Summary
Understanding lipid metabolism and its regulation requires information on the rates at which lipids are produced within the body, absorbed (dietary lipids) into the body, transported within the body, and utilized by various tissues. This article focuses on the use of stable isotope-labeled tracers for the quantitative evaluation of major pathways of fatty acid and triglyceride metabolism in humans in vivo. Adipose tissue lipolysis and free fatty acid appearance in plasma, fatty acid tissue uptake and oxidation, and hepatic very low-density lipoprotein triglyceride secretion are among the metabolic pathways that can be studied by using stable isotope labeled tracers, and will be discussed in detail. The methodology has been in use for many years and is constantly being refined. A variety of tracers and analytical approaches are available and can be used; knowing the advantages, assumptions, and limitations of each is essential for the planning of studies and the interpretation of data, which can provide unique insights into human lipid metabolism.
Keywords: lipid metabolism, lipolysis, oxidation, lipoprotein, adipose tissue, liver, mass spectrometry
Introduction
The most important function of lipids in the human body, traditionally, is to store energy in the form of triglyceride and provide energy through oxidation of triglyceride-derived fatty acids. In recent years, however, there has been an expansion of interest in fatty acid and triglyceride metabolism due to the recognition of lipids as important signaling molecules [1,2] and mediators of insulin resistance [3], and the adverse metabolic outcomes associated with ectopic fat accumulation [4,5]. Understanding lipid metabolism and its regulation requires quantitative information on the rates at which these compounds are produced within the body, taken up from the intestinal tract, transported within the body and utilized (e.g., fatty acid release from adipose tissue and uptake by muscle for energy production/oxidation). This article will provide an overview of stable isotope-labeled tracer methods for the quantitative evaluation of major pathways of fatty acid and triglyceride metabolism in humans in vivo.
General tracer theory and practice
A tracer is a compound that is administered (typically intravenously, sometimes orally) into the body to “trace” the metabolism of a specific compound of interest (the tracee); a tracer, therefore, must be metabolically indistinguishable from the tracee but distinct in some way that allows its detection. Typically one or more naturally occurring atoms in the molecule of the tracee are substituted with one or more isotopes (stable or radioactive) of that atom (Figure 1). Stable isotopes of the same element (e.g., 1H and 2H, 12C and 13C) have the same number of protons and different number of neutrons in their nucleus; hence they have different atomic mass (which is the property that allows their detection) but the same chemical properties. The major assumption underlying the use of stable isotope tracers in metabolic research is that the tracer and the tracee are indistinguishable with respect to their metabolic fate. However this may not always be true [6] and “isotope effects”, i.e., when the metabolic behavior of the tracer does not exactly mimic the metabolic behavior of the tracee, are most pronounced with heavy isotope loads leading to considerable changes in mass and when tracers are added in positions that are directly involved in a biochemical reaction.
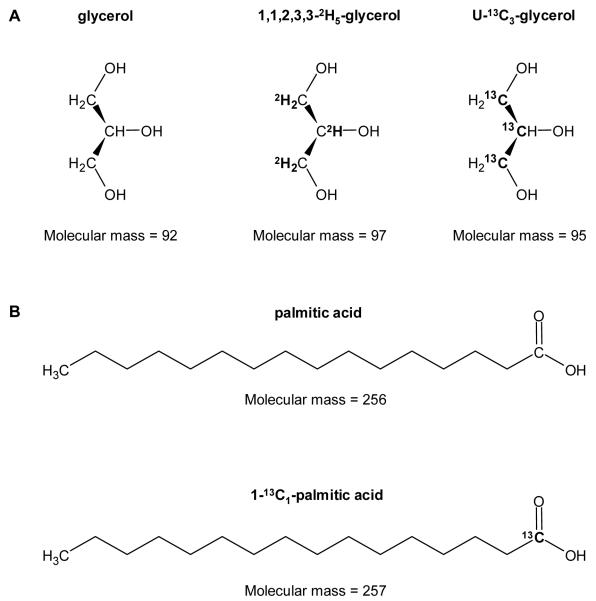
Figure 1.
Examples of stable isotope labeled tracers used in metabolic research. A: 1,1,2,3,3-2H5-glycerol where five hydrogen atoms (1H at positions 1, 2 and 3; atomic mass = 1 amu) were replaced with 2H (atomic mass = 2 amu), and 1,2,3-13C1-glycerol (or uniformly-labeled, U) where all three carbon atoms (12C at positions 1, 2, and 3; atomic mass = 12 amu) were replaced with 13C (atomic mass = 13 amu). B: 1-13C1-palmitic acid where one carbon atom (at position 1) was replaced with 13C.
Detection and quantification of stable isotope tracers relies on mass spectrometry (MS), which separates and measures compounds according to their mass; what is actually of interest is the amount of tracer relative to the trace, referred to as the enrichment [7]. Measures of enrichment are typically made as tracer-to-tracee ratio (TTR), which refers to the molar ratio of the labeled and unlabeled molecules in the biological sample (e.g., a TTR of 0.05 indicates that the amount of tracer is 5% of the amount of tracee), or mole percent excess (MPE) or atom percent excess (APE), which refer to the molar ratio of the labeled molecule/atom and the sum of the labeled and unlabeled molecules/atoms in the biological sample (e.g., a MPE or APE of 0.05 indicates that the amount of tracer is 5% of the total amount of tracee plus tracer molecules/atoms). The enrichment can also be assessed relative to a standard of known isotopic enrichment, in which case the enrichment of the biological sample is compared to the isotopic enrichment of a reference material and the data expressed as delta (i.e., the difference between the sample and standard); this is typically done for measuring the enrichment of pure gases (e.g., CO2). The TTR in biological samples is usually determined by using gas-chromatography (GC) MS in selected ion monitoring mode, where the abundances of a particular fragment of the molecule containing the heavier isotope (tracer) or not (tracee) are compared. Isotope ratio (IR) MS can be used to analyze very low TTR values in gases (e.g., CO2) and, recently, GC combustion IRMS has become available, combining the resolution capabilities of GC with the accuracy and precision of IRMS [7]. Tracers labeled with stable isotopes have several advantages over radioactive tracers, i.e., no exposure to potentially hazardous ionizing radiation, direct measurement of the TTR in biological samples, ability to readily determine the position of a label within a molecule with accuracy and precision, thereby expanding the number and type of possible measurements that can be made (e.g., simultaneous analysis of several labels within the same substrate), etc. [8]. On the other hand, because stable isotopes are naturally occurring, a correction needs to be made to account for the natural abundance of tracers in the system [9]. Also, since stable isotope-labeled tracers are used to provide quantitative information about the metabolic fate of the tracee, it is important that tracers are given in truly trace amounts so as not to perturb the system under study [8]. Large tracer doses are likely to perturb the endogenous kinetics or the pool size of the tracee [10] - simply imagine the metabolic effect of providing a significant amount of glucose.
Tracers are usually administered as a single bolus or via constant infusion (often combined with a bolus, in which case it is referred to as a primed constant infusion) (Figure 2) [9]. During bolus injection of the tracer, the total amount of tracer is administered as a single bolus, typically into the blood stream. In this case, the TTR of the substance of interest quickly builds up to a peak value and then declines over time in a manner that depends on the turnover of the tracee. Rapid appearance of tracee into the same pool leads to a rapid decline in enrichment whereas slow appearance of tracee extends the “wash-out”. Monitoring the rate at which the enrichment declines over time provides information regarding the rate of appearance of the tracee. In addition, valuable information can be gained regarding the system by evaluating the shape of the wash-out curve (i.e., the enrichment declines in a single exponential fashion in the case of a single pool, where the tracee appears from a single source). A drawback of this method, however, is that large amounts of tracer administered at once may perturb endogenous kinetics, and rapidly turning-over substrates (e.g., plasma free fatty acids, FFA) are difficult to monitor with this approach because they require impractically frequent sampling (i.e., several samples within a few minutes). During constant infusion, the tracer is infused at a constant rate, typically for several hours. In the simplest situation, the tracer is delivered directly into a homogenous, rapidly-mixing pool of the tracee at metabolic steady-state (i.e., the concentration of the tracee remains constant). This means that the tracee enters and leaves the pool at equal rates. When the tracer is first introduced into the system, its concentration starts to build up with time and the enrichment increases until it reaches a plateau (i.e., isotopic equilibrium or isotopic steady state). At this point, the enrichment of the substrate of interest (the proportional contribution of tracer and tracee) leaving the pool is identical to that within the pool (which can be measured), and also identical to that entering the pool. It is important to note that, in this case, determination of the kinetics of the tracee depends only on the value of TTR at isotopic plateau, and not on the time-course required to achieve it. Because it may take many hours to achieve isotopic steady state (for substrates with very slow turnover), the constant infusion of a tracer is usually combined with an initial bolus injection of the tracer. This involves administering a relatively large dose of the tracer as a single bolus (i.e., priming the pool) right before starting infusing the tracer at constant rate to accelerate the time required to reach isotopic equilibrium.
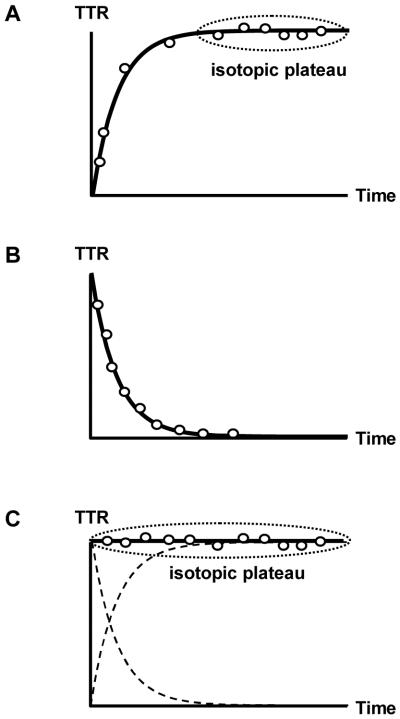
Figure 2.
