Abstract
Objective
We previously showed epidermal growth factor receptor (EGFR) transactivation as key mechanism in the regulation of resistance artery myogenic tone. Type 2 diabetes is associated with microvascular complications. We hypothesized that elevated EGFR phosphorylation contributes to resistance artery dysfunction in type 2 diabetes.
Methods-results
Diabetic db/db (diabetic) and non-diabetic (control) mice were treated with EGFR inhibitor (AG1478, 10 mg/kg/day) for 2 weeks. Isolated coronary artery (CA) and mesenteric resistance artery (MRA) were mounted in an arteriograph. Pressure-induced myogenic tone (MT) was increased in MRA and CA from diabetic mice and normalized by AG1478. Phenylephrine-induced contraction and nitric oxide donor-induced relaxation were similar in all groups. Relaxation endothelium-dependent in response to shear-stress and acetylcholine of MRA and CA from diabetic mice were altered and associated with reduced eNOS expression and phosphorylation. Treated diabetic mice with AG1478 improved CA and MRA endothelial function and restored eNOS expression. Immunostaining and western blot analysis showed increased endothelial and SMC EGFR phosphorylation of MRA and coronary from diabetic mouse, which was reduced by AG1478.
Primary cultured endothelial cells (EC), from resistance arteries, treated with high glucose for 48hrs showed an increase of EGFR phosphorylation associated with eNOS expression and phosphorylation decrease in response to calcium ionophore. Pretreatment of endothelial cells with AG1478 prevented the effect of high glucose.
Conclusion
This study provides evidence of the role of elevated EGFR phosphorylation in CA and MRA dysfunction in diabetic db/db mice. Therefore, EGFR should be a potential target for overcoming diabetic small arteries complications.
Keywords: resistance artery, myogenic tone, flow-induced dilation, eNOS, EGF receptor, Type 2 diabetes, diabetic db/db mice
INTRODUCTION
Resistance artery (RA) tone is mainly regulated by mechanical factors (pressure and flow) and vasoactive agents (1). In general, flow induces vasodilation endothelium-dependent (2). On the other hand, pressure-induced contraction (myogenic tone, MT) is generally endothelium-independent and is mediated by direct effect of intraluminal pressure on VSMC (1). Although the multi-factorial effects of type 2 diabetes on the regulatory mechanisms that dictate altered microvessel function are not well understood, it is likely that altered vascular reactivity is involved. Limited studies of the relationship between diabetes and altered vascular responsiveness have been conducted in the microvasculature from diabetic models and conflicting results have been obtained. For instance, skeletal muscle arterioles of streptozotocin treated rats exhibit enhanced pressure-induced myogenic responsiveness that is endothelium-independent but requires increased activation of L-type Ca2+ channels and protein kinase C (3; 4). (5) Similarly, Bagi et al. observed an increased basal tone of gracilis muscle arterioles from diabetic mice (6). On the other hand, small arteries (65–230 µm) from gluteal fat biopsies from patients with type 2 diabetes demonstrated decreased MT responsiveness (7).
EGFR is an 1186-amino-acid glycoprotein containing a single transmembrane domain with intracellular portion harboring the tyrosine kinase domain. Transactivation of EGFR by some G-protein coupled receptor (GPCR) agonists was originally reported by Daubet et al in 1996 (8). Importantly, we have shown in a previous study the importance of EGFR transactivation by mechanical factors in the development of myogenic tone (9). Although there is a plethora of information concerning the growth-promoting effects of EGFR, its role in the complications of resistance arteries in type 2 diabetes is unknown. Additionally, it has been shown that EGFR is regulated by high glucose through EGFR-N-glycosylation (10) and increased mitogenic response to heparin binding-EGF (11).
Type 2 diabetes is characterized by a chronic hyperglycemia due to inadequate insulin secretion in the setting of insulin resistance and is often associated with obesity, hypercholesterolemia and hyperlipidemia (12–14). The morbidity and mortality of diabetes are principally due to the development of both macrovascular and microvascular complications (15–17). Recently, it was reported that down-regulation of EGFR expression in the pancreas islet cells (altered β-cells proliferation) was responsible for the induction of diabetes in 1 to 8 weeks old mice (18) indicating the importance of EGFR as a protecting factor against the initiation of diabetes in the early stage of development. On the other hand, different studies stated the beneficial effect of EGFR inhibition in mature adult diabetic animals. For instance, it has been shown that diabetes is associated with an increase in advanced glycation end products (AGEs) formation. It is noteworthy that AGE-induced oxidative stress increase through EGFR trans-activation, which is suppressed in cells overexpressing AGEs receptor 1 (19) providing evidence for a relationship between diabetes, AGEs, EGFR and oxidative stress.
Thus, in the present study, we determined the role of elevated EGFR phoxphorylation in the dysfunction of resistance arteries observed in type 2 diabetes. Mesenteric resistance arteries and coronary arterioles function were studied in type 2 diabetic (db−/db−) mice with or without EGFR phosphorylation inhibitor.
METHODS
Animal Model
Obese homozygote (db−/db−) type 2 diabetic lacking the gene encoding for leptin receptor Leprdb−/db− (diabetic, 10 to 14 weeks old) and heterozygote Leprdb−/db+ (db−/db+) non-diabetic (control, 10 to 14 weeks old) adult male mice were obtained from Jackson Laboratory and maintained on a normal rodent show diet. Mice were divided into 4 groups: 1) control mice with no treatment (n=6); 2) control mice who received 10 mg/Kg/day of AG1478 in mini-osmotic pumps (n=7); 3) diabetic mice with no treatment (n=6); and 4) diabetic mice that received 10 mg/Kg/day of AG1478 in mini-osmotic pumps (n=8).
