Abstract
Background
Postural Tachycardia Syndrome (POTS) induces disabling chronic orthostatic intolerance with an excessive increase in heart rate (HR) upon standing. Beta-blockade is an appealing treatment approach, but there are conflicting preliminary reports. We tested the hypothesis that propranolol will attenuate the tachycardia and improve symptom burden in patients with POTS. In Protocol #1, a low dose (20mg) was compared to placebo, and the dose response was assessed in Protocol #2.
Methods and Results
In Protocol #1, patients with POTS (n=54) underwent acute drug trials of propranolol 20 mg orally and placebo, on separate mornings, in a randomized crossover design. Blood pressure, HR and symptoms were assessed while seated and after standing for up to 10 minutes prior to, and hourly, following study drug. Supine (P<0.001) and standing (P<0.001) HR were significantly lower following propranolol compared to placebo. The symptom burden improvement from baseline to 2 hours was greater with propranolol than placebo (median: −4.5 arbitrary units [au] vs. 0 au; P=0.044). In Protocol #2, 18 patients with POTS underwent similar trials of high dose (80 mg) vs. low dose (20 mg) propranolol. Although high dose elicited a greater decrease than low dose on standing HR (P<0.001) and orthostatic tachycardia (P<0.001), the improvement in symptoms at 2 hours was greater with low dose propranolol (−6 au vs. −2 au; P=0.041).
Conclusions
Low dose oral propranolol significantly attenuated tachycardia and improved symptoms in POTS. Higher dose propranolol did not further improve, and may worsen, symptoms.
Keywords: tachycardia; beta-adrenergic blocker; nervous system, autonomic; drugs; humans; orthostatic intolerance
INTRODUCTION
Postural tachycardia syndrome (POTS) is a disorder of chronic orthostatic intolerance that disproportionately affects women of childbearing age. It is characterized by symptoms (including palpitation, lightheadedness, chest discomfort, shortness of breath, blurred vision, and mental clouding) that occur during standing, but resolve with sitting or lying down 1–3. A most striking feature of this disorder is the excessive increase in heart rate (HR) that occurs upon standing in the absence of hypotension 4. Many patients with POTS have increased sympathetic nervous system tone measured with direct nerve recordings 5 or biochemically with an elevated plasma norepinephrine on standing 6–8. POTS is associated with a very poor quality of life and significant functional disability 9; there are a paucity of effective therapies. Due to the striking tachycardia that is central to this disorder, these patients are often referred to cardiologists for diagnosis and treatment.
Given that excessive tachycardia is a cardinal feature of this syndrome, a logical treatment strategy would be to reduce the HR with beta-adrenergic blockade. Beta-blockers have been reported to improve symptoms in case-reports 10, 11 and open-label studies 12, 13 and can decrease plasma norepinephrine levels in a hyperadrenergic state 14. Unfortunately, in experimental models of orthostatic intolerance, neither propranolol 15, 16 nor esmolol 17 were found to improve orthostatic tolerance. One reason for the limited effectiveness of beta-blockade might be that patients with POTS have a low stroke volume and might require an elevated HR in order to maintain their cardiac output 18. Conversely, a lower HR might allow for improved cardiac filling and increase the stroke volume.
Given the conflicting data regarding the effectiveness of beta-blockers in POTS, we conducted an acute single-blind cross-over trial to test the hypothesis that low dose propranolol would reduce orthostatic tachycardia and improve upright symptoms in patients with POTS. After the initial study, we conducted a second protocol to test the hypothesis that a higher dose of propranolol would be more effective in controlling HR and improving symptoms than a lower dose of propranolol in patients with POTS.
METHODS
Subjects
Patients referred to the Vanderbilt University Autonomic Dysfunction Center with postural tachycardia syndrome (POTS) between November 2003 and September 2008 were candidates for inclusion in this study. Patients were recruited for Protocol #1 (see Drug Trials below) during the entire time interval, and for Protocol #2 between January 2007 and September 2008. Individual patients were eligible to participate in both protocols. Patients met criteria for POTS 1, 4, 19 in that they developed symptoms of orthostatic intolerance accompanied by a HR rise ≥ 30 min−1 within the first 10 minutes of standing, in the absence of orthostatic hypotension (a fall in blood pressure [BP] > 20/10 mmHg). All patients had at least a 6-month history of symptoms in the absence of an additional chronic debilitating disorder or prolonged bed rest, were at least 18 years of age, were free of medications that could impair autonomic tone 1 and were not taking fludrocortisone for at least 5 days prior to testing. The Vanderbilt University Investigational Review Board approved this study and written informed consent was obtained from each subject prior to initiating the study. The data reported are a part of “The Treatment of Orthostatic Intolerance” study, which is registered with www.ClinicalTrials.gov (NCT00262470).
Study Diet and Baseline Characterization
Study investigations were performed in the Elliot V. Newman Clinical Research Center at Vanderbilt University. For at least 3 days prior to testing, subjects consumed a diet containing 150 mEq sodium/day and 70 mEq potassium/day. Chronic medications were discontinued 5 half-lives before the study. Fludrocortisone has an elimination half-life of 3.5 hours 20, but this was discontinued for at least 5 days. The diet was free of caffeine-containing beverages. HR, systolic BP (SBP), diastolic BP (DBP), and fractionated plasma catecholamines were assessed after overnight rest in the supine position and again after standing up to 30 minutes (as tolerated) as part of baseline characterization. For catecholamine measurements, blood was collected in plastic syringes, immediately transferred to chilled vacuum tubes with sodium heparin (BD, Franklin Lakes, NJ, USA) and placed on ice. Plasma was separated by centrifugation at −4°C and stored at −70°C in collection tubes with 6% reduced glutathione (Sigma-Aldrich Inc., St. Louis, MO, USA) until the assay was performed. Concentrations of norepinephrine and epinephrine were measured by batch alumina extraction followed by high performance liquid chromatography for separation with electrochemical detection and quantification 21. Plasma norepinephrine and epinephrine values are reported in SI units. To convert from nmol/l to the more conventional pg/ml, multiply by 169.18 for norepinephrine (1 nmol/l = 169.18 pg/ml) or by 183.2 for epinephrine (1 nmol/l = 183.2 pg/ml).
