Abstract
OBJECTIVES
The purpose of this study was to prospectively evaluate the diagnostic performance of 3.0T contrast-enhanced whole-heart coronary magnetic resonance angiography (CMRA) in patients with suspected coronary artery disease.
BACKGROUND
A slow-infusion, contrast-enhanced whole-heart CMRA approach has recently been developed at 3.0T. The accuracy of such technique has not yet been determined among patients with suspected coronary artery disease.
METHODS
3.0T contrast-enhanced whole-heart CMRA was performed in 69 consecutive patients. An ECG-triggered, navigator-gated, inversion-recovery prepared, segmented gradient-echo sequence was used to acquire isotropic whole-heart CMRA with slow infusion of 0.2 mmol/kg Gd-BOPTA. The diagnostic accuracy of whole-heart CMRA in detecting significant stenoses (≥50%) was evaluated using x-ray angiography as the reference.
RESULTS
The MR examinations were successfully completed in 62 patients. Acquisition time of whole-heart CMRA procedure was 9.0 ±1.9 min. 3T whole-heart CMRA correctly identified significant CAD in 32 patients and correctly ruled out CAD in 23 patients. The sensitivity, specificity, and accuracy of whole-heart CMRA for detecting significant stenoses were 91.6% (87/95), 83.1% (570/686), 84.1% (657/781), respectively, on a per-segment basis. These values were 94.1% (32/34), 82.1% (23/28), 88.7% (55/62), respectively, on a per-patient basis.
CONCLUSIONS
3.0T contrast-enhanced whole-heart CMRA allows for the accurate detection of coronary artery stenosis with high sensitivity and moderate specificity.
Keywords: Coronary disease, magnetic resonance imaging, contrast media, 3.0-Tesla
INTRODUCTION
Substantial progress has been made in coronary magnetic resonance angiography (CMRA) since the first reports of visualizing the ostia of coronary arteries in the late 1980’s[1, 2]. A prospective, multicenter study shows that three-dimensional (3D) CMRA using a spoiled gradient-echo sequence allows for accurate detection of coronary artery disease in the proximal and middle segments of coronary arteries at 1.5T [3]. Steady-state free precession (SSFP) imaging[4] was later shown to offer superior signal-to-noise ratio (SNR) and blood-myocardium contrast in CMRA. In recent years, improved gradient performance and radiofrequency (RF) receiving coils and advanced data acquisition techniques including navigator gating and parallel imaging[5, 6] allowed whole-heart CMRA within 10–15 min[7]. A recent study of 131 patients using the SSFP whole-heart CMRA approach at 1.5T demonstrates moderate sensitivity and high specificity for noninvasive detection of significant narrowing in coronary arterial segments of ≥ 2 mm in diameter [8, 9]. However, a comparative study is required to verify whether SSFP improves the diagnostic accuracy over the conventional GRE sequence.
Despite the substantial progress in imaging hardware and techniques, to date the clinical utilization of CMRA remains limited for the detection of coronary artery disease. Relatively low spatial resolution and long imaging time are the two major factors. 3.0T has been shown to be a promising platform for performing CMRA[10]. The theoretical doubling of SNR from 1.5T to 3.0T can be traded for improved spatial resolution and/or reduced imaging time. Nevertheless, the SSFP imaging technique that has gained wide acceptance at 1.5T is prone to imaging artifacts at 3.0T because of the increased magnetic field inhomogeneity and RF distortion at higher field strengths. In addition, energy deposition is increased by a factor of 4 from 1.5T to 3.0T.
A recent study has demonstrated the feasibility of whole-heart CMRA at 3.0T with slow infusion of a high relaxivity clinical contrast media Gd-BOPTA[11] using a spoiled gradient echo technique. Spoiled gradient-echo imaging is less sensitive to static and RF field inhomogeneities, and reduces RF power deposition and repetition time (TR) as compared to SSFP imaging. Contrast-enhanced data acquisition improves SNR and contrast-to-noise ratio (CNR). The purpose of this study was to prospectively evaluate the diagnostic performance of this 3.0T whole-heart CMRA technique on patients with suspected coronary artery disease.
