Abstract
[HIPTN3N]Mo(N2) (MoN2) ([HIPTN3N]3− = [(HIPTNCH2CH2)3N]3− where HIPT = 3,5-(2,4,6-i-Pr3C6H2)2C6H3) reacts with dihydrogen slowly (days) at 22 °C to yield [HIPTN3N]MoH2 (MoH2), a compound whose properties are most consistent with it being a dihydrogen complex of Mo(III). The intermediate in the slow reaction between MoN2 and H2 is proposed to be [HIPTN3N]Mo (Mo). In contrast, MoN2, MoNH3, and MoH2 are interconverted rapidly in the presence of H2, N2, and NH3, and MoH2 is the lowest energy of the three Mo compounds. Catalytic runs with MoH2 as a catalyst suggest that it is competent for reduction of N2 with protons and electrons under standard conditions. [HIPTN3N]MoH2 reacts rapidly with HD to yield a mixture of [HIPTN3N]MoH2, [HIPTN3N]MoD2, and [HIPTN3N]MoHD, and rapidly catalyzes H/D exchange between H2 and D2. MoH2 reacts readily with ethylene, PMe3, and CO to yield monoadducts. Reduction of dinitrogen to ammonia in the presence of 32 equivalents of added hydrogen (vs. Mo) is not catalytic, consistent with dihydrogen being an inhibitor of dinitrogen reduction.
Introduction
Dinitrogen is reduced to ammonia under mild conditions by nitrogenases, the most studied and best known being the FeMo nitrogenase.1 In all cases dihydrogen is also formed. The minimum amount of dihydrogen, approximately one equivalent per dinitrogen reduced, is formed in the FeMo nitrogenase system at high pressures (e.g. 50 atm N2).2 Formation of one equivalent of dihydrogen per two equivalents of ammonia amounts to a 75% yield of ammonia in terms of reducing equivalents employed. At 1 atm the yields of ammonia vary between 40 and 60%.3 How dihydrogen is formed and the extent to which its formation is coupled to the reduction of dinitrogen are still unknown. One possibility is that dihydrogen is formed through one or more metal-catalyzed reactions at sites that are different from where dinitrogen is reduced. Another view is that one equivalent of dihydrogen is formed at the same site where dinitrogen is reduced and one equivalent of dihydrogen must be formed for every dinitrogen reduced. What is known with certainty is that dihydrogen inhibits dinitrogen reduction.3 Furthermore, under a D2 atmosphere two equivalents of HD are formed during turnover of dinitrogen to ammonia, but no deuterium is incorporated into the solvent (H2O) or into the ammonia formed.1a
In 2003 it was shown that dinitrogen can be reduced catalytically to ammonia with protons and electrons by molybdenum complexes that contain the [HIPTN3N]3− ligand ([HIPTN3N]3− = [(HIPTNCH2CH2)3N]3− where HIPT is 3,5-(2,4,6-i-Pr3C6H2)2C6H3), examples being [HIPTN3N]Mo(N2), [HIPTN3N]Mo-N=NH, and [HIPTN3N]Mo(NH3).4 The only other abiological catalytic reduction of dinitrogen by protons and electrons requires molybdenum but produces hydrazine as a primary product, with ammonia being formed through a relatively common metal-catalyzed disproportionation of hydrazine to ammonia and dinitrogen.5 In the [HIPTN3N]Mo system dinitrogen binds end-on to a single Mo and is reduced by repeated alternate addition of a proton and an electron in the manner first proposed by Chatt.6 The experimental results in various triamidoamine molybdenum systems are now strongly supported by numerous theoretical studies, the most relevant being studies that employ the full [HIPTN3N]3− ligand.7 These and other theoretical results are consistent with the experimental findings and proposed mechanism.8 Ammonia is formed selectively (no hydrazine is detected) with an efficiency of 55–65% in electrons. The remaining reducing equivalents are used to form dihydrogen. The amount of ammonia increases from 63 % at 1 atm (15 psi) to 71 % at 30 psi when [HIPTN3N]Mo(N2) is employed.9 Experiments have not yet been conducted at pressures above 30 psi. The amount of dinitrogen that can be reduced is limited to ~4 equivalents under the conditions employed, in large part because the [HIPTN3N]3− ligand is removed from the metal in the presence of protons and reducing agent. It is clear that ammonia is an inhibitor as a consequence of the necessary conversion of [HIPTN3N]Mo(NH3) (MoNH3) to MoN2 via intermediate Mo(NH3)(N2) and because the equilibrium constant for this equilibrium ([MoN2][NH3]/[MoNH3][N2]) is 0.1 in benzene at 22°C.
Since dihydrogen is formed along with ammonia in the [HIPTN3N]Mo system we became interested in the dihydrogen chemistry of MoN2 and of other [HIPTN3N]Mo derivatives that have been isolated and characterized. We also wanted to understand why MoH is as efficient a catalyst precursor as [HIPTN3N]Mo complexes that are proposed to lie on the catalytic pathway, e.g., MoN2 or Mo-N=NH.10 Selected reactions that involve dihydrogen and various [HIPTN3N]Mo complexes are reported here.
Results
Synthesis of [HIPTN3N]Mo(H2)
[HIPTN3N]Mo(N2) (MoN2) reacts with dihydrogen slowly (2–3 days at 22 °C in C6D6) in solution to yield [HIPTN3N]Mo(H2) (MoH2). By 1H NMR after 48 hours the resonances corresponding to MoN2 are no longer visible if the atmosphere is removed and replaced with hydrogen after 24 hours. Addition of dinitrogen to MoH2 leads to slow reformation of MoN2. A magnetic susceptibility measurement on MoH2 in solution (Evans method; μeff = 1.4–1.5 BM) is consistent with S = ½ and therefore with MoH2 being either a d1 or a low spin d3 species. MoN2 reacts with D2 to yield MoD2 at the same rate as MoN2 reacts with H2. (A convenient method of distinguishing MoD2, MoH2, and MoHD is described later.) The ability to prepare MoD2 rules out any H/D scrambling that might result from reversible CH activation in the ligand and subsequent exchange of D for H. Attempts to prepare MoHD from MoN2 and HD resulted in formation of a 1:2:1 mixture of MoH2, MoHD and MoD2. No IR absorption assignable to some IR active MoH or MoD mode could be found for either MoH2 or MoD2 in pentane at 22°C. The absence of any readily observed MoH IR modes is more consistent with MoH2 being a Mo(III) dihydrogen complex than a Mo(V) dihydride.11
A persistent impurity present in early syntheses of MoH2 turned out to be MoCl, traces of which are formed upon oxidation of [MoN2] − with ZnCl2.12 We found that oxidation of [MoN2] − with zinc acetate instead of zinc chloride led to samples of MoN2 that were free of MoCl, and therefore samples of MoH2 that were free of MoCl. Unfortunately, we were not able to obtain crystals of MoH2 suitable for a complete X-ray structural study. Crystals obtained from a dilute pentane solution diffracted poorly, although it was clear from the preliminary structural solution that the [HIPTN3N]3− ligand was coordinated to the metal in the usual manner to give essentially a trigonal monopyramidal species, and nothing could be observed in the coordination pocket.