These studies conformed to the principles of the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”, and were approved by the LSU Institutional Animal Care and Use Committee.
Blood Glucose
Blood glucose measurements were obtained from tail vein samples using a blood glucose meter (Prestige Smart System HDI, Home Diagnostic, Inc) in all groups after a 5 hrs of fast (n=6).
Insulin Resistance
1 mL of Blood was obtained by cardiac puncture with a syringe containing heparin from mice after a 5 hrs of fast. Insulin resistance was determined by using the Mercodia Ultrasensitive Mouse Insulin Elisa protocol (Enzyme immunoassay), which estimates steady state insulin resistance (n=6) (20).
Mean arterial pressure measurement
1 - After 2 weeks of treatment, mice were anesthetized with ketamine/xylazine (45/2.5 mg/kg i.p. respectively) and a catheter (connected to a pressure transducer and recording system, www.livingsys.com) was placed in the left carotid. Mice were then subjected to a 15-minutes equilibration period before mean arterial pressure was measured.
2 - Telemetry: Briefly, mice were subjected to isofluorane anesthesia and a catheter connected to a radiotelemetry device was inserted in the left carotid artery to monitor heart rate and blood pressure by telemetry in conscious unrestrained conditions (Model PA-C10; Data Sciences International, St. Paul, MN). The body of the probe was placed in a subcutaneous pocket created in the subcutaneous abdominal area and the wound closed and sutured. After a recovery phase (24–48 hours), mean arterial blood pressures were collected for 10 sec. intervals every 10 minutes for 5 days. Data were stored and analyzed using Dataquest A.R.T 4.0 software (Data Sciences International).
Isolated coronary and mesenteric resistance artery
Mechanotransduction
Freshly isolated mesenteric resistance artery (MRA) and coronary artery (septal) (CA) were mounted onto two glass micropipettes in a vessel chamber and slowly pressurized to 100 mmHg using a pressure-servo-control perfusion (Living Systems Instruments, www.livingsys.com) in order to stretch the artery and set a constant artery length (9). Intraluminal pressure was then set at 50 mmHg for equilibration time (30–45 min). Vessel diameter was continuously monitored by a video image analyzer as previously described.(3; 9) Cannulated arterial segments were submerged in 2 ml of physiological salt solution (pH 7.4), oxygenated with (10%O2− 5%CO2 and 85%N2). The functional integrity of the endothelial and smooth muscle cell layer was assessed by relaxation endothelium-dependent and contraction in response to acetylcholine (1 µM) and phenylephrine (1 µM) respectively. Next pressure and shear stress diameter-(active diameter) relationships were performed to determine myogenic tone and flow-induced dilation. At the end of each experiment, MRA and CA were perfused and superfused with PSS containing 100 µM of exogenous nitric oxide donor (sodium nitroprusside, SNP) and 2 mM of EGTA in order to determine the maximum relaxation of artery (passive diameter). Myogenic tone is calculated as the percent between active and passive diameter. Flow-induced dilation was represented as percent of diameter in response to shear stress step increase.
Pharmacology studies
freshly isolated and mounted MRA and CA were equilibrated at 50 mmHg of intraluminal pressure for 30–45 minutes. Dose-response (10−10 to 10−5 M) curves phenylephrine, acetylcholine and nitric oxide donor diameter changes relationship were then performed.
Western blot analysis
Tissue
Freshly isolated MRA from all groups were immediately snap-frozen in liquid nitrogen. Frozen vessel segments were pulverized and re-suspended in ice-cold lysis buffer as described (21). Each sample was then subjected to immunoblotting with eNOS or EGFR antibodies (1:1000 dilution, Cell signaling). Blots were stripped and reprobed with the β-actin antibody to verify the equal loading between the samples.
Resistance Arteries Primary Cultured Endothelial Cells
After 80 % of confluency, endothelial cells were starved for 48 hrs in culture medium containing 1% of serum. Cultured cells were then stimulated with high glucose (22 mM) with and without dose-response AG1478 (1, 0.1 and 0.01 µM). Next, cells were stimulated for 5 min with calcium ionophore (eNOS activator), harvested and subjected to western blot analysis using phosphorylated (Ser1177 activator site and Thr495 negative regulatory site) and total eNOS, and EGFR antibodies (Tyr1173). Membranes were stripped and reprobed with total β-actin antibody to verify the loading of samples.
Immunohistochemistry
Freshly isolated MRA placed in mold tissue Teck and Formalin fixed heart embedded in paraffin were sectioned at 5 µm. Heart sections were heated at 58°C for 1 hr for antigen retrieval. Endogenous peroxidase activity was quenched in heart and MRA section by 10 min incubation with 3 % H2O2 in H2O. Heart sections were treated with sodium citrate buffer (10 mmol/L,pH 6.0) at 100°C for 10 minutes. After blocking with 5%bovine serum albumin in TBS, Sections (heart and MRA) were incubated overnight at 4°C with mouse monoclonal antibody against phosphorylated EGFR (Tyr1173; 1:200 dilution). For every section, a negative control without first antibody was processed simultaneously. After three 5 min washes in TBST (10 mM Tris-HCl, 0.15 M NaCl, 8 mM sodium azide, 0.05% Tween-20, pH 8.0), a secondary biotinylated antibody was added for 45 minutes at room temperature. After three 5 min washes in TBST, the avidin biotin-peroxidase complex (Vector labs) was applied for 30 minutes at room temperature. The color reaction was developed with the diaminobenzidine detection kit (Vector labs, 1 min incubation with the substrate for all the samples) and counterstained with hematoxylin. Staining was evaluated using fluorescent microscope.