Drug Trials
All drug trials were started in the morning at least 2 hours after breakfast (to avoid any acute hemodynamic effects from eating) in a post-void state. In protocol #1, the patients with POTS were given a tablet of propranolol 20 mg (Mylam Pharmaceuticals, Morgantown VW) or placebo in a randomized single-blind, cross-over fashion on separate days. This strength of propranolol represents a low dose. Placebo was prepared by the Vanderbilt Investigational Drug Services. The patients were seated comfortably in a chair for the duration of the data collection except during the prescribed periods of standing. Brachial cuff BPs and HRs were measured using an automated vital signs monitor (Dinamap Vital Signs Monitor™, Critikon Corp) and digitally acquired into a custom designed database (Microsoft Access, Microsoft Corporation, Redmond Washington). Immediately before, and every hour for 4 hours following study drug administration, each patient was asked to stand for 10 minutes while their standing HRs and BPs were recorded. The degree of orthostatic stress is not as great when standing from a seated position compared with standing from a supine position. While conducting the study while seated might decrease the magnitude of the orthostatic tachycardia, it does provide a clinically relevant and reproducible scenario.
In protocol #2, a high dose (80 mg) was compared to a low dose (20 mg) of propranolol (Mylam Pharmaceuticals, Morgantown VW) in a randomized single-blind, cross-over fashion on separate days, with study endpoints as in protocol #1.
Symptoms
Patients were asked to self-report their symptom burden immediately before, and at 2 and 4 hours following study drug administration using the Vanderbilt POTS Symptom Score 6. The patients were asked to rate the severity of 9 symptoms on a 0–10 scale (with 0 reflecting an absence of symptoms). The sum of the scores at each time point was used as a measure of symptom burden. The 9 symptoms were: mental clouding, blurred vision, shortness of breath, rapid heart beat, tremulousness, chest discomfort, headache, lightheadedness, and nausea. This symptom score has been previously used by our center 6 and the symptoms were chosen as they reflect common complaints of patients with POTS.
Individual missing hemodynamic data points were interpolated by taking the within-individual mean for the parameter at the data point for the hour immediately before and immediately after the missing data point. Hemodynamic data was not interpolated if either the baseline or 4 hour (final) value was missing, or if more than 1 consecutive hourly data point was missing. Only patients with paired sets of complete hemodynamic datasets (after interpolation) were included in these analyses.
Statistical Analysis
Our primary endpoint was the change in HR upon standing from a seated position (delta HR) 2 hours after study drug administration. The 2-hour time point was chosen because the peak effect of propranolol occurs at 90 minutes following a dose 22. We hypothesized that the 2-hour delta HR and 2-hour change in symptom score would be significantly lower on the propranolol day than the placebo day (Protocol #1). We used a 2-tailed paired t-test that compared the delta HR 2 hours after propranolol and placebo administration, respectively.
Repeated-measures analysis of variance (ANOVA) was used to compare HR, SBP, DBP and symptom scores over time on both the propranolol and placebo days. Repeated measures ANOVA was performed using a generalized estimating equation model with an identity link function and a normal random component. The Huber-White sandwich estimator was used to estimate the variance-covariance matrix of the model’s parameters. Confidence intervals for the difference in patient responses to propranolol and placebo were derived from these models. Tests for day-treatment interactions were performed using Wald tests of the appropriate interaction terms, and no significant day-treatment interactions were found.
The van Elteren test was used to assess differences in symptom scores of patients on propranolol and placebo stratified by time since baseline evaluation. As symptom score data were not normally distributed, paired comparisons between groups were performed using the non-parametric Wilcoxon signed-ranks test.
A similar data analysis plan was used for protocol #2. We hypothesized that the 2-hour delta HR and 2-hour change in symptom score would be significantly lower on the high dose (80 mg) propranolol day than the low dose (20 mg) propranolol day. Further, the changes in the individual component symptoms over 2 hours following each dose of propranolol were assessed with a paired t-test to determine if the change was different from 0.
Values are reported as means and standard deviation (SD) unless otherwise noted. Total Symptoms Scores are reported as median (25th percentile, 75th percentile). Probability (P) values of <0.05 were considered statistically significant, and all tests were two-tailed. Statistical analyses were performed with SPSS for Windows (version 16.0, SPSS), with the exception of the Generalized Estimating Equation and the van Elteren test which were performed with Stata Statistical Software (Release 10, StataCorp LP). Prism for Windows 4 (version 4.02, GraphPad Software Inc.) was used for graphical presentation.
RESULTS
Patient Characteristics
Demographic data and supine and standing parameters for each protocol are presented in Table 1. In protocol #1 (propranolol vs. placebo), 54 subjects with POTS had complete paired datasets suitable for analyses, as did 18 subjects in protocol #2 (propranolol 80 mg vs. propranolol 20 mg). As expected, subjects in both protocols were predominantly female with a mean age of 33–34 years.
Table 1.
Baseline demographics, and postural vital signs and catecholamines of the subjects with Postural Tachycardia Syndrome in the 2 protocols.
| Protocol #1 | Protocol #2 | |||
|---|---|---|---|---|
| (n=54) | (n=18) | |||
| Female (n) | 49 (91%) | 16 (89%) | ||
| Age (years) | 34 ± 10 | 33 ± 8 | ||
| Supine | ||||
| Heart Rate (min−1) | 76 ± 13 | 78 ± 13 | ||
| Systolic Blood Pressure (mmHg) | 110 ± 12 | 111 ± 16 | ||
| Diastolic Blood Pressure (mmHg) | 68 ±8 | 70 ± 10 | ||
| Norepinephrine (nmol/l) | 1.65 ± 1.41 | 1.86 ± 2.13 | ||
| Epinephrine (nmol/l) | 0.15 ± 0.14 | 0.19 ± 0.19 | ||
| Standing | ||||
| Heart Rate (min−1) | 122 ± 26 | ** | 130 ± 29 | ** |
| Systolic Blood Pressure (mmHg) | 115 ± 22 | 118 ± 24 | ||
| Diastolic Blood Pressure (mmHg) | 74 ± 14 | * | 79 ± 16 | * |
| Norepinephrine (nmol/l) | 4.94 ± 3.35 | ** | 3.93 ± 2.21 | * |
| Epinephrine (nmol/l) | 0.45 ±0.75 | * | 0.50 ± 1.05 | |
| Change from Supine to Standing | ||||
| Heart Rate (min−1) | 46 ± 24 | 52 ± 27 | ||
| Systolic Blood Pressure (mmHg) | 5 ± 19 | 7 ± 24 | ||
| Diastolic Blood Pressure (mmHg) | 6 ± 13 | 9 ± 15 | ||
| Norepinephrine (nmol/l) | 3.22 ± 3.22 | 1.80 ± 2.64 | ||
| Epinephrine (nmol/l) | 0.31 ± 0.68 | 0.31 ± 0.93 |
Data are presented as the mean ± standard deviation. Reported P values are for paired t-tests comparing supine and upright parameters.
