Abstract
Background
Newer behavior genetic methods can better elucidate gene-environment (G-E) interplay in the development of internalizing (INT) disorders (i.e., major depression and anxiety disorders). However, no study to date has conducted a comprehensive analysis examining multiple environmental risks with the purpose of delineating how general G-E mechanisms influence the development of INT disorders.
Methods
The sample consisted of 1315 male and female twin pairs participating in the age 17 assessment of the Minnesota Twin Family Study. Quantitative G-E interplay models were used to examine how genetic and environmental risk for INT disorders changes as a function of environmental context. Multiple measures and informants were employed to construct composite measures of INT disorders and 6 environmental risk factors including: stressful life events, mother-child and father-child relationship problems, antisocial and prosocial peer affiliation, and academic achievement and engagement.
Results
Significant moderation effects were detected between each environmental risk factor and INT such that in the context of greater environmental adversity, nonshared environmental factors became more important in the etiology of INT symptoms.
Conclusion
Our results are consistent with the interpretation that environmental stressors have a causative effect on the emergence of INT disorders. The consistency of our results suggests a general mechanism of environmental influence on INT disorders regardless of the specific form of environmental risk.
Internalizing (INT) disorders (i.e., major depression and anxiety disorders) are the most common forms of mental illness, and the prevalence of these disorders increases dramatically in late adolescence (Hankin et al., 1998; Kessler et al., 2005). Examining mechanisms of risk in late adolescence can provide important insights into their etiology and help curtail later negative outcomes. INT disorders are heritable (Kendler et al., 2003), but are also associated with various environmental risk factors such as stressful life events (Kendler et al., 1995), negative family relationships (Emery, 1982), and poor social support (e.g., friendship quality) (Kessler et al., 1992). Notably, these genetic and environmental risk factors are not independent. For example, persons who exhibit high levels of INT distress experience more stressful life events, and part of this association is genetically mediated (Silberg et al., 1999). Also, there is evidence that individuals at higher genetic risk are more likely to exhibit INT symptoms upon exposure to environmental stressors (Kendler et al., 1995, 2004).
While there has been a dramatic increase in the number of studies examining gene-environment (G-E) interplay in psychopathology research in recent years (Thapar et al., 2007), few studies have attempted to integrate findings across multiple disorders and multiple risk factors in an effort to answer fundamental questions regarding general principles of G-E influence in the development of psychiatric disorders. For example, does the mechanism of environmental influence differ for such risk factors as stressful life events, deviant peers, and parenting? Or, is there a general mechanism of environmental influence? Also, are the mechanisms of G-E interplay the same for all forms of psychopathology? For example, are the mechanisms of G-E influence the same or different for INT versus externalizing (EXT) disorders (i.e., antisocial behavior and substance use disorders)?
Two G-E mechanisms underlie the etiology of psychiatric disorders. The first process is G-E correlation, and refers to the fact that an individual’s exposure to environmental risk is in part due to his or her personal characteristics, decisions, and behaviors (Moffitt, 2005; Scarr & McCartney, 1983). For example, INT symptoms are associated with negative life events, especially events that are in some way “dependent” on the individual’s behavior such as interpersonal conflict, and these associations are to some extent due to common genetic risk factors (Silberg et al., 1999). G-E correlations also account for the finding that nearly all putatively “environmental” risk factors are to some extent heritable (Kendler & Baker, 2007).
G × E interactions are the second process, and refer to the fact that environmental stressors have a greater impact on a subset of individuals who are genetically at high-risk. For example, Kendler et al. (1995) found that adult women inferred to be at high genetic risk based on having a monozygotic (MZ) twin with a lifetime history of major depression were more likely to experience an episode of major depression following stressful life events than women who also experienced stressful events but whose MZ twin had no history of major depression. Similar results were found for adult men and women using scores on neuroticism as an index of genetic risk (Kendler et al., 2004). A few studies with adolescent twins have reported more direct tests of G × E interaction with genetic variance in INT symptoms increasing as a function of environmental stress such as negative life events and family conflict (Eaves et al., 2003; Lau & Eley, 2008; Rice et al., 2006; Silberg et al., 2001). Other analyses in both adult and child/adolescent samples, however, have found that the environmental rather the genetic variance of INT increased in the context of greater environmental adversity (Feinberg et al., 2007; Lau & Eley, 2008; Lau et al., 2007; South et al., 2009; South & Krueger, 2008).
Given these somewhat inconsistent findings, we took a more comprehensive approach to delineating G-E interplay in the emergence of INT symptoms by examining a variety of contextual domains in late adolescence that are associated with increased INT symptoms (Gerard & Buehler, 2004). Specifically, we sought to provide a fairly comprehensive assessment of environmental context during late adolescence by including a diverse set of measures that tap important dimensions of psychosocial functioning (Masten et al., 1995). These measures covered the domains of parent-child relationship quality, peer affiliation, academic functioning, and stressful life events. Our approach is based on the premise that general principles of G-E influence on INT would emerge by examining multiple risk factors in the same sample. Also, in a separate analysis, we have examined G-E interplay among the same environmental risk factors and an EXT composite (Hicks et al., in press). Thus, the two studies provide an excellent opportunity to examine convergent and discriminant patterns of G-E interplay by incorporating multiple environmental risk factors and multiple psychopathology constructs. An additional strength of our study was that both the environmental risk factors and INT were assessed using multiple methods and informants providing excellent measurement of the target constructs. Finally, mindful of the fact that a G-E correlation can confound the interpretation of a G × E interaction (Moffitt, 2005; Uher & McGuffin, 2007), we employed sophisticated quantitative twin models that incorporate both processes, thereby accounting for any selection effects (i.e., G-E correlation) in the presence of a G × E interaction (Purcell, 2002).