METHODS
Study Population
From April 2007 to July 2008, a total of 96 consecutive patients scheduled for conventional coronary angiography were prospectively recruited in this study. Exclusion criteria were general contraindications to MR examination (claustrophobia, pacemaker), unstable angina, atrial fibrillation, patients with coronary stents or bypass grafts, and renal insufficiency (estimated glomerular filtration rate assessed by creatinine clearance < 60 ml/min/1.73 m2). 27 patients were excluded for these reasons and sixty nine patients (36 men, age 61 ± 10) underwent whole-heart CMRA before conventional coronary angiography (figure 1). The average interval between CMRA and cardiac catheterization was 2 days, ranging from 0 to 12 days. No clinical cardiac events were reported between the examinations. The study protocol was approved by the institutional review board. Written informed consent was obtained from each patient.
Figure 1. Flow chart of patient inclusion.
CAD = Coronary Artery Disease. CMRA = Magnetic Resonance Coronary Angiography.
Patient Preparation
β-blocker (metoprolol tartrate, 25–50 mg) was given orally to patients with heart rate higher than 75 beats/min before CMRA. No nitroglycerin were given to the patients prior to the test.
Contrast-enhanced Whole-Heart CMRA
CMRA was performed on a 3.0T whole-body scanner (MAGNETOM Trio, A Tim System; Siemens AG Healthcare, Erlangen, Germany) with maximum slew rate of 200 mT/m/ms and maximum gradient strength of 40 mT/m. A twelve-element matrix coil (six anterior and six posterior elements) was activated for data collection. Patients were trained to perform regular, shallow breathing and to avoid changes in depth of breathing during the data acquisition. The R-wave acquired from a three-lead wireless vectorcardiogram was used to trigger the data acquisition. All images were collected under free breathing with patient in supine position. The procedures were as follows: Two dimensional (2D) scout images were first obtained in three orthogonal orientations to identify the position of the heart and diaphragm. To determine the optimal data acquisition window, retrospective ECG-triggered cine images (50 cardiac phases reconstructed) were acquired in a four-chamber view using a fast low-angle shot (FLASH) sequence during free breathing. The global cardiac motion was visually assessed from cine images to determine the patient-specific trigger-delay time and duration of data acquisition window per heartbeat. For whole-heart CMRA, 0.2 mmol/kg body weight of Gadobenate dimeglumine (MultiHance; Bracco Imaging SpA, Milan, Italy) was slowly infused using a power injector (Spectris, Medrad, Indianola, PA, USA) at a rate of 0.3 ml/sec, immediately followed by 20 ml saline at the same rate. Sixty seconds after the initiation of contrast administration, whole-heart CMRA data acquisition was started. The imaging volume was prescribed in the axial plane to cover the entire heart. A navigator-gated, ECG-triggered, fat-saturated, inversion-recovery prepared segmented 3D FLASH sequence was employed[11]. Prospective real-time adaptive motion correction was applied in the superior-inferior direction to compensate the respiratory motion with a correction factor of 0.6[12]. Imaging parameters included: TR /TE (echo time) = 3.0/1.4 ms, flip angle = 20°, readout bandwidth = 610 Hz/pixel, acquired voxel size = 1.3 × 1.3 × 1.3 mm3 and interpolated to 0.65 × 0.65 × 0.65 mm3. Data acquisition was accelerated by employing generalized autocalibrating partially parallel acquisitions (GRAPPA) in the phase-encoding direction with a factor of 2. A non-selective inversion pulse was applied prior to the navigator-gating and data acquisition to suppress background tissues. The inversion-recovery time (TI) was 200 msec.
Conventional Coronary Angiography
X-ray coronary angiography was performed in all patients and evaluated by QCA (QuantCor QCA, Siemens Healthcare) by two cardiologists in consensus who were blinded to the CMRA results. Standard 15-segment American Heart Association classification system was used. All coronary artery stenoses were graded in at least two orthogonal views and the measurement was performed in the projection that showed the highest degree of stenoses. Stenoses were quantitatively evaluated for segments with a reference diameter of 1.5 mm or more. Segments distal to complete occlusions were excluded for analysis. Significant coronary artery disease was defined as a luminal diameter reduction of ≥50% in coronary arteries.