MoH2 also can be prepared by heating solid samples of MoN2 under an atmosphere of dihydrogen at 60 °C for one week. Traces of an unidentified yellow species appear at temperatures above 70 °C. It is difficult to push the reaction to completion at 60 °C in the solid state unless the sample of MoN2 is ground to a fine powder and the atmosphere is refreshed periodically with dihydrogen. Even then, a trace (~1 %) of MoN2 (according to NMR spectra) always seems to be present in the final product. At 60 °C the risk of some irreversible decomposition is significant, so synthesis in solution at 22 °C is the preferred method.
MoH2 is extremely sensitive towards traces of oxygen and water and toward dinitrogen, both in solution and in the solid phase. Even in the solid state samples of MoH2 under dinitrogen at −30 °C slowly form some MoN2. MoH2 also decomposes slowly in vacuo at approximately the same rate as it reacts with dinitrogen to yield MoN2. For example, after three days in vacuo virtually all of the original MoH2 has decomposed. Attempts to crystallize and identify the decomposition product or products of MoH2 have been unsuccessful. The final decomposition product does not react with dihydrogen to reform MoH2 and no free ligand is found in NMR spectra of partially decomposed samples. Multiple attempts to obtain a satisfactory elemental analysis of MoH2 failed. We conclude that MoH2 is not stable over the long term in the absence of hydrogen. Therefore, samples are best prepared as needed, although they can be stored in solution or in the solid state under 1 atm of dihydrogen. The amount of MoH2 in solution can be followed conveniently through observation of a resonance for the three equivalent para protons on the central phenyl rings in the HIPT substituents (hereafter referred to as the Hp resonance) at 5.12 ppm at 22 °C. (See below for other NMR details.) Although various studies (see later section) suggest that Mo is the intermediate in the conversion of MoH2 into MoN2, and vice versa, no evidence for formation of a stable “naked” species, Mo, has yet been obtained. It should be noted that MoN2 does not decompose to any significant degree in vacuo under conditions where MoH2 does.
MoH2 reacts readily with ethylene, CO, and PMe3. Addition of ethylene to MoH2 results in formation of Mo(C2H4),13 with only a trace of H2 (~ 1% in solution) and ethane (~33% in solution versus an internal standard) being observed, according the NMR spectra of MoH2 under an ethylene atmosphere (C6D6, 22 °C, 12h). (The amounts of H2 and ethane in the gas phase were not determined.) The reaction is complete in a much shorter time than is required if the reaction were to proceed via formation of Mo. According to these results ethylene is hydrogenated to a significant extent. Since a solution of Mo(C2H4) in C6D6 at 22 °C under a hydrogen atmosphere is unchanged for weeks, ethane must be formed when ethylene attacks MoH2. Formation of ethane is most readily explained in classic terms, i.e., reaction of ethylene with a Mo(V) dihydride to give an ethyl hydride intermediate followed by reductive elimination. The Mo(V) dihydride need not be the ground state as long as it is readily accessible, i.e., from a Mo(III) dihydrogen complex.
MoCO13 is formed immediately upon exposure of a solution of MoH2 (or a solid sample more slowly) to an atmosphere of CO. However, the amount of dihydrogen formed as a product of this reaction when it was carried out in solution or in the solid state on samples that had been exposed to vacuum for some time was found to be less than one equivalent, typically between 0.30 and 0.70 equivalents, depending on the sample’s history. However, when freshly prepared samples of MoH2 in toluene were degassed and treated with CO, 0.95±0.05, 0.86±0.08, and 0.93±0.02 equivalents of H2 were found in the atmosphere above the solution in three different experiments. Therefore MoH2 clearly contains one equivalent of hydrogen.
A solution of MoH2 turned red after one hour upon addition of trimethylphosphine and red Mo(PMe3) was isolated in good yield (55% recrystallized) after 24 hours. The NMR spectrum of Mo(PMe3) is very similar to that of MoN2 and MoCO. Again less than one equivalent of dihydrogen was found in the gas phase when typically prepared MoH2 samples in toluene were treated with several equivalents of PMe3.
The results of the reactions between MoH2 and ethylene, CO, and PMe3 suggest that only one equivalent of dihydrogen is present in MoH2. However, the speed of these reactions also suggests that these reactions do not proceed through loss of dihydrogen to give intermediate Mo, but through attack by ethylene, CO, and PMe3 on MoH2. None of these results settles the question concerning whether MoH2 is a dihydrogen complex or a classic dihydride, something in between, or something else entirely. (See later.)
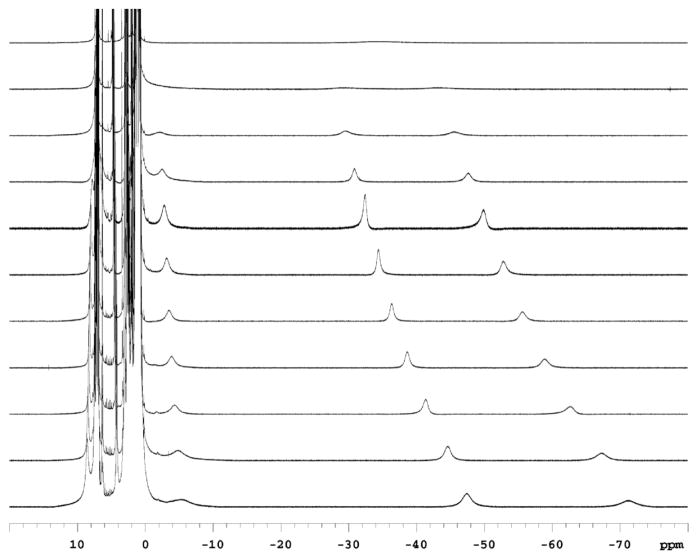
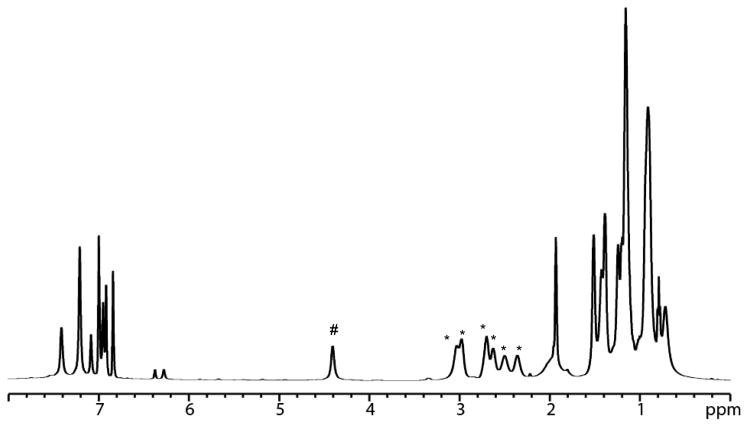
The variable temperature NMR spectrum of MoH2 is shown in Figure 1. At −60 °C four backbone methylene protons can be observed at 9 (barely visible), −4, −40, and −62 ppm as a consequence of a “locking” of the ligand backbone in one C3 conformation at low temperature, a known property of triamidoamine complexes.14 As shown in Figure 2 at −60 °C the Hp resonance is found at 4.4 ppm (#) and six isopropyl methine resonances (*) are present, consistent with only one type of HIPT substituent being present and each HIPT being in some locked, “twisted” configuration in which all methine protons are inequivalent in a given HIPT substituent. The Hp resonance remains sharp down to −90 °C. As the temperature is raised the methylene proton resonances shift as a consequence of Curie-Weiss behavior and broaden and merge as a result of an interconversion of the two possible C3 configurations of the ligand backbone to yield two backbone methylene resonances in a molecule with C3v symmetry on the NMR time scale.15 The methylene protons whose average resonance is found near −35 ppm equilibrate at approximately room temperature (see Figure 1). Above room temperature two methylene resonances emerge near −35 ppm and 3 ppm and two methine resonances are found in a ratio of 2:1, consistent with C3v symmetry on the NMR time scale and rapid rotation about the nitrogen-aryl bonds. This behavior is similar to that of other Mo(III) complexes of this type such as MoN2,12 MoCO,13 and MoNH3,16 and Mo(IV) species such as MoCl,12 [MoNH3]+,12 MoH,12 and MoMe.13 This behavior is also found for Mo(III) and Mo(IV) complexes that contain a [TMSN3N]3− or [C6F5N3N]3− ligand such as [TMSN3N]MoMe or [TMSN3N]MoCl.17,18
Figure 1.