Tracer administration techniques used in metabolic research. A: Constant infusion of the tracer, where the tracer-to-tracee ratio (TTR) increases with time until isotopic equilibrium (plateau) is reached. B: Bolus injection of the tracer, where the TTR decreases with time. C: Primed constant infusion of the tracer, where a large tracer dose is given as a single bolus before the constant infusion, and isotopic plateau is reached immediately (an ideal tracer prime is shown; the dashed lines represent the theoretical increase and decrease in TTR from constant infusion and bolus injection, respectively).
In tracer studies, sampling of the system needs to be made from an area that is directly accessible (for practical or ethical reasons); this typically refers to venous blood or breath, although arterial blood or tissue (e.g., muscle) can also be obtained. When the biochemical events of interest occur at areas inaccessible for direct or practical sampling (e.g., within various intra-cellular compartments or in sub-cellular organelles, or in tissues difficult to reach), several assumptions need to be made regarding the transport of material among the various pools of the system (i.e., until reaching the accessible pool). For example, several aspects of liver triglyceride metabolism are evaluated by administering a tracer fatty acid, glycerol, or amino acid into the systemic circulation, the tracer enters the liver, becomes incorporated into very low density lipoproteins (VLDL) in the liver, which are then secreted into the circulation. Sampling of blood and monitoring the changes in enrichment over time in VLDL provides information regarding the secretion rate of VLDL and its components (e.g., triglyceride, apolipoprotein B-100) [11,12].
There are three general ways in which isotopic tracers can be used to evaluate substrate metabolism: tracer dilution, tracer incorporation, and tracer conversion. Tracer dilution is widely used for measuring the rate of appearance (Ra) of the tracee in the circulation and requires the administration of a known amount of tracer (e.g., labeled glycerol) into a vein and monitoring its dilution by the appearance of tracee in the bloodstream (e.g., glycerol released during adipose tissue lipolysis) in blood samples collected from an artery or arterialized venous blood [13]. The Ra is usually a whole-body measurement that reflects the rate of release of the tracee into the bloodstream by all tissues of the body. When only a single tissue produces the tracee, the technique actually assesses regional metabolism; in more advanced applications, which require arterio-venous cannulation and blood sampling, the dilution technique can be applied to obtain information on specific body regions/tissues (e.g., whole leg, subcutaneous adipose tissue, splanchnic region) [14-19]. Tracer incorporation refers to the situation where the tracer serves as a precursor (e.g., labeled fatty acid) for the synthesis of a more complex compound (the product; e.g., triglyceride), and the rate at which this occurs is being measured by monitoring the amount of tracer in the product over time. Tracer conversion refers to measuring the rate at which a metabolic byproduct (e.g., labeled CO2) is being produced through metabolism (e.g., oxidation) of a more complex compound which contains a stable isotope (e.g., carbon-labeled fatty acid), or the rate at which a tracer is inter-converted; for example, the desaturation and elongation of fatty acids leading to the synthesis of very long-chain polyunsaturated fatty acids [20,21].
In the following sections, these approaches will be exemplified by presenting the use of stable isotope-labeled tracers for measuring adipose tissue lipolysis, release of FFA into and removal from the circulation, fatty acid oxidation, and hepatic VLDL-triglyceride secretion.
Adipose tissue lipolysis
Triglycerides stored within adipose tissue constitute the body’s major energy reserve. The balance between triglyceride storage and mobilization is regulated by complex hormonal and neuronal mechanisms. To measure the rate of adipose tissue lipolysis, one typically measures the Ra of glycerol in the bloodstream because the breakdown of triglyceride is usually complete, meaning that 1 mole of triglyceride gives rise to 1 mole of glycerol and 3 moles of fatty acids [22]. This has been demonstrated by measuring the ratio of FFA to glycerol release directly across adipose tissue [23]. Although measuring glycerol Ra onsite (i.e., by using arterio-venous differences across subcutaneous adipose tissue) provides the most direct measure of adipose tissue lipolysis, the application of this approach is limited by the technical challenges involved, which includes sampling from the venous outflow of adipose tissue and measuring adipose tissue blood flow. Fortunately, very similar results are obtained when using tracers to determine the ratio of systemic FFA to glycerol Ra at the whole-body level [23,24] which indicates that quantitatively, whole-body lipolysis reflects adipose tissue lipolysis [23]. However, the release of FFA and glycerol in plasma does not always occur in the theoretical ratio of 3:1 [10] because some fatty acids are re-esterified to triglyceride within adipocytes and do not enter the circulation; instead, glycerol is almost quantitatively released because adipose tissue contains little glycerol kinase activity [25]. Hence to measure lipolytic activity in adipose tissue, one typically measures the Ra of glycerol in the bloodstream; nonetheless, FFA Ra also provides a reasonable index of lipolysis under most circumstances [9,26].
The use of systemic glycerol Ra in plasma as an index of adipose tissue lipolysis depends on several reasonable assumptions, such as that glycerol is not produced from any metabolic pathway other than adipose tissue lipolysis (e.g., from glycolysis or the breakdown of lipids in other tissues) [24], that intracellular glycerol cannot be re-utilized to a significant extent within adipocytes [25], and that partial triglyceride hydrolysis is minimal [22]. Some assumptions are challenged, e.g., whether [27] or not [23] plasma glycerol is taken up by adipose tissue (which is relevant to the extent that adipose tissue may be able to utilize free glycerol and thus, not all glycerol liberated during lipolysis may be released into the circulation). And some are apparently not valid; for instance, plasma glycerol is taken up by and released from skeletal muscle [23,28,29]. This will confound the use of whole-body glycerol Ra as an index of adipose tissue lipolysis (since some unknown amount of muscle triglyceride lipolysis would be contributing to the measured Ra), especially in light of the rapid turnover of intramuscular triglyceride pool [30]. Nevertheless, part of glycerol (and FFA) released across human skeletal muscle tissue [23,28] may in fact be derived from adipocytes interspersed within muscle cells rather than from within the muscle itself [29]. Further uncertainty is introduced by the amount of glycerol derived from lipolysis of plasma triglycerides and the degree of splanchnic glycerol release [28]. Recently, Coppack et al. [31] developed a multicompartmental model of in vivo glycerol kinetics and demonstrated that, in the postabsorptive state, glycerol released during lipolysis of plasma triglycerides could account for 15-20% of total glycerol added to the bloodstream, leading to an overestimation of lipolysis rates when using systemic glycerol Ra [31]. At the same time, however, the inability to detect much of visceral fat lipolysis, as glycerol released into the portal vein is cleared by the liver [28,32], should lead to an underestimation of adipose tissue lipolysis by some 10-20% [31]. Fortuitously, these errors would more or less cancel out; therefore, measuring systemic plasma glycerol Ra probably provides a reasonable estimate of whole-body adipose tissue lipolysis rates under postabsorptive conditions. However, these phenomena might confound the results from studies in which for example between-group differences in plasma triglyceride turnover exist.
Glycerol flux has been measured by using either a bolus injection or a constant infusion of a glycerol tracer [33,34]. The rapid disappearance of the tracer after a bolus injection (half-life of ∼4 minutes) [33,34] requires frequent sampling over a very short time span and a very large tracer dose in order for plasma glycerol enrichment to remain at sufficiently high levels for accurate measurement. Thus, the primed, constant infusion technique (typically lasting 60-120 min) is preferred for measuring whole-body glycerol kinetics. The choice of tracer is not essential since 1,1,2,3,3-2H5-glycerol, 2-13C1-glycerol, and 1,2,3-13C3-glycerol all yield comparable results for endogenous glycerol Ra [9]. An infusion rate of 0.05-0.10 μmol per kg per min in the postabsorptive state can be used, whereas a priming dose equal to 15 times the infusion rate (i.e., 0.75-1.5 μmol per kg) is commonly given [10,23,35-37] in light of the large volume of distribution of glycerol [26]. However, priming the glycerol pool is not really necessary considering the rapid turnover of glycerol relative to its pool size in man, which is comparable to that of plasma FFA [38]. In fact, a 60-minute constant infusion of 1,1,2,3,3-2H5-glycerol, at a rate of 0.05 μmol per kg per min without a priming dose, has been used successfully to quantify glycerol kinetics in resting humans in the postabsorptive state [39].
Systemic plasma FFA availability
Many investigators interested in adipose tissue lipolysis prefer to measure plasma FFA (instead of glycerol) Ra [40] because it too provides a reasonable estimate of adipose tissue lipolytic activity under most circumstances [9,26,41], and often the amount and/or type of fatty acids released into the circulation is of greater interest than the actual rate of lipolysis. Similar to what was mentioned above for glycerol Ra, lipolysis of plasma triglyceride provides some fatty acids that escape tissue uptake and enter the systemic circulation, especially in the postprandial state [42,43]; however this is likely not an important source of total plasma FFA flux under postabsorptive conditions [44-46].
The approach most commonly used to measure the appearance of FFA in the circulation involves the constant infusion of a single fatty acid tracer bound to human albumin [9,47-49]. Although the bolus injection technique has been used in early human studies [50,51], the rapid turnover of plasma FFA (half-life of 1-4 minutes) [52,53] presents much of the same problems outlined above for glycerol when using stable isotopes. Binding to albumin is not only necessary because fatty acids are insoluble in aqueous solutions but also limits the initial volume of distribution of the tracer to the intravascular space (although a second, extravascular, slowly-mixing albumin pool does exist) [26] so that, although optimally-described by a two-pool model [51], plasma FFA kinetics during constant infusion of fatty acid tracers can be adequately described by a single-pool model and the endogenous Ra can be calculated by using simple steady-state equations [54]. Due to initial restriction of the tracer in the plasma compartment and the rapid turnover of FFA, no priming dose is necessary [9]. At an infusion rate of 0.03-0.04 μmol per kg per min, isotopic equilibrium is usually achieved within 30-60 minutes [9]. Tracers labeled with 2H are usually preferred over 13C-labeled tracers primarily due to lower cost. An alternative method makes use of uniformly (U)-labeled 13C16-palmitate (or any other U-labeled fatty acid for that matter) and analysis by GC combustion IRMS [55]. Due to the much greater instrument sensitivity, this method allows tracer (and thus albumin) infusion rates to be reduced by more than one order of magnitude.
