Abstract
Recent studies have demonstrated that green and black tea consumption can lower serum cholesterol in animals and in man, and suppression of hepatic cholesterol synthesis is suggested to contribute to this effect. To evaluate this hypothesis, we measured cholesterol synthesis in cultured rat hepatoma cells in the presence of green and black tea extracts and selected components. Green and black tea decreased cholesterol synthesis by up to 55% and 78%, respectively, as measured by a 3-h incorporation of radiolabeled acetate. Inhibition was much less evident when radiolabeled mevalonate was used, suggesting that the inhibition was mediated largely at or above the level of HMG-CoA reductase. Both extracts directly inhibited HMG-CoA reductase when added to microsomal preparations, although the extent of inhibition was considerably less than the decrease in cholesterol synthesis observed in whole cells. As HMG-CoA reductase activity also can be decreased by enzyme phosphorylation by AMP-kinase, the phosphorylation state of HMG-CoA reductase and AMP-kinase, which is activated by phosphorylation, was determined in lysates from cells treated with tea extracts. Both extracts increased AMP-kinase phosphorylation and HMG-CoA reductase phosphorylation by 2.5- to 4-fold, but with different time-courses: maximal phosphorylation with green tea was evident within 30 min of treatment, whereas with black tea phosphorylation was slower to develop, with maximal phosphorylation occurring 3 or more h after treatment. These results suggest that both green and black tea decrease cholesterol synthesis in whole cells by directly inhibiting HMG-CoA reductase and by promoting its inactivation by AMP-kinase.
Keywords: cholesterol, AMP-kinase, HMG-CoA reductase, hepatoma cells, tea
1. Introduction
Epidemiological studies have indicated that tea consumption is associated with a lower risk of cardiovascular disease. This decreased risk is attributed to the ability of tea to lower serum cholesterol levels, and several clinical studies have demonstrated that black tea can lower serum total- and LDL-cholesterol [1,2]. Green tea has been shown to be hypocholesterolemic in animal studies, with the bulk of evidence indicating that tea polyphenols reduce the absorption of dietary and biliary cholesterol and promote its fecal excretion [3]. Whether inhibition of cholesterol synthesis also plays a role in the hypocholesterolemic effect of tea is not yet clearly established.
Feeding studies have been equivocal on the ability of green tea extract to inhibit cholesterol synthesis. Although a recent study by Bursill and colleagues [4] showed a decrease in serum lathosterol (an indicator of whole body cholesterol synthesis) in rabbits fed a green tea extract, a similar study with rats by these investigators [5] was unable to demonstrate a decrease in this serum sterol, despite significant reductions in hepatic cholesterol levels and an increase in LDL receptor expression. A feeding study by Chan et al. [6] was similarly unable to demonstrate an effect of green tea extract on hepatic HMG-CoA reductase activity. Measuring cholesterol synthesis in vivo is difficult, whereas in vitro studies are more tractable. In this regard, Gebhardt and colleagues reported that several common polyphenols (luteolin, quercetin) were able to decrease cholesterol synthesis when added to cultured hepatocytes or hepatoma cell cultures [7,8]. This inhibition appeared to occur at the level of HMG-CoA reductase. Tea polyphenols [9], as well as the simple polyphenol resveratrol [10], have been shown to directly inhibit squalene monooxygenase, a rate-limiting downstream enzyme in cholesterol synthesis. Two studies by Bursill and colleagues [11,12] demonstrated an increase in HMG-CoA reductase and LDL-receptor mRNA in HepG2 cells incubated with green tea extract or its principal component, epigallocatechin gallate (EGCG), and a decrease in cellular lathosterol, indicating that cholesterol synthesis was inhibited in treated cells. Together, these studies suggest that green tea polyphenols are inhibitory to cholesterol synthesis, but the mechanism remains to be established. Moreover, the effect of black tea extract, which consists predominantly of a diverse mixture of polymerized polyphenols termed theaflavins and thearubigins, has not been examined, despite the recent clinical evidence that black tea can modestly reduce serum cholesterol levels.
Cholesterol synthesis is a highly regulated pathway and is subject to transcriptional, translational, and post-translational modulation [13]. Components of tea could act both directly to inhibit cholesterolgenic enzymes and indirectly to modulate the expression or activity of these enzymes. HMG-CoA reductase, the third enzyme in this pathway, is considered to be the primary regulatory step, and is subject to post-translational control through changes in the rate of degradation [13] and changes in its phosphorylation state; phosphorylation inactivates the enzyme and provides a means to rapidly modulate cholesterol synthesis in response to environmental and cellular changes [14].
AMP-kinase is the principal kinase involved in the phosphorylation-mediated inactivation of HMG-CoA reductase [15] and is itself activated by phosphorylation by upstream kinases [16,17]. In the present study we examined the effect of green and black tea extract and some individual components of green tea on cholesterol synthesis in hepatoma cells, and show that both extracts directly inhibit HMG-CoA reductase and activate AMP-kinase, leading to a concerted suppression of cholesterol synthesis at HMG-CoA reductase.