P<0.05;
P<0.001
Supine HR and BP were comparable in both protocols and supine plasma norepinephrine and epinephrine values were within the normal range in both groups (norepinephrine <2.81 nmol/l [<475 pg/ml] and epinephrine <0.41 nmol/l [<75 pg/ml]). On standing, there was a marked increase in HR (Protocol#1: 122±26 min−1, P<0.001; Protocol #2: 130±29 min−1, P<0.001) and norepinephrine (Protocol#1: 4.94±3.35 nmol/l, P<0.001; Protocol#2: 3.93±2.21 nmol/l, P=0.015) compared with respective supine values. The DBP also increased on standing (Protocol#1: 68±8 mmHg supine vs. 74±14 mmHg standing, P=0.001; Protocol#2: 70±11 mmHg supine vs. 79±16 mmHg standing, P=0.031). The mean differences and standard deviation of the mean differences are shown in Table 1.
Protocol #1: Seated and Standing HR and BP Measurements with Propranolol 20mg vs. Placebo
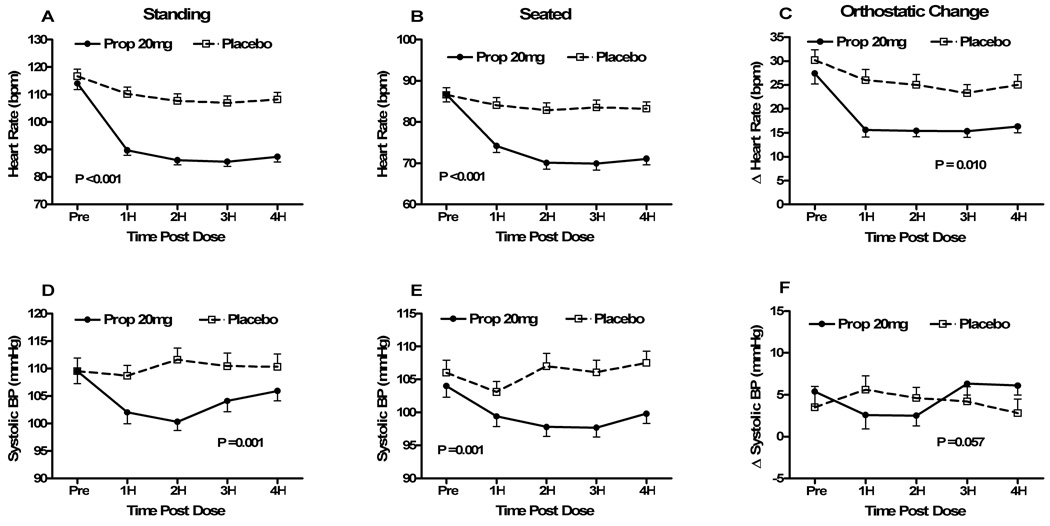
The summary of data from protocol #1 is presented in Table 2 and Figure 1. Standing HR prior to study drug administration was not different between placebo and propranolol 20mg (117±19 min−1 vs. 114±17 min−1; P=0.199; Fig 1a). Standing HR started to decrease by 1 hour post study drug in both groups, the first assessment post-baseline. Compared to placebo, propranolol 20mg effected a lower standing HR at 1 hour (90±13 min−1 vs. 110±18 min−1; P<0.001), and this difference was maintained at P<0.001 for the 4 hours following study drug administration. When the effect of propranolol 20mg vs. placebo on standing HR was analyzed over time, there was a significant difference in response based on study drug (P<0.001). Standing SBPs were similar at baseline for propranolol 20mg (110±17 mmHg) and placebo (110±18 mmHg; P=1.0). By 1 hour post study drug, propranolol 20mg had significantly lowered the standing SBP compared with placebo (102±15 mmHg vs. 109±14 mmHg; P=0.009; Fig 1d), and it remained significantly lower for 3 hours post study drug. When analyzed over time, standing SBP was lowered more with propranolol 20mg than placebo (P=0.001). Standing DBPs were similar at baseline, but were lowered more with propranolol 20mg than placebo (P=0.037) (all DBP data are presented in Supplemental Table 1).
Table 2.
Orthostatic hemodynamics and symptoms with propranolol 20mg and placebo (Protocol #1) in patients with Postural Tachycardia Syndrome (n=54).