Immunocytochemistry
Cultured endothelial cells were stimulated with high glucose for 48 hrs with and without EGFR tyrosine kinase inhibitor. Briefly, cells were fixed with methanol and incubated in PBS solution with 5% of BSA for 1 hr. Cells were then incubated with first antibody (phosphorylated EGFR tyrosine kinase antibody Tyr1173, dilution 1:200) for overnight at 4°C. The next day, cells were washed and incubated for 1 hr with fluorescent secondary antibody at room temperature. Staining was evaluated using fluorescent microscope.
In others slides of MRA, Verhoff Van Gieson (VVG) (22) staining was performed to determine the internal elastic lamina (IEL) and external elastic lamina (EEL). Briefly, the tissue is stained with a regressive hematoxylin, consisting of ferric chloride and iodine. The differentiating is accomplished by using excess mordant (ferric chloride) to break the tissue-mordant dye complex. The dye will be attracted to the larger amount of mordant in the differentiating solution and will be removed from the tissue. The elastic tissue has the strongest affinity of the iron-hematoxylin complex and will retain the dye longer than the other tissue elements.
Statistical analysis
Results are expressed as mean±sem, where n is the number of arterial segments and cultured cells studied. Significance of the differences between groups was determined by 1-repeated or 2-factor ANOVA, where appropriate. Differences were considered significant at P<0.05.
RESULTS
Blood pressure, measured under anesthesia and with telemetry (Table 1 and Figure 1A), was similar in all groups of mice (Table 1) indicating that db−/db− mice are normotensive. Blood glucose level was higher in diabetic compared to control mice (Table 1). Higher values of insulin resistance were found in plasma from type 2 diabetic compared to control mice (Table 1). The treatment of type 2 diabetic mice with AG1478 for two weeks had no effect on blood pressure, glucose level and insulin resistance (Table 1).
Table 1.
| Control | Diabetic | Diabetic + AG1478 | |
|---|---|---|---|
| Blood pressure in carotid under anesthesia (mmHg) | 97±3.2 | 96.5±4.4 | 95±5.1 |
| Blood pressure using Telemetry (mmHg) | 110±6.4 | 112±6.3 | - |
| Plasma glucose concentration (mg/dL) | 121±15 | 321±22* | 291±8.7* |
| Serum insulin concentration (µg/L) | 1.21±0.3 | 4.35±0.4* | 4.15±0.5* |
| Body weight | 26±3 g | 45±2.25* | - |
Figure 1.
Blood pressure measurements with telemetry and pressure-induced myogenic tone: In vivo blood pressure measurement with telemetry from diabetic and control mice, p>0.05, not statistically different; n=5 (1A). Pressure-induced myogenic tone relationship of mesenteric resistance artery (MRA) (1B), and coronary artery (CA) (1C) from all groups treated with and without EGFR tyrosine kinase inhibitor (AG1478, 10 mg/Kg/day); n=7, *P<0.05, statistically significant diabetic vs. control mice; diabetic + AG1478 vs. diabetic.
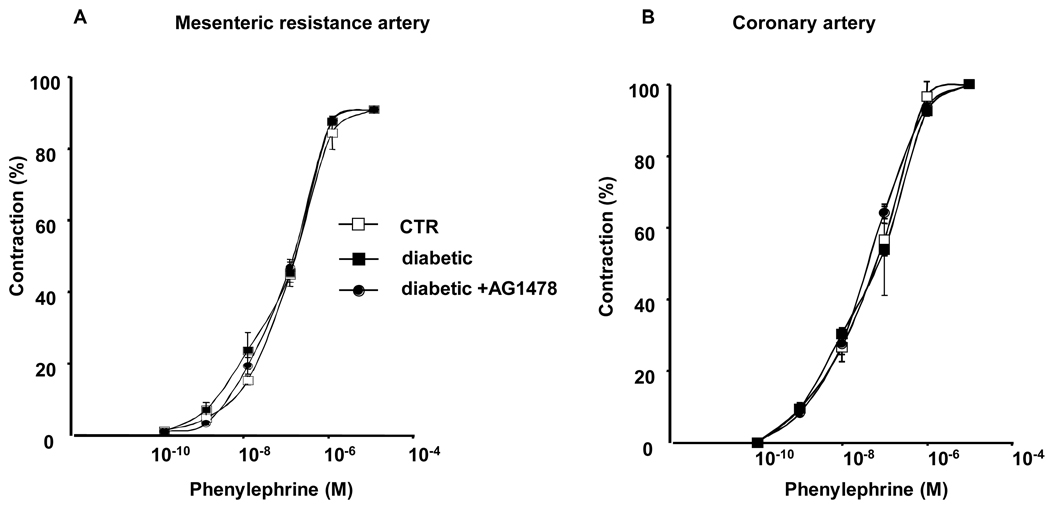
Freshly MRA and CA were isolated and mounted in an arteriograph. Stepwise increases intraluminal pressure induced myogenic tone (MT) development, which was significantly enhanced in MRA and CA from diabetic mice compared to control (Figure 1B, C). The AG1478 treatment for 2 weeks reduced the MT potentiation in MRA and CA from diabetic mice (Figure 1B, C) indicating that the exacerbation of EGFR phosphorylation is involved in the enhanced MT. Increasing doses of phenylephrine induced similar contractions of MRA and CA from all groups (Figure 2). On the other hand, relaxation-endothelium dependent was significantly altered in MRA and CA from diabetic mice compared to control mice as assessed with acetylcholine dose-response relaxation relationship (Figure 3) and flow-induced dilation (Figure 4A). Dysfunction of MRA from diabetic mice was associated with a decrease of endothelial nitric oxide synthesis (eNOS) phosphorylation and expression (Figure 4B). Phosphorylation at the negatively regulatory eNOS site Thr495 was similar in MRA from control and type 2 diabetic mice (data not shown).