VT NMR stack plot of MoH2 from −80 °C (bottom) to 20 °C (top) in steps of 10 °C.
Figure 2.
Proton NMR spectrum of MoH2 at −60 °C in toluene-d8 between 0 and 8 ppm. Only one resonance is observed for the HIPT Hp protons (#), which shifts with temperature. The presence of six isopropyl methine resonances (*) suggest that the six isopropyl groups in each HIPT group are inequivalent.
The value of ΔG‡ for equilibration of the methylene protons can be obtained by plotting the methylene chemical shifts versus 1/T and thereby obtaining the distance between two methylene resonances (Δνc) at Tc, the temperature of coalescence. From kc = πΔνc/√2 at Tc and kc = kBT/h exp(−ΔG‡/RT), a ΔG‡ value of 11.7 kcal/mol was obtained. Values of ΔG‡ for the C3 to C3v conversion in [TMSN3N]MoMe and [TMSN3N]MoCl were found to be 8.2 ± 0.2 kcal/mol and 9.2 ± 0.2 kcal/mol.19 For MoN2 a value of ΔG‡ = 8.5 ± 0.4 kcal/mol was found; the error is larger since the methylene resonances become relatively broad below Tc. For MoCl and MoCO no ΔG‡ could be obtained because the backbone signals were too broad or disappear into the baseline below Tc.20 The value of ΔG‡ for the C3 to C3v conversion in MoH2 is higher by ~2 kcal/mol than any other observed in a triamidoamine complex of this general type.
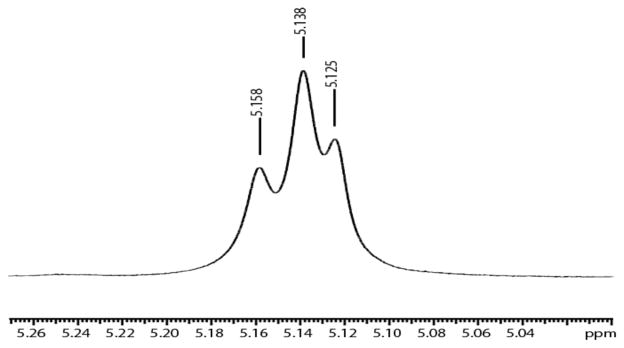
In the process of exploring MoH2, MoHD, and MoD2 species we noticed that the Hp protons could be observed (at 500 MHz) at 5.158 ppm for MoD2, 5.138 ppm for MoHD, and 5.125 ppm for MoH2 at 22 °C (Figure 3). Observation of only one MoHD resonance is consistent with a species that has C3v symmetry on the NMR time scale.
Figure 3.
Resonances for the Hp protons in a 1: 2: 1 mixture of MoD2 (at 5.158 ppm), MoHD (at 5.138 ppm), and MoH2 (at 5.125 ppm) at 22 °C. (Relative intensities were determined through modeling using gNMR.)
Cyclic voltammetry studies on MoH2 in PhF revealed no oxidation or reduction wave for MoH2 between + 1 and −2.8 V.
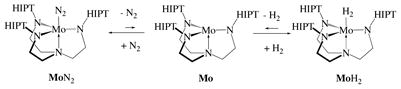
Details of reactions involving MoN2 or MoH2
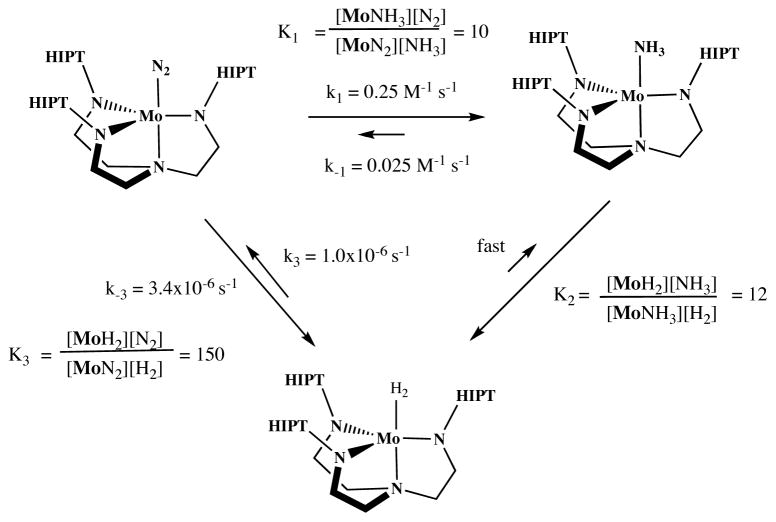
The reaction between MoN2 and H2 in C6D6 was followed in proton NMR studies with mesitylene as an internal standard at pressures between 1/3 and 3 atm. The rate of the reaction does not depend upon H2 pressure in this range. The rate constant (k−3, Scheme 1) was found to be 3.4 × 10−6 s−1 (t1/2 = 57 h). Therefore, we propose that the exchange of N2 for H2 consists of rate-limiting loss of dinitrogen to yield unobservable trigonal monopyramidal Mo (equation 1). Formation of MoH2 from MoN2 appears to be slower than exchange of 15N2 for N2 in Mo(15N2)
Scheme 1.
Exchange reactions relating MoN2, MoNH3, and MoH2.
 |
(1) |
(t1/2 = 35 h) in the presence of a large excess of normal N2, a reaction that also is not pressure dependent and is proposed to take place via formation of intermediate Mo.9 One would not expect such a discrepancy (t1/2 = 57 h vs. 35 h) to be ascribable to an 15N isotope effect, although the reaction of MoN2 with 15N2 has not been done. It should be noted that exchange of 15N2 in [HTBTN3N]Mo(15N2) (HTBT = hexa-t-butylterphenyl) is slower by a factor of 20 than what it is in Mo15N2,10 even though the νN2 values are identical in the two species. Details concerning dinitrogen exchange in various circumstances remain to be resolved. For the moment we will assume that the reaction of MoN2 with H2 proceeds via the Mo intermediate. The rate of reaction of MoN2 with H2 is the same in the presence of [Collidinium][BArF4] (ArF = 3,5-(CF3)2C6H3) and collidine = 2,4,6-NC5H2Me3) or CrCp*2.