Method
Sample
Participants were adolescent male and female twins taking part in the Minnesota Twin Family Study (MTFS), an ongoing population-based, longitudinal study of twins and their families (Iacono et al., 1999). Birth records and public databases were used to locate more than 90% of families that included a twin birth in the state of Minnesota from 1972 through 1984. Families were excluded from the study if they lived more than a day’s drive from our Minneapolis laboratory or if either twin had a cognitive or physical handicap that would preclude completing the daylong, in-person assessment. Seventy-eight percent of eligible families agreed to participate. Participating families were representative of Minnesota families for the target birth years in terms of socioeconomic status and history of mental health treatment though parents reported slightly more education (0.25 years) than parents of nonparticipating families. Reflecting the population of Minnesota during the target birth years, 96% of the sample was Caucasian. Parents and children gave informed consent or assent as appropriate, and an institutional review board approved all research protocols.
The MTFS utilizes an accelerated longitudinal design with participants first entering the study at either age 11 or 17, returning for follow-up assessments every 3–4 years. We utilized all available data for the assessment targeted to occur when the twins turned age 17, that is, the intake assessment for the older cohort and the second follow-up assessment for the younger cohort. The total sample included 1315 twin pairs with the following zygosity and gender composition: 437 MZ and 251 dizygotic (DZ) male twin pairs, and 418 MZ and 209 DZ female twin pairs. The mean age of the total sample at the time of assessment was 17.8 years (SD = .68 years). Zygosity was determined by agreement among 3 estimates: MTFS staff evaluations of the twins’ physical similarity; parents’ completion of a standard zygosity questionnaire; and twin similarity on an algorithm of ponderal and cephalic indices and fingerprint ridge count. A serological analysis was performed if the 3 estimates did not agree.
Measures
INT disorders were assessed via clinical interviews and a teacher rating of internalizing symptoms. Symptoms of major depressive disorder, social phobia, and simple phobia were assessed using a modified version of the Structural Clinical Interview for DSM-III-R (SCID-III-R; Spitzer et al., 1990), which was the diagnostic system in use at intake (panic disorder, agoraphobia, and generalized anxiety disorder were also assessed, but were not included in the analysis due to their low base rates). Interviewers held at least a bachelor-level degree in psychology or related discipline and completed intensive training in psychiatric assessment. Symptoms were assigned on a 3-point scale: absent, present at subthreshold, present at full threshold. Standard skip-out rules were applied such that participants had to report at least a subthreshold level of depressed mood or anhedonia for major depression and interference for the phobias to complete the remainder of that module of the interview. All interview data were reviewed in a clinical case conference by at least two clinical psychology graduate students, with members of the review team required to reach consensus before assigning symptoms. This assessment process yielded uniformly high diagnostic kappa reliabilities: .89 for major depression, .78 for social phobia, and .79 for simple phobia. The prevalence rates for the 3 disorders at definite (all criteria met) and probable (one symptom short of meeting full criteria) levels of certainty were: major depression 8.8% and 11.3%, social phobia 5.3% and 10.9%, and simple phobia 4.8% and 8.8%, respectively. The mean tetrachoric correlation among the disorders was .25 and .30 at a definite and probable-level of certainty, respectively.
Ratings were also obtained from up to 3 teachers on a 12-item scale of overall internalizing distress (e.g., falls apart under stress, worries about many things). The internal consistency reliability for the teacher rating was .85; the inter-rater reliability (intraclass correlation) for the mean of 2 raters was .66. In accordance with Minnesota-state policy, twins are placed in separate classrooms whenever possible, thereby minimizing the likelihood that a teacher would rate members of the same twin pair. All statistical analyses utilized an INT composite variable that was calculated by taking the mean z-score of the symptom counts of major depressive disorder, social phobia, simple phobia, and the teacher rating of internalizing distress.
Twin, mother, and teacher reports were also used to assess multiple dimensions of the twins’ environmental context. Measures of academic achievement and engagement included twin and mother reports of the twin’s cumulative grade point average (GPA), ratings of expectations regarding the twin’s ultimate educational attainment (e.g., high school, college), and a 7-item scale assessing attitudes and engagement in school (e.g., good attitude about school, enjoys attending school; α = .83) (Johnson et al., 2006). For statistical analyses, we used the mean z-score for ratings of GPA, academic expectations, and academic attitudes averaged across the twin and mother reports (r = .77).
Antisocial (e.g., friends drink alcohol, steal, get in fights) and prosocial (e.g., friends work hard in school, popular with other kids, liked by teachers) peer affiliation was assessed by twin reports on a 19-item questionnaire (α’s = .85 and .78 for antisocial and prosocial peer scales) and by teacher reports (α’s = .85 and .87 for ratings of antisocial and prosocial peer group characteristics) (Walden et al., 2004). The mean z-score for the twin and teacher reports (r = .40) was used to calculate composite measures of antisocial and prosocial peer affiliation.