| Pre | 2 Hours Post | 4 Hours Post | RM ANOVA | |
|---|---|---|---|---|
| P Value | ||||
| Delta (Standing-Seated) | ||||
| HR (min−1) | ||||
| Propranolol 20mg | 27.4 ± 16.1 | 15.4 ± 9.1 | 16.3 ± 9.4 | |
| Placebo | 30.2 ± 15.7 | 25.0 ± 16.3 | 25.0 ± 15.8 | |
| P value (between drugs) | NS | 0.033 | 0.028 | 0.076 |
| Standing HR (min−1) | ||||
| Propranolol 20mg | 114.0 ± 16.6 | 86.1 ± 12.2 | 87.3 ± 13.8 | |
| Placebo | 116.7 ± 18.6 | 107.7 ± 19.1 | 108.2 ± 19.3 | |
| P value (between drugs) | NS | <0.001 | <0.001 | <0.001 |
| Seated HR (min−1) | ||||
| Propranolol 20mg | 86.7 ± 13.0 | 70.1 ± 11.4 | 71.1 ± 10.6 | |
| Placebo | 86.6 ± 12.4 | 82.8 ± 13.8 | 83.2 ± 12.7 | |
| P value (between drugs) | NS | <0.001 | <0.001 | <0.001 |
| Delta (Standing-Seated) | ||||
| SBP (mmHg) | ||||
| Propranolol 20mg | 5.4 ± 12.8 | 2.5 ± 9.0 | 6.1 ± 8.2 | |
| Placebo | 3.5 ± 18.3 | 4.6 ± 9.6 | 2.8 ± 12.5 | |
| P value (between drugs) | NS | NS | NS | 0.057 |
| Standing SBP (mmHg) | ||||
| Propranolol 20mg | 109.5 ± 16.9 | 100.3 ± 11.7 | 105.9 ± 12.9 | |
| Placebo | 109.5 ± 17.8 | 111.6 ± 15.5 | 110.3 ± 17.1 | |
| P value (between drugs) | NS | <0.001 | NS | 0.001 |
| Sitting SBP (mmHg) | ||||
| Propranolol 20mg | 104.0 ± 12.5 | 97.8 ± 10.5 | 99.8 ± 10.5 | |
| Placebo | 106.0 ± 14.2 | 107.0 ± 14.5 | 107.5 ± 13.2 | |
| P value (between drugs) | NS | 0.001 | 0.005 | 0.002 |
| Symptom Score (au) | ||||
| [n=36] | ||||
| Propranolol 20mg | 17 (9, 29) | 9.5 (3.75, | 10 (4, 16) | |
| 16.25) | ||||
| Placebo | 16 (7.25, | 15 (6, 28.25) | 12.5 (5, 30) | |
| 28.5) | ||||
| P value (between drugs) | 0.385 | 0.012 | 0.007 | 0.035 (vE) |
HR – heart rate; SBP – systolic blood pressure; au – arbitrary units; NS – not significant. For hemodynamic parameters, data are presented as mean±standard deviation. Repeated measures analysis of variance (RM ANOVA) was used to determine the P value for the overall change between study drug and placebo and paired comparisons were made using within model contrasts. Symptoms data are presented as median (25th percentile, 75th percentile) and were analyzed using the van Elteren’s test (vE) overall and the Wilcoxon Signed Rank test for paired data. P<0.05 was considered significant.
Figure 1. Seated, standing and orthostatic changes in heart rates and blood pressures before and after propranolol 20mg vs. placebo (Protocol #1).
Heart rate (HR) and systolic blood pressure (SBP) data are presented immediately before (pre), and hourly for 4 hours (4H) following study drug administration for the propranolol 20mg day (“Prop 20”; solid circles) and the placebo day (open squares). Peak HR after standing for a maximum of 10 minutes (Fig 1A), seated HR immediately before standing (Fig 1B) and the changes in HR from sit to stand (Fig 1C) are shown in the top 3 panels. Similarly, the data for standing SBP (Fig 1D), seated SBP (Fig 1E) and the changes in SBP from sit to stand (Fig 1F) are shown in the bottom 3 panels. The error bars represent the standard error of the mean. The ANOVA P values are presented for the overall effect of the study drug over time. bpm – beats per minute; mmHg – millimeters of mercury.
Immediately prior to administration of the study drug, there was no difference in seated HR between the placebo day (87±12 min−1) and propranolol day (87±13 min−1; P=0.977; Fig 1b). Seated HR at 1 hour decreased significantly with propranolol 20mg (84±14 min−1) compared with placebo (74±12 min−1; P<0.001), and remained significantly lower for the 4 hours of follow-up. A RM ANOVA model also found that seated HR was lower over time following the propranolol 20mg (P<0.001). Seated SBPs were similar at baseline for propranolol 20mg (104±13 mmHg) and placebo (106±14 mmHg; P=0.249). By 2 hours post study drug, propranolol 20mg had significantly lowered seated SBP compared with placebo (98±11 mmHg vs. 107±15 mmHg; P=0.001; Fig 1e), and it remained significantly lower up to 4 hours post study drug. When analyzed over time, seated SBP was lowered more with propranolol 20mg than placebo (P=0.002). Seated DBP was lowered more with propranolol 20mg than placebo (P<0.001), when analyzed over time
Prior to study drug administration, the POTS patients had a large acute postural increase in HR with standing from a seated position (delta HR), a cardinal hemodynamic feature of the syndrome (propranolol 20mg day 27±16 min−1 vs. placebo day 30±16 min−1; P=0.193; Fig 1c). Delta HR decreased in both groups, but significantly more at 1 hour following propranolol 20mg (16±11 min−1) than placebo (26±17 min−1; P=0.004), and remained significantly lower with propranolol at each hour during follow-up (the primary study endpoint was delta HR at 2 hours following study drug [P=0.033]). When analyzed over time, there was a trend to a lower delta HR following propranolol 20mg than placebo (P=0.076). There was a similar non-significant effect of propranolol on delta SBP compared with placebo (P=0.057; Fig 1f). There was no difference in delta diastolic pressure between the 2 groups over time (P=0.148).