The reaction between MoH2 and N2 in C6D6 was followed by means of proton NMR with mesitylene as an internal standard at pressures less than or greater than one atmosphere. The reaction between MoH2 and N2 does not depend on N2 pressure, and the rate limiting step therefore is loss of H2 from MoH2. The reaction between MoH2 and N2 is relatively slow (t1/2 = 8 days). From a plot of ln[MoH2] versus time over a period of six days (five points) a value of k3 = 1.0 × 10−6 s−1 was obtained (cf. k−3 = 3.4 × 10−6 s−1 for the reaction between MoN2 and H2). The equilibrium constant for the reaction of MoN2 with hydrogen, Keq = K3 = [MoH2][N2]/[MoN2][H2], was found to be ~150 in C6D6 at 22 °C, i.e., dihydrogen exchanges with dinitrogen in MoN2 about ~3x faster than dinitrogen exchanges with dihydrogen in MoH2. Since the equilibrium between MoN2, MoH2, N2, and H2 is reached relatively slowly (days) at one atmosphere, direct interconversion of MoN2 and MoH2 almost certainly can be neglected during the time of a typical catalytic reduction of N2 (6 h).4a These results suggest also that protonation of MoH to give [MoH2]+, reduction of [MoH2]+ to MoH2, and conversion of MoH2 to MoN2 cannot be the mechanism by which MoH is drawn into a successful catalytic for dinitrogen reduction.10
In contrast to the slow reaction between MoN2 and H2 (and vice versa), MoNH3 reacts with H2 within minutes to yield MoH2. After 10 minutes, conversion of MoNH3 to MoH2 under one atmosphere of H2 in C6D6 was more than 80% complete according to NMR spectra. The reverse reaction is also fast. Roughly 4 equivalents of NH3 (dried over a freshly prepared sodium mirror) were added to a C6D6 solution of MoH2 from which all N2 had been removed. Since the concentrations of NH3, H2, MoNH3, and MoH2 can all be determined in solution, Keq (= K2 = [MoH2][NH3]/[MoNH3][H2]) for the reaction between MoNH3, and MoH2 could be determined; K2 was found to be ~12 in C6D6 at 22 °C. Since K1 = [MoNH3][N2]/[MoN2][NH3] = 10,16 K3 (which equals K1K2) therefore should be ~120, consistent with the measured value for K3 (150; see above).
A 1:5 mixture of MoNH3 and MoH2 in C6D6 under 1 atm of dinitrogen was examined by proton NMR. Almost all MoNH3 had disappeared after five minutes and a 2:1 mixture of MoH2 and MoN2 was formed. This result is to be expected since a large excess of dinitrogen is present and MoH2 is the most stable of the three adducts. The relationships between MoN2, MoNH3, and MoH2 are shown in Scheme 1. The two reactions that involve ammonia are second order overall, while the exchange of hydrogen and nitrogen (in the absence of ammonia) is first order overall.
Catalytic runs with MoH2 as a catalyst suggest that it is competent for reduction of N2 with protons and electrons under standard conditions.4 Ammonia is formed in 52% yield (from N2) relative to reducing equivalents added (cf. 60–65% yield from N2 with other catalysts such as MoN2.) The lower yield of ammonia, along with the data shown in Scheme 1 and discussed above could be taken as evidence that dihydrogen inhibits reduction of dinitrogen, i.e., ~1 equivalent of dihydrogen is present from the beginning of the experiment. Both ammonia and dihydrogen remove MoN2 from the system to yield MoNH3 and MoH2. Therefore turnover to give ammonia (relative to dihydrogen) should slow as ammonia and dihydrogen are formed. Since the rate constants for MoH2→MoNH3 and MoNH3 →MoN2 are of the order or faster than the time frame of the catalytic reduction (6 hours), a catalytic reaction in which MoH2 is present in the system is viable.
Catalytic runs employing Mo≡N were carried out in a modified reactor designed to allow a small amount of gas to be injected before a standard reduction. After demonstrating that the reactor can be used successfully outside the inert atmosphere box in a standard run, including one in which additional dinitrogen was injected, two runs were carried out after injection of 5% and 10% of dihydrogen. These runs produced approximately one equivalent of ammonia from Mo≡N, but no ammonia from dinitrogen. Therefore we conclude that a large amount of dihydrogen (5% is approximately 32x the amount of Mo) prevents formation of any ammonia from dinitrogen. It remains to be seen whether relatively small amounts of dihydrogen also lead to observable inhibition, as proposed in the experiment noted immediately above.
Reactions that involve HD or D2
When a solution that contains 0.1% of MoH2 is placed under a 1:1 mixture of H2 and D2, an equilibrium 1:2:1 ratio of H2, HD, and D2 is formed in 2 h (equation 2). When a solution of MoH2 is exposed to a large excess of HD a 1:2:1 ratio of H2, HD, and D2 also forms rapidly. This fast H/D exchange process is the reason why it is not possible to obtain the MoHD complex; only a mixture of MoH2, MoHD, and MoD2 is formed, as noted earlier. An attempt to prepare MoHD from MoN2 and HD in the solid phase also yielded a 1:2:1 mixture of MoH2, MoHD, and MoD2, but relatively inefficiently; one week at 60 °C was required when starting from MoN2.
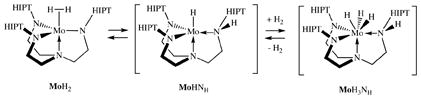
Exactly how H-H, D-D, and H-D scission (equation 2) is accomplished is not known. It seems doubtful that hydrogen could attack the metal in a Mo(III) dihydrogen complex or a Mo(V) dihydride to give some sort of “MoH4” species, since the last remaining orbital of the three available to bind H2 in the coordination pocket of Mo, or to form a dihydride, would contain a single electron. One interesting possibility is that scission is accomplished through a heterolytic cleavage to give an intermediate in which H or D is bound to one amido nitrogen, as shown for MoH2 in equation 3; reversible oxidation of MoHNH to a Mo(V) trihydride, MoH3NH, would then
 |
(3) |
 |
(4) |
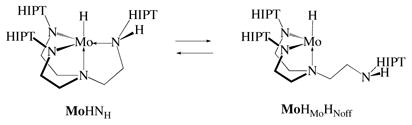
result in H/D scrambling. This mechanism is sufficient to account for rapid exchange if MoHNH is formed rapidly and reversibly from MoH2 and if MoHNH reacts rapidly and reversibly with HxD2−x. Dissociation of one arm in MoHNH to give MoHMoHNoff (equation 4) would also open positions at the metal for coordination and exchange of H and D, as well as H/D exchange reactions that are bimolecular in Mo. Whether formation of MoHMoHNoff would be fast enough to account for the observed rapid HD exchange between H2, D2, and HD is not known. In any case, we can at least have some confidence that heterolytic splitting of dihydrogen is a requirement for rapid H/D exchange, either via MoHNH or MoHMoHNoff. Heterolytic splitting of dihydrogen or especially formation of MoHMoHNoff (equation 4) could plausibly be involved in processes that lead to decomposition of MoH2 in the absence of hydrogen. It does not seem likely that MoHNH is the ground state, however, since we have not been able to find a Mo-H or N-H stretch in the solution IR spectrum.
