .746]. Additionally, although there was a a significant effect of time [F(2,38) = 49.9, P < .0001] and a significant interaction between dose and time [F(8,152) = 7.6, P < .0001] there was no significant interaction between drug and time nor between drug, dose and time. The dose and time interaction refected the fact that food intake scores increased with time for lower, but not higher drug doses. An additional analysis was computed using individual ED50 food intake suppression scores (data not presented) which also did not reveal significant differences between the drug conditions of this study [F(3,22) = 0.541, P = 0.660].
4. Discussion
The present study used a novel apparatus and concurrent method (Wellman et al., 2005) to compare the impact of the amphetamine-like molecules PAL-287, PAL-313, and PAL-353 with that of (+)-amphetamine on eating and on locomotion. Each of these drugs exerts a similar degree of releasing activity at NET and at DAT, but have widely varying releasing activity at SERT (Negus et al., 2007; Rothman et al., 2001; Wee et al., 2005). PAL-353 has minimal releasing activity at SERT in contrast to PAL-313 and PAL-287. Amphetamine and PAL-353 thus exert quantitatively similar profiles of action at the biogenic amine transporters.
The three amphetamine-like drugs of the present study exerted dissimilar effects on forward locomotion. PAL-353 and amphetamine exerted significant increases in total distance traveled scores, whereas PAL-287 and PAL-313 did not. These results imply that variation in SERT activity modulates locomotor activity, such that compounds with higher SERT activity (PAL-287 and PAL-313) induce less psychostimulation than do compounds with lower SERT activity (PAL-353 and amphetamine). These results confirm and extend an earlier study in which PAL-287 induced less motor activation than did amphetamine in rats (Rothman et al., 2005). Activity at SERT may interact so as to diminish or attenuate drug reinforcement associated with activity at DAT/NET (Hall et al., 2004; Roberts et al., 1999; Rothman et al., 2002; Rothman et al., 2005; Wee et al., 2005). It should be noted that PAL-287 is not readily self-administered in rhesus monkeys (Rothman et al., 2005). Although the capacity of a drug to stimulate forward locomotion in the rat is an indicator of abuse potential (Wise and Bozarth, 1987), it is clear that forward locomotion may not always correlate with abuse potential (Donovan et al., 1999; Rocha et al., 1998). In the present study, PAL-313 did not activate forward locomotion, yet PAL-313 (and PAL-353) are readily self-administered in monkeys (Wee et al., 2005).
Moreover, the present results also bear on questions as to whether the capacity of a psychostimulant drug to suppress eating reflects altered motor behavior that in turn impairs eating. PAL-353 and amphetamine exerted similar significant increases in locomotion that were greater than those of PAL-287 and PAL-313, yet all four drugs exerted similar suppressions of eating. The overall dose-effect curves were similar and an analysis of doses required to suppress eating by 50% did not reveal significant diffferences between amphetamine and the three PAL drugs. These results suggest non-overlapping substrates for hypophagia and hyperlocomotion and that activation of locomotion by a psychostimulant does not contribute to the magnitude of hypophagia induced by that drug. Additionally, this pattern of results indicates that although serotonergic drugs can act to suppress eating (Blundell and Lawton, 1995; Carr, 2002; Carroll and Meisch, 1981; Halford et al., 2005), variation in SERT activity did not result in differential changes in hypophagia in the present study
In this project, we utilized a testing paradigm in which eating and locomotion are examined concurrently in rats without the use of food deprivation. The mash diet used in the present study is highly palatable and generates a moderate degree of baseline eating. One issue associated with the use of the palatable mash diet is the extent to which a palatable diet per se can alter drug-induced hypophagia. Examination of the extant literature, however, suggests that diet palatability can augment, diminish, or not alter drug-induced hypophagia. For example, nicotine induces greater hypophagia in rats that have been fed a high-fat diet, relative to rats fed a chow pellet diet (Wellman et al., 2005). Blundell and Lawton noted that the serotonergic agent dexfenfluramine produced stronger suppressions of food intake and weight gain in rats fed a high-fat diet (50–60% as fat) relative to a low-fat (4–5%) diet (Blundell and Lawton, 1995). With regard to amphetamine, Wellman et al., (1982) reported no change in hypophagia after systemic amphetamine in rats fed a palatable high-fat diet relative to that of rats fed a chow pellet diet (Wellman, 1982). In contrast other studies have noted a diminished hypophagic response in rats fed a palatable high-fat diet in response to an acute ICV administration of drugs that activate MC3/MC4 receptors extensively in brain (Clegg et al., 2003).