Protocol #2: Comparison of Low-Dose Propranolol (20mg) vs. High Dose Propranolol (80mg)
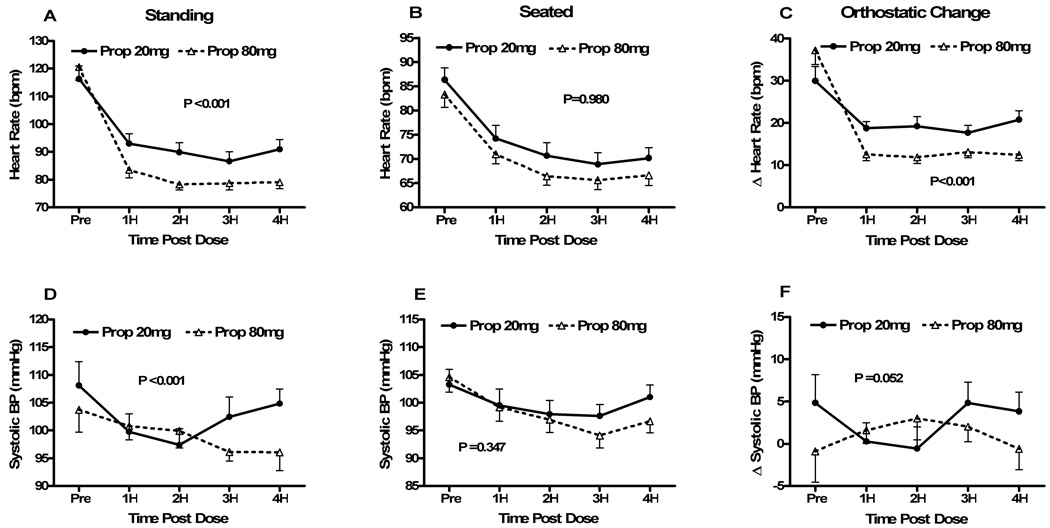
The summary of the data from protocol #2 can be found in Table 3 and Figure 2. Standing HR prior to study drug administration was not different between low dose and high dose propranolol (116±20 min−1 vs. 121±20 min−1; P=0.366; Fig 2a). Standing HR at 1-hour was lower with high dose (84±12 min−1) than low dose propranolol (93±15 min−1; P<0.001), and this significant difference persisted through the 4 hour follow-up. When the effect of propranolol dose on standing HR was analyzed over time, there was a significantly lower HR with high dose propranolol (P<0.001). Standing SBPs were similar at baseline for both low dose (108±18 mmHg) and high dose propranolol (104±17 mmHg; P=0.216; Fig 2d). Although standing SBP following propranolol decreased for both low dose and high dose, this was significantly greater for the high dose (P<0.001). Standing DBP was not different between doses at baseline, and although there was a slight trend to a lower DBP with high dose propranolol, this was not statistically significant (P=0.080).
Table 3.
Orthostatic hemodynamics and symptoms with low dose propranolol (20mg) vs. high dose propranolol (80mg) (Protocol #2) in patients with Postural Tachycardia Syndrome (n=18).
| Pre | 2 Hours Post | 4 Hours Post | RM ANOVA | |
|---|---|---|---|---|
| P Value | ||||
| Delta (Standing-Seated) | ||||
| HR (min−1) | ||||
| Propranolol Low Dose | 29.9 ± 14.4 | 19.2 ± 9.8 | 20.8 ± 9.1 | |
| Propranolol High Dose | 37.2 ± 14.4 | 11.9 ± 6.4 | 12.4 ± 5.8 | |
| P value (between drugs) | NS | <0.001 | <0.001 | <0.001 |
| Standing HR (min−1) | ||||
| Propranolol Low Dose | 116.3 ± 20.0 | 89.9 ± 14.5 | 90.9 ± 15.0 | |
| Propranolol High Dose | 120.5 ± 20.3 | 78.3 ± 8.5 | 79.1 ± 9.9 | |
| P value (between drugs) | NS | <0.001 | <0.001 | <0.001 |
| Seated HR (min−1) | ||||
| Propranolol Low Dose | 86.3 ± 10.5 | 70.6 ± 11.3 | 70.2 ± 9.0 | |
| Propranolol High Dose | 83.3 ± 11.2 | 66.4 ± 7.7 | 66.6 ±9.0 | |
| P value (between drugs) | NS | NS | NS | 0.980 |
| Delta (Standing-Seated) | ||||
| SBP (mmHg) | ||||
| Propranolol Low Dose | 4.8 ± 14.2 | −0.6 ± 10.9 | 3.8 ± 9.7 | |
| Propranolol High Dose | −0.9 ± 15.5 | 3.0 ± 10.8 | −0.6 ± 10.4 | |
| P value (between drugs) | NS | NS | NS | 0.052 |
| Standing SBP (mmHg) | ||||
| Propranolol Low Dose | 108.1 ± 18.3 | 97.4 ± 12.4 | 104.8 ± 11.3 | |
| Propranolol High Dose | 103.7 ± 17.0 | 99.9 ± 13.2 | 96.1 ± 14.0 | |
| P value (between drugs) | NS | 0.05 | NS | <0.001 |
| Sitting SBP (mmHg) | ||||
| Propranolol Low Dose | 103.3 ± 11.6 | 97.9 ± 10.6 | 101.0 ± 9.3 | |
| Propranolol High Dose | 104.6 ± 11.3 | 96.9 ± 9.8 | 96.7 ± 8.8 | |
| P value (between drugs) | NS | NS | NS | 0.347 |
| Symptom Score (au) | ||||
| [n=16] | ||||
| Propranolol Low Dose | 17.5 (8, 27.5) | 7 (1.5, 13.25) | 6 (3, 11.75) | |
| Propranolol High Dose | 12 (3.5, 26.5) | 8 (2, 18) | 5 (1, 13.5) | |
| P value (between drugs) | NS | NS | NS | 0.527 (vE) |
SD – standard deviation; HR – heart rate; SBP – systolic blood pressure; au – arbitrary units; NS – not significant. For hemodynamic parameters, data are presented as mean±standard deviation. Repeated measures analysis of variance (RM ANOVA) was used to determine the P value for the overall change between high and low doses of propranolol and paired comparisons were made using within model contrasts. Symptoms data are presented as median (25th percentile, 75th percentile) and were analyzed using the van Elteren’s test (vE) overall and using the Wilcoxon signed-rank test for paired data. P<0.05 was considered significant.
Figure 2. Seated, standing and orthostatic changes in heart rates and blood pressures before and after Low Dose (20mg) Propranolol vs. High Dose (80mg) Propranolol (Protocol #2).
Heart rate (HR) and systolic blood pressure (SBP) data are presented immediately before (pre), and hourly for 4 hours (4H) following study drug administration for the propranolol 20mg day (“Prop 20”; solid circles) and the propranolol 80mg day (“Prop 80”; open triangles). Peak HR after standing for a maximum of 10 minutes (Fig 1A), seated HR immediately before standing (Fig 1B) and the changes in HR from sit to stand (Fig 1C) are shown in the top 3 panels. Similarly, the data for standing SBP (Fig 1D), seated SBP (Fig 1E) and the changes in SBP from sit to stand (Fig 1F) are shown in the bottom 3 panels. The error bars represent the standard error of the mean. The ANOVA P values are presented for the overall effect of the study drug over time. bpm – beats per minute; mmHg – millimeters of mercury.