Mother-child and father-child relationship problems were assessed using the Parental Environment Questionnaire (PEQ; Elkins et al., 1997), a 50-item questionnaire that assesses various aspects of the parent-child relationship (e.g., conflict, involvement; scale α’s range from .69 to .82). Twins completed separate PEQ forms for each parent, and parents rated their relationship with each twin as well as the relationship between each twin and their co-parent (i.e., up to 3 ratings were available for each parent-child dyad). Our measure of mother-child and father-child relationship problems was the average of the 3 informant ratings on the first principal component of the PEQ scales (the scales exhibit a dominant 1st component; mean correlation across raters was .41).
Finally, twins completed a structured interview to assess stressful life events (Billig et al., 1996). We limited our analyses to “independent” life events, that is, events that are largely independent of the individual’s behavior (e.g., parent attempted suicide) rather than being dependent on the twin’s behavior (e.g., suspended from school). For our analyses, we used a tally of 20 life events covering the domains of parental divorce and discord, and family money, legal, and mental health problems. Because members of a twin pair should be concordant for these events, the correlation between twin reports provided an estimate of reliability (r = .81; inter-rater reliability was .89).
Statistical analyses
Biometric modeling was utilized to evaluate G-E interplay between the INT composite and the 6 environmental risk factors. First, we fit univariate models to estimate the relative genetic and environmental contributions to the variance of each variable. These models parse variance in each measure into additive genetic (A), shared environmental (C), and nonshared environmental (E) components. Estimates of the ACE variance components are derived based on differences in MZ and DZ twin similarity. That is, greater MZ (who share 100% of their genes) relative to DZ (who share on average 50% of their segregating genes) similarity is attributed to A (rMZ > rDZ), while within pair similarity due to environmental effects is attributed to C (rDZ > ½ rMZ). The extent to which twins differ is attributed to E (rMZ < 1.0; this includes measurement error).
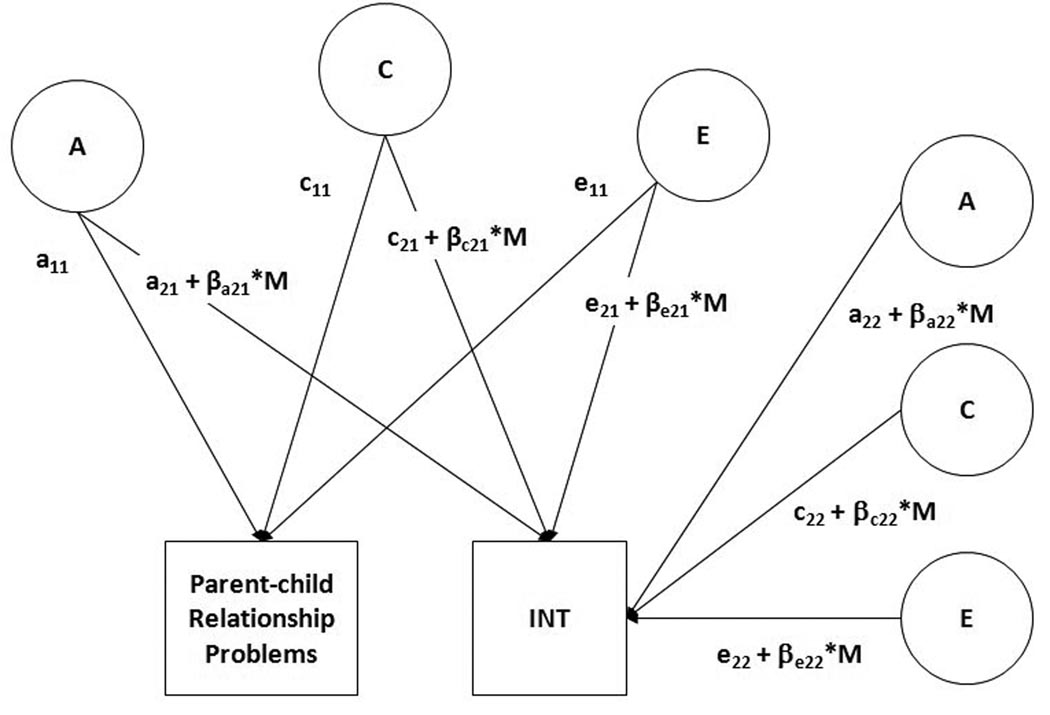
Next, we examined whether the ACE effects on the INT composite varied across different environmental contexts as operationalized by different levels of the 6 environmental risk factors. This was accomplished by fitting a G-E interplay model (depicted in Figure 1), which can be conceptualized as a bivariate extension of the univariate model with the added complexity of moderation terms on the ACE effects influencing INT (Purcell, 2002). First, this model estimates the ACE contributions to the covariance between INT and a given environmental risk factor (e.g., the product a11 × a21 is the genetic covariance) as well as the ACE effects unique to INT (e.g., a22). This allows for the estimation of the G-E correlation between INT and the environmental variable, thereby accounting for any selection processes between INT and exposure to the environmental stressor.
Figure 1.
INT = Internalizing disorders composite. Full model of moderation of the genetic and environmental influences on INT as a function of different levels of Parent-child Relationship Problems. A refers to additive genetic effects, C to shared environmental effects, and E to nonshared environmental effects. The parameters a21, c21, and e21 include genetic and environmental influences that overlap between Parent-child Relationship Problems and INT (i.e., can be used to derive the genetic and environmental covariance), while a22, c22, and e22 are genetic and environmental influences unique to INT. Parent-child Relationship Problems can moderate either the common variance with INT or the unique variance of INT. The β’s indicate the direction (+ or −) and magnitude of any moderation effects on the paths from the ACE effects to INT, while M indicates the level of the moderators, that is, the number of parent-child relationship problems.