CMRA image analysis
All CMRA images were transferred to an external workstation (MMWP, Siemens AG Healthcare, Erlangen, Germany) and patient information was removed. CMRA images were independently assessed by two experienced readers who were blinded to the patient information. Axial source images, curved multiplanar reformations (MPR), and thin-slab maximum-intensity projections (MIP) images were assessed on a per-segment basis. CoronaViz software (Siemens Corporate Research, Princeton, NJ, USA) were used for CMRA images to project multi vessels onto a single image. MR image quality was graded on a 4-point scale (1, non-assessable with severe image artifacts, poor vessel contrast; 2, assessable with moderate image artifacts, fair vessel contrast; 3, assessable with minor artifacts, good vessel contrast; 4, assessable with no apparent artifacts, excellent vessel contrast)[3]. The severity of luminal diameter reduction as being < 50% or ≥ 50% was visually assessed by two readers independently. The disagreement of diagnosis between the two readers was settled by a consensus reading.
Statistical analysis
All statistical analysis was performed using statistical software (SAS version 9.1, SAS Institute Inc., Cary, North Carolina). Quantitative variables were expressed as mean value ± standard deviation, and categorical variables as percentages. The diagnostic performance of CMRA for the detection of significant coronary artery stenosis (sensitivity, specificity, positive predictive value, negative predictive value, and accuracy with 95% confidence intervals) were calculated on a per-segment, per-vessel and per-patient basis using invasive x-ray coronary angiography as reference standard. In the primary analysis, only the assessable segments were included and calculation was performed on per-patient, per-vessel, and per-segment basis, respectively. In the second analysis, the intention-to-diagnose approach [13] was used and non-assessable segments were considered to have a stenosis[14]. Interobserver agreement was assessed on segmental basis for the image quality grading and stenosis analysis by using weighted and unweighted kappa, respectively.
RESULTS
The characteristics of the study population are summarized in Table 1. CMRA was successfully completed in 62 of 69 (90%) patients. Seven patient studies were aborted due to poor ECG signal (n=3), or extremely low respiratory gating efficiency (navigator efficiency < 20% by the time half of the imaging data were collected) (n=4). Acquisition time of whole-heart CMRA was 9.0 ±1.9 min. Mean heart rate during CMRA was 67±7 beats/min. CMRA was acquired during diastole in 53 patients (acquisition window 135±33 ms) and during systole in 9 patients (acquisition window 89±8 ms). The trigger-delay time was 554 ± 143 ms. The average navigator efficiency was 35%. The average duration of contrast injection was 1.5 minutes. Twenty (29%) patients received oral β-blocker before CMRA. Figure 2 shows representative CMRA images from a patient with normal coronary arteries.
Table 1.
Characteristics of the study population
| Characteristics | Patients Who Underent Cronary MRA (n =69) |
Patients With Successful Coronary MR (n =62) |
|---|---|---|
| Age (y) | 61±10 | 61±11 |
| Range | 38–82 | 38–82 |
| Sex (male/female) | 36/33 | 30/32 |
| Mean Heart rate during CMRA(beats/min) | 67±6 | 67±7 |
| Range | 52–83 | 52–83 |
| Body mass index (kg/m2) | 24.2±2.8 | 24.1±2.8 |
| Hypertension, n (%) | 31 (45%) | 29 (47%) |
| Hypercholesterolemia, n (%) | 37 (54%) | 34 (55%) |
| Diabetes mellitus | 19 (28%) | 19 (31%) |
| Current or prior cigarette smoking, n (%) | 27 (39%) | 24 (39%) |
| Chest pain | 45 (65%) | 40 (65%) |
| Prior myocardial infarction | 11 (16%) | 9 (15%) |
| Stenosis on x-ray coronary angiography, n (%) | 38 (55%) | 34 (55%) |
| One vessel | 11 | 10 |
| Two vessel | 18 | 16 |
| Three vessel | 5 | 4 |
| Four vessel | 4 | 4 |
Figure 2. 3T contrast-enhanced whole-heart CMRA images of a 71 year-old woman with normal coronary arteries.
A. Volume rendered (VR) image providing an overview of coronary anatomy, clearly depicts RCA, LAD and diagonal branches. B. Curved MPR image using CoronaViz software shows LM, LAD, LCX and RCA
Image quality of the whole-heart CMRA
The CMRA image quality of 62 patients is summarized in Table 2. Ninety-three of 781 segments (12%) with a reference luminal diameter ≥ 1.5 mm on QCA were evaluated as non-assessable. The reasons for these segments were poor contrast-to-noise ratio (n = 27), motion artifacts (n=39), and small diameter (n=27). Most segments were assessable in LM (98%, 61/62), followed by RCA (90%, 260/288), LAD (89%, 220/246), and the least in the LCX (80%, 147/185). The image score was 2.8 ± 1.0. Weighted Kappa value for interobserver agreement for image quality grading was 0.82.
