Introduction
The number of cancer survivors is steadily on the rise. The American Cancer Society (ACS) recently reported that the 5-year relative survival rate for all cancers combined is 66% (1). The National Cancer Institute estimates that there are approximately 11 million cancer survivors, defined as individuals with a cancer diagnosis regardless of the course of the illness, until the end of their life (2). These numbers are projected to double by 2050 (3). Cancer is currently the leading cause of death among individuals under the age of 85 years (4,5). Overall, it is the second leading cause of death responsible for 1 in 4 deaths in the United States (U.S.) (4). In fact, cancer is expected to be the top cause of death in the U.S. by 2010 surpassing cardiovascular disease (6).
The prevalence of obesity has increased in parallel to cancer, reaching epidemic proportions in the country, with about 70% of the population being obese or overweight (7). Scientific evidence suggests that obesity is predictive of poor cancer prognosis among cancer survivors. Adipocytes, once thought of as passive depots of excess fat, are now collectively recognized as an active endocrine organ that secretes adipokines. Adipokines are a group of proteins with signaling properties and include leptin, pro-inflammatory proteins and adiponectin, each of which is hypothesized to play a role in cancer biology (8).
Insulin resistance, characterized by hyperinsulinemia, and frequently accompanied by impaired glucose tolerance, is also associated with obesity (9). Insulin resistance is estimated to be prevalent among approximately 1 in 3 American adults (9). Insulin has been implicated in cancer progression by virtue of its proliferative and pro-inflammatory properties (8). Epidemiologic evidence collectively suggests that obesity and insulin resistance may be a ‘high-risk’ state for disease progression among cancer survivors (discussed below), implying that the trajectory of care within this population needs urgent attention. The Institute of Medicine has identified ‘cancer surveillance and control’ as a top public health priority (10).
Modifiable lifestyle interventions targeting energy balance and insulin resistance are measures that may be used as important cancer control tools. In the sections below, an overview of the hypothesized underlying biological mechanisms that link obesity and insulin resistance with cancer progression are provided, and epidemiologic evidence of studies that have investigated these associations is presented. Further, potential practice implications are suggested for all dietetic practitioners, board certified specialists in oncology nutrition (CSOs), and other healthcare providers to alter dietary, lifestyle and behavioral factors, in an attempt to delay cancer progression or mortality among the very large at-risk population of cancer survivors.
Putative biological mechanisms
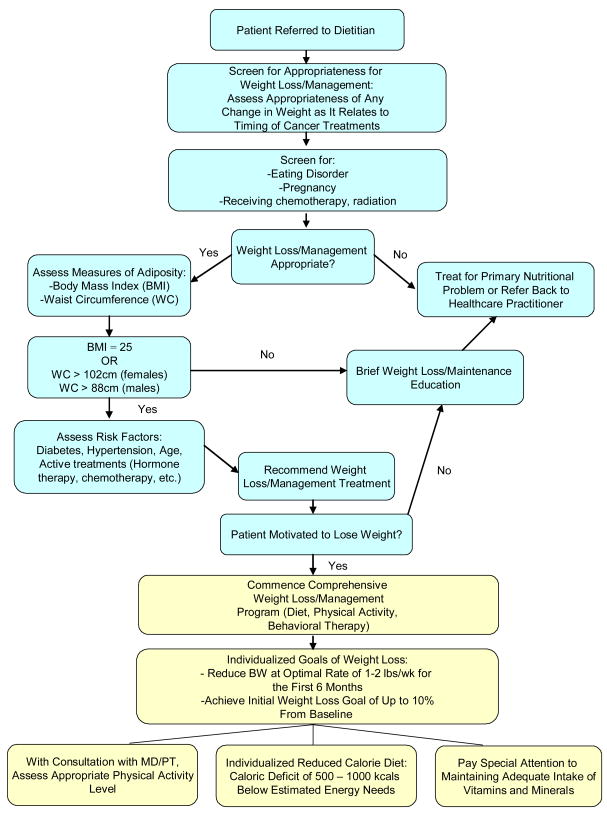
Several adipocyte-derived cytokines have been suggested to act as a link between obesity, insulin resistance and cancer. The potential biological role of insulin and these adipokines in cancer progression, and how they might work in concert to promote tumor growth are summarized (Figure 1):
Figure 1. Weight Loss/Management Screening and Treatment Algorithm for Cancer Survivors.