Additionally, in the presence of a G × E interaction, the initial ACE effects on INT derived from the bivariate model are adjusted based on the direction (+ or −) and magnitude of the interaction (specified by the moderation weight, βxij) and the level of the moderator (M; e.g., parent-child relationship problems). Moderation effects may be present on either the ACE effects shared with INT and the environmental risk factor (e.g., a21 + βa21*M) or those effects that are unique to INT (e.g., a22 + βa22*M). For example, a positive sign for βa22 would indicate that genetic variance unique to INT increases in the context of more severe parent-child relationship problems.
All models were fit to the raw data using full information maximum-likelihood in the Mx software system (Neale, Boker, Xie, & Maes, 2003), a procedure that relies on the assumption that data are missing at random and yields less biased parameter estimates than other procedures (Little & Rubin, 2002). The INT composite was log transformed to better approximate normality (skewness = 1.013, kurtosis = .492 after log transformation). We followed standard analytic procedures regarding correcting for potential biases by regressing all variables on sex, age, age2, and their interactions prior to analyses (McGue & Bouchard, 1984). Model fit was evaluated using the difference in −2 × loglikelihood (-2LL) between nested models and several information theoretic indices that balance overall fit with model parsimony including Akaike’s Information Criterion (AIC), the Bayesian Information Criterion (BIC), the sample-size adjusted BIC, and the Deviance Information Criterion (DIC) with lower (or more negative) values indicative of better fit. We tested for G-E moderation using the likelihood ratio test between the full and no moderation models, which yields a χ2 statistic. If the full moderation model provided a better fit, follow-up model trimming analyses were conducted to identify the best fitting model based on consistency among the information theoretic indices, retaining only the significant moderation terms in the model.
Results
Phenotypic Correlations, Twin Correlations, and Univariate Biometric Analyses
Table 1 provides the phenotypic correlations among the study variables. Each environmental variable was significantly correlated with INT with effect sizes ranging from small to moderate. All environmental risk factors were also significantly correlated with each other (mean r = .34), indicating stress in one domain tended to be associated with problems in all other domains.
Table 1.
Phenotypic Correlations for Internalizing and Environmental Risk Factors.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Internalizing | -- | |||||
| 2. Academic Achievement & Engagement | .21 | -- | ||||
| 3. Antisocial Peers | .17 | .49 | -- | |||
| 4. Prosocial Peers | .26 | .48 | .40 | -- | ||
| 5. Mother-child Relationship Problems | .18 | .31 | .24 | .24 | -- | |
| 6. Father-child Relationship Problems | .22 | .29 | .27 | .24 | .57 | -- |
| 7. Stressful Life Events | .21 | .20 | .25 | .20 | .18 | .38 |
Note. All correlations are significant at p < .001. Academic Achievement & Engagement and Prosocial Peers have been reverse scored.
Table 2 presents the MZ and DZ twin correlations and ACE estimates from the univariate biometric models for each variable. INT exhibited moderate heritability with a non-significant shared environmental effect. Each environmental variable also exhibited significant heritable variance though there was a wide range (.08 to .72). Each environmental variable except academic achievement and engagement also exhibited significant shared environmental effects.
Table 2.
Twin Correlations and Univariate ACE estimates (95% Confidence Intervals).
| Variable | MZ | DZ | A | C | E |
|---|---|---|---|---|---|
| Internalizing | .49 | .33 | .38 (.20, .54) | .12 (.00, .28) | .50 (.45, .55) |
| Academic Achievement & Engagement | .77 | .42 | .72 (.57, .79) | .05 (.00, .19) | .23 (.20, .26) |
| Antisocial Peers | .70 | .51 | .42 (.28, .58) | .28 (.13, .42) | .30 (.26, .33) |
| Prosocial Peers | .66 | .44 | .41 (.25, .59) | .24 (.08, .39) | .34 (.31, .38) |
| Mother-child Relationship Problems | .79 | .56 | .49 (.38, .62) | .30 (.17, .41) | .21 (.18, .23) |
| Father-child Relationship Problems | .83 | .75 | .23 (.15, .31) | .62 (.54, .69) | .15 (.14, .18) |
| Stressful Life Events | .81 | .80 | .08 (.01, .15) | .75 (.68, .80) | .17 (.16, .20) |
Note. MZ = monozygotic; DZ = dizygotic; A = additive genetic; C = shared environment; E = nonshared environment.
G-E Interplay of INT and Environmental Risk Factors
Table 3 provides the fit statistics for the models of G-E interplay between INT and each environmental risk factor. Each environmental variable exerted significant moderation effects on INT as evidenced by the significant likelihood ratio tests between the full and no moderation models. Follow-up model trimming analyses to identify the significant moderation effects revealed a consistent mechanism of G-E interplay such that for each environmental variable, the unique nonshared environmental variance in INT increased with higher levels of environmental adversity.1 For antisocial peers, there was also a modest moderation effect (same direction) on the common nonshared environmental variance with INT.
Table 3.