As noted earlier, treatment of MoH2 in solution with a large excess of D2 results in formation of MoD2. The reaction does not depend upon the pressure of D2 at the pressures explored (up to 4 atm) and has a half-life of ~24 hours at one atmosphere of D2 (k = 8.0 × 10−6 s−1). The reverse reaction has the same k within one significant figure, so the isotope effect, if any, is too small to measure with any accuracy under the conditions employed. Since the reaction between MoH2 and N2 proceeds with a first order k−3 = 1.0×10−6 s−1, while the reaction between MoH2 and D2 to form MoD2 proceeds with a first order k = 8.0 × 10−6 s−1, Mo cannot be a common intermediate in the two reactions. In the presence of a large excess of D2 only MoD2 is formed from MoH2 through mass action. We propose that formation of MoD2 in a reaction between MoH2 and a large amount of D2 proceeds via a mechanism that is the same as that shown in equation 3.
A solution that contains MoH2 and MoD2 under argon yields MoHD after several days. Equilibrium is reached with a half reaction time of ~28 hours for a 24 mM solution of total molybdenum species. The rate of MoHD formation from MoH2 and MoD2 was found to increase as the molybdenum concentration increased. If we assume that the exchange reaction is bimolecular in Mo, then an apparent rate constant of k−1 = 6.8 × 10−4 M−1 s−1 can be derived for the disappearance of MoHD.21 One could propose that a reaction that is actually bimolecular in Mo is required, e.g., MoHMoHNoff (equation 4) could form a {Mo(μ2-H)}2 dimer, {MoHMoHNoff}2, which would result in H/D exchange. The steric feasibility of forming {MoHMoHNoff}2 is supported through molecular mechanics simulations (Spartan). It remains to be seen whether this “slow” H/D exchange reaction actually could take place via loss of some H2 from MoH2 (or D2 from MoD2) followed by HD exchange as shown in equation 3 under circumstances where the rate depends upon Mo concentration but does not involve a reaction that is actually bimolecular in Mo. A more worrisome possibility, especially since the long term stability of MoH2 is not secure, is that this relatively slow “background” H/D exchange reaction could involve products of decomposition of MoH2. Therefore the precise nature of the slow background H/D reaction remains in doubt.
Discussion and Conclusions
One of the important findings from this work is that MoN2, MoNH3, and MoH2 are in ready equilibrium in the presence of H2, N2, and NH3, with MoH2 being the lowest energy of the three species (Scheme 1). Therefore, both ammonia and dihydrogen should inhibit turnover, with dihydrogen being the most effective inhibitor since it converts MoNH3 into MoH2, which results in conversion of more MoN2 into MoNH3. Dihydrogen does not directly compete with dinitrogen for the Mo(III) center since interconversion of MoN2 and MoH2 is slow in the presence of only dinitrogen and dihydrogen. In a typical catalytic reaction the amount of ammonia and dihydrogen relative to dinitrogen is miniscule, so there is some question as to how much the MoN2 concentration decreases under catalytic conditions as NH3 and H2 are formed and therefore the degree to which turnover actually is inhibited by NH3 and H2. Loss of ligand from the metal under catalytic conditions is likely to be the more serious and ultimately disasterous problem.
The second important finding is that H/D exchange at MoH2 is facile. Therefore if a catalytic dinitrogen reduction were to be carried out in the presence of a large amount of D2 relative to Mo, dinitrogen reduction should be inhibited and twice as much HD should be formed as H2 is formed in the absence of D2. Both inhibition (by H2 or D2) and formation of HD (in the presence of D2) are observed in the natural FeMo system during turnover.1 Further experiments are planned that are aimed at investigating inhibition of dinitrogen reduction and possible formation of HD in the presence of D2 in the system that we have described here.
The mechanism by which MoH functions as a catalyst precursor has not been settled as a consequence of the studies reported here. Since MoH is known to react with lutidinium to yield [Mo(Lut)]+,16 protonation of MoH to give [MoH2]+, followed by reduction of [MoH2]+ to MoH2 is an insufficient explanation, since the direct reaction between MoH2 and dinitrogen to give MoN2 we now know is slow. Exchange of dihydrogen in [MoH2]+ for dinitrogen to yield [MoN2]+, followed by reduction of [MoN2]+ to MoN2 is still a viable option.
Reactions that involve H2 or D2 have been studied to some degree in triamido amine systems other than the one described here. For example, MoH was found to react slowly with D2 to yield MoD and HD,16 while [(TMSNCH2CH2)3N]WH was found to react slowly with H2 to give isolable [(TMSNCH2CH2)3N]WH3.22 Finally, relatively slow H/D exchange in [(C6F5NCH2CH2)3N]ReH2 was observed upon heating samples in solution under mixtures of H2 and D2.15 Exchange in the Re system was proposed to involve formation of intermediate 18 electron [(CH2CH2NC6F5)2NCH2CH2NHC6F5]ReH, followed by dissociation of the amine donor in the CH2CH2NHC6F5 arm, thereby exposing the 16e metal center to oxidative addition to Re(III) to give a Re(V) trihydride. “Arm-off” species also have been observed in triamidoamine systems. For example, addition of MeMgCl to {[N(CH2CH2NTMS)2(CH2CH2NMe2)]Mo(NNTMS)}OTf produced structurally characterized [N(CH2CH2NTMS)2(CH2CH2NMe2)]Mo(CH3)(NNTMS) in which the amine donor in the CH2CH2NMe2 arm was not coordinated to the metal.23 Experimental evidence suggests that an amido nitrogen in MoN2 is protonated to give cationic {[(HIPTNHCH2CH2)N(CH2CH2NHIPT)2]MoN2}+, which is much more susceptible to reduction to [(HIPTNHCH2CH2)N(CH2CH2NHIPT)2]MoN2, and that the ligand eventually is removed from the metal, thereby limiting catalytic turnover to approximately four under the conditions employed. Therefore, there is some precedent for formation of MoNMoHNoff at some stage (equation 2).
Splitting of dihydrogen heterolytically across a metal-ligand bond (often a Ru-N bond24) has been proposed or observed in many circumstances. For example, in Noyori-type Ru-based ketone hydrogenation catalysts the dihydrogen(amido) complexes in the catalytic cycle are calculated to be too high in energy to be observed; only the hydride(amine) forms are observed.25 External bases can act as a catalyst proton shuttle of a proton between the metal and the ligand.26 Heterolytic splitting has also been proposed for complexes that contain metals other than ruthenium.27 These findings provide support for the proposed heterolytic splitting of dihydrogen across a Mo-Namido bond to form MoHNH (equation 4). Evidence suggests that this heterolytic splitting is relatively rapid on the chemical time scale (allowing rapid H/D exchange at Mo), and may even be rapid on the NMR time scale at room temperature. However, as mentioned earlier, (negative) IR data do not support the ground state being MoHNH. Therefore, we still favor the proposal that MoH2 is a Mo(III) dihydrogen complex, a Mo(V) dihydride, or something in between, and that MoHNH can be formed in a rapid equilibrium. Formation of MoHNH seems to be a likely contributor to the instability of MoH2 under some conditions.