Table 2.
Image quality of 62 patients with successful CMRA
| No. of Segments >1.5 mm On QCA |
No. of Assessable Segments on MRA |
Causes of non-assessibility | Image quality of MRA |
|||
|---|---|---|---|---|---|---|
| Poor opacification |
Motion artifacts |
Small caliber |
||||
| LM | 62 | 61 (98%) | 0 | 1 | 0 | 3.3±0.7 |
| LAD | 0 | |||||
| Proximal | 62 | 61 (98%) | 0 | 1 | 0 | 3.2±0.7 |
| Mid | 60 | 58 (97%) | 1 | 1 | 0 | 3.1±0.7 |
| Distal | 57 | 51 (90%) | 3 | 1 | 2 | 2.8±1.1 |
| Diagonal branches | 67 | 50 (75%) | 6 | 4 | 7 | 2.6±0.9 |
| LCX | ||||||
| Proximal | 62 | 60 (97%) | 0 | 2 | 0 | 2.8±0.8 |
| Distal | 50 | 41 (82%) | 2 | 4 | 3 | 2.4±1.1 |
| Marginal branches | 73 | 46 (63%) | 6 | 13 | 8 | 2.1±1.0 |
| RCA | ||||||
| Proximal | 62 | 61 (98%) | 0 | 1 | 0 | 3.3±0.7 |
| Mid | 61 | 60 (98%) | 0 | 1 | 0 | 3.2±0.8 |
| Distal | 58 | 54 (93%) | 2 | 2 | 0 | 2.7±1.1 |
| PDA/PL | 107 | 85 (79%) | 7 | 8 | 7 | 2.3±1.1 |
| Total | 781 | 688(88%) | 27 | 39 | 27 | 2.8±1.0 |
QCA = quantitative coronary angiography; LAD = left anterior descending coronary artery; LCX = left circumflex coronary artery; LM = left main coronary artery; RCA = right coronary artery; pro = proximal: mid = middle; dis = distal.
Diagnostic performance of CMRA compared with QCA
3T contrast enhanced CMRA correctly identified significant CAD (presence of at least 1 stenosis) in 32 out of 34 patients (sensitivity 94.1%) and correctly ruled out CAD in 23 out of 28 patients (specificity 82.1%). CMRA failed to detect CAD in two patients (two missed single-vessel disease, one in first diagonal branch and the other in distal RCA). In 3 patients CMRA detected CAD despite normal QCA (2 distal LCX, 1 posterior descending artery, 1 distal LAD). Two patients had no significant coronary artery disease on QCA were regarded as false positive due to non-assessable segments included for analysis.
In a total of 688 assessable coronary segments, QCA detected a total of 91 lesions (≥50%). CMRA correctly identified 83 of these lesions (sensitivity 91.2%). In 570 segments, stenosis was ruled out correctly by CMRA (specificity 95.5%). The main reasons for false-positive were poor opacification and motion artifacts (89%). For the intention-to-diagnose analysis, the specificity decreased to 83.1% (570/686). A detailed overview of the diagnostic performance of 3.0T CMRA compared with QCA is summarized in Table 3. Figure 3 and Figure 4 show examples illustrating the detection of significant stenoses CMRA with correlation to QCA. Kappa value for interobserver agreement for coronary artery stenosis detection with CMRA was 0.84.
Table 3.