Fit Statistics for G-E Interplay Models of INT and Environmental Moderators
| Model | -2LL | df | AIC | BICadj | DIC | BIC | Δχ2 | df | p |
|---|---|---|---|---|---|---|---|---|---|
| Academic Achievement & Engagement | |||||||||
| Full G−E moderation | 12,188.69 | 4700 | 2788.69 | −3061.40 | −6206.84 | −10,525.85 | -- | -- | -- |
| No moderation | 12,222.41 | 4706 | 2810.41 | −3056.23 | −6205.68 | −10,530.21 | 33.72 | 6 | <.001 |
| Moderation effects for best fit: unique E |
12,195.16 | 4705 | 2785.16 | −3067.91 | −6216.69 | −10,540.29 | 6.47 | 5 | 0.263 |
| Antisocial Peers | |||||||||
| Full G−E moderation | 11,333.99 | 4372 | 2589.99 | −2692.49 | −5618.14 | −9635.74 | -- | -- | -- |
| No moderation | 11,370.63 | 4378 | 2614.63 | −2685.64 | −5615.30 | −9638.42 | 36.64 | 6 | <.001 |
| Moderation effects for best fit: unique and common E |
11,339.29 | 4376 | 2587.29 | −2697.49 | −5625.81 | −9647.09 | 5.30 | 4 | 0.258 |
| Prosocial Peers | |||||||||
| Full G-E moderation | 11,749.67 | 4501 | 2747.67 | −2795.90 | −5807.99 | −9944.13 | -- | -- | -- |
| No moderation | 11,795.92 | 4507 | 2781.92 | −2784.33 | −5800.43 | −9942.09 | 46.25 | 6 | <.001 |
| Moderation effects for best fit: unique E |
11,757.82 | 4506 | 2745.82 | −2801.46 | −5816.89 | −9957.63 | 8.15 | 5 | 0.148 |
| Mother−Child Relationship Problems | |||||||||
| Full G-E moderation | 12,617.11 | 4913 | 2791.11 | −3369.96 | −6658.12 | −11,172.87 | -- | -- | -- |
| No moderation | 12,676.37 | 4919 | 2838.37 | −3352.15 | −6644.33 | −11,164.59 | 59.26 | 6 | <.001 |
| Moderation effects for best fit: unique E |
12,626.45 | 4918 | 2790.45 | −3375.14 | −6666.65 | −11,185.99 | 9.34 | 5 | 0.096 |
| Father−Child Relationship Problems | |||||||||
| Full G-E moderation | 12,155.34 | 4909 | 2337.34 | −3590.98 | −6876.46 | −11,387.53 | -- | -- | -- |
| No moderation | 12,236.08 | 4915 | 2406.08 | −3562.42 | −6851.92 | −11,368.50 | 80.74 | 6 | <.001 |
| Moderation effects for best fit: unique E |
12,163.65 | 4914 | 2335.65 | −3596.67 | −6885.50 | −11,401.16 | 8.31 | 5 | 0.140 |
| Stressful Life Events | |||||||||
| Full G-E moderation | 12,177.89 | 4891 | 2395.78 | −3534.37 | −6807.79 | −11,302.31 | -- | -- | -- |
| No moderation | 12,235.69 | 4894 | 2447.69 | −3511.31 | −6786.74 | −11,284.02 | 57.80 | 3 | <.001 |
| Moderation effects for best fit: unique E |
12,177.96 | 4893 | 2391.86 | −3538.27 | −6813.02 | −11,309.39 | 0.07 | 2 | 0.966 |
Note. Full G-E refers to the gene-environment interplay model with all possible moderation parameters. No moderation refers to a bivariate biometric model with no moderation parameters. Lower values for all fit indices are indicative of better fit. E = nonshared environment.
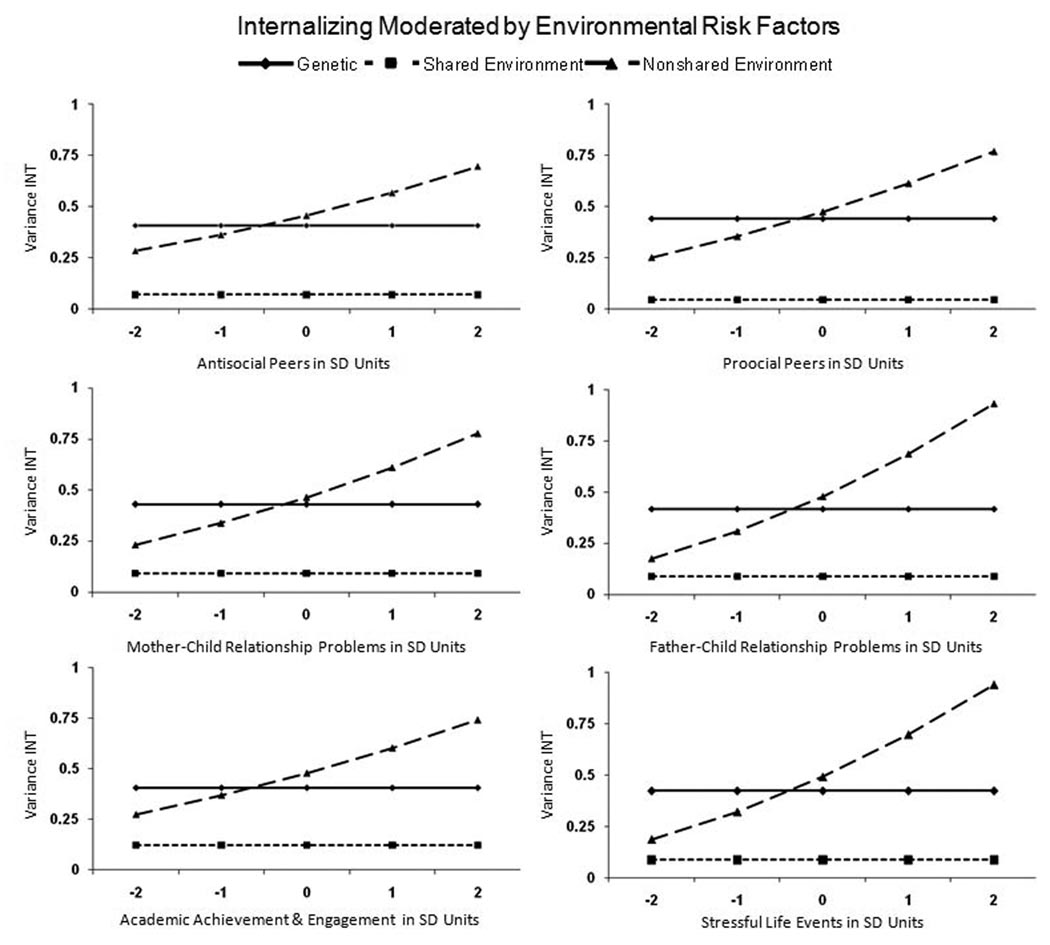
Figure 2 provides a graphical display of the G-E moderation effects on INT for each environmental variable. In each case, the nonshared environmental variance of INT increased with greater environmental adversity while the additive genetic and shared environmental variance remained stable across different levels of the environmental risk factors. As a consequence, the heritability of INT (i.e., the ratio of heritable to non-heritable variance) was greatest at low levels of environmental stress. That is, for INT, the mean a2 = .57 and .32 in the context of low (−2 SD) and high (+2 SD) environmental stress, respectively (consequently, mean e2 = .30 and .62 at low and high levels of environmental stress).
Figure 2.
Changes in the variance of Internalizing (INT) as a function of environmental risk factors for the best fitting model. The x-axis provides the level of the environmental moderator in standard deviation (SD) units with each variable coded so that higher values indicate greater environmental adversity. The y-axis provides the level of the unstandardized ACE variance components for INT.