2. Methods and Materials
2.1 Materials
Tea extract from black tea, polyphenols from green tea, (-)-epicatechin, (-)-epicatechin gallate (ECG); (-)-epigallocatechin (EGC); (-)-epigallocatechin gallate (EGCG), ellagic acid, and gallic acid were obtained from Sigma Chemical Co. (St. Louis, MO) and were dissolved in sterile water at 1 mg/ml; epicatechin was dissolved in a 1:1 mixture of actone:water, and ellagic acid was dissolved in 1 M sodium hydroxide. All were used fresh or stored at -20°C. Lipoprotein-free calf serum, acadesine (AICAR), 1,1-dimethylbiguanide hydrochloride (Metformin), and protease inhibitor cocktail were also obtained from Sigma. HALT phosphatase inhibitor and the BCA protein assay kit were purchased from Pierce Biotechnology, Inc. (Rockford, IL). Dulbecco's modified media (DMEM), penicillin-streptomycin-glutamine (PSG), and trypsin were obtained from GIBCO (Invitrogen, Carlsbad, CA). Fetal bovine serum was obtained from BioWhittaker, Inc. (Walkersville, MD) or GIBCO. Radiochemicals (14C-acetate at 56 mCi/mmol, 14C-mevalonate at 65 mCi/mmol, and 14C-Hydroxymethylglutaryl-CoA at 55 mCi/mmol) were purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). McARH7777 rat hepatoma cells were obtained from American Type Culture Collection and used between passages 1-40.
2.2 Cytotoxicity assays
McARH7777 rat hepatoma cells were grown in DMEM supplemented with 10% FBS and 1.0% PSG in 6-well plates at 37°C under a humidified atmosphere of 5% CO2. The cells were used between passages 1 and 40. After 48 h the media was replaced with fresh media with the addition of the selected tea compound or extract and incubation was continued for 3 h. Control cells received an equal volume of vehicle. The cells were detached with trypsin, suspended in DMEM containing 0.2% trypan blue, and counted in a hemocytometer. Leakage of lactate dehydrogenase from cells was determined by measuring NADH oxidation from added pyruvate spectrophotometrically at 340 nm and compared to total lactate dehydrogenase activity from cells lysed with 0.1% Triton X100.
2.3 Determination of cholesterol synthesis
Hepatoma cells were cultured for 48 h in 6-well plates, at which time the media was replaced and appropriate concentrations of the tea extracts or compounds were added along with 1 μCi of 14C-acetate or 14C-mevalonate. Incubations were carried out for 3 h, after which time the cells were washed twice with phosphate-buffered saline, harvested by trypsinization or scraping, resuspended in 20 mM Tris buffer, pH 7.4, containing 0.1% Triton X100, and lysed by sonication (Sonic Dismembrator, Fisher Scientific, Pittsburgh, PA) at medium setting on ice with ten 8-sec pulses, separated by 30 sec each. Lipids were extracted into 5 ml of chloroform:methanol (2:1), the solvent was removed by evaporative centrifugation, and the lipids were resuspended in 50 μl of chloroform/methanol and spotted onto silica thin layer plates (Whatman). Chromatography was carried out in petroleum ether:ethyl ether:acetic acid (60:40:1). Cholesterol was identified by co-chromatography of an authentic standard visualized by iodine vapor staining and quantified by electronic autoradiography (Packard Instant Imager).
2.4 Determination of 14C-acetate uptake
Hepatoma cells treated with tea extract were incubated for 3 h with 1 μCi of 14C-acetate or 14C-mevalonate, after which time the media was removed, the cells were washed twice with phosphate-buffered saline, harvested by trypsinization or scraping, resuspended in 20 mM Tris buffer, pH 7.4, containing 0.1% Triton X100, and lysed by sonication (Sonic Dismembrator, Fisher Scientific) at medium setting on ice with ten 8-sec pulses, separated by 30 sec each. Radioactivity was counted by scintillation spectrophotometry and normalized to protein concentration, as determined by BCA method (Pierce Chemical, Inc., Rockford, IL).
2.5 Determination of squalene and lanosterol synthesis
For the determination of squalene and lanosterol synthesis, cells were incubated as described above for cholesterol synthesis with the inclusion of 60 μM terbinafine (100× in ethanol), an inhibitor of squalene monooxygenase (for the determination of squalene) or 10 μM ketoconazole (100× in methanol), an inhibitor of lanosterol demethylase (for the determination of lanosterol), along with the selected tea extract or component. Lipids were saponified by addition of 0.5 ml of 10% methanolic potassium hydroxide and incubated at 80°C for 1 h. For the determination of squalene the neutral lipids were extracted into 5 ml of petroleum ether, the solvent was removed by centrifugal evaporation, and the samples were resuspended in 50 μl of petroleum ether and resolved by silica thin-layer chromatography in 5% ethyl acetate in hexane. Lanosterol was determined as described for cholesterol. Authentic standards for squalene and lanosterol were visualized by iodine-vapor staining.