Diagnostic performance of 3T contrast-enhanced whole-heart CMRA
| All Segments | Per patient n=62 | Per vessel n=248 | Per segment n=781 |
|---|---|---|---|
| Accuracy | 88.7 (55/62) [78.1,95.3] | 89.9 (223/248) [85.5,93.4] | 84.1(657/781) [81.4,86.6] |
| Sensitivity | 94.1 (32/34) [80.3,99.3] | 92.9 (65/70) [84.1,97.6] | 91.6 (87/95) [84.1,96.3] |
| Specificity | 82.1 (23/28) [63.1,93.9] | 88.8 (158/178) [83.2,93.0] | 83.1 (570/686) [80.1,85.8] |
| PPV | 86.5 (32/37) [71.2,95.5] | 76.5 (65/85) [66.0,85.0] | 42.9(87/203) [36.0,50.0] |
| NPV | 92.0 (23/25) [74.0,99.0] | 96.9(158/163) [93.0,99.0] | 98.6 (570/578) [97.3,99.4] |
| Assessable Segments | n=60 | n=234 | n=688 |
| Accuracy | 91.7 (55/60) [81.6,97.2] | 93.6 (219/234) [89.7,96.4] | 94.9 (653/688) [93.0,96.4] |
| Sensitivity | 94.1 (32/34) [80.3,99.3] | 92.4 (61/66) [83.2,97.5] | 91.2 (83/91) [83.4,96.1] |
| Specificity | 88.5 (23/26) [69.9,97.6] | 94.1 (158/168) [89.3,97.1] | 95.5 (570/597) [93.5,97.0] |
| PPV | 91.4 (32/35) [76.9,98.2] | 85.9 (61/71) [75.6,93.0] | 75.5 (83/110) [66.3,83.2] |
| NPV | 92.0 (23/25) [74.0,99.0] | 96.9 (158/163) [93.0,99.0] | 98.6(570/578) [97.3,99.4] |
Note.—Data are percentages, with raw data in parentheses and 95% confidence intervals in brackets.
Figure 3. 3T contrast-enhanced whole-heart CMRA images of a 54 year-old female patient.
MIP image (A) of MRA detects a significant stenosis in the middle RCA (arrow) with good correlation with x-ray angiography (B). Both readers interpreted this as significant stenosis based on CMRA images.
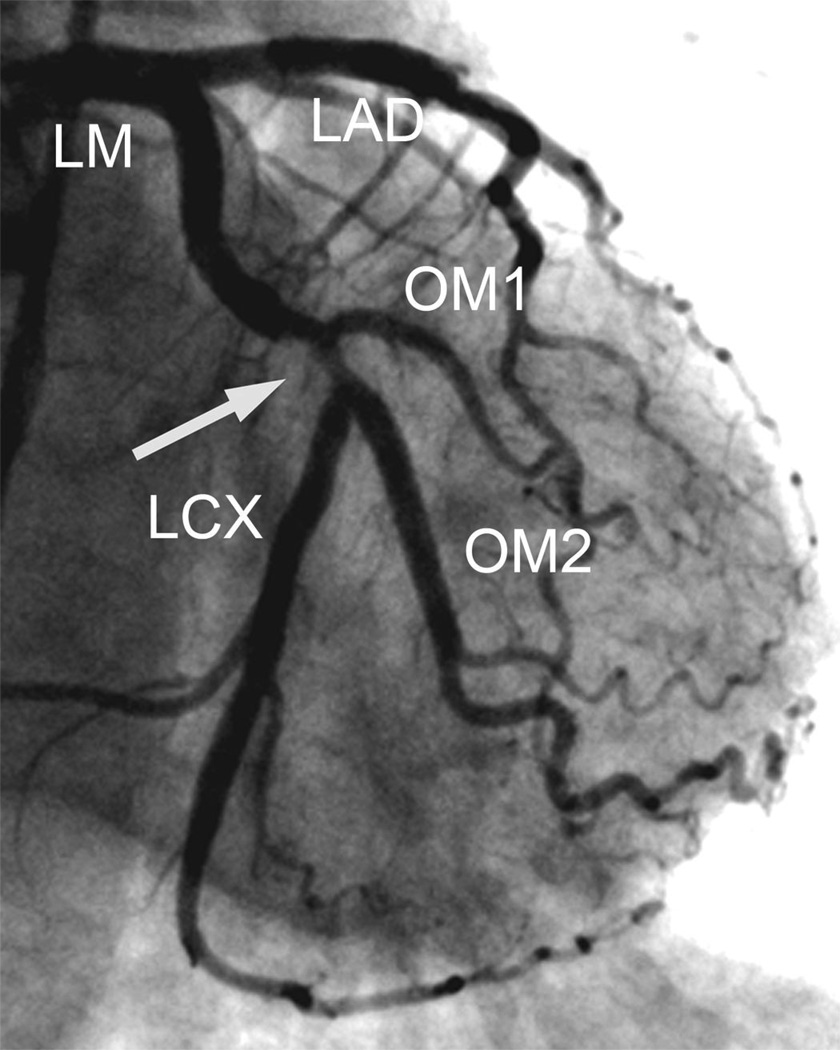
Figure 4. 3T contrast-enhanced whole-heart CMRA images of a 75 year-old male patient with atypical chest pain.