Each environmental risk factor exhibited a small to moderate genetic correlation (rA) with INT ranging from .15 to .47 (mean rA = .29). Because the nonshared environmental variance of INT was moderated, the nonshared environmental (rE) correlations also varied with levels of the environmental risk factors though they were of small magnitude. The mean rE = .17 and .05 at low (−2 SD) and high (+2 SD) levels of environmental stress, respectively.
To summarize, our results are consistent with a social causation mechanism of environmental influence on INT symptoms. That is, greater environmental adversity was associated with greater mean-levels and variance in INT, and the greater variance in INT was nonshared environmental in origin. Also, selection effects do not seem to account for these associations as evinced by the dual finding that moderation effects were present for the unique variance in INT rather than the common variance between INT and the environmental risk factors, and by the declining overlapping variance (indicated by the downward shift in rE) as environmental adversity increases (Johnson, 2007).
Discussion
Our analyses revealed a surprisingly consistent pattern of G-E interplay2 in the emergence of INT symptoms during late adolescence. Specifically, across several environmental risk factors, nonshared environmental variance in INT increased in the context of greater environmental adversity. While no methodology relying on observational data ensures unambiguous causal interpretations, our results are consistent with a direct causal mechanism of environmental stress on the expression of INT symptoms. Three findings are consistent with this interpretation. First, as Table 1 and Figure 2 indicate, greater environmental adversity was associated with greater mean-levels and variance in INT symptoms. Second, the greater variance in INT was due to an increase in nonshared environmental variance. Finally, there was little evidence that social selection accounted for these associations.
A corollary to our findings is that in the context of low environmental stress, genetic factors will play a relatively greater role in the etiology of INT symptoms (i.e., the heritability of INT will be higher). This finding does not suggest that individuals who carry genetic risk factors for INT disorders should avoid low stress environments; rather it indicates that in the absence of environmental stressors inherited characteristics will play a greater role in the emergence of INT symptoms. A consequence for molecular genetic studies of INT, however, is that detecting significant associations with specific genes could be more difficult in the context of environmental adversity as the increased environmental variance will create “noise” around the genetic signal.
Our findings are consistent with the majority of previous studies examining G-E interplay in the emergence of depressive symptoms in adolescent samples. Specifically, two independent studies reported that the nonshared environmental variance in depressive symptoms increased as a function of maternal punitive discipline (Lau& Eley, 2008) and overall parental negativity (Feinberg et al., 2007). Additionally, recent analyses utilizing a nationwide sample of middle-aged twins found that the nonshared environmental variance in INT distress (composite of major depression, generalized anxiety, panic, and neuroticism) was greater in the contexts of poorer marital relationship quality, lower income, and worse physical health (South et al., 2008; South & Krueger, in press). Taken in concert with the consistency of our findings, this emerging literature suggests a general mechanism of environmental influence on the expression of INT distress regardless of the particular form of environmental risk. This consistency is especially noteworthy given the diversity of the content of the environmental measures, the use of multiple informants and methods of assessment, differences in heritability of the environmental measures, and the different sample characteristics.