Figure 2. Pharmacology approach.
Phenylephrine dose-response (10−10 to 10−5 µM) induced contraction of mesenteric resistance artery (2A) and coronary artery (2B) from control (CTR), diabetic and diabetic treated with EGFR tyrosine kinase inhibitor (AG1478, 10 mg/Kg/day), n=7, P>0.05, not statistically different.
Figure 3.
Changes in diameter in response to dose-response (10−10 to 10−5 M) of acetylcholine in mesenteric resistance artery (3A) and coronary artery (3B) from control (CTR), diabetic and diabetic with EGFR tyrosine kinase inhibitor (AG1478, 10 mg/kg/day), n=6, *P<0.001 diabetic vs. control; diabetic vs. diabetic.
Figure 4.
4A: Changes in diameter in response to step increases of flow in mesenteric resistance artery isolated from control (CTR), diabetic (Db) and diabetic treated with EGFR tyrosine kinase inhibitor (AG: AG1478, 10 mg/kg/day), *P<0.001 diabetic vs. control, diabetic vs. diabetic+AG1478; 4B: In vivo eNOS phosphorylation and expression in freshly isolated mesenteric resistance artery from control, diabetic and diabetic treated with AG1478, *P<0.001 diabetic vs. control, and diabetic+AG; 4C: Changes in diameter in response to dose-response (10−10 to 10−5 µM) of exogenous nitric oxide donor (SNP) in mesenteric resistance artery from control, diabetic and diabetic treated with EGFR tyrosine kinase inhibitor (AG: AG1478, 10 mg/kg/day), n=6–8, P>0.05 not statistically different.
The treatment of diabetic mice with AG1478 for 2 weeks significantly improved the relaxation-endothelium dependent in response to shear stress and acetylcholine (dose-response)-induced relaxation in MRA and CA (Figure 3, Figure 4A) and normalized the eNOS phosphorylation and expression (figure 4B).
The relaxation endothelium-independent of MRA and CA from diabetic mice to exogenous nitric oxide donor was similar in all groups indicating that the sensitivity of SMC to nitric oxide was not altered in type 2 diabetic mice (Figure 4C).
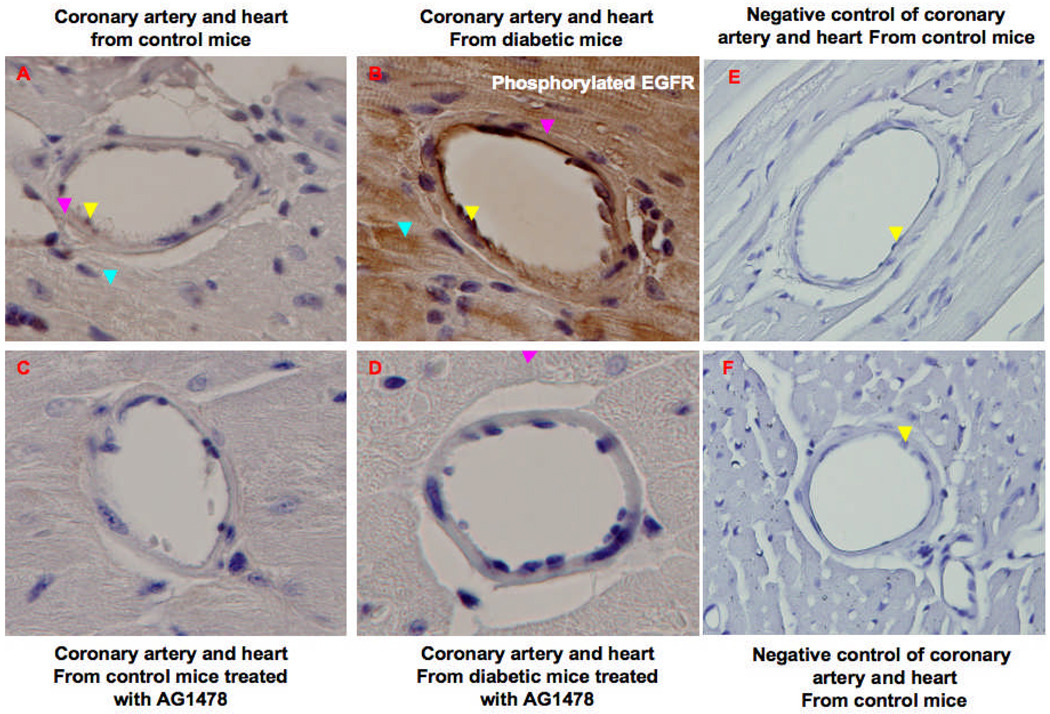
Immunohistochemistry revealed an increase of EGFR phosphorylation of MRA and CA from diabetic compared to control and diabetic treated with AG1478 (Figure 5, Figure 6). In other MRA slides, IEL and EEL staining was performed (Figure 5D, E F).
Figure 5.
Immunhistochemical staining showing the in vivo EGFR phosphorylation of mesenteric resistance arteries (MRA) from control (5A), diabetic (5B) and diabetic treated with EGFR inhibitor (AG: AG1478) (5C). The brown staining indicated by yellow and magenta arrows show the phosphorylated EGFR on endothelial and SMC of MRA, respectively. Figures 5D, 5E and 5F show the internal elastic lamina (IEL, red arrow) and the external elastic lamina (EEL, turquoise arrow) of different MRA from control, diabetic and diabetic mice treated with AG1478, respectively. Each figure is a representative of 5 experiments.
Figure 6.