(Adapted from ADA Evidence Analysis Library Weight Management Screening Algorithm)
Figure 1 is a flow chart to serve as a guide to dietitians/CSOs counseling cancer survivors for weight management. Patient needs for weight loss as well as the appropriate level of intervention can be assessed using this chart.
Insulin, Insulin-like growth factor-1 (IGF-1) and IGF-1 binding protein 3 axis (IGFBP3)
Insulin is an anabolic hormone that may stimulate cell proliferation and cancer progression via multiple pathways: 1) insulin can increase production of IGF-1 via the growth hormone mechanism, (8). 2) Insulin increases free IGF-1 levels, making more IGF-1 available to bind to its receptors on normal and cancer cells. This receptor-bound IGF-1 modulates cell proliferation and survival. 3) Together insulin and IGF-1 stimulate production of adipocyte-derived vascular endothelial growth factor (VEGF), a critical angiogenic factor, which influences endothelial cell survival and migration (11). Furthermore, IGFBP-3, the most predominant IGF binding protein in serum, regulates the function of IGF-1 through the inhibition of the IGF-1 receptor. IGFBP-3 is hypothesized to act like a tumor suppressor gene and inhibits cell growth by sequestering free IGFs as well by inhibiting IGF-independent effects on cell growth (12).
The interplay of several factors seem to contribute to insulin resistance and may include sedentary lifestyles, body weight, high caloric diets, saturated fat intake, higher socioeconomic status, and inflammation, specifically tumor necrosis factor-α(TNF-α) (13,14). Additionally, higher concentrations of free fatty acids among obese individuals have also been implicated in decreasing insulin sensitivity (15).
Leptin
Leptin, an adipokine, found among obese individuals at higher concentrations, is the key player in energy balance and appetite control (16). Leptin induces expression of VEGF, which is responsible for cancer progression via its potent pro-angiogenic effect (11) and has been shown to induce cell migration and expression of growth factors in human prostate cancer cells in vitro (17). Laboratory evidence shows that leptin promotes proliferation and invasiveness of endometrial and prostate cells (18). Furthermore, it interferes with insulin signaling and is associated with insulin resistance (19), which has proliferative properties.
Proinflammatory adipokines
It has been hypothesized that phases of cancer progression are intimately linked to persistent inflammation and a dysfunctional cytokine network (20). Furthermore, obese individuals have higher levels of inflammatory markers accompanied by derangements of insulin metabolism (21). Proinflammatory factors produced by adipocytes include prostaglandin E2, TNF-α, interleukin-6 (IL-6), IL-8, IL-10, macrophage inflammatory protein-1, and monocyte chemoattractant protein-1 (22,23). These factors produce a cellular environment, conducive to survival and growth of cancer cells. Additionally, TNF-α stimulates VEGF production, which is key to tumor growth, via angiogenesis (8). One epidemiologic study is supportive of the link between cancer mortality and higher levels of inflammatory markers (24).
Adiponectin
Adiponectin, another major adipokine, which plays a role in the metabolism of fatty acids and glucose, is a key regulator of inflammatory response and insulin sensitivity. Adiponectin concentrations are inversely associated with insulin resistance, body weight and inflammatory response (23). Recent evidence from laboratory studies suggest that adiponectin is an important inhibitor of proliferation, either through its anti-inflammatory role, influence on insulin sensitivity, or by acting directly on cancer cells to increase apoptosis (25). Some studies suggest that adiponectin might have an anti-angiogenic potential (25). Evidence from human studies, although limited, is consistent with the laboratory evidence. For example, results from several small case-control studies (n=30 to 236), revealed that lower adiponectin concentrations were associated with more advanced breast cancer (26) and prostate cancer (27,28).