2.6 Determination of HMG-CoA reductase activity in microsomes
The microsomal fraction (100,000×g pellet resuspended at ∼15 mg protein/ml) was prepared by standard procedures from the livers of untreated male Harlan Sprague-Dawley rats (∼200 g). HMG-CoA reductase activity was determined by the procedure of Brown and Goldstein [18] as follows: Microsomes (100 μg) were incubated at 37°C in a final volume of 200 μl of 0.1 M potassium phosphate buffer, pH 7.5, containing the indicated tea extract or component, 20 mM glucose 6-phosphate, 2.5 mM NADP+, 1 unit of glucose-6-phosphate dehydrogenase, 5 mM dithiothreitol, and 0.2 μCi 14C-HMG-CoA.
The reaction was stopped after 3 h by the addition of 25 μl of 6M HCl. Mevalonate was converted to the lactone by standing at 37°C for 30 min, extracted into 5 ml of ethyl acetate, and brought to dryness by evaporative centrifugation. The sample was redissolved in 50 μl of ethyl acetate and fractionated by silica thin-layer chromatography with benzene:acetone (1:1). Mevalonolactone was identified by co-migration with authentic mevalonolactone visualized by iodine vapor staining and quantified by electronic autoradiography.
2.7 Immunoquantitation of AMP-kinase and HMG-CoA reductase
Hepatoma cells between passages 12-15 were cultured in 6 cm plates for 2-3 days, until reaching confluence, with daily changes of media. Cells were treated with the indicated tea extract or AMP-kinase activator (AICAR or metformin, 1 mM final concentration) for 3 hr in fresh media, after which cells were washed once with phosphate-buffered saline, scraped from the plates, pelleted by low-speed centrifugation, and lysed by two cycles of freeze-thawing in 0.25 M Tris buffer, pH 7.5, containing protease and phosphatase inhibitor cocktails at 2× standard concentration. The lysates were cleared by centrifugation (18,300 × g, 10 min, at 4°C) and the supernatant was stored as aliquots at -80°C. Thirty μg of protein (determined by BCA analysis) was fractionated by sodium dodecyl sulfate-polyacrylamide gel (8%) electrophoresis and electroblotted to nitrocellulose. The membrane was blocked with 0.05% Tween-20 and 5% defatted milk, and then incubated in this same buffer with rabbit antibody to total AMP-kinase (Anti-AMPK α-pan, 1:2000; Upstate USA, Inc., Charlottesville, VA) or to phosphorylated AMP-kinase (Anti-phospho-AMPKα, 1:500; Upstate USA, Inc.) overnight at 4°C. The immunoblot was developed with a secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature and the chemiluminescent image (Supersignal West Pico Chemiluminescent Substrate, Pierce Biotechnology, Inc.) captured by autoradiography. Band intensity was measured with Image J software on the scanned image with background subtraction. For estimation of HMG-CoA reductase phosphorylation, lysates were incubated for 1 hr at 4° with 20 μl of mouse monoclonal antibody to phosphoserine/phosphothreonine/phosphotyrosine (Abcam Inc., Cambridge, MA) and antibody conjugates were precipitated with 35 μl of protein G Plus-Agarose from Calbiochem (EMD Chemicals, La Jolla, CA) by following an Amersham Biosciences immunoprecipitation protocol. Immunoprecipitated protein was released by boiling in gel loading buffer and fractionated by SDS-gel electrophoresis, followed by electroblotting to nitrocellulose. HMG-CoA reductase was detected and quantified with an antibody to the enzyme (Upstate USA, Inc.) followed by a secondary antibody conjugated with horseradish peroxidase as described above.
3. Results
The addition of a green or black tea extract to the media of McARH7777 rat hepatoma cell cultures decreased 14C-acetate incorporation into cholesterol in a 3-h assay (Fig. 1). Black tea extract at 100 μg/ml resulted in a 78% reduction in cholesterol synthesis, while green tea extract yielded a 55% decrease at 250 μg/ml. EGCG, which constitutes about 70% of the polyphenols in green tea extract, produced a 36% decrease at 458 μg/ml (1 mM), suggesting that other components of this extract contribute significantly to the observed inhibition. However, a number of related polyphenols found in green tea (epicatechin, epicatechin gallate, and epigallocatechin), and ellagic acid were not inhibitory at concentrations up to 1 mM. Epicatechin stimulated acetate incorporation into cholesterol by 47% at 1 mM, and was the only compound found to do so. None of the above compounds, nor the tea extracts, were cytotoxic at maximal concentrations tested as determined by trypan blue exclusion and lactate dehydrogenase release assays (data not shown). Gallic acid was also tested but found to be strongly toxic at sub-millimolar concentrations. The inhibition by the tea extracts could be readily reversed by removing the media, rinsing twice with phosphate-buffered saline, and adding back fresh media.