CMRA MIP images (A and B) show a significant stenosis in the proximal LCX and a non-significant stenosis in the middle RCA (arrows), respectively. VR images (syngo InSpace, Siemens AG Healthcare, Erlangen, Germany) (C and D)have the same findings in LCX and RCA, which were consistent with the conventional coronary angiography (E and F).
DISCUSSION
In this work, we have prospectively examined the diagnostic value of contrast-enhanced whole-heart CMRA at 3.0T on patients suspected of coronary artery disease. Using an inversion recovery-prepared, navigator-gated spoiled gradient-echo sequence, CMRA was able to depict significant stenoses with an overall sensitivity of 91.6%, 92.9%, and 94.1% based on per-segment, per-vessel and per-patient analyses, respectively. The negative predictive values was 98.6%, 96.9%, and 92.0%, respectively, indicating that the technique can reliably rule out significant stenoses, consistent with findings from previous studies[8, 9]. By including all false positive non-assessable segments, the positive predictive values were lower (42.9%, 76.5%, 86.5%), however, they still represent an improvement over recent study using the SSFP technique at 1.5T (14%, 38%, and 50%, respectively)[14]. The reduced incidents of false positives could be attributed to the potential benefits of contrast-enhanced data acquisition, which is T1-weighted, doesn’t depend on blood inflow, and is less prone to signal loss due to complex flow as compared to SSFP data acquisition.
Previous studies using whole-heart CMRA at 1.5T have demonstrated promising clinical results, particularly high negative predictive value. However, long scan time and relatively low spatial resolution have prevented its wide clinical acceptance as a routine test for coronary artery stenosis detection. 3.0T systems have the potential to improve SNR by a factor of 2 as compared to 1.5T with the same imaging sequence.
SSFP has been the sequence of choice for CMRA at 1.5T. However, there are substantial technical challenges of using SSFP imaging for CMRA at 3.0T because of increased Bo and B1 field inhomogeneities and power deposition, despite various improvements in recent years[15–17]. Contrast-enhanced data acquisition overcomes many problems associated with SSFP and allows faster imaging because of its shorter TR.
Reduced imaging time is critically important for whole-heart CMRA as long scan times tend to cause lower image quality from increased motion artifacts and reduced coronary SNR. 3.0T imaging and contrast-enhancement combined with inversion-recovery preparation allow high contrast between blood and background tissue. The depiction of distal coronary artery segments can be improved as a result. In this study, we were able to assess coronary artery segments with diameter greater than 1.5 mm, as compared to 2.0 mm in previous 1.5T studies[9].
Multi-slice CT has emerged as a noninvasive method for imaging the coronary arteries for several years. However, it has several disadvantages of requiring rapid injection of iodinated contrast medium and of exposing patients to ionizing radiation. In addition, blooming artifact from calcification leads to false positive diagnosis in many cases. A recent study by Liu et al[18] demonstrated that CMRA has advantages over CTA in the depiction of coronary lumen with severe calcification.
A major challenge for CMRA remains to be respiration-induced motion artifacts. Adaptive navigator-gating and motion correction is an effective method for reducing respiratory motion artifacts. However, the effectiveness of the method is related to patient’s breathing pattern. Patient training and practice before data acquisition for maintaining regular breathing should be useful to improve the gating efficiency and image quality of CMRA.
Study limitations
Several important limitations exist in the current study. First, a fixed delay time of 60 sec was applied between initiation of contrast agent infusion and start of imaging data acquisition. Such a setting may not necessarily be optimal for every patient due to variations in physiological conditions (e.g., cardiac output, heart rate, blood pressure, respiratory gating efficiency) and contrast kinetics. Automatic triggering of data acquisition based on real-time tracking of signal enhancement [19] tailors such delay time to individual subject which may potentially optimize signal enhancement for each patient. Second, compared to competing techniques including x-ray angiography and CTCA, the imaging time for CMRA is still long and the spatial resolution is relatively low. Combined with dedicated 32- or even 128-channel phased-array coils, 2D parallel imaging with higher acceleration factors should allow further improvement in imaging speed and/or spatial resolution [17, 20]. Third, 3.0 T imaging relies on slow injection of contrast media. Coronary veins are also enhanced as a result, which may impair the depiction of coronary arteries. Fourth, usage of contrast media results in additional study cost as well as potential side effects, particularly for patients with impaired renal function. It is also difficult to repeat the scan in the same imaging session if the acquisition is aborted for some reason. Further development of non-contrast [21] or reduced dose [22] CMRA techniques will alleviate these problems.