One concern regarding the consistency of our findings is that these environmental measures would have the same moderating effects on the genetic and environmental risk for any form of psychopathology. However, in a separate analysis utilizing the same study design to examine G-E interplay for EXT disorders, we found that it was primarily the genetic variance that increased as a function of our environmental risk factors such that in the context of environmental adversity, genetic factors actually became more important in the etiology of EXT symptoms (Hicks et al., in press). Taken together, our findings for INT and EXT provide impressive evidence of convergent and discriminant validity, and indicate that while environmental stressors have a general effect of increasing the mean-levels and variance of psychopathology, the mechanisms of their influence will differ depending on the nature of the psychopathological condition.
Another question regarding our findings is how our results relate to the literature of measured gene × measured environment studies in risk for INT disorders. In particular, the finding that certain serotonin transporter genes (5-HTT) increase risk for major depression as a function of stressful life events (Uher & McGuffin, 2007), which would predict that the genetic variance would increase in the context of greater environmental adversity. However, our results are consistent with at least one investigation that found that serotonin receptor genes (HTR2A) decreased risk for depressive symptoms in the context of high maternal nurturance (Jokela et al., 2007). That is, in the “good” environment the genetic effect was stronger (i.e., higher heritability) while the mean-level and overall variance of depression increased in the “bad” environment. Interestingly, findings for the serotonin transporter genes are more consistent with our findings for EXT, and these genes have been associated with externalizing phenotypes (e.g., suicidal behavior, violence, and substance abuse; for a review see Uher & McGuffin, 2007) suggesting serotonin transporter genes may confer risk for a general vulnerability characterized by negative affect and impulsivity that cuts across diagnostic categories. While these conjectures require further research to test, they provide an example of how integrating findings from quantitative twin modeling and measured gene × measured environment designs can help to forge a greater understanding of mechanisms underlying psychopathology.
Our results must be considered in light of certain limitations. First, while the sample is representative of the Minnesota population from which it was drawn, it is not representative of the broader United States population, limiting generalizability to other racial and ethnic groups. Second, the causal direction of the moderation effects is unclear. For example, it is possible that INT could moderate the genetic and environmental effects on the environmental variables. Additionally, the presence of genetic correlations between INT and the environmental variables indicates that genetic risk for INT also contributes to exposure to environmental risk factors, which then contributes to greater levels of INT. Future studies that utilize the longitudinal data of the MTFS will further clarify the causal links between INT and these environmental risk factors. Another potential limitation is that our assessment of internalizing disorders allowed skipping-out of the interview if the participant did not endorse gating symptoms for at least a subthreshold level. Replication of our results using a method that does not employ skip-out rules would further demonstrate the robustness of our findings. Finally, while our results have been replicated in one sample of middle-aged individuals (South et al., 2009; South & Krueger, 2008), future studies are needed to determine if the mechanisms of G-E interplay we observed are present throughout development.
To conclude, our results have important implications for understanding the etiology of INT disorders, gene association and measured gene × measured environment studies, and for intervention efforts. Specifically, our results are consistent with a causal role for environmental adversity in the emergence of INT distress, and suggest that the amelioration of environmental stress will result in relief of INT symptoms. The consistency of our findings across the different environmental risk factors is especially interesting and suggests a ubiquity of environmental influence on the expression of INT disorders. Future studies that examine the consistency of our findings with different environmental risk factors, utilize prospective designs to further tease apart direction of causation, and examine the interplay of measured genes and environments will provide additional insights into the etiological mechanisms underlying INT disorders.
Internalizing (INT) disorders (major depression and anxiety disorders) are the most common forms of psychopathology
INT disorders are heritable but are also associated with environmental risk factors
Using G × E interaction models with a large twin sample and examining the effects of 6 environmental risk factors, we found that environmental variance in INT increased as a function of environmental adversity.
Our results are consistent both with a general mechanism of environmental influence on INT and that environmental stressors have a causative effect on the emergence of INT disorders.
Acknowledgements
This research was supported in part by USPHS Grants AA 09367, DA 05147, and DA 024417.
Footnotes
Because members of a twin pair should not differ on our measure of independent stressful life events, this results in the unrealistic situation of no genetic effect on stressful life events. Therefore, it is inappropriate to estimate a G-E correlation with and moderation on the common variance with INT. Thus, for the stressful life events measure, we only allowed for moderation of the unique variance in INT. While not ideal, this approach allowed us to examine partial moderation of important environmental stressors (e.g., parental discord, poverty, family legal and mental health problems) and whether the mechanism of their effect was consistent with other environmental risk factors.