Epidemiologic evidence of obesity and insulin resistance and cancer progression, disease recurrence and mortality
Obesity
Evidence suggests that obesity is associated with cancer progression, recurrence of disease and mortality. Perhaps the best evidence of obesity and cancer mortality comes from a large, prospective, national study (n=900,000) in an ACS cohort, with a 16 year follow-up, which reported approximately a 50–60% higher prevalence of mortality from all cancers combined and multiple site-specific cancers among obese (body mass index (>40) vs. participants within the normal weight range, after adjusting for demographic, dietary and lifestyle factors (29). Similarly, in another study of two large ACS cohorts of men (n=81,6268), there was a approximately a 25% increase in prostate cancer mortality in obese (body mass index >30) vs. non-obese men, after adjusting for age, race, exercise, smoking status and family history of prostate cancer (30). Furthermore, a 35-year prospective cohort study among London-based men (n=18,403), noted an increased risk of dying from cancers of the colon, rectum, liver, and lymphoma among men with increased body weight, with hazard ratios (HR) ranging from 1.3–2.9 after adjusting for a range of covariates including socioeconomic status and physical activity (31). Studies have suggested that excess body fat is a negative prognostic factor for prostate cancer progression, with patients having faster biochemical failure, positive surgical margins and mortality (32–34).
Insulin resistance
Although there are several plausible biologic mechanisms that link insulin resistance to cancer progression or mortality, there are fewer epidemiologic studies that have investigated these associations. The existing evidence collectively reports detrimental associations between insulin resistance and cancer biology. For example there is robust evidence from the Risk Factors and Life Expectancy Project (1972–1987), a prospective study among adults 20–69 years, pooled from 9 large epidemiologic studies (n=62,285), suggesting ~3-fold increased risk (HR:2.99; 95% Confidence Interval (CI):1.27–7.02) of colorectal cancer mortality among participants with insulin resistance or associated biochemical abnormalities after adjusting for age, alcohol consumption, and smoking (35). Similarly, findings from the Chicago Heart Association Detection Project in Industry reported a 50% increased risk of colon cancer mortality among participants having the insulin resistance syndrome (≥3 of 5 biochemical abnormalities), after adjusting for race, age, education, sex and height (36). In a prospective cohort study evaluating the late effects of cancer among 512 women with breast cancer, insulin resistance was associated with a 2–3 fold increased risk of distant recurrence of disease (HR:2.0; 95% CI:1.2–3.3) and death from the disease (HR:3.1; 95% CI:1.7–5.7) after adjusting for tumor and treatment-related variables (37). Similarly, in a 10-year prospective Korean study (n=12,98,385), after controlling for smoking and alcohol use, individuals with higher versus lower fasting serum glucose concentrations (proxy for insulin resistance) were ~30% more likely to die of cancer (all cancers combined) (HR: 1.29; 95% CI: 1.22–1.37), and these detrimental associations persisted across all body mass index categories, suggesting an independent role of insulin beyond excess body weight (38).
In summary, the large, well-powered studies collectively suggest that both obesity and hyperinsulinemia may be ‘high-risk’ conditions for the late effects of cancer. It is noteworthy that the detrimental direction of the associations were not limited to site-specific cancers, but observed for most cancers (except lung cancer because of the confounding effects of smoking); these may possibly be explained by the biochemical reverberations of obesity and insulin resistance, which create a conducive environment for cancer cells to proliferate and survive, regardless of cancer type.
However, the existing literature on obesity and insulin resistance does not answer some important questions about survivorship. Although there is convincing evidence in the literature that obesity is associated with increased risk of developing cancer and death from it, the impact of obesity among cancer survivors is not well-studied. The relationships of insulin resistance and cancer progression or recurrence require additional studies among survivors. Further, insulin resistance is the hallmark of metabolic syndrome, a constellation of related biochemical abnormalities, which may act synergistically to influence tumor growth. At this time, the relationships of the full metabolic syndrome with cancer progression are unclear. Next, the majority of the studies have used mortality, as a proxy for long-term or late consequences of cancer.
Overall, the investigation of obesity, insulin resistance and metabolic syndrome, all of which are current public health concerns in the U.S., warrant further investigation in the context of surveillance of cancer survivors and cancer prognosis. Addressing these issues may teach important lessons to health professionals at cancer centers, and motivate them to design and implement special lifestyle and weight management intervention programs, to prevent late consequences of cancer among the very large at-risk population of cancer survivors.