Seated HR was not significantly different between high dose and low dose propranolol at any time point or when analyzed over time (P=0.981; Fig 2b). Seated SBPs were similar at baseline for high dose (105±11 mmHg) and low dose propranolol (103±12 mmHg; P=0.604; Fig 2e). When analyzed over time, the effects of propranolol dose over time on seated SBP was not statistically significant (P=0.347). Seated DBP was not different between doses at baseline, and although there was a slight trend to a lower DBP with high dose propranolol, this was not statistically significant (P=0.125).
Prior to study drug administration, there was a trend toward a larger change in HR with high dose propranolol (37±14 min−1) than low dose propranolol (30±14 min−1; P=0.052; Fig 2c). While delta HR decreased in both groups, it was significantly lower at 1 hour following high dose propranolol (13±7 min−1) than low dose propranolol (19±7 min−1; P=0.001), and remained significantly lower with high dose propranolol (at P<0.002). The primary endpoint in this protocol was delta HR at 2 hours following study drug (High Dose: 12±6 min−1 vs. Low Dose: 19±10 min−1;P<0.001). When analyzed over time, the orthostatic increase in HR was significantly lower following high dose propranolol than low dose propranolol (P<0.001). The effect of propranolol dose on orthostatic change in SBP did not achieve statistical significance (P=0.052; Fig 2f). There were no differences in orthostatic changes in DBP by the propranolol dose.
Symptoms
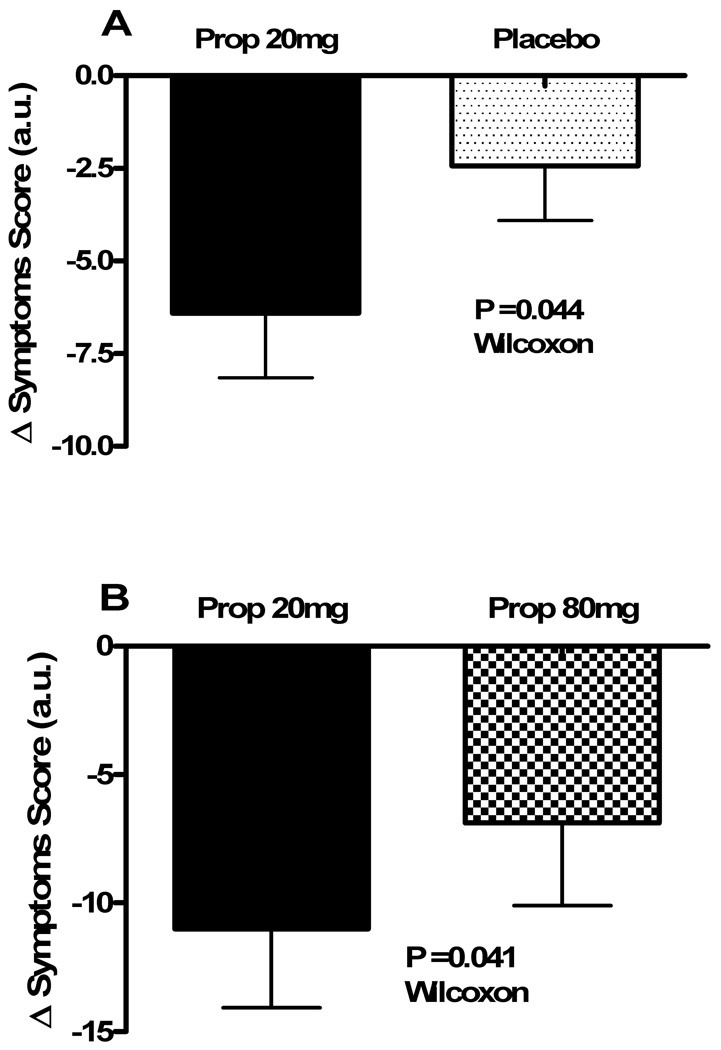
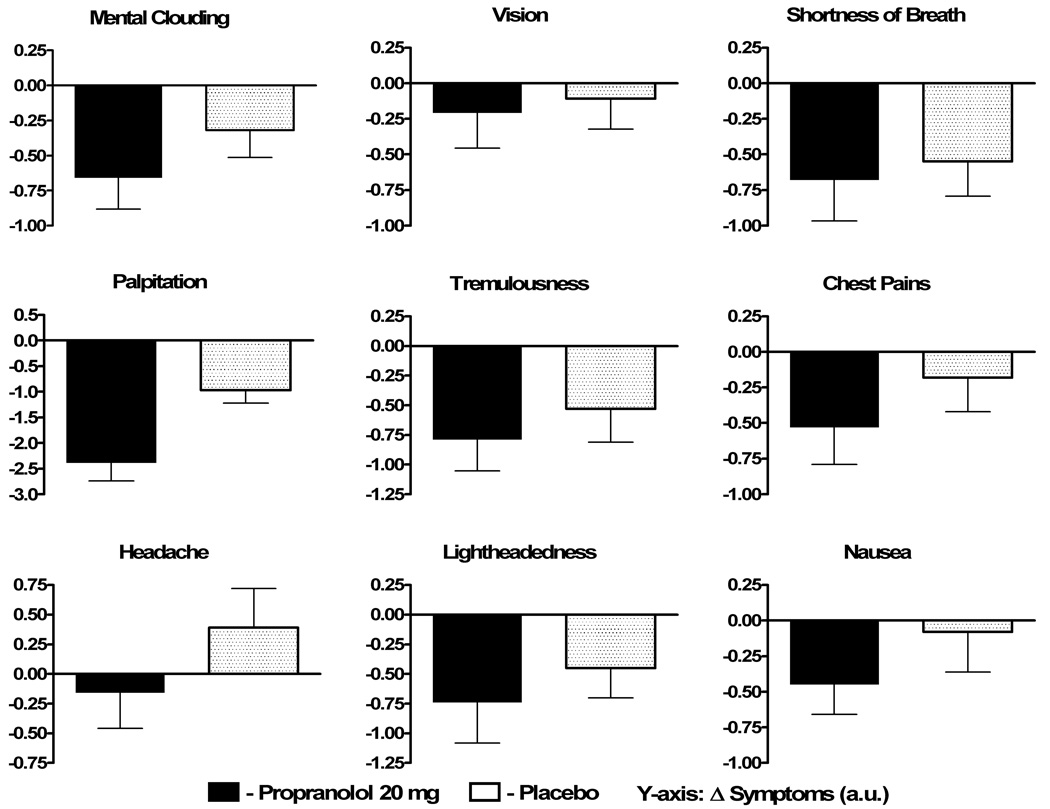
In Protocol #1, the symptom scores were completed for both propranolol and placebo by 36 patients with POTS, who comprised the analysis pool. Data are shown in Table 2 and Fig 3a. There was no difference in total symptom scores at baseline between the propranolol day and the placebo day. The decrease in total symptom burden from baseline to 2 hours post study drug was significantly greater with propranolol 20mg than placebo (−4.5 [−14.5, 0] arbitrary units [au] vs. 0 [−3, 2] au; P=0.044; Fig 3a). When analyzed over the entire 4 hours, the total symptom burden was significantly lower following propranolol than placebo (P=0.045). Propranolol decreased each component symptom more than placebo (Figure 4). Exclusive of palpitation, a major symptom in POTS, there was a non-significant trend towards an improvement in symptoms with low dose propranolol (P=0.096).