Because the moderation parameters on the additive genetic variance of INT were not significant, technically, we did not detect a G × E interaction between INT and the environmental risk factors. Therefore, we use the broader term “G-E interplay” to refer to moderation of either the genetic or environmental variance of INT.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington, D.C: American Psychiatric Press; 1987. Revised. [Google Scholar]
- Billig JP, Hershberger SL, Iacono WG, McGue M. Life events and personality in late adolescence: genetic and environmental relations. Behavior Genetics. 1996;26:543–554. doi: 10.1007/BF02361227. [DOI] [PubMed] [Google Scholar]
- Eaves L, Silberg J, Erkanli A. Resolving multiple epigenetic pathways to adolescent depression. Journal of Child Psychology and Psychiatry. 2003;44:1006–1014. doi: 10.1111/1469-7610.00185. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Genetic and environmental influences on parent-son relationships: evidence for increasing genetic influence during adolescence. Developmental Psychology. 1997;33:351–363. doi: 10.1037//0012-1649.33.2.351. [DOI] [PubMed] [Google Scholar]
- Emery RE. Interpersonal conflict and the children of discord and divorce. Psychological Bulletin. 1982;92:310–330. [PubMed] [Google Scholar]
- Feinberg ME, Button TMM, Neiderhiser JM, Reiss D, Heatherington EM. Parenting and adoleslcent antisocial behavior and depression: Evidence of genotype x parenting environment interaction. Archives of General Psychiatry. 2007;64:457–465. doi: 10.1001/archpsyc.64.4.457. [DOI] [PubMed] [Google Scholar]
- Gerard JM, Buehler C. Cumulative environmental risk and youth problem behavior. Journal of Marriage and Family. 2004;66:702–720. [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hicks BM, South SC, DiRago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Archives of General Psychiatry. doi: 10.1001/archgenpsychiatry.2008.554. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Johnson W. Genetic and environmental influences on behavior: Capturing all the interplay. Psychological Review. 2007;114:423–440. doi: 10.1037/0033-295X.114.2.423. [DOI] [PubMed] [Google Scholar]
- Johnson W, McGue M, Iacono WG. Genetic and environmental influences on academic achievement trajectories during adolescence. Developmental Psychology. 2006;42:514–532. doi: 10.1037/0012-1649.42.3.514. [DOI] [PubMed] [Google Scholar]
- Jokela M, Keltikangas-Jarvinen L, Kivimaki M, Puttonen , Elovainio M, Rontu R, Lehtimaki Serotonin receptor 2A gene and the influence of childhood maternal nurturance on adulthood depressive symptoms. Archives of General Psychiatry. 2007;64:356–360. doi: 10.1001/archpsyc.64.3.356. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Baker JH. Genetic influences on measures of the environment: A systematic review. Psychological Medicine. 2007;37:615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean CJ, Neale MC, Heath AC, Eaves LJ. Stressful life events, genetic liability and onset of an episode of major depression in women: Evidence of genetic control of sensitivity to the environment. American Journal of Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The inter-relationship of neuroticism, sex, and stressful life events in predictions of episodes of major depression. American Journal of Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Kendler KS, Heath AC, Neale MC, Eaves LJ. Social support, depressed mood, and adjustment to stress: A genetic epidemiologic investigation. Journal of Personality & Social Psychology. 1992;62:257–272. doi: 10.1037//0022-3514.62.2.257. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Eley TC. Disentangling gene-environment correlations and interactions on adolescent depressive symptoms. Journal of Child Psychology and Psychiatry. 2008;49:142–150. doi: 10.1111/j.1469-7610.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Gregory AM, Goldwin MA, Pine DS, Eley TC. Assessing gene-environment interactions on anxiety symptom subtypes across childhood and adolescence. Development & Psychopathology. 2007;19:1129–1146. doi: 10.1017/S0954579407000582. [DOI] [PubMed] [Google Scholar]
- Little RJ, Rubin DB. Statistical analysis with missing data. Second edition. New York: John Wiley & Sons; 2002. [Google Scholar]
- Masten AS, Coatsworth JD, Neemann J, Gest SD, Tellegen A, Garmezy N. The structure and coherence of competence from childhood through adolescence. Child Development. 1995;66:1635–1659. [PubMed] [Google Scholar]
- McGue M, Bouchard TJ. Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14:325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. The new look of behavioral genetics in developmental psychopathology: Gene-environment interplay in antisocial behaviors. Psychological Bulletin. 2005;131:533–554. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th Edition. VCU Box 900126, Richmond, VA 23298: Department of Psychiatry; 2003. [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Shelton KH, Thapar A. Family conflict interacts with genetic liability in predicting childhood and adolescent depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:841–848. doi: 10.1097/01.chi.0000219834.08602.44. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype => environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, Carbonneau R, Murrelle L, Foley D, Eaves L. The influence of genetic factors and life stress on depression among adolescent girls. Archives of General Psychiatry. 1999;56:225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- Silberg J, Rutter M, Neale M, Eaves L. Genetic moderation of environmental risk for depression and anxiety in adolescent girls. British Journal of Psychiatry. 2001;179:116–121. doi: 10.1192/bjp.179.2.116. [DOI] [PubMed] [Google Scholar]
- South SC, Krueger RF. Martial quality moderates genetic and environmental influences on the internalizing spectrum. Journal of Abnormal Psychology. 2008;117:826–837. doi: 10.1037/a0013499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South SC, Krueger RF, Johnson W. Socioeconomic status and health problems moderate genetic and environmental influences on internalizing pathology. 2009 Manuscript in preparation. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R, Patient Edition/Non-patient Edition (SCID-P/SCID-NP) Washington, DC: American Psychiatric Press, Inc; 1990. [Google Scholar]
- Thapar A, Harold G, Rice F, Langley K, O'Donovan M. The contribution of gene-environment interaction to psychopathology. Development & Psychopathology. 2007;19:989–1004. doi: 10.1017/S0954579407000491. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: Review and methodological analysis. Molecular Psychiatry. 2007;12:1–16. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Walden B, McGue M, Iacono WG, Burt SA, Elkins I. Identifying shared environmental contributions to early substance use: the respective roles of peers and parents. Journal of Abnormal Psychology. 2004;113:440–450. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]