Fig. 1.
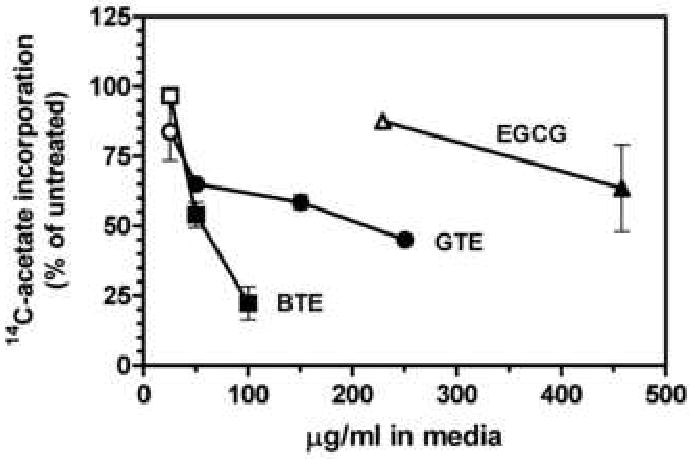
Inhibition of 14C-acetate incorporation into cholesterol by tea. Cholesterol synthesis from 14C-acetate was determined in 3-h assays with hepatoma cells in the presence of tea extracts or selected components. Values represent the mean and standard error of two to three experiments carried out in duplicate. Closed symbols are statistically different from untreated controls as determined by one-way analysis of variance with Dunnett's post-hoc test, p < 0.05. BTE, black tea extract; GTE, green tea extract.
The inhibition observed with 14C-acetate was much less evident when 14C-mevalonate was used to radiolabel cholesterol, as shown in Fig. 2. Inhibition with mevalonate was no greater than 15% at concentrations of green tea extract that yielded a 42% decrease in 14C-acetate incorporation (Fig. 2A). As mevalonate is the product of HMG-CoA reductase, the second and principal regulatory enzyme for cholesterol biosynthesis, this result indicates that green tea extract inhibits cholesterol synthesis at or prior to this enzyme. Total 14C-acetate uptake and incorporation into cellular components follows a similar course (Fig 2A, dashed line), suggesting that at higher concentrations green tea suppresses other biosynthetic pathways that utilize acetate as well. Uptake and incorporation of mevalonate was not affected by green tea extract (data not shown), consistent with the more limited role of mevalonate in biosynthetic pathways. However, at the highest concentration of green tea extract tested, 250 μg/ml, mevalonate incorporation into cholesterol was decreased to the level seen with 14C-acetate, suggesting that green tea may act via multiple and sequential mechanisms to suppress cholesterol synthesis. Similar results were obtained with black tea extract (Fig. 2B). Inhibition of 14C-acetate incorporation was evident at 35 μg/ml, and reached 88% at 100 μg/ml. In contrast, inhibition of 14C-mevalonate incorporation did not exceed 15% at the highest concentration tested, arguing that black tea acted at or prior to HMG-CoA reductase. As with green tea, black tea reduced 14C-acetate, but not 14C-mevalonate uptake and incorporation into cells (data not shown).
Fig. 2.
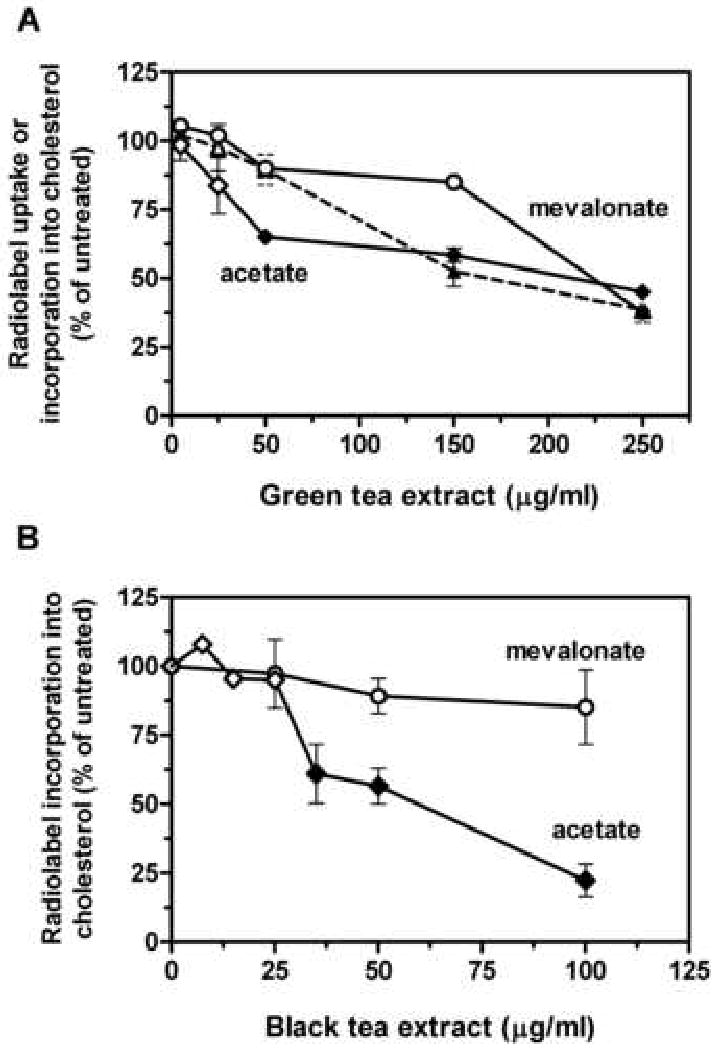