There are many different fatty acids circulating in plasma, which vary in chain length and structure. When using a single fatty acid tracer (e.g., palmitate) to measure FFA flux, the assumption is made that it is representative of all plasma FFA with regard to turnover [49]. Total plasma FFA flux can then be calculated by dividing the endogenous Ra of that fatty acid by its relative contribution to total plasma FFA concentration. This requires measuring the concentration of all individual fatty acids in plasma by quantitative GC [56]. Early studies in humans, however, suggest that there may be significant differences in the metabolic behavior and kinetics of individual fatty acids in plasma [50,57], which means that not all fatty acids are suitable for use as a tracer of plasma FFA. In a recent study, we measured the Ra of five fatty acids (myristate, palmitate, stearate, oleate, and linoleate) into plasma, which together represent ∼97% of all FFA in the circulation, during the basal postabsorptive state as well as during conditions that stimulated (epinephrine infusion) and inhibited (insulin infusion) adipose tissue lipolysis and thus the release of FFA into the circulation [41]. We was demonstrated that palmitate, oleate and linoleate each provided good estimates (within 10-15%) of total FFA flux across a physiological range of adipose tissue lipolytic rates, whereas myristate and stearate consistently overestimated and underestimated respectively, total FFA flux [41]. Alternatively, mixtures of labeled fatty acids resembling in composition that of plasma FFA are commercially available and can be used as more direct measures of total FFA Ra [16] - however, this is costly and the analysis is cumbersome.
Total FFA Ra (in μmol per min) reflects the total amount of fatty acids released into the circulation. In an attempt to help explain different physiological issues, and to account for differences in body size, systemic FFA flux is often expressed in several different ways [40]. Typically, FFA Ra is normalized for fat-free mass to provide an index of fatty acids released into plasma in relationship to tissues of the body that consume fatty acids for use as a fuel (e.g., skeletal muscle) or for other purposes (e.g., synthesis of triglyceride in the liver); FFA Ra normalized for fat-mass is used to provide an index of fatty acids released into plasma with respect to endogenous adipose tissue triglyceride stores. However, the use of ratios can be misleading because the relationship between FFA Ra and fat-free mass, fat mass, or total body mass is characterized by regression lines that have y and x intercepts that are significantly different from zero [40] (and Mittendorfer et al., unpublished data). Therefore, lipolytic rates normalized for body composition will be different between groups who differ in body composition because of mathematical bias rather than true differences in fatty acid metabolism. Hence careful attention should be paid in cross-sectional comparisons or longitudinal studies with exercise or weight loss, etc., as putative differences in body composition need to be taken into account when interpreting FFA Ra data. Furthermore, differences in tissue perfusion and possibly also in FFA extraction and/or uptake, e.g., between individual skeletal muscles [58,59], likely lead to disproportionate distribution of total FFA flux amongst fat-free tissues or different adipose tissue regions, and therefore further confound the interpretation of ratios. It is also important to note that, probably due to the pulsatile/oscillatory nature of adipose tissue lipolysis [60,61], the intra-individual day-to-day variability of plasma FFA turnover rates is considerable, ∼15-30% [62-64]. Therefore, careful attention to details and standardization of the experimental conditions (prior diet and physical activity, fasting duration, sample timing, etc.) is required if accurate and reproducible estimates of plasma FFA Ra are to be obtained.
When combined with arterio-venous balance measurements across body regions or tissues, fatty acid tracers can be used to provide information on the tissue-specific uptake and release of FFA. In this case, the fractional extraction of fatty acids across the tissue of interest is calculated from the concentration and TTR of fatty acids in arterial and venous blood, and local tissue uptake is then calculated as fractional extraction times arterial concentration times blood flow through the tissue. Local tissue release is readily estimated since net arterio-venous balance equals uptake minus release. This technique has been successfully used to measure local uptake and release rates of FFA across skeletal muscle, adipose tissue and the splanchnic region [16,23,27-29]. Measuring the uptake of FFA by a particular tissue is essential for further assessing fatty acid utilization (e.g., oxidation) within that tissue [16,27,29].
Fatty acid oxidation
Fat is a major energy fuel for the human body. Popular methods such as indirect calorimetry provide an estimate of total fat oxidation rate, including fatty acids taken up from plasma and fatty acids released from intracellular triglyceride that are oxidized directly without entering the circulation. Stable isotope tracers can be used to determine plasma FFA oxidation rate, and in combination with indirect calorimetry, this measure can provide an estimate of intracellular triglyceride oxidation. In addition to whole-body plasma FFA oxidation, using arterio-venous balances across tissues allows for determination of regional fatty acid oxidation, e.g., in skeletal muscle and the splanchnic region [16,27,29]. Traditionally, a constant infusion of a 13C-labeled fatty acid has been used to estimate plasma FFA oxidation, the general idea being that as the fatty acid tracer is oxidized the labeled carbon appears in breath (or in the venous effluent of a tissue when regional metabolism is assessed) as 13C-labeled CO2 [26]. Dividing the TTR of breath CO2 by the infusion rate of the fatty acid tracer at steady-state gives the fractional rate of fatty acid oxidation (i.e., percent uptake of fatty acid that was oxidized to CO2). The absolute rate of oxidation (in μmol per min) can then be calculated by multiplying the rate of disappearance (Rd) of the fatty acid (which equals Ra when in steady-state) by the fractional oxidation rate. Although this provides a reasonable estimate of plasma FFA oxidation, a single fatty acid tracer is not likely truly representative of total plasma FFA oxidation, because there are substantial differences between the oxidation rates of individual fatty acids [65-67], especially between major plasma FFA such as palmitate and oleate (∼20% difference) [66].
When infusing a carbon-labeled fatty acid, some 8 hours of infusion or more are required for an isotopic equilibrium in expired CO2 to be achieved, in part due to slow isotopic exchange between the labeled CO2 (from the oxidation of the fatty acid tracer) and the unlabeled bicarbonate pool [68]. This would introduce substantial error in the calculation of plasma FFA oxidation rates during studies lasting only a few hours (< 4 h) [69]. This problem, common to all substrates being oxidized, was partly resolved by priming the bicarbonate pool, which allowed for considerably shortening the infusion protocol [70,71]. Nonetheless, it was further discovered that the rate of labeled carbon entry via acetyl-CoA in the tricarboxylic acid cycle does not correspond to the rate of labeled carbon appearing in breath, not only due to bicarbonate retention, but also and predominantly due to label fixation occurring during the exchange reactions of the tricarboxylic acid cycle [72]. Subsequently, an acetate correction factor was introduced for use in tracer estimations of plasma FFA oxidation [73]. Acetate enters the tricarboxylic acid cycle directly as acetyl-CoA, so the difference between the amount of labeled carbon infused and that recovered in breath gives the absolute amount of label fixed both via tricarboxylic acid cycle exchange reactions and the bicarbonate pool. Hence the fraction of the label recovered in breath CO2 after labeled acetate infusion represents the appropriate correction factor for use in oxidation calculations [73]. The correction is applied by dividing the calculated rate of plasma FFA oxidation by the acetate correction factor. Although the acetate recovery factor is highly reproducible, there is large inter-individual variability [74], which implies that, ideally, the acetate correction factor needs to be determined in every subject, under similar conditions as those used for the tracer-derived determination of substrate oxidation rates [75]. Simply put, either a separate labeled acetate infusion study, mimicking all conditions of the actual labeled fatty acid infusion study, is required to determine the acetate recovery factor, or stable and radioactive isotope tracers need to be used simultaneously (e.g., 1-14C1-acetate and 1-13C1-palmitate) [73-76]. Because the recovery of labeled CO2 is different for acetate labeled in positions 1 and 2 [77], it is important to use the corresponding acetate and fatty acid tracers (e.g., 1-13C1-acetate and 1-13C1-palmitate or 1,2-13C2-acetate and U-13C16-palmitate).
The limitations and the considerable burden associated with the use of carbon-labeled fatty acid tracers for the determination of plasma FFA oxidation has led to the development of another approach for measuring fat oxidation in vivo, utilizing U-2H31-palmitate [78]. The general idea is that as the fatty acid is being oxidized, deuterium appears as water and mixes with the body water pool; hence, the enrichment of label in body fluids (preferably by sampling blood but also urine or even saliva) can be used effectively to calculate the cumulative recovery of 2H and hence the amount of fat oxidized over a period of time. It was shown that the recovery of 2H in urine 10 hours after dose administration was essentially the same as that for acetate-corrected 13C (the mean difference in percentage recovery of the two labels was ∼0.5%), leading to similar estimates of fat oxidation rates [78]. The method was subsequently validated for estimating fat oxidation rates during exercise [79]. The use of 2H-labeled fatty acid tracers does not require the determination of the acetate correction factor due to minimal sequestration of the label in the tricarboxylic acid cycle [78,79]. Furthermore, there is no need for frequent sampling, not even for tracer infusion; instead, the tracer can be administered orally, which makes the method ideal for use in free-living conditions and for estimation of dietary fatty acid oxidation rates [80]. In fact, this method had been developed and validated for measuring dietary fatty acid oxidation, not endogenous plasma FFA oxidation. However, note should be made of the fact that the U-2H31-palmitate tracer contains 31 atoms of deuterium and the relative increase in mass compared with the native tracee is considerable (>10%); it is not known whether this results in significant isotope effects in vivo.