There is circumstantial evidence that other types of “arm-off” species might be formed under catalytic conditions since an amido nitrogen in MoN2 is known to be protonated and the ligand ultimately stripped from the metal. Among the puzzling results that might be explained through addition of H to an amido nitrogen and formation of “arm-off” species are the following: (i) Mo-N=NH is known to decompose slowly to MoH;12a (ii) Mo=NH is known to decompose to yield a mixture of Mo≡N and MoNH2;16 (iii) [Hybrid]Mo-N=NH species, in which the “hybrid” ligand is significantly smaller than [HIPTN3N]3−, are relatively unstable toward 3,5-lutidine, the conjugate base of the acid employed in a typical catalytic reduction of dinitrogen; hydrogen is evolved and [hybrid]MoN2 species are formed; (iv) [Hybrid]Mo-15N=15NH species have been observed to exchange with N2 to yield [Hybrid]Mo-N=NH species at a rate slightly faster than the rate at which they decompose to yield [hybrid]MoN2.28 Species in which an H is present on an amido nitrogen, whether the resulting amine donor is still coordinated to the metal or not, are still possible intermediates during catalytic turnover, although formation of “arm-off” species is a logical first step that ultimately leads to catalyst decomposition.
Experimental section
General
All manipulations of air- and moisture-sensitive compounds were carried out by standard Schlenk and glovebox techniques under an atmosphere of nitrogen or argon using flame- and oven dried glassware, including NMR tubes. Ether, pentane, methylene chloride and toluene were purged with nitrogen, passed through activated alumina columns, and freeze-pumpthaw degassed three times if necessary; THF, heptane and C6D6 were distilled from a dark purple Na/benzophenone ketyl solutions; PhF was distilled from P2O5 under N2 and THF-d8 was used as received; all dried and deoxygenated solvents were stored over molecular sieves in a nitrogen or argon-filled glovebox. LiN(TMS)2 (sublimed) and [CoCp*2]PF6 were used as received, unless indicated otherwise. CrCp*2 was prepared via literature procedures using LiCp* instead of NaCp*.29 CoCp*2 was prepared from [CoCp*2]PF6 by reduction with crushed sodium and purified by sublimation.29 [2,4,6-collidinium]BArF was prepared in a procedure similar to [2,6-lutidinium]BArF. Syntheses of MoN2, MoH, MoCO, Mo(C2H4) and [MoNH3]+ have been published.12,13,16 All metal complexes were stored in the dark, under N2 or Ar at −35 °C. All NMR spectra were recorded on a Varian Mercury 300 or 500 MHz spectrometer and referenced to the residual protio solvent peaks.
Synthesis of [HIPTN3N]MoH2
MoN2 (379 mg) was dissolved in 15 mL toluene in the inert atmosphere box and the solution was freeze-pump-thawed 4 times in a Schlenk flask. H2 was introduced into the flask and the solution was heated for two days at 70 °C. The atmosphere in the flask was refreshed repeatedly by freeze-pump-thawing the solution several times and refilling the flask with H2. The solvent was evaporated from the mixture. The residue was extracted with pentane under an argon atmosphere and the mixture was quickly passed through a frit. A brown powder precipitated from the filtrate. The product was filtered off; yield 225 mg of MoH2 (60%): 1H NMR (C6D6, 20°C) δ 7.28 (s, 12H, 3,5,3″,5″-H), 5.15 (s, 3H, 2′-H), 2.98 (septet, JHH = 6.9 Hz, 6H, 4,4″-CHMe2), 2.88 (br septet, 12H, 2,6,2″,6″-CHMe2), 1.39 (d, JHH = 6.9 Hz, 36H, 4,4″-CH(CH3)2), 1.3 (very br s, 36H, 2,6,2″,6″-CH(CH3)2), 1.17 (br s, 36H,,6,2″,6″-CH(CH3)2). The 4′,6′-H resonance has not been observed, and the NCH2 signals are not observed at room temperature due to coalescence. In spite of many attempts at elemental analysis of MoH2 no satisfactory and repeatable results were obtained.
Alternative synthesis of [HIPTN3N]MoH2
A sample of very finely crushed MoN2 was evacuated and placed under an H2 atmosphere. The solid sample was heated at 60 °C for a week, during which time the atmosphere was refreshed several times with H2, leading to formation of MoH2. The NMR spectrum of the material from this reaction is identical to that above. MoD2 was prepared using the same procedure using D2 instead of H2.
Quantitation of hydrogen in [HIPTN3N]MoH2
A Schlenk flask of known volume is loaded with 31 mg of [HIPTN3N]MoN2 in 1.0 mL of toluene. The flask is then sealed with a rubber septum. A separate 500 mL Schlenk flask is then filled with H2 and frozen in liquid N2 for 20 minutes. The Schlenk flask containing the [HIPTN3N]MoN2 solution is freeze/pump/thaw degassed and connected to the Schlenk bomb containing H2 still immersed in liquid N2. The two are allowed to equilibrate for 10 minutes and isolated from one another. The is repeated after 24 and 48 hours. By 1H NMR the resonances corresponding to MoN2 are no longer visible within 48 hours. After 72 hours the sample of [HIPTN3N]MoH2 is frozen in liquid N2 and purged with a flow of N2 gas for 10 minutes. The solution was then removed from liquid N2 and allowed to warm under a flow of N2 gas for an additional 10 minutes. The sample was then sealed and 2.0 mL of CO was injected to form [HIPTN3N]MoCO and hydrogen was measured in the atmosphere after five minutes. Four GC measurements employing 20 μL samples yielded an average of 0.95 ± 0.05 equivalents of H2 per metal center. A run employing 36 mg of [HIPTN3N]MoH2 gave an average (four measurements) of 0.86 ± 0.08 equivalents of H2, while a third employing 61 mg of [HIPTN3N]MoH2 gave an average of 0.93 ± 0.02 equivalents of H2 (five measurements).
Gas chromatographic quantitation of hydrogen
An HP 6890 Series GC equipped with a 50 m × 0.530 mm, 25 μm, HP MoleSieve column and a TCD (Thermal Conductivity Detector) is used for the detection and quantification of H2. An injection temperature of 150° C, an oven temperature of 200° C, and a detector temperature of 250° C are used with a flow rate of carrier gas (N2) at 8 psi or 5 mL/min. Under these conditions with sample volumes of 20 μL, H2 is detectable at 1.12 minutes. The sample volumes were corrected for the partial pressure of toluene and the volume of CO added.
Synthesis of [HIPTN3N]Mo(PMe3)
MoH2 (220 mg) was dissolved in toluene and treated with a slight excess (30 μL) of PMe3. The reaction mixture turned red within an hour. After 24 h the toluene was removed in vacuo and the red product was recrystallized from pentane to give 125 mg of Mo(PMe3) (55 % yield): 1H NMR (C6D6, 20 °C) δ 19.2 (br s, 9H, PMe3), 15.8 (br s, 6H, NCH2), 6.55 (s, 12H, 3,5,3″,5″-H), 2.43 (br septet, 6H, 4,4″-CHMe2), 1.8 (br s, 36H, 2,6,2″,6″-CH(CH3)2), 0.94 (d, 36H, 4,4″-CH(CH3)2), 0.88 (br s, 15H, 2,6,2″,6″-CHMe2 and 2′-H), 0.55 (br s, 36H, 2,6,2″,6″-CH(CH3)2), −7.3 (br s, 6H, 4′,6′-H), −25.9 (br s, 6H, NCH2). Anal. Calcd for C117H168MoN4P: C, 79.91; H, 9.69; N, 5.46. Found: C, 79.50; H, 9.87; N, 5.06.