Figure 3. Change in symptoms with study medication.
The changes in the total Vanderbilt POTS symptom score (in arbitrary units [au]) are presented from immediately before to 2 hours following study drug administration. Panel A shows the changes for Protocol #1, with data for the propranolol 20mg day (solid black) and the placebo day (black dots) for the 36 subjects that completed symptom reporting for both interventions. Panel B shows the changes for Protocol #2, with data for the propranolol 20mg day (solid black) and the propranolol 80mg (checkered) for the 18 subjects. A negative score reflects a reduction in symptom burden. The error bars represent standard error of the mean. The P value was generated using a Wilcoxon rank-sum test.
Figure 4. Changes in individual symptoms in Protocol #1.
The changes in the 9 individual components of the Vanderbilt POTS symptom score (in arbitrary units [au]) are presented from immediately before to 2 hours following study drug administration for propranolol 20mg (solid black) and placebo (black dots). A negative number represents an improvement in symptoms.
In Protocol #2, all 18 patients completed the symptom scores at baseline and 2 hours post propranolol dose, but only 16 patients completed the 4 hour symptom score. Both low dose and high dose propranolol lowered total symptoms over time (Table 3), but there was no significant overall difference in symptom burden between low vs. high dose propranolol (P=0.527). In contrast to the effect of propranolol dose on HR response, the decrease in total symptom burden from baseline to 2 hours post study drug was significantly greater with low dose (20mg) propranolol than high dose (80mg) propranolol (−6 [−21.5, −2.5] au vs. −2 [−12.5, 1.5] au; P=0.041; Fig 3b).
In order to better understand the contribution of changes to the individual component symptoms to the overall improvement in symptoms score with propranolol, the 2 hour change in score for each of the 9 component symptoms were compared to a reference change of 0 (presented in Supplemental Table 2 for both low and high dose propranolol). Palpitation and tremulousness decreased significantly with both high and low dose propranolol while chest pains, headache, nausea and visual disturbance did not change significantly with either dose of propranolol. Interestingly, 3 symptoms (lightheadedness, mental clouding and shortness of breath) improved significantly only with low dose (20mg) propranolol, but not with high dose (80mg) propranolol.
DISCUSSION
This paper is the first placebo controlled trial of a beta-blocker in patients with POTS. We found that: (1) low doses of propranolol significantly decreased the standing HR and orthostatic tachycardia of patients with POTS acutely compared to placebo, and that this was associated with a symptomatic improvement; (2) higher dose propranolol (more complete beta-blockade) can further restrain HR and orthostatic tachycardia but does not further improve symptoms in patients with POTS; and (3) some symptoms (mental clouding, lightheadedness and dyspnea) improved after patients received low dose propranolol, but not after they received higher dose propranolol.
Acute Hemodynamic Arguments Against Propranolol are Incorrect
Hemodynamic arguments can be made both for why beta-adrenergic receptor blockers, such as propranolol could be beneficial or deleterious in patients with POTS. At its most basic level, the disorder is associated with a sometimes striking increase in HR in response to upright posture. It has been recognized for many years that beta-blockade will lower HR. The value of controlling HR, however, will depend on whether an elevated HR is needed to maintain hemodynamic stability. Masuki et al. 18 have reported that patients with POTS have a lower stroke volume than healthy control subjects both at rest and in response to exercise. Their data suggest that the excessive HR seen in patients with POTS might be required to maintain an adequate cardiac output in the setting of a diminished stroke volume. Supporting this line of reasoning, Stewart et al. 17 have reported that IV esmolol does not improve orthostatic tolerance during tilt table testing in patients with POTS.
Our current data with propranolol demonstrate a marked clinical improvement acutely in patients with POTS. While orthostatic tolerance was not formally assessed as an endpoint (since we only asked subjects to stand for 10 minutes), the symptom scores significantly improved with propranolol as compared to placebo. This was in the setting of a significant reduction in HR. These data suggest that not all of the orthostatic HR increase seen in POTS is needed to avoid a hemodynamic collapse.
Less is More: Benefits of Low Dose Propranolol
There are many anecdotal reports from our patients with POTS that they have “failed” or not tolerated beta-blockers. Consequently the magnitude of improvement in both hemodynamics and in reported symptoms in our first protocol comparing a low dose of propranolol to placebo was surprising. This raised questions about whether the anecdotal patient reports related to the use of excessive beta-blockade. We chose to compare our low dose of 20 mg of propranolol to a high dose of 80mg, which is the highest dose available in a single tablet 22. The higher dose of propranolol did further restrain orthostatic tachycardia in the patients with POTS, but there was no further improvement in symptoms. In fact, the relationship between propranolol dose and symptoms is more complicated. Some individual symptoms did improve with high dose propranolol (such as palpitation and tremulousness; see Table 4), while other symptoms did not improve (lightheadedness, mental clouding, and shortness of breath). These data suggest that symptoms in POTS are not just due to orthostatic tachycardia or an elevated HR. One possibility is that the elevated HR does not cause symptoms directly, but that both the HR and the symptoms are worsened by a common underlying cause (such as norepinephrine). Another possibility is that an underlying cause worsens some symptoms directly, while other symptoms are mediated through the elevated HR. In this case, more effective beta-blockade might help the HR mediated symptoms, but not the other symptoms.