Hepatic VLDL-triglyceride secretion
During postabsorptive conditions, most plasma triglyceride circulates in the core of VLDLs, which are produced and secreted by the liver, thereby providing energy dense substrates to peripheral tissues, while at the same time buffering excess amounts of fatty acids, which could otherwise be cytotoxic [81]. VLDL is assembled within hepatocytes in a two-step process, which involves the partial lipidation of a newly synthesized apolipoprotein B-100 molecule and the fusion of this precursor with a large triglyceride droplet to form mature VLDL [82]. Each VLDL particle contains a single molecule of apolipoprotein B-100 [83], whereas the availability of core triglyceride varies considerably and in part determines the metabolic fate of the particle itself [84,85]. Fatty acids used for hepatic triglyceride synthesis originate primarily from the systemic plasma FFA pool, as well as from several other, non-systemic fatty acid sources, e.g., hepatic de novo lipogenesis, lipolysis of intrahepatic and intra-abdominal triglyceride [86]. By using tracer techniques, one can study a variety of different aspects of VLDL metabolism and gain unique insights into the system. For example, one can evaluate VLDL-triglyceride kinetics (discussed below) to find out the secretion rate of triglycerides within the VLDL particle core, or one can evaluate VLDL-apolipoprotein B-100 kinetics to find out the metabolic behavior of VLDL particles themselves. The latter techniques are based on protein labeling (e.g., by radio-iodination or by using amino acid tracers) and are only touched upon herein; more detailed reviews are available [11,87]. It is important to point out that studying the kinetics of one VLDL component cannot be used to draw conclusions regarding the kinetics of other components or the whole particle. For example, differences in VLDL-triglyceride secretion between groups or in response to an intervention (e.g., changes in macronutrient composition of the diet, weight loss) are not always mirrored by differences VLDL-apolipoprotein B-100 secretion due to differences in triglyceride loading of VLDL particles [88-90].
The hepatic secretion rate of VLDL-triglyceride can be measured with a variety of different approaches, e.g., splanchnic arterio-venous balance technique, ex vivo labeling of VLDL and re-infusion (as a bolus or constant infusion), and many in vivo labeling techniques with radioisotopes and stable isotopes [12]. The least demanding (for subjects and investigators) and thus most commonly used approach relies on the administration of a labeled precursor (glycerol or fatty acids) and monitoring its incorporation in the labeled product (VLDL-triglyceride) [12]. Constant infusions of glycerol or palmitate tracers have been used to measure VLDL-triglyceride flux by fitting the data to a monoexponential rise-to-plateau model [91-94]. However, this method does not account for the considerable tracer recycling that takes place during a prolonged constant infusion. Recycling of the tracer can occur when plasma glycerol or palmitate is incorporated into another pool (e.g., hepatic triglyceride), from which it is later released and incorporated into VLDL-triglyceride without reappearing in plasma, or tracer initially incorporated in VLDL-triglyceride and subsequently released by lipolysis in plasma, taken up by the liver and re-incorporated in VLDL-triglyceride. During a constant infusion of labeled palmitate, for instance, there is considerable recycling of the tracer within the various hepatic lipid pools (∼15% of all fatty acids in VLDL-TG in healthy subjects [95]), thereby not allowing for adequate resolution of VLDL-triglyceride kinetic parameters [96]. Still, this approach remains useful in that it allows for the determination of the relative contribution of fatty acids from different sources to total VLDL-TG secretion (see later on). Another common approach is the bolus injection of the tracer (usually glycerol but also fatty acid) and the determination of the monoexponential slope of decline in VLDL-triglyceride enrichment [97-102]. These data are used to calculate the fractional turnover rate (FTR) of VLDL-triglyceride, i.e., the fraction of the VLDL-triglyceride pool that enters or leaves the pool per unit of time. The secretion rate of VLDL-triglyceride is then calculated as the FTR times the pool size of VLDL-triglyceride (concentration times plasma volume). However, this approach too does not account for tracer recycling and therefore can lead to substantial underestimation of the true turnover rate.
Compartmental modeling analysis can be used to improve the accuracy of measuring VLDL-triglyceride kinetics by accounting for tracer recycling. By simultaneously administering a bolus of 1,1,2,3,3-2H5-glycerol and a bolus of 1-13C1-palmitate or a constant infusion of 2,2-2H2-palmitate to human subjects in the postabsorptive state, and monitoring the enrichment in VLDL-triglyceride-bound glycerol and palmitate for ∼12 hours, we have recently demonstrated that monoexponential data analysis underestimates the turnover of VLDL-triglyceride compared with compartmental modeling [96]. The latter effect was most prominent when VLDL-triglyceride turnover was high; when the turnover rate is sufficiently slow (<0.5 pools per hour), monoexponential analysis provides similar results with those from compartmental modeling [96]. Monoexponential analysis may thus be accurate under some circumstances [103], though this would require a priori knowledge of the VLDL-triglyceride FTR. There are no differences in the measured VLDL-triglyceride turnover rates when using a fatty acid or glycerol tracer bolus injection in conjunction with compartmental modeling analysis; however, a fatty acid tracer gives lower rates of VLDL-triglyceride turnover than a glycerol tracer when using a bolus injection of the tracer in conjunction with monoexponential slope analysis, most likely due to differences in the magnitude of tracer recycling (less for 2H5-glycerol than fatty acids). Constant infusion of a fatty acid tracer and compartmental data analysis does not overcome this problem because the model, unlike after bolus injection of the same tracer, cannot adequately resolve the extent of recycling.
Modeling, or any of the other approaches available, does not require treating VLDL as a single pool, but can be applied to VLDL subfractions, for instance, the large and triglyceride-rich VLDL1 and small and triglyceride-poor VLDL2, as well [104]. This necessitates separation of VLDL into its subclasses by using preparative ultracentrifugation, thereby likely inflating total measurement variability (20-35%) [105] compared to when the VLDL fraction is treated as a whole (15-25%) [62]. However, it can provide novel insights, e.g., evidence of an inverse association between the hepatic secretion of VLDL1 (decreases) and VLDL2 (increases) in response to insulin [106]. The latter observation is only one of many examples illustrating that the triglyceride content of VLDL is a major factor influencing the metabolism of VLDL particles [84,85]. Stable isotope-labeled tracers for measuring VLDL-triglyceride [12] and VLDL-apolipoprotein B-100 [107] kinetics can be used simultaneously to provide information on the triglyceride content of nascent VLDL particles. Since each VLDL particle contains a single molecule of apolipoprotein B-100 [83], by dividing the secretion rate of VLDL-triglyceride by the secretion rate of VLDL-apolipoprotein B-100 (in molar units), one can obtain an estimate of the average number of triglyceride molecules secreted in each VLDL particle which is an index of nascent VLDL particle size [88,90,108]. This provides a unique insight into VLDL metabolism because it refers to the time of VLDL secretion into the circulation, i.e., before any intravascular remodeling takes place.
Tracers can also be used to provide information about the origin of the fatty acids used for VLDL-triglyceride secretion. By using a constant infusion of labeled palmitate, and monitoring the enrichment in plasma free palmitate and VLDL-triglyceride-bound palmitate for several (∼10-12) hours, in conjunction with multicompartmental modeling, we have demonstrated that it possible to estimate the contribution of systemic plasma FFA and non-systemic fatty acids to total VLDL-triglyceride production [88,90,108-111]. By fitting the isotopic enrichments of plasma free palmitate and palmitate in VLDL-triglyceride to the model, one can derive a dilution factor which indicates the extent to which the plasma palmitate enrichment was diluted by unlabeled sources of palmitate before being incorporated into VLDL-triglyceride. These unlabeled, non-systemic fatty acids in VLDL-triglyceride are derived from pools of fatty acids that are not labeled with tracer during the infusion period, and include: i) fatty acids released from pre-existing, slowly turning over lipid stores in the liver and tissues draining directly into the portal vein, ii) fatty acids liberated from lipolysis of plasma lipoproteins that are taken up by the liver, and iii) fatty acids derived from hepatic de novo lipogenesis. The remaining fatty acids in VLDL-triglyceride (systemic plasma FFA) represent FFA from the systemic circulation that are taken up by the liver and directly incorporated into VLDL-triglyceride, or temporarily incorporated into rapidly turning over intra-hepatic and intra-peritoneal triglyceride stores before incorporation into VLDL-triglyceride.
The relative contribution of fatty acids derived from hepatic de novo lipogenesis (i.e., the synthesis of fatty acids from acetate) to total VLDL-triglyceride production can be determined by using mass isotopomer distribution analysis (MIDA), which evaluates the label incorporation pattern into palmitate in VLDL-triglyceride during constant infusion of a 13C-acetate tracer (1-13C1, 2-13C1, or 1,2-13C2) [112-115]. MIDA relies on combinatorial probabilities in the biosynthesis of polymers from repeating monomer units (e.g., synthesis of a fatty acid molecule from many acetate molecules). For determining the rate of de novo lipogenesis, the acetate tracer is initially distributed in a precursor pool (cytosolic acetyl-CoA in the liver) which is held at constant enrichment throughout the experimental period (hence the need for constant infusion). The fatty acid produced by this precursor pool contains a certain number of precursor monomers (e.g., 8 for palmitate). These monomers are assumed to be randomly selected from the precursor pool when the product is synthesized, so a probability function is constructed to predict the distribution of newly formed fatty acid molecules that will contain from 0 to 8 labeled monomers (in the case of palmitate); the variously-labeled versions of the product are called isotopomers. Since the precursor pool is inaccessible for direct sampling, the precursor enrichment is determined by examining the relative distribution pattern of enriched isotopomers in the product, and the fractional rate of lipogenesis (i.e., the fraction of fatty acids in VLDL-triglyceride derived from de novo synthesis) is calculated from the rate at which enrichment increases in the product [112,115]. The contribution of de novo synthesized fatty acids to total VLDL-triglyceride turnover is typically extremely small (in healthy subjects it contributes <10% to total VLDL-triglyceride production in the fed state and <5% during fasting) [95,113,114,116]. However, there are pathological conditions, such as non-alcoholic fatty liver disease, where de novo lipogenesis can account for as much as ∼15-25% of total VLDL-triglyceride production [116,117].