Effect of tea extract on radiolabel uptake and incorporation into cholesterol. Incorporation of 14C-acetate (acetate, diamond symbols) or 14C-mevalonate (mevalonate, circle symbols) into cholesterol was determined in 3-h assays with hepatoma cells in the presence of the indicated concentrations of green tea extract (A) or black tea extract (B). Values represent the mean and standard error of one to five experiments carried out in duplicate. The dashed line represents total 14C-acetate content in cells. Closed symbols are statistically different from untreated controls as determined by one-way analysis of variance with Dunnett's post-hoc test, p < 0.05.
To determine if green and black tea directly inhibited HMG-CoA reductase, microsomes were prepared from rat livers and mevalonate formation from 14C-HMG-CoA was assayed in the presence of tea extracts or EGCG. As shown in Fig. 3, green tea extract was inhibitory only at the highest concentration tested, 100 μg/ml, where it decreased HMG-CoA reductase activity by 34%. Black tea extract was inhibitory at 25 μg/ml, and yielded a 43% decrease in HMG-CoA reductase activity at 100 μg/ml. No inhibition was evident with EGCG at concentrations that produced a modest decrease in cholesterol synthesis from 14C-acetate (see Fig. 1). These results indicate that green and black tea contain compounds capable of directly inhibiting HMG-CoA reductase. However, the extent of inhibition is less than that seen in whole cells (up to 88% inhibition with black tea at 100 μg/ml, as noted above), suggesting that additional mechanisms of inhibition are also operant with tea.
Fig. 3.
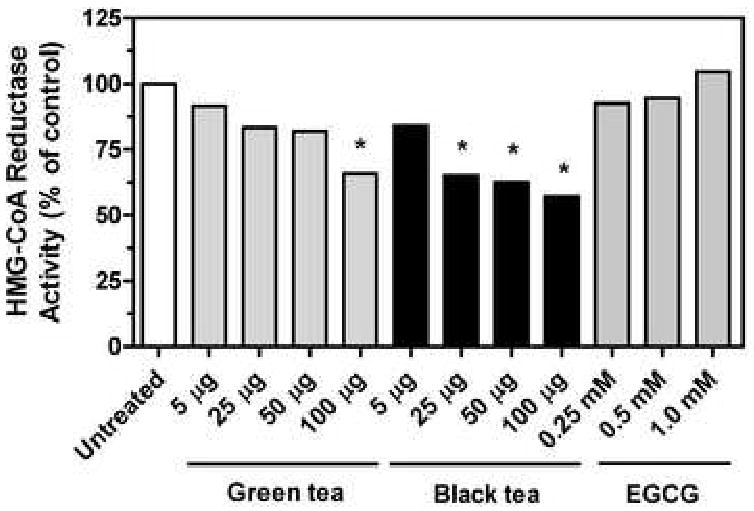
Inhibition of HMG-CoA reductase by tea extract. HMG-CoA reductase activity in rat liver microsomes was determined in the presence of the indicated tea extracts or component. Values represent the mean and standard error of two to four experiments carried out in duplicate. Asterisks indicate values statistically different from the untreated controls as determined by one-way analysis of variance with Dunnett's post-hoc test, p < 0.05.
To determine if tea extracts might be down-regulating the activity of HMG-CoA reductase, the ability of tea to activate AMP-kinase by promoting its phosphorylation was determined. Both green and black tea extracts increased AMP-kinase phosphorylation by 6.6- and 3.3-fold, respectively, with maximal stimulation at 10 μg/ml (Fig. 4A). These increases were comparable to the increases seen with AICAR and metformin, both of which are known to activate AMP-kinase by phosphorylation [19]. Treatment with green tea extract rapidly activated AMP-kinase, with phosphorylation peaking at 30 min and staying elevated through 3 h (Fig. 4B). In contrast, phosphorylation was slower to develop with black tea extract, reaching a maximum at 3 h and remaining elevated through 6 h.