Implications for Practice
Identification of “high-risk” cancer survivors
Because high body mass index and insulin resistance are predictive of poor cancer prognosis, the multidisciplinary healthcare team including oncologists, primary care physicians and the dietitians or CSOs, should identify obese patients and screen for insulin resistance to stratify individuals by risk of cancer progression. In addition to the traditional body weight assessment and laboratory tests, assessment of some obesity-related phenotypic manifestations may also be done. These includes hyperpigmentation of the skin, specifically on the back of the neck, (a condition known as acanthosis nigricans) (39), caused primarily due to insulin resistance, or the presence of the “buffalo hump” (or cervical lipomatosis) and a double-chin, which is predictive of the insulin resistance syndrome (40). It seems reasonable to offer patients in the “high-risk” strata, appropriate counseling referrals to dietitians and fitness experts for dietary, behavioral and lifestyle changes (specifically weight loss), as an integral part of the therapy. This could serve as an important secondary cancer control measure. Additionally, promotion of lifestyle changes maybe the key to improved treatment outcomes and successful therapy among individuals undergoing cancer treatment (41).
Cancer control though lifestyle modifications: implications for the dietetic practitioner
The dietitian or CSO plays a pivotal role in the care of cancer survivors through the course of the disease and during routine follow-up care. The Institute of Medicine report outlines three “seasons” of cancer survivorship: 1) acute survival, which begins with cancer diagnosis, and consists primarily of cancer therapy, 2) extended survival, which is a post-treatment period during which a person goes into remission and 3) longer-term survival which is usually tantamount with cure (10). During these periods of survival, individuals may live cancer-free for several years, or with intermittent bouts of cancer, have persistent disease, or die rapidly of late disease. Registered dietitians and CSOs are key players in improving survival and quality of life, as well as in reducing complications and risks that may arise during these “seasons” of survival, during and after treatment from co-morbidities associated with being overweight or obese, through dietary and lifestyle modification. In fact, a recent ACS report identified that over 50% of cancer deaths could be prevented by modifiable lifestyle factors including diet and exercise (42). Additionally, evidence from two large studies suggests that modification of lifestyle behaviors, including low energy diets and increased physical activity to control weight may be prevent cancer progression and mortality (43,44).
Cancer patients may also have weight management challenges due to disease- or treatment-related weight gain. During treatment, patients may experience fatigue and depression due to neutropenia, insomnia, decreased oral intake, side effects of medication, and changes in body composition, which may lead to decreased physical activity levels, resulting in an involuntary weight gain (45). Certain chemotherapy regimens, hormone therapy and steroid medications can also cause changes in body composition and undesirable weight gain (46). After considering all the clinical aspects and the potential for disease-related weight loss, a customized plan with an energy deficit of 500–1000 calories per day may be recommended by a dietitian, with the expectation of a weight loss of 1–2 pounds/week per the American Dietetic Association guidelines (47). This regime must be followed in conjunction with the physician’s care plan, provided it does not interfere with treatment or coincide with periods of time during treatment deemed high risk for any involuntary weight loss. Cancer survivors should be encouraged to follow the current guidelines on nutrition and physical activity proposed by the American Institute of Cancer Research (48) and the ACS (45) (Table titled “Current guidelines on nutrition and physical activity for cancer prevention” on American Dietetic Association website for more details). Table 1 summarizes some practice points for dietitians.
Table 1. Current guidelines on nutrition and physical activity for cancer prevention and survivorship.
| American Cancer Society Guidelines | American Institute for Cancer Research/World Cancer Research Fund Guidelines |
|---|---|
|
|
Adapted from: Kushi LH, Byers T, Doyle C, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention: Reducing the Risk of Cancer With Healthy Food Choices and Physical Activity. Vol 56; 2006:254–281.
World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington DC; AICR, 2007.
Prolonged hyperinsulinemia may be caused in part by specific types of carbohydrates and fatty acids. Quicker absorption of dietary glucose into the bloodstream from the intestines, regardless of source, results in a stronger stimulus for insulin secretion (49). Typical American diets characterized by higher intakes of red meat, processed meat, high-fat dairy products, sweets, refined grains and lower intakes of whole grains and fiber, are associated with hyperinsulinemia (50). Saturated and trans-fatty acids may increase insulin levels, whereas polyunsaturated fatty acids, especially omega-3 fatty acids, appear to be protective against hyperinsulinemia (51). However, it is more likely that the overall dietary pattern would influence insulin levels.