One limitation of the aforementioned techniques is the use of labeled precursors to investigate VLDL-triglyceride metabolism, which requires metabolic steady-state and makes measurement of kinetics under non-steady-state conditions problematic, because of the ongoing tracer appearance and disappearance at unknown rates [38]. This limitation can perhaps be circumvented with direct administration of labeled VLDL tracers. There have been several early attempts to measure VLDL-triglyceride turnover with in vivo or, most commonly ex vivo production of labeled VLDL-triglyceride with radioisotopes [12]. One of the first such approaches in humans involves ingestion of U-13C3-glycerol to in vivo label the glycerol in VLDL-triglyceride, plasmapheresis and isolation of the newly 13C-labeled VLDL from plasma, and administration within the next 2-3 days via a primed constant autologous re-infusion [118]. More recently, a modification of this technique was presented, involving ex vivo preparation of a VLDL tracer using radiolabeled triglyceride [44]. Although the latter method may be difficult to adapt for use with stable isotopes, it was demonstrated that ex vivo labeled VLDL particles are likely very similar in respect to their kinetic behavior with native, endogenous VLDL particles [44]; more research is warranted to evaluate the metabolism (including the shelf-life) of exogenously prepared VLDL tracers under a variety of physiological conditions. Still, development of such direct methods for measuring VLDL-triglyceride may make it possible to perform kinetic studies under non-steady-state conditions. There remain, however, important limitations, e.g., the autologous VLDL tracer needs to be used within a short period of time (e.g., 2-7 days), thereby precluding longer-term investigations. Also, the risk associated with experimental procedures is relatively high, and there is substantial added burden to subjects.
Postprandial conditions represent a prime example of a metabolic non-steady-state, owing to the dynamic and short-lived changes in lipid metabolism after meal consumption, i.e., the entry of chylomicrons into the circulation, removal of triglycerides from chylomicrons and subsequently chylomicron particle removal from the circulation, concomitant changes in VLDL metabolism, suppression of lipolysis by insulin, etc. Only a few techniques have been developed in an attempt to describe VLDL-triglyceride kinetics under non-steady-state conditions. For example, by taking advantage of the observation that large VLDL accumulate in plasma after a bolus injection of exogenous fat emulsion [119], likely due to saturation of lipoprotein lipase by exogenous chylomicron-like particles [120], Al-Shayji et al. [121] recently described a simple, non-isotopic method for estimating VLDL-triglyceride (and apolipoprotein B-100) kinetics under conditions that mimic the postprandial state. Still, intravenous administration of a fat emulsion as a single bolus does not adequately represent true postprandial conditions, during which chylomicrons enter gradually in the circulation. Other investigators developed techniques to quantify other aspects of VLDL-triglyceride metabolism following ingestion of a meal [17,122,123]. For instance, by adding U-13C16-palmitate to a test meal to label chylomicron-triglycerides and simultaneous intravenous infusion of 2H2-palmitate to label VLDL-triglycerides in conjunction with arterio-venous blood sampling, Bickerton et al. [122] compared the extraction of triglycerides from these two sources across adipose tissue and skeletal muscle in the postprandial period. They observed that both chylomicron- and VLDL-triglyceride are cleared across adipose tissue and muscle, with greater fractional extraction of the chylomicron-triglyceride, confirming in vivo what has previously only been demonstrated in vitro[124], i.e., that lipoprotein lipase preferentially hydrolyzes triglycerides in larger and triglyceride-richer lipoproteins. Furthermore, by modeling the fate of fatty acids released by lipoprotein lipase, they provided evidence of spillover of fatty acids liberated from the lipolysis of lipoprotein-triglyceride into the circulation (as opposed to tissue uptake) [122]. Similar evidence has been presented earlier by Miles et al. [46] using radioactive isotope tracers. In a more recent study, Hodson et al. [125] used various U-13C-labeled fatty acids in a test meal and examined the incorporation of dietary fatty acids into various lipid pools (e.g., VLDL-triglyceride, plasma cholesteryl esters and phospholipids) in human subjects. These and future studies are a much needed contribution to the field and will surely increase our understanding of lipid metabolism in the postprandial state.
Conclusion
Stable isotope-labeled tracers can be safely administered to healthy and diseased individuals to determine the rates at which lipids are handled within the human body, thereby overcoming limitations associated with static metabolic measurements (i.e., concentration) without the potentially negative health effects of radioactive tracers. Stable isotope tracers also offer more flexibility in analysis (e.g., positional specificity allowing multiple labels in a single substrate) and, unlike with radioactive tracers, multiple stable isotope labeled tracers can be used simultaneously (whether of the same or different species). Several aspects of fatty acid and triglyceride metabolism can be investigated and thus enhance our understanding of the normal regulation (e.g., swings throughout the day brought on by physical activity and food intake) but also the dysregulation of lipid metabolism, which is common in obesity [40], insulin resistance [3], severe injury [126] and several other disease states [127]. Application of tracers at the whole-body level is relatively simple, often though the underlying assumptions are limiting the interpretation of the data; regional information can be obtained if only a single tissue is responsible for the whole-body measurement (e.g., VLDL-triglyceride secretion) [7]. The use of tracers for evaluating metabolism across individual tissues requires simultaneous arterio-venous balance measurements which are technically more challenging and add burden to the subjects. There is no ideal tracer or method; the choice of tracer and analytical technique need to be decided on the basis of available resources, desired outcome, and subject population, etc.
Future perspective
Continuous refinement of established stable isotope-labeled tracer techniques and availability of novel new methodologies will provide unique insights into human lipid metabolism in vivo. Further development, including optimal tracer selection and sampling sites, improved tracer isolation and measurement, and better mathematical treatment of the data, as well as combination of tracer methodology with other techniques for the investigation of human metabolism in vivo [128] will expand our understanding of lipid metabolism in health and disease.
Executive summary
Adipose tissue lipolysis and systemic plasma FFA availability
Adipose tissue lipolysis rates and release of FFA in plasma can be readily determined by short-term constant infusions of glycerol and/or fatty acid tracers.
Fatty acid oxidation
Measurement of plasma FFA oxidation rates with carbon-labeled fatty acid tracers necessitates determination of the acetate recovery factor, usually on a separate occasion, because of the loss of labeled carbon before it appears in breath CO2. There are differences between the oxidation of different fatty acids, so a single fatty acid tracer may not be representative of total plasma FFA oxidation.
Measurement of dietary fatty acid oxidation with deuterium-labeled fatty acid tracers is associated with much less burden to the subject and the investigator, when compared with the determination of plasma FFA oxidation.
Hepatic VLDL-triglyceride secretion
Evaluation of VLDL-triglyceride kinetics, especially when using multiple labeled precursors simultaneously, is a burdensome process; however, it provides valuable information on several aspects of hepatic triglyceride and fatty acid metabolism. In vivo or ex vivo production of VLDL tracers for human use is in its infancy, but should enable measurements under a greater variety of circumstances including non-steady-state conditions (e.g., postprandial state).
Conclusions
Stable isotope-labeled tracers make it possible to obtain quantitative information on substrate flux at the whole-body level and across individual tissues (the latter usually when combined with arterio-venous balance measurements).
Tracer methods are associated with many advantages but there are also several underlying assumptions and inherent limitations; these should be taken into account when interpreting kinetic data.
Acknowledgements
The authors’ original work presented in this review has been supported by grants from the American Heart Association (0365436Z and 0510015Z), grant number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH grants AR 49869, HD 057796, DK 56341 (Clinical Nutrition Research Unit), and RR 00954 (Biomedical Mass Spectrometry Resource).
Footnotes
Financial disclosure
None.
Conflicts of interest
None.
Bibliography
*of interest
**of considerable interest
- 1.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55(Suppl 2):S16–23. doi: 10.2337/db06-s003. [DOI] [PubMed] [Google Scholar]
- 2.Eyster KM. The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Adv Physiol Educ. 2007;31:5–16. doi: 10.1152/advan.00088.2006. [DOI] [PubMed] [Google Scholar]
- 3.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Uses a variety of stable isotope tracer methods for the study of metabolism.
- 5.Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86:205–243. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- 6.Ludke SM, Flenker U, Schanzer W, Schomburg D. Stable carbon isotope discrimination by human 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Lipid Res. 2008;49:2620–2626. doi: 10.1194/jlr.M800313-JLR200. [DOI] [PubMed] [Google Scholar]
- *.Shows how stable isotope tracers may be discriminated in the body.
- 7.Patterson BW. Use of stable isotopically labeled tracers for studies of metabolic kinetics: an overview. Metabolism. 1997;46:322–329. doi: 10.1016/s0026-0495(97)90260-2. [DOI] [PubMed] [Google Scholar]
- *.Provides a comprehensive review of instrumentation and methodology used in tracer studies.
- 8.Coggan AR, Mittendorfer B, Klein S. Evaluating substrate metabolism. In: Schuster DP, Powers WJ, editors. Translational and Experimental Clinical Research. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. pp. 435–449. [Google Scholar]
- 9.Wolfe RR. Principles and Practice of Kinetic Analysis. Wiley-Liss; New York, NY: 1992. Radioactive and Stable Isotope Tracers in Biomedicine. [Google Scholar]
- **.A reference book on stable isotope tracer methodology. Ideal for those currently using or planning to use stable isotope labeled tracers for the study of metabolism in vivo.
- 10.Beylot M, Martin C, Beaufrere B, Riou JP, Mornex R. Determination of steady state and nonsteady-state glycerol kinetics in humans using deuterium-labeled tracer. J Lipid Res. 1987;28:414–422. [PubMed] [Google Scholar]
- 11.Burnett JR, Barrett PH. Apolipoprotein B metabolism: tracer kinetics, models, and metabolic studies. Crit Rev Clin Lab Sci. 2002;39:89–137. doi: 10.1080/10408360208951113. [DOI] [PubMed] [Google Scholar]
- **.Provides an overview of methods for measuring apolipoprotein B-100 kinetics in VLDL and LDL.
- 12.Magkos F, Sidossis LS. Measuring very low density lipoprotein-triglyceride kinetics in man in vivo: how different the various methods really are. Curr Opin Clin Nutr Metab Care. 2004;7:547–555. doi: 10.1097/00075197-200409000-00007. [DOI] [PubMed] [Google Scholar]
- **.Provides an overview of methods for measuring VLDL-triglyceride kinetics.