Fig. 4.
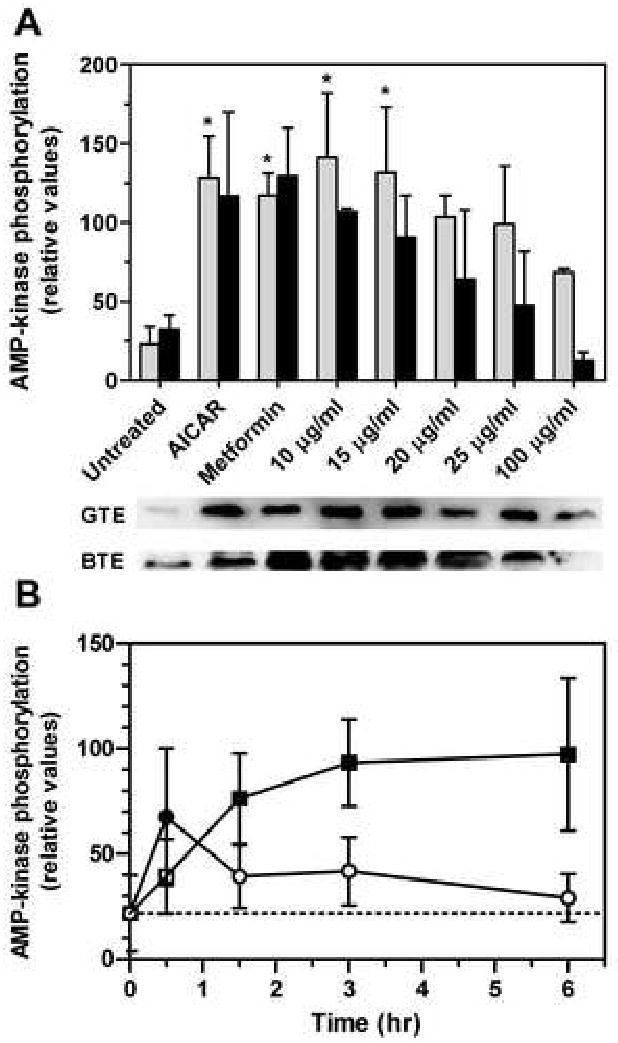
Phosphorylation of AMP-kinase by tea extract. Phosphorylated AMP-kinase was measured by immunoquantitation with an antibody specific for the phosphorylated enzyme. A, Enzyme phosphorylation in hepatoma cells after a 3-h treatment with green (grey bars) or black (black bars) tea extract; each data set included an untreated sample and samples treated with the AMP-kinase activators AICAR or metformin (1 mM each). Values represent the mean and standard deviation of 2 experiments; values that are statistically different from untreated controls are indicated with asterisks. The immunoblots below the graph illustrate the increase in AMP-kinase phosphorylation with each treatment (GTE, green tea extract; BTE, black tea extract). B, Enzyme phosphorylation in hepatoma cells at various times after treatment with 15 μg/ml of green (circles) or black (boxes) tea extract. Values represent the mean and standard deviation of 4 experiments; closed symbols are statistically different from untreated controls. Statistical significance was determined by one-way analysis of variance with Dunnett's post-hoc text, p < 0.05.
The activation of AMP-kinase by the tea extracts led to the concomitant phosphorylation of HMG-CoA reductase, as shown in Fig. 5. The amount of phosphorylated HMG-CoA reductase increased by about 50% after 3 h of treatment with green or black tea, and was increased by greater than 250% at 6 h. Treatment of cells with AICAR yielded a similar increase in immunoprecipitable HMG-CoA reductase.
Fig. 5.