Immunhistochemical staining showing the in vivo EGFR phosphorylation of coronary artery from control (6A), diabetic (6B), control + AG1478 (6C) and diabetic treated with EGFR inhibitor (AG1478) (6D). The brown staining indicated by yellow, magenta and ice arrows show the phosphorylated EGFR on endothelial, SMC of coronary artery and cardio-myocytes respectively. 6E and 6F show negative control sections of coronary artery from control and diabetic and diabetic mice treated with AG1478. Each figure is a representative of 5 experiments.
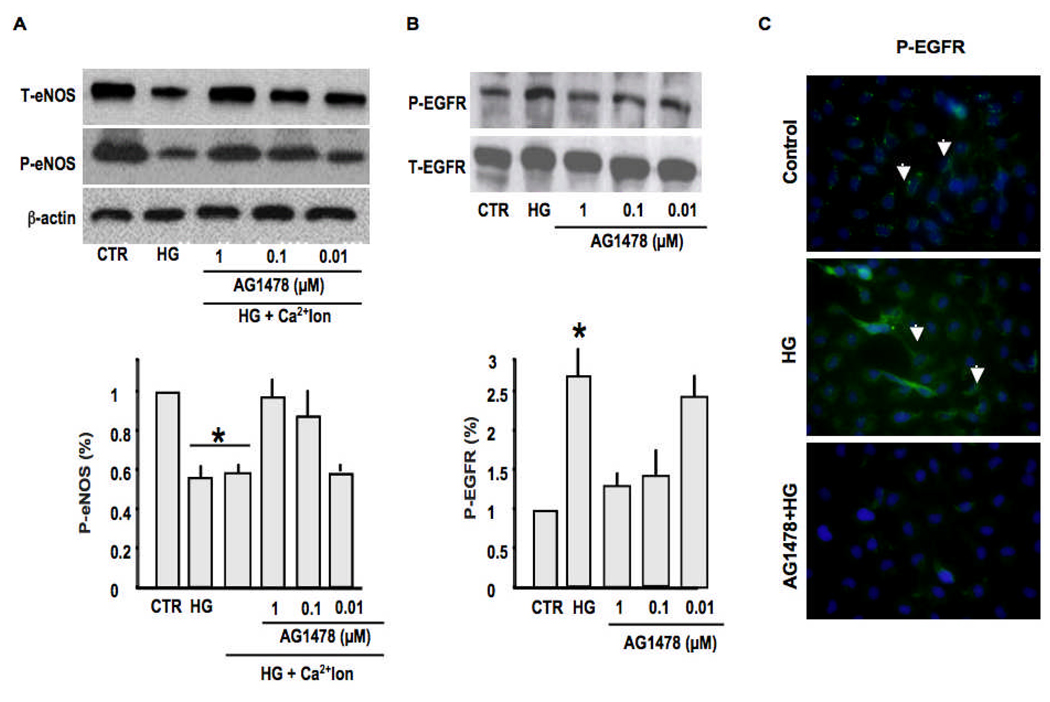
To strengthen our approach, we used resistance artery primary cultured endothelial cells treated with high glucose (20 mM) with or without dose-response of AG1478 (1, 0.1 and 0.01 µM) for 48 hrs. The treatment of endothelial cells with high glucose induced an increase in EGFR phopshorylation and an alteration in eNOS expression and phosphorylation in response to calcium ionophore (Figure 7A), which were restored when endothelial cells were pretreated with AG1478 (Figure 7A). Western blot analysis and immunostaining show an increase in phosphorylated EGFR on endothelial cells stimulated with high glucose for 48hrs (Figure 7B–C). The specificity of the primary antibody was confirmed by subjecting tissue section and primary cultured cells to immunostaining without the primary antibody as no signal was observed (data not shown). The control and pretreated endothelial cells with AG1478 showed low background staining of EGFR phosphorylation in response to high glucose (Figure 7C).
Figure 7.
7A: Western blot analysis and cumulative data showing the effect of high glucose (HG), with or without AG1478 dose-response (1, 0.1 and 0.01 µM), on total eNOS and phosphorylated eNOS in response to calcium ionophore of primary cultured endothelial cells, n=8, *P<0.001 CTR vs. high glucose; high glucose vs. high glucose + AG1478; 7B: Western blot analysis and cumulative data showing total and phosphorylated EGFR tyrosine kinase of primary cultured endothelial cells in response to high glucose, with or without AG1478 dose-response (1, 0.1 and 0.01 µM); n=8, *P<0.001 CTR vs. high glucose; high glucose vs. high glucose + AG1478 (1 µM); 7C: Immunostaining showing EGFR tyrosine kinase phosphorylation on primary cultured endothelial cells membrane under control, high glucose and AG1478 + high glucose conditions. The green staining indicated by arrows show the phosphorylated EGFR on cultured endothelial cells. Each figure is a representative of 5 experiments.
DISCUSSION
In the present study, we provide strong evidence supporting the involvement of elevated EGFR phosphorylation in dysfunction of mesenteric resistance artery (MRA) and coronary arteriole (CA) (alteration of endothelial and smooth muscle cells “SMC”) from db−/db− mouse, which is a widely accepted model for obesity and type 2 diabetes. Endothelial cells dysfunction is associated to a decrease in eNOS expression and phosphorylation, while SMCs are hypersensitive to pressure changes leading to the potentiation of myogenic tone in MRA and CA from diabetic mice. Interestingly, endothelial and SMC function was significantly improved in MRA and CA from diabetic mice treated with EGFR phosphorylation inhibitor.