Existing evidence suggests that exercise is safe and feasible for patients during cancer treatment (45). Physical activity and survival have especially been investigated in breast and colon cancers and appear to reduce the risk of mortality (52,53). The Nurses’ Health Study consisting of women diagnosed with breast cancer between 1984–1998 (n=2,987), noted that physical activity reduced the risk of recurrence and death from the disease, with the greatest benefit occurring in women who walked 3–5 hours per week (43).
Additionally, a prospective randomized study among patients with advanced colorectal cancer (n=832), found that increasing levels of physical activity reduced risk of recurrence and cancer mortality, in a multivariate analyses including adjustment for disease-free survival and chemotherapy (52). Findings from a previous laboratory study suggests that healthier diet and physical activity reduced tumor growth and increased apoptosis by modulating the insulin/IGF-1 pathway (54). For counseling purposes, decisions on the type, frequency, duration, and intensity of physical activity should be individualized to the survivor’s age, previous fitness activities, type of cancer, stage of treatment, type of therapy, and co-morbid conditions.
Although cancer diagnosis is described as a “teachable moment” to empower cancer patients to make lifestyle changes, a recent study found that only a minority of cancer survivors are meeting the ACS guidelines for physical activity and consuming the recommended ≥5 servings of fruits and vegetables daily (55). A major challenge for the dietitian/CSO is to plan the trajectory of care among cancer survivors with a vision of both the long- and short-term consequences of cancer diagnosis, while empowering them to adhere to healthier diets and lifestyles.
Conclusions
The prevalence of obesity and cancer have been increasing in parallel. The number of cancer survivors has increased in the past three decades, with a projected doubling by 2050 (3). Furthermore, about a third of the U.S. population has insulin resistance. Biochemical reverberations of both obesity and insulin resistance, specifically higher concentrations of leptin, pro-inflammatory factors, and a disturbed insulin-IGF-1 axis, may cooperatively contribute to tumor growth and cancer progression (8). Although, additional studies are warranted to investigate this issue specifically in the context of surveillance of cancer survivors and cancer prognosis, existing epidemiologic evidence support poor prognostic potential of obesity and hyperinsulinemia with cancer biology, suggesting a need for updated clinical guidelines.
This has several implications for the dietetic practitioner for behavioral counseling and weight management among cancer patients through the course of the illness, and beyond treatment, to routine follow-up care. Clinicians should work closely with dietitians to identify obese cancer survivors, and screen for insulin resistance during treatment and follow-up visits, to ascertain individuals who are at potentially higher risk of cancer progression. These cancer survivors should be offered appropriate counseling referrals, specifically for weight management, to prevent repercussions of obesity on their existing cancer. The American Dietetic Association, ACS and American Institute of Cancer Research guidelines on diet and physical activity should be referred to by dietitians, CSOs and healthcare professionals, to delay or prevent development of new cancers, disease recurrence or mortality.
In conclusion, increased body weight and prolonged insulin resistance, both associated with chronic low-grade inflammation, have been implied in poor prognosis among the rapidly growing population of cancer survivors. It is time to consider management of these modifiable risk factors, through healthier lifestyles, as an important secondary cancer control tool.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts and Figures. [Accessed on February 24, 2009];American Cancer Society, 2008. 2008 http://www.cancer.org/docroot/stt/content/stt_1x_cancer_facts_and_figures_2008.asp.
- 2.Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK. SEER Cancer Statistics Review 1975–2005. National Cancer Institute; Bethesda, MD: 2007. [Google Scholar]
- 3.Edwards BK, Howe HL, Ries LA, Thun MJ, Rosenberg HM, Yancik R, Wingo PA, Jemal A, Feigal EG. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94(10):2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 5.US Mortality Data, 1960–2004, National Center for Health Statistics, Centers for Disease Control and Prevention, 2006.