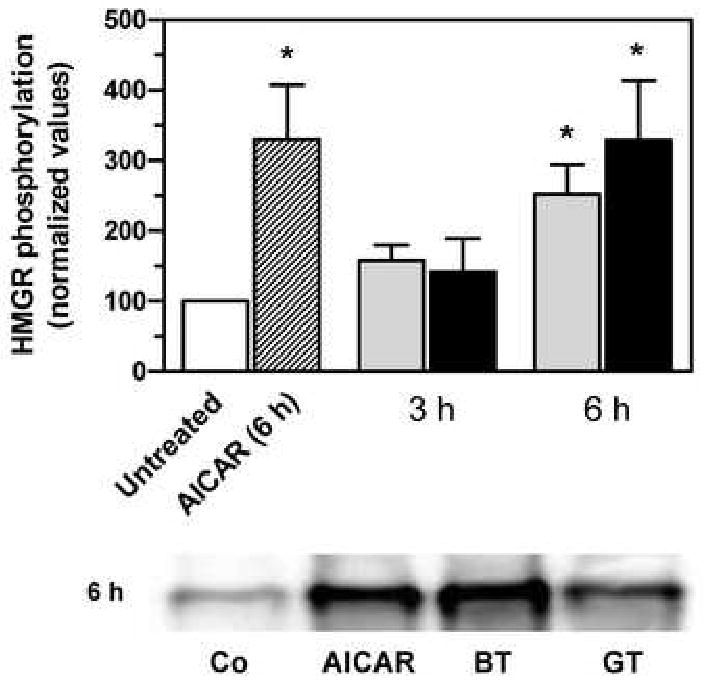
Phosphorylation of HMG-CoA reductase by tea extract. Phosphorylated HMG-CoA reductase (HMGR) was immunoprecipitated from hepatoma cell lysates with an antibody to phosphoserine/phosphothreonine/phosphotyrosine and then quantified by immunoblotting with an antibody to HMG-CoA reductase. Cells were treated with 15 μg/ml of green (grey bars) or black (black bars) tea extract or 1 mM AICAR for 3 or 6 h, as indicated. Values represent the mean and standard deviation of 3 or 4 experiments. Asterisks indicate values statistically different from the untreated cells as determined by one-way analysis of variance with Dunnett's post-hoc test, p < 0.05. The image below the graph shows a representative immunoblot of immunoprecipitated HMG-CoA reductase after 6 h of treatment; Co, untreated; BT, black tea, GT, green tea.
4. Discussion
The ability of tea to decrease serum cholesterol levels in animals and man has been attributed largely to its ability to decrease dietary cholesterol absorption and increase fecal excretion [3], although other mechanisms have not been excluded. In the present studies we show that green and black tea extracts decrease the synthesis of cholesterol in cultured rat hepatoma cells, suggesting that inhibition of cholesterol synthesis may contribute to this salutary effect of tea in vivo. The inhibition appears to be mediated largely at the level of HMG-CoA reductase, as inhibition was evident with radiolabeled acetate but not with radiolabeled mevalonate, the product of HMG-CoA reductase. Moreover, addition of either green or black tea extract to microsomal preparations directly inhibited the conversion of HMG-CoA to mevalonate by HMG-CoA reductase. Lastly, addition of either tea extract to cells in culture caused a pronounced increase in the amount of phosphorylated AMP-kinase, the principal regulatory kinase for HMG-CoA reductase, and increased the amount of phosphorylated HMG-CoA reductase. As phosphorylation of HMG-CoA reductase decreases its activity [20], this indicates that the tea extracts act both directly and indirectly to decrease HMG-CoA reductase activity and suppress cholesterol synthesis. Notably, several non-tea derived polyphenols, including apigenin and resveratrol, have been shown to activate AMP-kinase and lower intracellular lipid levels in human hepatoma cells [21]. The mechanism by which tea extracts activate AMP-kinase remains to be determined, but LKB1, PPARγ, and sirtuin all have been suggested to be involved in the activation of AMP-kinase by these other polyphenols [21].
Although there was some precedent in the literature to anticipate that polyphenols present in tea might prove inhibitory to cholesterol synthesis [4,7-10,12], it was not anticipated that black tea would prove more effective than green tea, as found here. Most studies to date have been carried out with green tea, and the assumption has been that epigallocatechin gallate (EGCG), which constitutes 70% of the mass of green tea polyphenols, was the active principal for most of the biological effects attributed to green tea. Surprisingly, we found EGCG to be about 5-fold less potent than green tea extract with whole cells, and largely ineffective in inhibiting HMG-CoA reductase activity in isolated microsomes. This result suggests that other, minor components of green tea are the active principal(s); however, a complete inventory of the constituents of green tea is lacking and only a limited number of the known compounds are commercially available in pure form. Of the available green tea components that we tested, epicatechin, epicatechin gallate, epigallocatechin, ellagic acid, and gallic acid, none were effective inhibitors of cholesterol synthesis, leaving the active principal(s) unidentified.
As noted above, of greater surprise was the marked ability of black tea to inhibit cholesterol synthesis. Black tea was 2-3-fold more potent than green tea in inhibiting cholesterol synthesis in whole cells and in inhibiting HMG-CoA reductase in microsomes, and equally effective in activating AMP-kinase, leading to the phosphorylation of HMG-CoA reductase. Black tea was also more efficacious in reducing cholesterol synthesis in cells than green tea, yielding a 78% decrease at 100 μg/ml vs a 55% decrease for green tea at 250 μg/ml. Although both tea extracts appeared equally effective in activating AMP-kinase at relatively low concentrations (10-15 μg/ml), the time-course of activation was somewhat slower with black tea, reaching a maximum at 3 h as compared to the rapid, 30-min onset with green tea. We attribute this to the predominance of larger polymerized and oxidized polyphenols in black tea, which are likely to be slower to cross cell membranes and reach active sites within the cells. Nonetheless, they appear to be more effective inhibitors of HMG-CoA reductase, leading to a greater overall suppression of cholesterol synthesis.