Blood pressure, measured under anesthesia and with telemetry, was normal and similar in all groups indicating that db−/db− mice are not hypertensive. Our data are not in agreement with Bagi et al study showing an increase of systolic blood pressure in diabetic mice measured by tail-cuff method (6). However, our data are in agreement with a previous study showing a normal blood pressure in diabetic compared to control mice (20; 23). Blood glucose and serum insulin levels were increased in type 2 diabetic mice compared to control, and were not affected by AG1478 treatment indicating that elevated EGFR phosphorylation in type 2 diabetic mice is a consequence to the onset type 2 diabetes. These data are in agreement with previous studies showing an increased of plasma glucose and serum insulin from db−/db− mice (20).
Resistance arteries play a crucial role in the control of local blood flow and subsequently tissue perfusion. These resistance arteries develop tone, which is mainly regulated by mechanical factors (pressure and shear stress) and hormonal factors (24; 25).
It has been reported that type 2 diabetes is associated with small arteries complications (26). Only limited studies have been conducted to establish the relationship between diabetes and altered vascular responsiveness in the microvasculature from diabetic models with conflicting results. Lagaud et al demonstrate increased myogenic tone in mesenteric resistance arteries from 12- and 16-week diabetic mice compared to the control that was independent of endothelium removal (5). In contrast Bagi et al. showed no significant increase in myogenic tone in coronary arterioles from 12-week db−/db− mice (27). Small arteries (65–230 µm) from patients with Type 2 diabetes demonstrated decreased myogenic responsiveness (7). The reasons for these discrepancies are unclear but could be related to differences in vascular beds and species.
Emerging evidences suggest the existence of a new mode of EGFR signalling pathway in which activated receptor undergoes nuclear translocalization and subsequently regulates gene expression and potentially mediates other cellular processes. This signalling route is distinct from the better-characterized EGFR pathway that involves transduction of mitogenic signals through activation of multiple signalling cascades (28; 29). Our data show increased MT in MRA and CA from diabetic compared to their control, which are in accordance with a study conducted by Lagaud et al (5). Interestingly, treatment with EGFR tyrosine kinase inhibitor reduces the MT potentiation of MRA and CA from diabetic mice indicating that elevated EGFR phosphorylation is a key element in the enhanced MT in type 2 diabetic mice. Contraction and relaxation-endothelium independent of MRA and CA in response to G-protein coupled receptor (phenylephrine) and nitric oxide donor were similar in all groups. In agreement with our study, Malik et al have shown that vasoconstriction to phenylephrine and angiotensin II was similar in small arteries from patients with or without type 2 diabetes mellitus (30). This study strengthens our data indicating that MRA and CA do not develop hypersensitivity to vasconstrictors G-protein coupled receptor-dependent in type 2 diabetes. The mechanism leading to increased EGFR tyrosine kinase phosphorylation in type 2 diabetes is unknown. In a previous study, we have shown that myogenic tone development is dependent on EGFR tyrosine kinase activation through metalloproteinases 2/9 (MMPs) activity. We suggest that MMPs 2/9 and hyperglycemia signaling such oxidative stress and advanced glycation end products could be involved in the increased EGFR tyrosine kinase phosphorylation in type 2 diabetes. Further studies are needed to determine and emphasize the role of MMPs 2/9, AGEs and oxidative stress in the increased EGFR phosphorylation in type 2 diabetes and microvessels dysfunction.
Endothelial dysfunction has been demonstrated to occur in small arteries from patients with type 2 diabetes (31–33). Our data revealed that relaxation endothelium-dependent assessed with flow changes was significantly altered in MRA from type 2 diabetic compared to control mice. Our data are concordant with others studies showing a dysfunction of endothelial cells in diabetes (27; 34). To strengthen our conclusion, we used acetylcholine dose-response induced relaxation, which was significantly decreased in MRA and CA from type 2 diabetic mice compared to control mice. Interestingly, the treatment of type 2 diabetic mice with EGFR tyrosine kinase inhibitor significantly improved relaxation to shear stress and acetylcholine stimulation and no effect was observed in control mice. The endothelium dysfunction was associated with a decrease in eNOS activator site (Ser1177) phosphorylation and expression in type 2 diabetic mice; the inhibitory eNOS site phosphorylation Thr495 was not affected. Ohashi et al showed a decrease of eNOS at the mRNA level in KKAy mice, which develop a maturity-onset obesity, type 2 diabetes and hypertension (35). Similarly, it has been reported a reduction in eNOS phsophorylation and expression in kidney from diabetic rats (36). Together, these data provide evidence of a link between resistance arteries endothelium dysfunction and alteration of eNOS phosphorylation and expression in type 2 diabetic mice. Interestingly, the treatment of type 2 diabetic mice with EGFR tyrosine kinase inhibitor improved resistance arteries relaxation in response to flow and acetylcholine, and restored eNOS phosphorylation and expression. These data strongly support the link between EGFR tyrosine kinase and resistance artery relaxation through, in part, eNOS pathway. It is important to mention that endothelium of resistance artery is a source of different vasoactive compounds such nitric oxide, prostacyclin I2 and endothelium derived hyperpolarizing factor involved in SMC relaxation. Thus, the treatment of type 2 diabetic mice with AG1478 partially improved the relaxation, while totally restored the phosphorylation and expression of eNOS, indicating that type 2 diabetes could also affect prostacyclin and/or EDHF pathways. Further studies are needed to explore the molecular mechanisms linking EGFR and vasoactive compounds in small arteries from type 2 diabetic mice.
The mechanism responsible for the elevated EGFR tyrosine kinase phosphorylation and its link to eNOS in resistance arteries endothelial cells from type 2 diabetic mice is unknown. It is likely that EGFR tyrosine kinase signaling such oxidative stress could be involved in eNOS pathway alteration in type 2 diabetes. Additionally, it has been reported that AGEs could have a potential role since it can also increase oxidative stress generation through their receptors. Future studies are needed to determine the mechanism leading to increased EGFR tyrosine kinase and its link to eNOS in type 2 diabetes.