- 6.Boyle PL, Levin B. World Cancer Report 2008. World Health Organization, International Agency for Research on Cancer; 2008. [Google Scholar]
- 7.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 8.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114(1):71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 9.Ioannou GN, Bryson CL, Boyko EJ. Prevalence and trends of insulin resistance, impaired fasting glucose, and diabetes. J Diabetes Complications. 2007;21(6):363–370. doi: 10.1016/j.jdiacomp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Hewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. The National Academies Press; Washington D.C.: 2005. [Google Scholar]
- 11.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82(3):925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 12.Lee HY, Chun KH, Liu B, Wiehle SA, Cristiano RJ, Hong WK, Cohen P. Insulin-like growth factor binding protein-3 inhibits the growth of non-small cell lung cancer. Cancer Res. 2002;62(12):3530–3537. [PubMed] [Google Scholar]
- 13.Noguchi Y, Yoshikawa T, Marat D, Doi C, Makino T, Fukuzawa K, Tsuburaya A, Satoh S, Ito T, Mitsuse S. Insulin resistance in cancer patients is associated with enhanced tumor necrosis factor-alpha expression in skeletal muscle. Biochem Biophys Res Commun. 1998;253(3):887–892. doi: 10.1006/bbrc.1998.9794. [DOI] [PubMed] [Google Scholar]
- 14.Riserus U, Arnlov J, Berglund L. Long-term predictors of insulin resistance: role of lifestyle and metabolic factors in middle-aged men. Diabetes Care. 2007;30(11):2928–2933. doi: 10.2337/dc07-0360. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52(9):2191–2197. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 16.Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J Clin Endocrinol Metab. 2004;89(2):447–452. doi: 10.1210/jc.2003-031005. [DOI] [PubMed] [Google Scholar]
- 17.Frankenberry KA, Somasundar P, McFadden DW, Vona-Davis LC. Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. Am J Surg. 2004;188(5):560–565. doi: 10.1016/j.amjsurg.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13(2):629–640. doi: 10.1677/erc.1.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer S, Hanefeld M, Haffner SM, Fusch C, Schwanebek U, Köhler C, Fücker K, Julius U. Insulin-resistant patients with type 2 diabetes mellitus have higher serum leptin levels independently of body fat mass. Acta Diabetol. 2002;39(3):105–110. doi: 10.1007/s005920200027. [DOI] [PubMed] [Google Scholar]
- 20.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 21.Choi KM, Ryu OH, Lee KW, Kim H, Seo JA, Kim SG, Kim NH, Choi DS, Baik SH. Serum adiponectin, interleukin-10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res Clin Pract. 2006;75(2):235–40. doi: 10.1016/j.diabres.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145(5):2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 23.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Il’yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 25.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86(3):s858–866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 26.Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, Noguchi S. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9(15):5699–5704. [PubMed] [Google Scholar]
- 27.Freedland SJ, Sokoll LJ, Platz EA, Mangold LA, Bruzek DJ, Mohr P, Yiu SK, Partin AW. Association between serum adiponectin, and pathological stage and grade in men undergoing radical prostatectomy. J Urol. 2005;174(4 Pt 1):1266–1270. doi: 10.1097/01.ju.0000173093.89897.97. [DOI] [PubMed] [Google Scholar]
- 28.Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology. 2005;65(6):1168–1172. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 29.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10(4):345–353. [PubMed] [Google Scholar]
- 31.Batty GD, Shipley MJ, Jarrett RJ, Breeze E, Marmot MG, Smith GD. Obesity and overweight in relation to organ-specific cancer mortality in London (UK): findings from the original Whitehall study. Int J Obes (Lond) 2005;29(10):1267–1274. doi: 10.1038/sj.ijo.0803020. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqui SA, Inman BA, Sengupta S, Slezak JM, Bergstralh DJ, Leibovich BC, Zincke H, Blute ML. Obesity and survival after radical prostatectomy: A 10-year prospective cohort study. Cancer. 2006;107(3):521–529. doi: 10.1002/cncr.22030. [DOI] [PubMed] [Google Scholar]