The present results are allied with several clinical studies in which black tea or theaflavin consumption effectively lowered serum cholesterol levels [1,2]. Whether black tea theaflavins and thearubigins are absorbed from the gut in sufficient quantity to suppress hepatic cholesterol synthesis, or are mainly acting to limit intestinal cholesterol absorption, remains to be determined.
Acknowledgments
Supported in part by grant AT-003488 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maron DJ, Lu GP, Cai NS, Wu ZG, Li YH, Chen H, Zhu JQ, Jin XJ, Wouters BC, Zhao J. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: a randomized controlled trial. Arch Intern Med. 2003;163:1448–53. doi: 10.1001/archinte.163.12.1448. [DOI] [PubMed] [Google Scholar]
- 2.Davies MJ, Judd JT, Baer DJ, Clevidence BA, Paul DR, Edwards AJ, Wiseman SA, Muesing RA, Chen SC. Black tea consumption reduces total and LDL cholesterol in mildly hypercholesterolemic adults. J Nutr. 2003;133:3298S–302S. doi: 10.1093/jn/133.10.3298S. [DOI] [PubMed] [Google Scholar]
- 3.Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J Nutr Biochem. 2007;18:179–83. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bursill CA, Abbey M, Roach PD. A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis. 2007;193:86–93. doi: 10.1016/j.atherosclerosis.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 5.Bursill CA, Roach PD. A green tea catechin extract upregulates the hepatic low-density lipoprotein receptor in rats. Lipids. 2007;42:621–7. doi: 10.1007/s11745-007-3077-x. [DOI] [PubMed] [Google Scholar]
- 6.Chan PT, Fong WP, Cheung YL, Huang Y, Ho WK, Chen ZY. Jasmine green tea epicatechins are hypolipidemic in hamsters (Mesocricetus auratus) fed a high fat diet. J Nutr. 1999;129:1094–101. doi: 10.1093/jn/129.6.1094. [DOI] [PubMed] [Google Scholar]
- 7.Gebhardt R. Variable influence of kaempferol and myricetin on in vitro hepatocellular cholesterol biosynthesis. Planta Med. 2003;69:1071–4. doi: 10.1055/s-2003-45184. [DOI] [PubMed] [Google Scholar]
- 8.Gebhardt R. Inhibition of cholesterol biosynthesis in primary cultured rat hepatocytes by artichoke (Cynara scolymus L.) extracts. J Pharmacol Exp Ther. 1998;286:1122–8. [PubMed] [Google Scholar]
- 9.Abe I, Seki T, Umehara K, Miyase T, Noguchi H, Sakakibara J, Ono T. Green tea polyphenols: novel and potent inhibitors of squalene epoxidase. Biochem Biophys Res Commun. 2000;268:767–71. doi: 10.1006/bbrc.2000.2217. [DOI] [PubMed] [Google Scholar]
- 10.Laden BP, Porter TD. Resveratrol inhibits human squalene monooxygenase. Nutr Res. 2001;21:747–53. [Google Scholar]
- 11.Bursill C, Roach PD, Bottema CD, Pal S. Green tea upregulates the low-density lipoprotein receptor through the sterol-regulated element binding Protein in HepG2 liver cells. J Agric Food Chem. 2001;49:5639–45. doi: 10.1021/jf010275d. [DOI] [PubMed] [Google Scholar]
- 12.Bursill CA, Roach PD. Modulation of cholesterol metabolism by the green tea polyphenol (-)-epigallocatechin gallate in cultured human liver (HepG2) cells. J Agric Food Chem. 2006;54:1621–6. doi: 10.1021/jf051736o. [DOI] [PubMed] [Google Scholar]
- 13.Espenshade PJ, Hughes AL. Regulation of sterol synthesis in eukaryotes. Annu Rev Genet. 2007;41:401–27. doi: 10.1146/annurev.genet.41.110306.130315. [DOI] [PubMed] [Google Scholar]
- 14.Beg ZH, Stonik JA, Brewer HB., Jr Modulation of the enzymic activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase by multiple kinase systems involving reversible phosphorylation: a review. Metabolism. 1987;36:900–17. doi: 10.1016/0026-0495(87)90101-6. [DOI] [PubMed] [Google Scholar]
- 15.Carling D, Clarke PR, Zammit VA, Hardie DG. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem. 1989;186:129–36. doi: 10.1111/j.1432-1033.1989.tb15186.x. [DOI] [PubMed] [Google Scholar]
- 16.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–83. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 17.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–41. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 18.Brown MS, Goldstein JL. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J Biol Chem. 1974;249:7306–14. [PubMed] [Google Scholar]
- 19.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beg ZH, Stonik JA, Brewer HB., Jr 3-Hydroxy-3-methylglutaryl coenzyme A reductase: regulation of enzymatic activity by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1978;75:3678–82. doi: 10.1073/pnas.75.8.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–91. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]