To corroborate our functional studies, we performed immunostaining and found an increase of EGFR tyrosine kinase phosphorylation in endothelial and SMC of MRA and CA from type 2 diabetic mice compared to control mice, which was reduced with the EGFR tyrosine kinase inhibitor treatment. Together, these data strengthen a link between the increased EGFR tyrosine kinase phosphorylation and alteration of resistance arteries function in type 2 diabetic mice.
To determine the contribution of hyperglycemia in endothelium dysfunction of resistance arteries from type 2 diabetic mice, we used primary cultured endothelial cells prepared from resistance artery and treated with high glucose for 48 hrs. Cells lysates subjected to western blot analysis showed an increase of EGFR tyrosine kinase phosphorylation associated with alterations in eNOS expression and phosphorylation strengthen our in vivo observation indicating that hyperglycemia participates in the dysfunction of small arteries endothelial cells. EGFR phosphorylation increase in response to hyperglycemia was supported by immunostaining. The pretreatment of endothelial cells with EGFR tyrosine kinase phosphorylation inhibitor prevented the effect of high glucose. This indicates that in type 2 diabetic mice, hyperglycemia-induced EGFR tyrosine kinase phosphorylation increase, to some extent, responsible for endothelial cells dysfunction. It is well established that type 2 diabetes is characterized with hyperglycemia and insulin resistance, and it is likely that insulin resistance might have an impact on resistance artery endothelial and SMC dysfunction. Further studies are indeed needed to investigate the insulin resistance in the absence of hyperglycemia on resistance artery reactivity.
Our data suggest that, in type 2 diabetic mice, elevated EGFR tyrosine kinase phosphorylation is involved in resistance artery enhanced myogenic tone and alteration of endothelial cells through the nitric oxide synthesis pathway. The treatment of diabetic mice with AG1478 improved the MRA and CA functions. We strongly believe that additional factors are indeed implicated in the alteration of resistance arteries in type 2 diabetes. In addition, we believe that a myriad of signaling pathways involved. The discovery of EGFR tyrosine kinase as regulator of endothelial and SMC dysfunction of resistance arteries might be relevant not only for type 2 diabetes small arteries complications but also for many other pathological conditions in which EGFR has been suggested to play a role.
ACKNOWLEDGEMENTS
This work was supported by the SDG National American Heart Association, 0430278N (PI: K Matrougui); Enhancement Research Phase II Award Tulane University (PI: K Matrougui); P20RR017659, HL26371 (PI: Dr. L Navar, NCRR); COSEHC Warren Trust Fellowship Award (RA. Gonzalez-Villalobos, PhD) and R01 HL072889 from the NIH (PI: HA. Boulares).
REFERENCES
- 1.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 2.Shimokawa H, Takeshita A. Endothelium-dependent regulation of the cardiovascular system. Intern Med. 1995;34:939–946. doi: 10.2169/internalmedicine.34.939. [DOI] [PubMed] [Google Scholar]
- 3.Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens. 2005;23:571–579. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- 4.Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res. 1991;68:359–367. doi: 10.1161/01.res.68.2.359. [DOI] [PubMed] [Google Scholar]
- 5.Lagaud GJ, Masih-Khan E, Kai S, van Breemen C, Dube GP. Influence of type II diabetes on arterial tone and endothelial function in murine mesenteric resistance arteries. J Vasc Res. 2001;38:578–589. doi: 10.1159/000051094. [DOI] [PubMed] [Google Scholar]
- 6.Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, Kaley G. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol. 2005;25:1610–1616. doi: 10.1161/01.ATV.0000172688.26838.9f. [DOI] [PubMed] [Google Scholar]
- 7.Schofield I, Malik R, Izzard A, Austin C, Heagerty A. Vascular structural and functional changes in type 2 diabetes mellitus: evidence for the roles of abnormal myogenic responsiveness and dyslipidemia. Circulation. 2002;106:3037–3043. doi: 10.1161/01.cir.0000041432.80615.a5. [DOI] [PubMed] [Google Scholar]
- 8.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 9.Lucchesi PA, Sabri A, Belmadani S, Matrougui K. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation. 2004;110:3587–3593. doi: 10.1161/01.CIR.0000148780.36121.47. [DOI] [PubMed] [Google Scholar]
- 10.Konishi A, Berk BC. Epidermal growth factor receptor transactivation is regulated by glucose in vascular smooth muscle cells. J Biol Chem. 2003 doi: 10.1074/jbc.M304913200. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda K, Kawata S, Inui Y, Higashiyama S, Matsuda Y, Igura T, Tamura S, Taniguchi N, Matsuzawa Y. High concentration of glucose increases mitogenic responsiveness to heparin-binding epidermal growth factor-like growth factor in rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17:1962–1968. doi: 10.1161/01.atv.17.10.1962. [DOI] [PubMed] [Google Scholar]
- 12.Del PratoS, Marchetti P, Bonadonna RC. Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes. 2002;51 Suppl 1:S109–S116. doi: 10.2337/diabetes.51.2007.s109. [DOI] [PubMed] [Google Scholar]