- 33.Freedland SJ, Isaacs WB, Mangold LA, Yiu SK, Grubb KA, Partin AW, Epstein JI, Walsh PC, Platz EA. Stronger association between obesity and biochemical progression after radical prostatectomy among men treated in the last 10 years. Clin Cancer Res. 2005;11(8):2883–2888. doi: 10.1158/1078-0432.CCR-04-2257. [DOI] [PubMed] [Google Scholar]
- 34.Amling CL, Riffenburgh RH, Sun L, Moul JW, Lance RS, Kusuda L, Sexton WJ, Soderdahl DW, Donahue TF, Foley JP, Chung AK, McLeod DG. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22(3):439–445. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 35.Trevisan M, Liu J, Muti P, Misciagna G, Menotti A, Fucci F. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev. 2001;10(9):937–941. [PubMed] [Google Scholar]
- 36.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11(4):385–391. [PubMed] [Google Scholar]
- 37.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, Hood N. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20(1):42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 38.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293(2):194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 39.Monajemi H, Stroes E, Hegele RA, Fliers E. Inherited lipodystrophies and the metabolic syndrome. Clin Endocrinol (Oxf) 2007;67(4):479–484. doi: 10.1111/j.1365-2265.2007.02906.x. [DOI] [PubMed] [Google Scholar]
- 40.Misra A, Jaiswal A, Shakti D, Vikram NK, Pandey RM, Kondal D, Bhushan B. Novel phenotypic markers and screening score for the metabolic syndrome in adult Asian Indians. Diabetes Res Clin Pract. 2008;79(2):e1–5. doi: 10.1016/j.diabres.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Griggs JJ, Sabel MS. Obesity and cancer treatment: weighing the evidence. J Clin Oncol. 2008;26(25):4060–4062. doi: 10.1200/JCO.2008.17.4250. [DOI] [PubMed] [Google Scholar]
- 42.American Cancer Society: Half the cancer deaths could be prevented. CA Cancer J Clin. 2005;55:209–210. [Google Scholar]
- 43.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 44.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy M, Goodman MT, Giuliano AE, Karanja N, McAndrew P, Hudis C, Butler J, Merkel D, Kristal A, Caan B, Michaelson R, Vinciguerra V, Del Prete S, Winkler M, Hall R, Simon M, Winters BL, Elashoff RM. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 45.Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, McTiernan A, Rock CL, Thompson C, Gansler T, Andrews KS. The 2006 Nutrition, Physical Activity and Cancer Survivorship Advisory Committee; American Cancer Society. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56(6):323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 46.Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, Mason MD, Cockcroft JF, Scanlon MF, Davies JS. The Effects of Induced Hypogonadism on Arterial Stiffness, Body Composition, and Metabolic Parameters in Males with Prostate Cancer. J Clin Endocrinol Metab. 2001;86(9):4261–4267. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 47.Seagle HM, Strain GW, Makris A, Reeves RS. Position of the American Dietetic Association: weight management. J Am Diet Assoc. 2009;109(2):330–346. doi: 10.1016/j.jada.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 48.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 49.Wolever TM, Jenkins DJ, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr. 1991;54(5):846–854. doi: 10.1093/ajcn/54.5.846. [DOI] [PubMed] [Google Scholar]
- 50.Fung TT, Rimm EB, Spiegelman D, Fifai N, Tofler GH, Willett WC, Hu FB. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73(1):61–67. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 51.Rivellese AA, De Natale C, Lilli S. Type of dietary fat and insulin resistance. Ann N Y Acad Sci. 2002;967:329–335. doi: 10.1111/j.1749-6632.2002.tb04288.x. [DOI] [PubMed] [Google Scholar]
- 52.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Ruchs CS. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 53.Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, Baumgartner RN, Baumgartner KB, Bernstein L. Influence of Pre- and Postdiagnosis Physical Activity on Mortality in Breast Cancer Survivors: The Health, Eating, Activity, and Lifestyle Study. J Clin Oncol. 2008;26(24):3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ngo TH, Barnard RJ, Leung PS, Cohen P, Aronson WJ. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003;144(6):2319–2324. doi: 10.1210/en.2003-221028. [DOI] [PubMed] [Google Scholar]
- 55.Blanchard CM, Courneya KS, Stein K American Cancer Society’s SCS-II. Cancer Survivors’ Adherence to Lifestyle Behavior Recommendations and Associations With Health-Related Quality of Life: Results From the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]