- 13.Jensen MD, Heiling VJ. Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism. 1991;40:406–409. doi: 10.1016/0026-0495(91)90152-m. [DOI] [PubMed] [Google Scholar]
- 14.Mittendorfer B, Sidossis LS, Walser E, Chinkes DL, Wolfe RR. Regional acetate kinetics and oxidation in human volunteers. Am J Physiol. 1998;274:E978–983. doi: 10.1152/ajpendo.1998.274.6.E978. [DOI] [PubMed] [Google Scholar]
- 15.Sidossis LS, Mittendorfer B, Walser E, Chinkes D, Wolfe RR. Hyperglycemia-induced inhibition of splanchnic fatty acid oxidation increases hepatic triacylglycerol secretion. Am J Physiol. 1998;275:E798–805. doi: 10.1152/ajpendo.1998.275.5.E798. [DOI] [PubMed] [Google Scholar]
- 16.Sidossis LS, Mittendorfer B, Chinkes D, Walser E, Wolfe RR. Effect of hyperglycemia-hyperinsulinemia on whole body and regional fatty acid metabolism. Am J Physiol. 1999;276:E427–434. doi: 10.1152/ajpendo.1999.276.3.E427. [DOI] [PubMed] [Google Scholar]
- 17.Evans K, Burdge GC, Wootton SA, Clark ML, Frayn KN. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes. 2002;51:2684–2690. doi: 10.2337/diabetes.51.9.2684. [DOI] [PubMed] [Google Scholar]
- **.Uses stable isotope tracers to examine the fate of dietary fatty acids in the postprandial period.
- 18.Roberts R, Bickerton AS, Fielding BA, et al. Reduced oxidation of dietary fat after a short term high-carbohydrate diet. Am J Clin Nutr. 2008;87:824–831. doi: 10.1093/ajcn/87.4.824. [DOI] [PubMed] [Google Scholar]
- 19.Frayn KN. Macronutrient metabolism of adipose tissue at rest and during exercise: a methodological viewpoint. Proc Nutr Soc. 1999;58:877–886. doi: 10.1017/s0029665199001184. [DOI] [PubMed] [Google Scholar]
- 20.Burdge GC. Metabolism of alpha-linolenic acid in humans. Prostaglandins Leukot Essent Fatty Acids. 2006;75:161–168. doi: 10.1016/j.plefa.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Emken EA. Stable isotope approaches, applications, and issues related to polyunsaturated fatty acid metabolism studies. Lipids. 2001;36:965–973. doi: 10.1007/s11745-001-0807-4. [DOI] [PubMed] [Google Scholar]
- 22.Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19:471–482. doi: 10.1016/j.beem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Coppack SW, Persson M, Judd RL, Miles JM. Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue in vivo. Am J Physiol. 1999;276:E233–240. doi: 10.1152/ajpendo.1999.276.2.E233. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe RR, Peters EJ. Lipolytic response to glucose infusion in human subjects. Am J Physiol. 1987;252:E218–223. doi: 10.1152/ajpendo.1987.252.2.E218. [DOI] [PubMed] [Google Scholar]
- 25.Reshef L, Olswang Y, Cassuto H, et al. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278:30413–30416. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- 26.Klein S, Wolfe RR. The use of isotopic tracers in studying lipid metabolism in human subjects. Baillieres Clin Endocrinol Metab. 1987;1:797–816. doi: 10.1016/s0950-351x(87)80006-x. [DOI] [PubMed] [Google Scholar]
- 27.Van Hall G, Bulow J, Sacchetti M, Al Mulla N, Lyngso D, Simonsen L. Regional fat metabolism in human splanchnic and adipose tissues; the effect of exercise. J Physiol. 2002;543:1033–1046. doi: 10.1113/jphysiol.2002.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen MD. Regional glycerol and free fatty acid metabolism before and after meal ingestion. Am J Physiol. 1999;276:E863–869. doi: 10.1152/ajpendo.1999.276.5.E863. [DOI] [PubMed] [Google Scholar]
- 29.Van Hall G, Sacchetti M, Radegran G, Saltin B. Human skeletal muscle fatty acid and glycerol metabolism during rest, exercise and recovery. J Physiol. 2002;543:1047–1058. doi: 10.1113/jphysiol.2002.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagstrom-Toft E, Qvisth V, Nennesmo I, et al. Marked heterogeneity of human skeletal muscle lipolysis at rest. Diabetes. 2002;51:3376–3383. doi: 10.2337/diabetes.51.12.3376. [DOI] [PubMed] [Google Scholar]
- 31.Coppack SW, Chinkes DL, Miles JM, Patterson BW, Klein S. A multicompartmental model of in vivo adipose tissue glycerol kinetics and capillary permeability in lean and obese humans. Diabetes. 2005;54:1934–1941. doi: 10.2337/diabetes.54.7.1934. [DOI] [PubMed] [Google Scholar]
- **.Uses a combination of methods, including stable isotope tracers, microdialysis, and arterio-venous differences, to describe human adipose tissue lipolysis.
- 32.Havel RJ, Kane JP, Balasse EO, Segel N, Basso LV. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest. 1970;49:2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Pivotal study of VLDL-triglyceride kinetics using the spanchnic arterio-venous balance technique.
- 33.Himms-Hagen J. The turnover of glycerol in plasma. Life Sci. 1963;2:170–174. doi: 10.1016/0024-3205(63)90046-8. [DOI] [PubMed] [Google Scholar]
- 34.Kallai-Sanfacon MA, Norwich KH, Steiner G. A new approach to the measurement of glycerol turnover. Can J Physiol Pharmacol. 1978;56:934–939. doi: 10.1139/y78-148. [DOI] [PubMed] [Google Scholar]
- 35.Bortz WM, Paul P, Haff AC, Holmes WL. Glycerol turnover and oxidation in man. J Clin Invest. 1972;51:1537–1546. doi: 10.1172/JCI106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein S, Young VR, Blackburn GL, Bistrian BR, Wolfe RR. Palmitate and glycerol kinetics during brief starvation in normal weight young adult and elderly subjects. J Clin Invest. 1986;78:928–933. doi: 10.1172/JCI112682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickner RC, Racette SB, Binder EF, Fisher JS, Kohrt WM. Effects of 10 days of endurance exercise training on the suppression of whole body and regional lipolysis by insulin. J Clin Endocrinol Metab. 2000;85:1498–1504. doi: 10.1210/jcem.85.4.6550. [DOI] [PubMed] [Google Scholar]
- 38.Miles JM, Nelson RH. Lipid kinetics. In: Roden M, editor. Clinical Diabetes Research. Methods and Techniques. John Wiley & Sons; Chichester: 2007. pp. 151–168. [Google Scholar]
- 39.Wolfe RR, Klein S, Carraro F, Weber JM. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol. 1990;258:E382–389. doi: 10.1152/ajpendo.1990.258.2.E382. [DOI] [PubMed] [Google Scholar]
- 40.Koutsari C, Jensen MD. Free fatty acid metabolism in human obesity. J Lipid Res. 2006;47:1643–1650. doi: 10.1194/jlr.R600011-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes. 2003;52:1641–1648. doi: 10.2337/diabetes.52.7.1641. [DOI] [PubMed] [Google Scholar]
- **.Uses a variety of fatty acid tracers and demonstrates that not all can be used to measure total free fatty acid rate of appearance in plasma.
- 42.Teusink B, Voshol PJ, Dahlmans VE, et al. Contribution of fatty acids released from lipolysis of plasma triglycerides to total plasma fatty acid flux and tissue-specific fatty acid uptake. Diabetes. 2003;52:614–620. doi: 10.2337/diabetes.52.3.614. [DOI] [PubMed] [Google Scholar]
- 43.Evans K, Clark ML, Frayn KN. Effects of an oral and intravenous fat load on adipose tissue and forearm lipid metabolism. Am J Physiol. 1999;276:E241–248. doi: 10.1152/ajpendo.1999.276.2.E241. [DOI] [PubMed] [Google Scholar]
- 44.Gormsen LC, Jensen MD, Nielsen S. Measuring VLDL-triglyceride turnover in humans using ex vivo-prepared VLDL tracer. J Lipid Res. 2006;47:99–106. doi: 10.1194/jlr.M500205-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Wolfe RR, Shaw JH, Durkot MJ. Effect of sepsis on VLDL kinetics: responses in basal state and during glucose infusion. Am J Physiol. 1985;248:E732–740. doi: 10.1152/ajpendo.1985.248.6.E732. [DOI] [PubMed] [Google Scholar]
- 46.Miles JM, Park YS, Walewicz D, et al. Systemic and forearm triglyceride metabolism: fate of lipoprotein lipase-generated glycerol and free fatty acids. Diabetes. 2004;53:521–527. doi: 10.2337/diabetes.53.3.521. [DOI] [PubMed] [Google Scholar]
- *.Demonstrates that there is escape of fatty acids liberated from lipoprotein-triglyceride lipolysis into the circulation (using radioactive tracers).
- 47.Armstrong DT, Steele R, Altszuler N, Dunn A, Bishop JS, De Bodo RC. Regulation of plasma free fatty acid turnover. Am J Physiol. 1961;201:9–15. doi: 10.1152/ajplegacy.1961.201.1.9. [DOI] [PubMed] [Google Scholar]
- 48.Barter PJ, Nestel PJ. Plasma free fatty acid transport during prolonged glucose consumption and its relationship to plasma triglyceride fatty acids in man. J Lipid Res. 1972;13:483–490. [PubMed] [Google Scholar]
- 49.Wolfe RR, Evans JE, Mullany CJ, Burke JF. Measurement of plasma free fatty acid turnover and oxidation using [1-13C]palmitic acid. Biomed Mass Spectrom. 1980;7:168–171. doi: 10.1002/bms.1200070407. [DOI] [PubMed] [Google Scholar]
- 50.Fredrickson DS, Gordon RS., Jr. The metabolism of albumin-bound C14-labeled unesterified fatty acids in normal human subjects. J Clin Invest. 1958;37:1504–1515. doi: 10.1172/JCI103742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eaton RP, Berman M, Steinberg D. Kinetic studies of plasma free fatty acid and triglyceride metabolism in man. J Clin Invest. 1969;48:1560–1579. doi: 10.1172/JCI106122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nikkila EA. Transport of free fatty acids. Prog Biochem Pharmacol. 1971;6:102–129. [Google Scholar]
- 53.Spector A. Metabolism of free fatty acids. Prog Biochem Pharmacol. 1971;6:130–176. [Google Scholar]
- 54.Miles JM, Ellman MG, McClean KL, Jensen MD. Validation of a new method for determination of free fatty acid turnover. Am J Physiol. 1987;252:E431–438. doi: 10.1152/ajpendo.1987.252.3.E431. [DOI] [PubMed] [Google Scholar]
- 55.Guo Z, Nielsen S, Burguera B, Jensen MD. Free fatty acid turnover measured using ultralow doses of [U-13C]palmitate. J Lipid Res. 1997;38:1888–1895. [PubMed] [Google Scholar]
- *.Describes a method for measuring very low enrichments of plasma fatty acids, in order to enable administration of very small tracer doses.