- 13.Pfeiffer A, Spranger J, Meyer-Schwickerath R, Schatz H. Growth factor alterations in advanced diabetic retinopathy: a possible role of blood retina barrier breakdown. Diabetes. 1997;46 Suppl 2:S26–S30. doi: 10.2337/diab.46.2.s26. [DOI] [PubMed] [Google Scholar]
- 14.Sharma K, Ziyadeh FN. Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes. 1995;44:1139–1146. doi: 10.2337/diab.44.10.1139. [DOI] [PubMed] [Google Scholar]
- 15.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 16.Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1:1–15. doi: 10.1016/s0047-6374(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai S, Yonekura H, Yamamoto Y, Watanabe T, Tanaka N, Li H, Rahman AK, Myint KM, Kim CH, Yamamoto H. The AGE-RAGE system and diabetic nephropathy. J Am Soc Nephrol. 2003;14:S259–S263. doi: 10.1097/01.asn.0000077414.59717.74. [DOI] [PubMed] [Google Scholar]
- 18.Miettinen PJ, Ustinov J, Ormio P, Gao R, Palgi J, Hakonen E, Juntti-Berggren L, Berggren PO, Otonkoski T. Downregulation of EGF receptor signaling in pancreatic islets causes diabetes due to impaired postnatal beta-cell growth. Diabetes. 2006;55:3299–3308. doi: 10.2337/db06-0413. [DOI] [PubMed] [Google Scholar]
- 19.Cai W, He JC, Zhu L, Lu C, Vlassara H. Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc Natl Acad Sci U S A. 2006;103:13801–13806. doi: 10.1073/pnas.0600362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 21.Palen DI, Belmadani S, Lucchesi PA, Matrougui K. Role of SHP-1, Kv.1.2, and cGMP in nitric oxide-induced ERK1/2 MAP kinase dephosphorylation in rat vascular smooth muscle cells. Cardiovasc Res. 2005;68:268–277. doi: 10.1016/j.cardiores.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Hu JJ, Ambrus A, Fossum TW, Miller MW, Humphrey JD, Wilson E. Time Courses of Growth and Remodeling of Porcine Aortic Media During Hypertension: A Quantitative Immunohistochemical Examination. J Histochem Cytochem. 2007 doi: 10.1369/jhc.7A7324.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, Kikuchi T, Lapointe N, Pojoga L, Williams GH, Ricchiuti V, Adler GK. Mineralocorticoid Receptor Antagonist Reduces Renal Injury in Rodent Models of Type 1 and 2 Diabetes Mellitus. Endocrinology. 2006 doi: 10.1210/en.2006-0944. [DOI] [PubMed] [Google Scholar]
- 24.Matrougui K, Levy BI, Henrion D. Tissue angiotensin II and endothelin-1 modulate differently the response to flow in mesenteric resistance arteries of normotensive and spontaneously hypertensive rats. Br J Pharmacol. 2000;130:521–526. doi: 10.1038/sj.bjp.0703371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matrougui K, Eskildsen-Helmond YE, Fiebeler A, Henrion D, Levy BI, Tedgui A, Mulvany MJ. Angiotensin II stimulates extracellular signal-regulated kinase activity in intact pressurized rat mesenteric resistance arteries. Hypertension. 2000;36:617–621. doi: 10.1161/01.hyp.36.4.617. [DOI] [PubMed] [Google Scholar]
- 26.Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vasc Surg. 2005;42:574–581. doi: 10.1016/j.jvs.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Bagi Z, Koller A, Kaley G. PPARgamma activation, by reducing oxidative stress, increases NO bioavailability in coronary arterioles of mice with Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2004;286:H742–H748. doi: 10.1152/ajpheart.00718.2003. [DOI] [PubMed] [Google Scholar]
- 28.Navolanic PM, Steelman LS, McCubrey JA. EGFR family signaling and its association with breast cancer development and resistance to chemotherapy (Review) Int J Oncol. 2003;22:237–252. [PubMed] [Google Scholar]
- 29.Grant S, Qiao L, Dent P. Roles of ERBB family receptor tyrosine kinases, and downstream signaling pathways, in the control of cell growth and survival. Front Biosci. 2002;7:d376–d389. doi: 10.2741/grant. [DOI] [PubMed] [Google Scholar]
- 30.Malik RA, Schofield IJ, Izzard A, Austin C, Bermann G, Heagerty AM. Effects of angiotensin type-1 receptor antagonism on small artery function in patients with type 2 diabetes mellitus. Hypertension. 2005;45:264–269. doi: 10.1161/01.HYP.0000153305.50128.a1. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald SM, Kemp-Harper BK, Tare M, Parkington HC. Role of endothelium-derived hyperpolarizing factor in endothelial dysfunction and during diabetes. Clin Exp Pharmacol Physiol. 2005;32:482–487. doi: 10.1111/j.1440-1681.2005.04216.x. [DOI] [PubMed] [Google Scholar]
- 32.Guerci B, Bohme P, Kearney-Schwartz A, Zannad F, Drouin P. Endothelial dysfunction type 2 diabetes. Part 2: altered endothelial function and the effects of treatments in type 2 diabetes mellitus. Diabetes Metab. 2001;27:436–447. [PubMed] [Google Scholar]
- 33.Guerci B, Kearney-Schwartz A, Bohme P, Zannad F, Drouin P. Endothelial dysfunction and type 2 diabetes. Part 1:physiology and methods for exploring the endothelial function. Diabetes Metab. 2001;27:425–434. [PubMed] [Google Scholar]
- 34.Avogaro A, Fadini GP, Gallo A, Pagnin E, de Kreutzenberg S. Endothelial dysfunction in type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2006;16 Suppl 1:S39–S45. doi: 10.1016/j.numecd.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 36.Komers R, Schutzer WE, Reed JF, Lindsley JN, Oyama TT, Buck DC, Mader SL, Anderson S. Altered endothelial nitric oxide synthase targeting and conformation and caveolin-1 expression in the diabetic kidney. Diabetes. 2006;55:1651–1659. doi: 10.2337/db05-1595. [DOI] [PubMed] [Google Scholar]