Finally, we suggest that the present method may be useful for the screening of psychostimulant drugs intended for use as appetite suppressants. Presumably drugs can be identified using this method that inhibit consumption of the mash diet, but that do not enhance forward locomotion (i.e. PAL-287). The method may allow for the dissociation of pharmacological and neurochemical mechanisms through which psychostimulants act to modulate eating and locomotion. Additionally, the method may be useful in identifying drugs that significantly stimulate forward locomotion but do not alter feeding. Such drugs might be used as replacement medications for abused psychostimulants (Negus et al., 2007).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70:S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behavioural Brain Research. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Balopole DC, Hansult CD, Dorph D. Effect of cocaine on food intake in rats. Psychopharmacology. 1979;64:121–122. doi: 10.1007/BF00427356. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology. 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Berthold CW, 3rd, Gonzales RA, Moerschbaecher JM. Prazosin attenuates the effects of cocaine on motor activity but not on schedule-controlled behavior in the rat. Pharmacology, Biochemistry & Behavior. 1992;43:111–115. doi: 10.1016/0091-3057(92)90646-w. [DOI] [PubMed] [Google Scholar]
- Blosser JC, Barrantes M, Parker RB. Correlation between anorectic potency and affinity for hypothalamic (+)-amphetamine binding sites of phenylethylamines. European Journal of Pharmacology. 1987;134:97–103. doi: 10.1016/0014-2999(87)90136-1. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Lawton CL. Serotonin and dietary fat intake: effects of dexfenfluramine. Metabolism. 1995;44:33–37. doi: 10.1016/0026-0495(95)90207-4. [DOI] [PubMed] [Google Scholar]
- Brady JV, Griffiths RR. Behavioral procedures for evaluating the relative abuse potential of CNS drugs in primate. Fed Proc. 1976;35:2245–2253. [PubMed] [Google Scholar]
- Burridge SB, Blundell JE. Amphetamine anorexia: Antagonism by typical, but not atypical neuroleptics. Neuropsychopharmacology. 1979;18:453–457. doi: 10.1016/0028-3908(79)90069-8. [DOI] [PubMed] [Google Scholar]
- Carlton PL. Cholinergic mechanisms in the control of behavior by the brain. Psychol Rev. 1963;70:19–39. doi: 10.1037/h0045063. [DOI] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Determinants of increased drug self-administration due to food deprivation. Psychopharmacology. 1981;74:197–200. doi: 10.1007/BF00427092. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Thompson HS. Drugs and sport. Research findings and limitations. Sports Med. 1997;24:366–384. doi: 10.2165/00007256-199724060-00003. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Benoit SC, Air EL, Jackman A, Tso P, D'Alessio D, Woods SC, Seeley RJ. Increased dietary fat attenuates the anorexic effects of intracerebroventricular injections of MTII. Endocrinology. 2003;144:2941–2946. doi: 10.1210/en.2002-0218. [DOI] [PubMed] [Google Scholar]
- Cochrane C, Malcolm R, Brewerton T. The role of weight control as a motivation for cocaine abuse. Addict Behav. 1998;23:201–207. doi: 10.1016/s0306-4603(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Cole SO. Hypothalamic feeding mechanisms and amphetamine anorexia. Psychol Bull. 1973;79:13–20. doi: 10.1037/h0033795. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, van der Hoek GA. Cocaine: a microstructural analysis of its effects on feeding and associated behaviour in the rat. Brain Res. 1993;608:45–51. doi: 10.1016/0006-8993(93)90772-f. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Miner LL, Perry MP, Revay RS, Sharpe LG, Przedborski S, Kostic V, Philpot RM, Kirstein CL, Rothman RB, Schindler CW, Uhl GR. Cocaine reward and MPTP toxicity: alteration by regional variant dopamine transporter overexpression. Brain Res Mol Brain Res. 1999;73:37–49. doi: 10.1016/s0169-328x(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Drouin C, Blanc G, Villegier AS, Glowinski J, Tassin JP. Critical role of alpha1-adrenergic receptors in acute and sensitized locomotor effects of D-amphetamine, cocaine, and GBR 12783: influence of preexposure conditions and pharmacological characteristics. Synapse. 2002;43:51–61. doi: 10.1002/syn.10023. [DOI] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2000;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res. 2003;11:845–851. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend. 2003;70:S41–S54. doi: 10.1016/s0376-8716(03)00098-x. [DOI] [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Lawton CL, Blundell JE. Serotonin (5-HT) drugs: effects on appetite expression and use for the treatment of obesity. Curr Drug Targets. 2005;6:201–213. doi: 10.2174/1389450053174550. [DOI] [PubMed] [Google Scholar]
- Hall FS, Sora I, Drgonova J, Li XF, Goeb M, Uhl GR. Molecular mechanisms underlying the rewarding effects of cocaine. Ann N Y Acad Sci. 2004;1025:47–56. doi: 10.1196/annals.1316.006. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF. Amphetamine: possible site and mode of action for producing anorexia in the rat. Brain Res. 1975;84:160–167. doi: 10.1016/0006-8993(75)90811-2. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate "agonist" medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, Davies H. Self-administration of cocaine analogs by rats. Psychopharmacology. 1999;144:389–397. doi: 10.1007/s002130051022. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;479:23–40. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Appetite suppressants as agonist substitution therapies for stimulant dependence. Ann N Y Acad Sci. 2002;965:109–126. doi: 10.1111/j.1749-6632.2002.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Stevens Negus S, Mello N, Roth BL, Baumann MH. Development of a rationally designed, low abuse biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther ms. 2005 doi: 10.1124/jpet.104.082503. in press. [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Norepinephrine and the control of food intake. Nutrition. 2000;16:837–842. doi: 10.1016/s0899-9007(00)00415-9. [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Modulation of eating by central catecholamine systems. Current Drug Targets. 2005;6:191–199. doi: 10.2174/1389450053174532. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Bellinger LL, Cepeda-Benito A, Susabda A, Ho DH, Davis KW. Meal patterns and body weight after nicotine in male rats as a function of chow or high-fat diet. Pharmacol Biochem Behav. 2005;82:627–634. doi: 10.1016/j.pbb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Ho DH, Cepeda-Benito A, Bellinger LL, Nation JR. Cocaine-induced hypophagia and hyperlocomotion in rats attenuated by prazosin. Eur J Pharmacol. 2002;455:117–126. doi: 10.1016/s0014-2999(02)02616-x. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Ho DH, Davis K. Concurrent measures of feeding and locomotion. Physiol Behav. 2005;84/5:769–774. doi: 10.1016/j.physbeh.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Miller DK, Ho DH. Noradrenergic modulation of ephedrine-induced hypophagia. Synapse. 2003;48:18–24. doi: 10.1002/syn.10182. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Pittenger DJ, Wikler KC. Diet palatability and amphetamine anorexia. Physiological Psychology. 1982;10:117–121. [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psych Rev. 1987;94:469–492. [PubMed] [Google Scholar]