General procedures for reductions of dinitrogen in the presence of dihydrogen
A catalytic apparatus is set up in the glove box according to previously published methods4a using a receiving flask fitted with a side arm containing a 0–4 K Teflon plug, and a rubber septum for gas sampling or addition. The apparatus is then taken from the glove box and connected to a Schlenk line where the internal pressure is equilibrated to atmosphere under an N2 flow. The apparatus is then removed from the line and attached to the syringe drive. A pressure-Lok® syringe is flushed three times and filled with H2 drawn from a flask under a constant H2 purge. The syringe is locked and brought to the catalytic apparatus where it is opened and H2 slowly driven out until the desired volume is reached. In a continuous motion the syringe is injected into the septum being careful not to over-pressurize the side arm. The plug is then opened and the desired volume of H2 is injected into the system and mixed by pumping the syringe three times. The system is then allowed to equilibrate for 10 minutes, and the Teflon stopper is then closed. After 10 minutes the syringe is started and the run completed as previously described.4a Analysis of ammonia is also done by the previously reported indophenol method.4a
Catalytic reduction of dinitrogen employing the modified reactor
The catalytic apparatus described above had a total volume of 82.0 mL. To a receiving flask containing 9.0 mg (5.31 μmol) of [HIPTN3N]MoN and 321 mg (0.325 mmol) of Collidinium{BAr′4}1.0 mL of heptane is added. The syringe barrel is then loaded with 81 mg (0.249 mmol) of Cp2 *Cr in 9.1 mL of heptane. With the apparatus having a remaining headspace of 72.9 mL, 4.5 mL (0.200 mmol) of N2 was added to the system as described above. Addition of 4.5 mL of N2 increases the internal pressure of the apparatus by 6.3%. Upon completion of the catalytic run and indophenol analysis 6.4 equivalents of ammonia were obtained relative to molybdenum.
Catalytic reduction of dinitrogen in the presence of dihydrogen
To a catalytic reaction as described above 4.5 mL (0.200 mmol) of H2 were added. Addition of 4.5 mL (0.200 mmol) of H2 increased the internal pressure of the apparatus by 6.3 %, and H2 accounted for 6.3 % of the N2/H2 mixture, or 32 equivalents of H2 versus Mo. Upon completion of the catalytic run and indophenol analysis an average of 0.87 equivalents of ammonia were obtained relative to molybdenum, or 0 from dinitrogen.
In a second run 9.0 mL (0.400 mmol) of H2 were added. The internal pressure increased by 12.3% and H2 to give a N2/H2 mixture in which 11.6 % consisted of dihydrogen, or 65 equivalents of H2 versus Mo. Upon completion and analysis of the catalytic run 0.89 equivalents of ammonia were obtained relative to molybdenum, or 0 from dinitrogen.
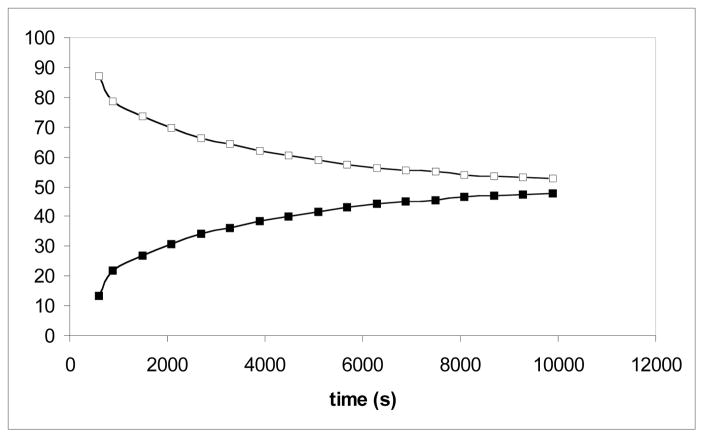
Figure 4.
Conversion of a mixture of H2 plus D2 (□) into HD (■) in C6D6 in the presence of 0.1 % MoH2 as a catalyst in C6D6 (y axis = %).
Acknowledgments
Research support from the National Institutes of Health (GM 31978) is gratefully acknowledged. D.G.H.H. thanks the Netherlands Organization for Scientific Research (NWO-CW) for a postdoctoral fellowship and Prof. Budzelaar for a copy of gNMR. R.R.S. thanks R. H. Morris for consultations concerning heterolytic splitting of dihydrogen. We thank Nathaniel Szymczak in the group of Professor Jonas Peters for assistance with dihydrogen measurements.
References
- 1.(a) Burgess BK, Lowe DJ. Chem Rev. 1996;96:2983. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]; (b) Eady RR. Chem Rev. 1996;96:3013. doi: 10.1021/cr950057h. [DOI] [PubMed] [Google Scholar]; (c) Howard JB, Rees DC. Chem Rev. 1996;96:2965. doi: 10.1021/cr9500545. [DOI] [PubMed] [Google Scholar]; (d) Dos Santos PC, Igarashi RY, Lee H-I, Hoffman BM, Seefeldt LC, Dean DR. Acc Chem Res. 2005;38:208. doi: 10.1021/ar040050z. and references therein. [DOI] [PubMed] [Google Scholar]; (e) Hardy RWF, Bottomley F, Burns RC. A Treatise on Dinitrogen Fixation. Wiley-Interscience; New York: 1979. [Google Scholar]; (f) Veeger C, Newton WE. Advances in Nitrogen Fixation Research. Dr. W. Junk/Martinus Nijhoff; Boston: 1984. [Google Scholar]; (g) Coughlan MP. Molybdenum and Molybdenum-containing Enzymes. Pergamon; New York: 1980. [Google Scholar]; (h) Burgess BK. Chem Rev. 1990;90:1377. [Google Scholar]
- 2.Simpson FB, Burris RH. Science. 1984;224:1095. doi: 10.1126/science.6585956. [DOI] [PubMed] [Google Scholar]
- 3.(a) Wherland S, Burgess BK, Stiefel EI, Newton WE. Biochemistry. 1981;20:5132. doi: 10.1021/bi00521a006. [DOI] [PubMed] [Google Scholar]; (b) Burgess BK, Wherland S, Newton WE, Stiefel EI. Biochemistry. 1981;20:5140. doi: 10.1021/bi00521a007. [DOI] [PubMed] [Google Scholar]; (c) Fisher K, Dilworth MJ, Newton WE. Biochemistry. 2000;39:15570. doi: 10.1021/bi0017834. [DOI] [PubMed] [Google Scholar]
- 4.(a) Yandulov DV, Schrock RR. Science. 2003;301:76. doi: 10.1126/science.1085326. [DOI] [PubMed] [Google Scholar]; (b) Schrock RR. Acc Chem Res. 2005;38:955. doi: 10.1021/ar0501121. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Schrock RR. Chem Commun. 2003:2389. doi: 10.1039/b307784p. [DOI] [PubMed] [Google Scholar]