- 56.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 57.Hagenfeldt L. Turnover of individual free fatty acids in man. Fed Proc. 1975;34:2246–2249. [PubMed] [Google Scholar]
- 58.Hannukainen JC, Nuutila P, Kaprio J, et al. Relationship between local perfusion and FFA uptake in human skeletal muscle-no effect of increased physical activity and aerobic fitness. J Appl Physiol. 2006;101:1303–1311. doi: 10.1152/japplphysiol.00012.2006. [DOI] [PubMed] [Google Scholar]
- 59.Kalliokoski KK, Scheede-Bergdahl C, Kjaer M, Boushel R. Muscle perfusion and metabolic heterogeneity: insights from noninvasive imaging techniques. Exerc Sport Sci Rev. 2006;34:164–170. doi: 10.1249/01.jes.0000240018.07502.48. [DOI] [PubMed] [Google Scholar]
- 60.Getty L, Panteleon AE, Mittelman SD, Dea MK, Bergman RN. Rapid oscillations in omental lipolysis are independent of changing insulin levels in vivo. J Clin Invest. 2000;106:421–430. doi: 10.1172/JCI7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karpe F, Fielding BA, Coppack SW, Lawrence VJ, Macdonald IA, Frayn KN. Oscillations of fatty acid and glycerol release from human subcutaneous adipose tissue in vivo. Diabetes. 2005;54:1297–1303. doi: 10.2337/diabetes.54.5.1297. [DOI] [PubMed] [Google Scholar]
- **.Demonstrates that adipose tissue lipolysis in humans occurs in bursts, which has a direct implication for determining isotopic steady-state in tracer studies.
- 62.Magkos F, Patterson BW, Mittendorfer B. Reproducibility of stable isotope-labeled tracer measures of VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics. J Lipid Res. 2007;48:1204–1211. doi: 10.1194/jlr.D600048-JLR200. [DOI] [PubMed] [Google Scholar]
- **.Describes day-to-day variability of plasma lipid kinetics in human subjects.
- 63.Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes. 1989;38:1595–1601. doi: 10.2337/diab.38.12.1595. [DOI] [PubMed] [Google Scholar]
- 64.Nielsen S, Guo Z, Albu JB, Klein S, O’Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest. 2003;111:981–988. doi: 10.1172/JCI16253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr. 2000;72:905–911. doi: 10.1093/ajcn/72.4.905. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt DE, Allred JB, Kien CL. Fractional oxidation of chylomicron-derived oleate is greater than that of palmitate in healthy adults fed frequent small meals. J Lipid Res. 1999;40:2322–2332. [PubMed] [Google Scholar]
- 67.Jones PJ, Pencharz PB, Clandinin MT. Whole body oxidation of dietary fatty acids: implications for energy utilization. Am J Clin Nutr. 1985;42:769–777. doi: 10.1093/ajcn/42.5.769. [DOI] [PubMed] [Google Scholar]
- 68.Issekutz B, Jr., Paul P, Miller HI, Bortz WM. Oxidation of plasma FFA in lean and obese humans. Metabolism. 1968;17:62–73. doi: 10.1016/s0026-0495(68)80008-3. [DOI] [PubMed] [Google Scholar]
- 69.Leijssen DP, Elia M. Recovery of 13CO2 and 14CO2 in human bicarbonate studies: a critical review with original data. Clin Sci (Lond) 1996;91:665–677. doi: 10.1042/cs0910665. [DOI] [PubMed] [Google Scholar]
- 70.Allsop JR, Wolfe RR, Burke JF. Tracer priming the bicarbonate pool. J Appl Physiol. 1978;45:137–139. doi: 10.1152/jappl.1978.45.1.137. [DOI] [PubMed] [Google Scholar]
- 71.Yki-Jarvinen H, Puhakainen I, Saloranta C, Groop L, Taskinen MR. Demonstration of a novel feedback mechanism between FFA oxidation from intracellular and intravascular sources. Am J Physiol. 1991;260:E680–689. doi: 10.1152/ajpendo.1991.260.5.E680. [DOI] [PubMed] [Google Scholar]
- 72.Sidossis LS, Coggan AR, Gastaldelli A, Wolfe RR. Pathway of free fatty acid oxidation in human subjects. Implications for tracer studies. J Clin Invest. 1995;95:278–284. doi: 10.1172/JCI117652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sidossis LS, Coggan AR, Gastaldelli A, Wolfe RR. A new correction factor for use in tracer estimations of plasma fatty acid oxidation. Am J Physiol. 1995;269:E649–656. doi: 10.1152/ajpendo.1995.269.4.E649. [DOI] [PubMed] [Google Scholar]
- **.Describes the development of acetate correction factor, which improves estimates of plasma fatty acid oxidation in humans.
- 74.Schrauwen P, van Aggel-Leijssen DP, van Marken Lichtenbelt WD, van Baak MA, Gijsen AP, Wagenmakers AJ. Validation of the [1,2-13C]acetate recovery factor for correction of [U-13C]palmitate oxidation rates in humans. J Physiol. 1998;513:215–223. doi: 10.1111/j.1469-7793.1998.215by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schrauwen P, Blaak EE, Van Aggel-Leijssen DP, Borghouts LB, Wagenmakers AJ. Determinants of the acetate recovery factor: implications for estimation of [13C]substrate oxidation. Clin Sci (Lond) 2000;98:587–592. [PubMed] [Google Scholar]
- 76.van Loon LJ, Koopman R, Schrauwen P, Stegen J, Wagenmakers AJ. The use of the [1,2-13C]acetate recovery factor in metabolic research. Eur J Appl Physiol. 2003;89:377–383. doi: 10.1007/s00421-003-0810-x. [DOI] [PubMed] [Google Scholar]
- 77.Wolfe RR, Jahoor F. Recovery of labeled CO2 during the infusion of C-1-vs C-2-labeled acetate: implications for tracer studies of substrate oxidation. Am J Clin Nutr. 1990;51:248–252. doi: 10.1093/ajcn/51.2.248. [DOI] [PubMed] [Google Scholar]
- 78.Votruba SB, Zeddun SM, Schoeller DA. Validation of deuterium labeled fatty acids for the measurement of dietary fat oxidation: a method for measuring fat-oxidation in free-living subjects. Int J Obes Relat Metab Disord. 2001;25:1240–1245. doi: 10.1038/sj.ijo.0801672. [DOI] [PubMed] [Google Scholar]
- **.Describes a method for measuring dietary fatty acid oxidation in human subjects.
- 79.Raman A, Blanc S, Adams A, Schoeller DA. Validation of deuterium-labeled fatty acids for the measurement of dietary fat oxidation during physical activity. J Lipid Res. 2004;45:2339–2344. doi: 10.1194/jlr.M400289-JLR200. [DOI] [PubMed] [Google Scholar]
- 80.Westerterp KR, Smeets A, Lejeune MP, Wouters-Adriaens MP, Westerterp-Plantenga MS. Dietary fat oxidation as a function of body fat. Am J Clin Nutr. 2008;87:132–135. doi: 10.1093/ajcn/87.1.132. [DOI] [PubMed] [Google Scholar]
- 81.Gibbons GF, Wiggins D, Brown AM, Hebbachi AM. Synthesis and function of hepatic very-low-density lipoprotein. Biochem Soc Trans. 2004;32:59–64. doi: 10.1042/bst0320059. [DOI] [PubMed] [Google Scholar]
- 82.Shelness GS, Sellers JA. Very-low-density lipoprotein assembly and secretion. Curr Opin Lipidol. 2001;12:151–157. doi: 10.1097/00041433-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 83.Elovson J, Chatterton JE, Bell GT, et al. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. J Lipid Res. 1988;29:1461–1473. [PubMed] [Google Scholar]
- 84.Packard CJ. Understanding coronary heart disease as a consequence of defective regulation of apolipoprotein B metabolism. Curr Opin Lipidol. 1999;10:237–244. doi: 10.1097/00041433-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Gibbons GF, Islam K, Pease RJ. Mobilisation of triacylglycerol stores. Biochim Biophys Acta. 2000;1483:37–57. doi: 10.1016/s1388-1981(99)00182-1. [DOI] [PubMed] [Google Scholar]
- 86.Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8:146–153. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 87.Parhofer KG, Barrett PH. What we have learned about VLDL and LDL metabolism from human kinetics studies. J Lipid Res. 2006;47:1620–1630. doi: 10.1194/jlr.R600013-JLR200. [DOI] [PubMed] [Google Scholar]
- 88.Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J Clin Endocrinol Metab. 2007;92:1311–1318. doi: 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- **.Similar to ref. 4; uses a combination of tracers to describe sex differences in lipid metabolism.