In conclusion, whole-heart CMRA at 3.0T with slow infusion of contrast agent allows for noninvasive detection of significant coronary artery stenosis with high sensitivity and specificity. Improved SNR and CNR from high field strength and contrast-enhancement warrant further development of CMRA to allow for whole-heart coverage with higher spatial resolution and/or shorter imaging time. Finally, it is possible to integrate first-pass perfusion, CMRA, and delayed-enhancement in the same imaging session at 3.0 T for a comprehensive examination.
Acknowledgments
The study was partially support by National Institutes of Health Grant number NIBIB (EB002623), National Natural Science Foundation of China (30828009), and Bracco Diagnostics, Inc.
Abbreviations
- CAD
coronary artery disease
- CMRA
coronary magnetic resonance angiography
- LM
left main coronary artery
- LAD
left anterior descending coronary artery
- LCX
left circumflex coronary artery
- RCA
right coronary artery
- QCA
quantitative coronary angiography
- SSFP
steady-state free precession
- SNR
signal-to-noise ratio
- CNR
contrast-to-noise ratio
- RF
radiofrequency
- TR
repetition time
- FLASH
fast low-angle shot
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
Dr Kuncheng Li reports receiving lecture fees, and/or grant support from Siemens Medical Solutions and Bracco Diagnostics Imaging. Dr Debiao Li reports receiving research support from Siemens Medical Solutions, Bracco Diagnostics. Xiaoming Bi, Jing An, Al Zhang and Renate Jerecic are employees of Siemens. The remaining authors report no conflicts.
References
- 1.Lieberman JM, Botti RE, Nelson AD. Magnetic resonance imaging of the heart. Radiol Clin North Am. 1984;22(4):847–858. [PubMed] [Google Scholar]
- 2.Paulin S, von Schulthess GK, Fossel E, Krayenbuehl HP. MR imaging of the aortic root and proximal coronary arteries. AJR Am J Roentgenol. 1987;148(4):665–670. doi: 10.2214/ajr.148.4.665. [DOI] [PubMed] [Google Scholar]
- 3.Kim WY, Danias PG, Stuber M, Flamm SD, Plein S, Nagel E, Langerak SE, Weber OM, Pedersen EM, Schmidt M, Botnar RM, Manning WJ. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345(26):1863–1869. doi: 10.1056/NEJMoa010866. [DOI] [PubMed] [Google Scholar]
- 4.Deshpande VS, Shea SM, Laub G, Simonetti OP, Finn JP, Li D. 3D magnetization-prepared true-FISP: A new technique for imaging coronary arteries. Magn Reson Med. 2001;46(3):494–502. doi: 10.1002/mrm.1219. [DOI] [PubMed] [Google Scholar]
- 5.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. [PubMed] [Google Scholar]
- 6.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 7.Weber OM, Martin AJ, Higgins CB. Whole-heart steady-state free precession coronary artery magnetic resonance angiography. Magn Reson Med. 2003;50(6):1223–1228. doi: 10.1002/mrm.10653. [DOI] [PubMed] [Google Scholar]
- 8.Sakuma H, Ichikawa Y, Suzawa N, Hirano T, Makino K, Koyama N, Van Cauteren M, Takeda K. Assessment of coronary arteries with total study time of less than 30 minutes by using whole-heart coronary MR angiography. Radiology. 2005;237(1):316–321. doi: 10.1148/radiol.2371040830. [DOI] [PubMed] [Google Scholar]
- 9.Sakuma H, Ichikawa Y, Chino S, Hirano T, Makino K, Takeda K. Detection of coronary artery stenosis with whole-heart coronary magnetic resonance angiography. J Am Coll Cardiol. 2006;48(10):1946–1950. doi: 10.1016/j.jacc.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 10.Stuber M, Botnar RM, Fischer SE, Lamerichs R, Smink J, Harvey P, Manning WJ. Preliminary report on in vivo coronary MRA at 3 Tesla in humans. Magn Reson Med. 2002;48(3):425–429. doi: 10.1002/mrm.10240. [DOI] [PubMed] [Google Scholar]