- 5.(a) Bazhenova TA, Shilov AE. Coord Chem Rev. 1995;144:69. [Google Scholar]; (b) Shilov AE. Russ Chem Bull Int Ed. 2003;52:2555. [Google Scholar]
- 6.Chatt J, Dilworth JR, Richards RL. Chem Rev. 1978;78:589. [Google Scholar]
- 7.(a) Reiher M, Le Guennic B, Kirchner B. Inorg Chem. 2005;44:9640. doi: 10.1021/ic0517568. [DOI] [PubMed] [Google Scholar]; (b) Schenk S, Le Guennic B, Kirchner B, Reiher M. Inorg Chem. 2008;47:3634. doi: 10.1021/ic702083p. [DOI] [PubMed] [Google Scholar]; (C) Schenk S, Kirchner B, Reiher M. Chem Eur J. 2009;15:5073. doi: 10.1002/chem.200802438. [DOI] [PubMed] [Google Scholar]
- 8.Schrock RR. Angew Chem Int Ed. 2008;47:5512. doi: 10.1002/anie.200705246. [DOI] [PubMed] [Google Scholar]
- 9.Weare WW, Dai X, Byrnes MJ, Chin JM, Schrock RR, Müller P. Proc Nat Acad Sci. 2006;103:17099. doi: 10.1073/pnas.0602778103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritleng V, Yandulov DV, Weare WW, Schrock RR, Hock AR, Davis WM. J Am Chem Soc. 2004;126:6150. doi: 10.1021/ja0306415. [DOI] [PubMed] [Google Scholar]
- 11.(a) Morris RH. Coord Chem Rev. 2008;252:2381. [Google Scholar]; (b) Heinekey DM, Oldham WM., Jr Chem Rev. 1993;93:913. [Google Scholar]; (c) Heinekey DM, Lledos A, Lluch Jose M. Chem Soc Rev. 2004;33:175. doi: 10.1039/b304879a. [DOI] [PubMed] [Google Scholar]
- 12.(a) Yandulov DV, Schrock RR, Rheingold AL, Ceccarelli C, Davis WM. Inorg Chem. 2003;42:796. doi: 10.1021/ic020505l. [DOI] [PubMed] [Google Scholar]; (b) Yandulov DV, Schrock RR. J Am Chem Soc. 2002;124:6253. doi: 10.1021/ja020186x. [DOI] [PubMed] [Google Scholar]
- 13.Byrnes MJ, Dai X, Schrock RR, Hock AS, Müller P. Organometallics. 2005;24:4437. [Google Scholar]
- 14.Schrock RR. Acc Chem Res. 1997;30:9. [Google Scholar]
- 15.Reid SM, Neuner B, Schrock RR, Davis WM. Organometallics. 1998;17:4077. [Google Scholar]
- 16.Yandulov DV, Schrock RR. Inorg Chem. 2005;44:1103. doi: 10.1021/ic040095w. [DOI] [PubMed] [Google Scholar]
- 17.Schrock RR, Seidel SW, Mösch-Zanetti NC, Shih KY, O’Donoghue MB, Davis WM, Reiff WM. J Am Chem Soc. 1997;119:11876. [Google Scholar]
- 18.(a) Greco GE, O’Donoghue MB, Seidel SW, Davis WM, Schrock RR. Organometallics. 2000;19:1132. [Google Scholar]; (b) Kol M, Schrock RR, Kempe R, Davis WM. J Am Chem Soc. 1994;116:4382. [Google Scholar]; (c) Seidel SW, Schrock RR, Davis WM. Organometallics. 1998;17:1058. [Google Scholar]; (d) Mösch-Zanetti N, Schrock RR, Davis WM, Wanninger K, Seidel SW, O’Donoghue MB. J Am Chem Soc. 1997;119:11037. [Google Scholar]
- 19.Schrock RR, Seidel SW, Mösch-Zanetti NC, Shih KY, O’Donoghue MB, Davis WM, Reiff WM. J Am Chem Soc. 1997;119:11876. [Google Scholar]
- 20.The coalescence temperatures for N3NMo species other than MoH2 lie between −30 °C and −40 °C.
- 21.Moore JW, Pearson RG. Kinetics and Mechanism. 3. John Wiley & Sons; New York: 1981. (b) From the equilibrium rate law, ln {x[ab−(a+b)xe]+abxe/ab(xe−x)}= kt{2ab− (a+b)xe}/xe, where x equals the amounts of MoH2 and MoD2, 2x equals the amount of MoHD that is formed, a is the starting concentration of MoH2, b is the starting concentration MoD2, and xe is x at equilibrium, v1 = k1[MoH2][MoD2] and v−1 = k−1[MoHD]2, k−1 = 6.8 × 10−4 M−1 s−1 was determined. Since K = [MoHD]2/[MoH2][MoD2] = 4 = k1/k−1, k1 = 2.7 × 10−3 M−1 s−1.
- 22.Dobbs DA, Schrock RR, Davis WM. Inorg Chim Acta. 1997;263:171. [Google Scholar]
- 23.O’Donoghue MB, Davis WM, Schrock RR. Inorg Chem. 1998;37:5149. [Google Scholar]
- 24.Clapham SE, Hadzovic A, Morris RH. Coord Chem Rev. 2004;248:2201. [Google Scholar]
- 25.Abdur-Rashid K, Clapham SE, Hadzovic A, Harvey JN, Lough AJ, Morris RH. J Am Chem Soc. 2002;124:15104. doi: 10.1021/ja016817p. [DOI] [PubMed] [Google Scholar]
- 26.(a) Ito M, Hirakawa M, Murata K, Ikariya T. Organometallics. 2001;20:379. [Google Scholar]; (b) Hedberg C, Källström K, Arvidsson PI, Brandt P, Andersson PG. J Am Chem Soc. 2005;127:15083. doi: 10.1021/ja051920q. [DOI] [PubMed] [Google Scholar]; (c) Hadzovic A, MacLaughlin CM, Song D, Morris RH. Organometallics. 2007;26:5987. [Google Scholar]; (d) Maire P, Büttner T, Breher F, Le Floch P, Grützmacher H. Angew Chem Int Ed. 2005;44:6318. doi: 10.1002/anie.200500773. [DOI] [PubMed] [Google Scholar]
- 27.(a) Koelliker R, Milstein D. J Am Chem Soc. 1991;113:8524. [Google Scholar]; (b) Crabtree RH, Lavin M, Bonneviot L. J Am Chem Soc. 1986;108:4032. [Google Scholar]; (c) Schlaf M, Morris RH. J Chem Soc, Chem Commun. 1995:625. [Google Scholar]; (d) Schlaf M, Lough AJ, Morris RH. Organometallics. 1996;15:4423. [Google Scholar]; (e) Jessop PG, Morris RH. Inorg Chem. 1993;32:2236. [Google Scholar]; (f) Lough AJ, Park S, Ramachandran R, Morris RH. J Am Chem Soc. 1994;116:8356. [Google Scholar]
- 28.Weare WW, Schrock RR, Hock AS, Müller P. Inorg Chem. 2006;45:9185. doi: 10.1021/ic0613457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins JL, Edelstein N, Spencer B, Smart JC. J Am Chem Soc. 1982;104:1882. [Google Scholar]