- 89.Melish J, Le NA, Ginsberg H, Steinberg D, Brown WV. Dissociation of apoprotein B and triglyceride production in very-low-density lipoproteins. Am J Physiol. 1980;239:E354–362. doi: 10.1152/ajpendo.1980.239.5.E354. [DOI] [PubMed] [Google Scholar]
- 90.Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab. 2003;284:E549–556. doi: 10.1152/ajpendo.00379.2002. [DOI] [PubMed] [Google Scholar]
- 91.Boberg J, Carlson LA, Freyschuss U. Studies on the total and splanchnic turnover of plasma free fatty acids and plasma triglycerides in man by means of isotopic and chemical methods. Prog Biochem Pharmacol. 1969;5:149–159. [Google Scholar]
- 92.Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J Clin Invest. 1999;104:1087–1096. doi: 10.1172/JCI6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Siler SQ, Neese RA, Parks EJ, Hellerstein MK. VLDL-triglyceride production after alcohol ingestion, studied using [2-13C1] glycerol. J Lipid Res. 1998;39:2319–2328. [PubMed] [Google Scholar]
- 94.Wang W, Basinger A, Neese RA, et al. Effect of nicotinic acid administration on hepatic very low density lipoprotein-triglyceride production. Am J Physiol Endocrinol Metab. 2001;280:E540–547. doi: 10.1152/ajpendo.2001.280.3.E540. [DOI] [PubMed] [Google Scholar]
- 95.Vedala A, Wang W, Neese RA, Christiansen MP, Hellerstein MK. Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J Lipid Res. 2006;47:2562–2574. doi: 10.1194/jlr.M600200-JLR200. [DOI] [PubMed] [Google Scholar]
- **.Uses various stable isotope tracers intravenously and orally to determine the contribution of fatty acids from different sources to VLDL-triglyceride.
- 96.Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res. 2002;43:223–233. [PubMed] [Google Scholar]
- **.Compares various methods for measuring VLDL-triglyceride kinetics in humans using stable isotopes, and develops and validates a novel multicompartmental approach.
- 97.Kekki M. Plasma triglyceride turnover in 92 adult normolipaemic and 30 hypertriglyceridaemic subjects-the effect of age, synthesis rate and removal capacity on plasma triglyceride concentration. Ann Clin Res. 1980;12:64–76. [PubMed] [Google Scholar]
- 98.Sane T, Nikkila EA. Very low density lipoprotein triglyceride metabolism in relatives of hypertriglyceridemic probands-Evidence for genetic control of triglyceride removal. Arteriosclerosis. 1988;8:217–226. doi: 10.1161/01.atv.8.3.217. [DOI] [PubMed] [Google Scholar]
- 99.Farquhar JW, Gross RC, Wagner RM, Reaven GM. Validation of an incompletely coupled two-compartment nonrecycling catenary model for turnover of liver and plasma triglyceride in man. J Lipid Res. 1965;6:119–134. [PubMed] [Google Scholar]
- 100.Reaven GM, Hill DB, Gross RC, Farquhar JW. Kinetics of triglyceride turnover of very low density lipoproteins of human plasma. J Clin Invest. 1965;44:1826–1833. doi: 10.1172/JCI105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nikkila EA, Kekki M. Polymorphism of plasma triglyceride kinetics in normal human adult subjects. Acta Med Scand. 1971;190:49–59. doi: 10.1111/j.0954-6820.1971.tb07395.x. [DOI] [PubMed] [Google Scholar]
- 102.Lemieux S, Patterson BW, Carpentier A, Lewis GF, Steiner G. A stable isotope method using a [2H5]glycerol bolus to measure very low density lipoprotein triglyceride kinetics in humans. J Lipid Res. 1999;40:2111–2117. [PubMed] [Google Scholar]
- 103.Magkos F, Tsekouras YE, Prentzas KI, et al. Acute exercise-induced changes in basal VLDL-triglyceride kinetics leading to hypotriglyceridemia manifest more readily after resistance than endurance exercise. J Appl Physiol. 2008;105:1228–1236. doi: 10.1152/japplphysiol.90761.2008. [DOI] [PubMed] [Google Scholar]
- 104.Adiels M, Packard C, Caslake MJ, et al. A new combined multicompartmental model for apolipoprotein B-100 and triglyceride metabolism in VLDL subfractions. J Lipid Res. 2005;46:58–67. doi: 10.1194/jlr.M400108-JLR200. [DOI] [PubMed] [Google Scholar]
- **.Develops a novel multicompartmental approach for measuring the kinetics of triglyceride and apolipoprotein B-100 in VLDL subfractions.
- 105.Gill JM, Brown JC, Bedford D, et al. Hepatic production of VLDL1 but not VLDL2 is related to insulin resistance in normoglycaemic middle-aged subjects. Atherosclerosis. 2004;176:49–56. doi: 10.1016/j.atherosclerosis.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 106.Adiels M, Westerbacka J, Soro-Paavonen A, et al. Acute suppression of VLDL1 secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia. 2007;50:2356–2365. doi: 10.1007/s00125-007-0790-1. [DOI] [PubMed] [Google Scholar]
- 107.Watts GF, Moroz P, Barrett PH. Kinetics of very-low-density lipoprotein apolipoprotein B-100 in normolipidemic subjects: pooled analysis of stable-isotope studies. Metabolism. 2000;49:1204–1210. doi: 10.1053/meta.2000.8621. [DOI] [PubMed] [Google Scholar]
- 108.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Similar to ref. 4; uses a combination of tracers to describe the effect of intra-hepatic fat on lipid metabolism.
- 109.Magkos F, Patterson BW, Mittendorfer B. No effect of menstrual cycle phase on basal very-low-density lipoprotein triglyceride and apolipoprotein B-100 kinetics. Am J Physiol Endocrinol Metab. 2006;291:E1243–1249. doi: 10.1152/ajpendo.00246.2006. [DOI] [PubMed] [Google Scholar]
- 110.Magkos F, Patterson BW, Mohammed BS, Mittendorfer B. A single 1-h bout of evening exercise increases basal FFA flux without affecting VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics in untrained lean men. Am J Physiol Endocrinol Metab. 2007;292:E1568–1574. doi: 10.1152/ajpendo.00636.2006. [DOI] [PubMed] [Google Scholar]
- 111.Magkos F, Wright DC, Patterson BW, Mohammed BS, Mittendorfer B. Lipid metabolism response to a single, prolonged bout of endurance exercise in healthy young men. Am J Physiol Endocrinol Metab. 2006;290:E355–362. doi: 10.1152/ajpendo.00259.2005. [DOI] [PubMed] [Google Scholar]
- 112.Hellerstein MK, Christiansen M, Kaempfer S, et al. Measurement of de novo hepatic lipogenesis in humans using stable isotopes. J Clin Invest. 1991;87:1841–1852. doi: 10.1172/JCI115206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Describes the use of stable isotopes for measuring hepatic de novo lipogenesis in humans.
- 113.Aarsland A, Chinkes D, Wolfe RR. Contributions of de novo synthesis of fatty acids to total VLDL-triglyceride secretion during prolonged hyperglycemia/hyperinsulinemia in normal man. J Clin Invest. 1996;98:2008–2017. doi: 10.1172/JCI119005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab. 2006;91:1446–1452. doi: 10.1210/jc.2005-1709. [DOI] [PubMed] [Google Scholar]
- **.Uses a variety of tracers to determine the contribution of fatty acids from different sources to VLDL-triglyceride, during both the fasting and fed conditions.
- 115.Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr. 1996;16:523–557. doi: 10.1146/annurev.nu.16.070196.002515. [DOI] [PubMed] [Google Scholar]
- *.Provides a good background on mass isotopomer distribution analysis (MIDA).
- 116.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 117.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sidossis LS, Magkos F, Mittendorfer B, Wolfe RR. Stable isotope tracer dilution for quantifying very low-density lipoprotein-triacylglycerol kinetics in man. Clin Nutr. 2004;23:457–466. doi: 10.1016/j.clnu.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 119.Bjorkegren J, Packard CJ, Hamsten A, et al. Accumulation of large very low density lipoprotein in plasma during intravenous infusion of a chylomicron-like triglyceride emulsion reflects competition for a common lipolytic pathway. J Lipid Res. 1996;37:76–86. [PubMed] [Google Scholar]
- 120.Karpe F, Bickerton AS, Hodson L, Fielding BA, Tan GD, Frayn KN. Removal of triacylglycerols from chylomicrons and VLDL by capillary beds: the basis of lipoprotein remnant formation. Biochem Soc Trans. 2007;35:472–476. doi: 10.1042/BST0350472. [DOI] [PubMed] [Google Scholar]
- 121.Al-Shayji IA, Gill JM, Cooney J, Siddiqui S, Caslake MJ. Development of a novel method to determine very low density lipoprotein kinetics. J Lipid Res. 2007;48:2086–2095. doi: 10.1194/jlr.D600044-JLR200. [DOI] [PubMed] [Google Scholar]
- 122.Bickerton AS, Roberts R, Fielding BA, et al. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes. 2007;56:168–176. doi: 10.2337/db06-0822. [DOI] [PubMed] [Google Scholar]
- **.Uses a dual-labeling technique (oral and intravenous) to describe the tissue-specific fate of lipoprotein-triglyceride in the postprandial state.
- 123.Hodson L, Bickerton AS, McQuaid SE, et al. The contribution of splanchnic fat to VLDL triglyceride is greater in insulin-resistant than insulin-sensitive men and women: studies in the postprandial state. Diabetes. 2007;56:2433–2441. doi: 10.2337/db07-0654. [DOI] [PubMed] [Google Scholar]
- 124.Fisher RM, Coppack SW, Humphreys SM, Gibbons GF, Frayn KN. Human triacylglycerol-rich lipoprotein subfractions as substrates for lipoprotein lipase. Clin Chim Acta. 1995;236:7–17. doi: 10.1016/0009-8981(95)06032-3. [DOI] [PubMed] [Google Scholar]
- 125.Hodson L, McQuaid SE, Karpe F, Frayn KN, Fielding BA. Differences in partitioning of meal fatty acids into blood lipid fractions: a comparison of linoleate, oleate and palmitate. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.90730.2008. doi:10.1152/ajpendo.90730.92008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cree MG, Wolfe RR. Postburn trauma insulin resistance and fat metabolism. Am J Physiol Endocrinol Metab. 2008;294:E1–9. doi: 10.1152/ajpendo.00562.2007. [DOI] [PubMed] [Google Scholar]
- 127.Betteridge DJ, Illingworth DR, Shepherd J, editors. Lipoproteins in Health and Disease. Arnold Publishers; London: 1999. [Google Scholar]
- 128.Magkos F, Sidossis LS. Methodological approaches to the study of metabolism across individual tissues in man. Curr Opin Clin Nutr Metab Care. 2005;8:501–510. doi: 10.1097/01.mco.0000170756.71114.78. [DOI] [PubMed] [Google Scholar]