- 11.Bi X, Carr JC, Li D. Whole-heart coronary magnetic resonance angiography at 3 Tesla in 5 minutes with slow infusion of Gd-BOPTA, a high-relaxivity clinical contrast agent. Magn Reson Med. 2007;58(1):1–7. doi: 10.1002/mrm.21224. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Ehman RL. Retrospective adaptive motion correction for navigator-gated 3D coronary MR angiography. J Magn Reson Imaging. 2000;11(2):208–214. doi: 10.1002/(sici)1522-2586(200002)11:2<208::aid-jmri20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Dewey M, Teige F, Schnapauff D, Laule M, Borges AC, Wernecke KD, Schink T, Baumann G, Rutsch W, Rogalla P, Taupitz M, Hamm B. Noninvasive detection of coronary artery stenoses with multislice computed tomography or magnetic resonance imaging. Ann Intern Med. 2006;145(6):407–415. doi: 10.7326/0003-4819-145-6-200609190-00004. [DOI] [PubMed] [Google Scholar]
- 14.Pouleur A-C, Waroux J-BlPd, Kefer J, Pasquet A, Vanoverschelde J-L, Gerber BL. Direct Comparison of Whole-Heart Navigator-Gated Magnetic Resonance Coronary Angiography and 40- and 64-Slice Multidetector Row Computed Tomography to Detect the Coronary Artery Stenosis in Patients Scheduled for Conventional Coronary Angiography. Circ Cardiovasc Imaging. 2008;1:114–121. doi: 10.1161/CIRCIMAGING.107.756304. [DOI] [PubMed] [Google Scholar]
- 15.Nezafat R, Stuber M, Ouwerkerk R, Gharib AM, Desai MY, Pettigrew RI. B1-insensitive T2 preparation for improved coronary magnetic resonance angiography at 3 T. Magn Reson Med. 2006;55(4):858–864. doi: 10.1002/mrm.20835. [DOI] [PubMed] [Google Scholar]
- 16.Huber ME, Kozerke S, Pruessmann KP, Smink J, Boesiger P. Sensitivity-encoded coronary MRA at 3T. Magn Reson Med. 2004;52(2):221–227. doi: 10.1002/mrm.20062. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt M, Potthast A, Sosnovik DE, Polimeni JR, Wiggins GC, Triantafyllou C, Wald LL. A 128-channel receive-only cardiac coil for highly accelerated cardiac MRI at 3 Tesla. Magn Reson Med. 2008;59(6):1431–1439. doi: 10.1002/mrm.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Zhao X, Huang J, Francois CJ, Tuite D, Bi X, Li D, Carr JC. Comparison of 3D free-breathing coronary MR angiography and 64-MDCT angiography for detection of coronary stenosis in patients with high calcium scores. AJR Am J Roentgenol. 2007;189(6):1326–1332. doi: 10.2214/AJR.07.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai P, Bi X, Bhat H, Larson A, Jerecic R, Li D. Contrast-Enhanced Whole-heart Coronary MRA with Self-Timing and Respiratory Self-Gating. Proceedings 16th Scientific Meeting, International Society for Magnetic Resonance in Medicine; April, 2008; Toronto. [Google Scholar]
- 20.Niendorf T, Hardy CJ, Giaquinto RO, Gross P, Cline HE, Zhu Y, Kenwood G, Cohen S, Grant AK, Joshi S, Rofsky NM, Sodickson DK. Toward single breath-hold whole-heart coverage coronary MRA using highly accelerated parallel imaging with a 32-channel MR system. Magn Reson Med. 2006;56(1):167–176. doi: 10.1002/mrm.20923. [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Shankaranarayanan A, Pohost G, Nayak K. Improved Coronary MRA Using Wideband SSFP at 3 Tesla with Sub-millimeter Resolution. Proceedings 16th Scientific Meeting, International Society for Magnetic Resonance in Medicine; April, 2008; Toronto. [Google Scholar]
- 22.Yang Q, Li K, Bi X, An J, Renate J, Li D. 3T contrast-enhanced whole heart coronary MRA using 32-channel cardiac coils for the detection of coronary artery disease. Journal of Cardiovascular Magnetic Resonance. 2009;11 Suppl 1:O5. [Google Scholar]