Propranolol is a non-selective beta-blocker with good penetration of the blood-brain barrier. From our data, we cannot conclude whether the beneficial effects of propranolol are due purely to its peripheral effects on the heart and vasculature or whether some of the benefits of low dose propranolol stem from central effects in lowering sympathetic activity and hence peripheral norepinephrine 14 (this latter effect is controversial 23). If all of the benefits are due to peripheral actions, then other beta-blockers that do not cross the blood-brain barrier might be preferable, as they may be less likely to cause some central adverse effects. This hypothesis, however, remains to be tested.
Vanderbilt POTS Symptom Score
We used the Vanderbilt POTS Symptom Score to assess orthostatic symptom burden in our patients with POTS. This score has been used in prior studies by our group 6. It involves subjective rating by the patient at multiple time points for a number of symptoms that were commonly reported among our patients with POTS. Since there is no objective anchor to these symptoms, one individual’s rating may vary greatly from that of another individual (e.g. one person’s rating of 4 may reflect more severe symptoms than another person’s rating of 6). Like serial chest pain rating scales, however, we expect serial intrapatient reports to use a more constant frame of reference. This is one reason that all of our symptom-based analyses were done using paired comparisons within an individual. There are historical disease-specific (Orthostatic Grading Scale 24) and general health-related quality of life tools available (SF-36 25 or EurQol 26), but these are not designed for acutely repeated assessments over hours, as were done in these protocols.
Clinical Significance
These are the first blinded data comparing oral beta-blocker therapy to placebo in POTS. Low doses (20 mg) of propranolol were very effective in reducing HR and orthostatic tachycardia in patients with POTS. This is physiologically noteworthy. Of more clinical importance, however, is that low doses of propranolol also significantly improved symptom burden in these patients. The clinician should be careful not to be too aggressive by targeting complete beta-blockade as this may not further improve symptoms, and may paradoxically worsen some symptoms. The findings from this controlled study match our clinical experience. Many of our patients are clinically prescribed propranolol 10–20 mg per dose, and most patients tolerated this dose quite well.
Limitations
Our studies were conducted with the patient and the principal investigator blind to study drug order until after an individual’s study completion, but the research center nurse was not blind to the intervention. The study was thus single-blind, and not double-blind. It is noteworthy, however, that the research center nurse did not directly collect the data presented in this report. The hemodynamic data were collected by an automated oscillometric BP cuff that was connected to a personal computer for digital data collection, and the symptoms were all self-reported by the blinded patient.
Another limitation of this study is the relatively small sample of patients in protocol #2, although it is comparable to other reported POTS trials. The sample size in protocol #1 is, in fact, quite large by the standard of interventional trials in POTS.
The short duration of follow-up in this study (4 hours) makes it difficult to intelligently project the long-term efficacy of this treatment. While our anecdotal clinical experience is that low dose propranolol is well tolerated in this patient population, a longer duration clinical trial would be required in order to show chronic efficacy.
Conclusion
We have found that low dose propranolol is a highly effective agent in acutely decreasing the orthostatic tachycardia and improving symptoms in patients with POTS. Symptoms did not further improve with higher doses of propranolol, suggesting that caution should be used by clinicians in titrating up the propranolol dose.
Clinical Summary
Postural tachycardia syndrome (POTS) is a disorder of chronic orthostatic intolerance that disproportionately affects women of childbearing age. It is characterized by a constellation of symptoms that occur during standing, but resolve with sitting or lying down. A most striking feature of this disorder is the excessive increase in heart rate that occurs upon standing in the absence of hypotension. Given the striking tachycardia, beta-adrenergic blockers would seem like ideal treatments, but prior anecdotal and experimental experience has been disappointing. We report the first placebo controlled trial of propranolol in POTS. A low dose of propranolol (20 mg) acutely decreased heart rate and orthostatic tachycardia and improved the orthostatic symptoms in patients with POTS. A higher dose of propranolol (80 mg) elicited more complete beta-blockade with a further lowering of heart rate, but did not further improve symptoms, and may have made some symptoms worse. These data suggest that while low doses of propranolol are of benefit in POTS, that higher doses might be counter-productive. These data also offer a potential explanation for the conflicting prior results of beta-blockers in POTS.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank our patients. Without their participation this research project could not have been performed. We would also like to recognize the highly professional care provided by the Elliot V. Newman Clinical Research Center nursing and nutrition staff.
RESEARCH FUNDING
Supported in part by NIH grants K23 RR020783 (to SRR), R01 HL071784 (DR), R01 NS055670 (to IB), P01 HL56693 (to DR), 1 UL1 RR024975 (Clinical and Translational Science Award), and the Paden Dysautonomia Center.
Footnotes
Clinical Trials Registration: NCT00262470 (http://clinicaltrials.gov/ct2/show/NCT00262470)
Conflicts of Interest
None
Reference List
- 1.Raj SR. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart JM. Chronic orthostatic intolerance and the postural tachycardia syndrome (POTS) J Pediatr. 2004;145:725–730. doi: 10.1016/j.jpeds.2004.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grubb BP, Karabin B. Cardiology patient page. Postural tachycardia syndrome: perspectives for patients. Circulation. 2008;118:e61–e62. doi: 10.1161/CIRCULATIONAHA.107.761650. [DOI] [PubMed] [Google Scholar]
- 4.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 5.Muenter SN, Charkoudian N, Dotson RM, Suarez GA, Low PA. Baroreflex control of muscle sympathetic nerve activity in postural orthostatic tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2005;289:H1226–H1233. doi: 10.1152/ajpheart.01243.2004. [DOI] [PubMed] [Google Scholar]
- 6.Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111:2734–2740. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- 7.Jacob G, Biaggioni I, Mosqueda-Garcia R, Robertson RM, Robertson D. Relation of blood volume and blood pressure in orthostatic intolerance. Am J Med Sci. 1998;315:95–100. doi: 10.1097/00000441-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- 9.Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77:531–537. doi: 10.4065/77.6.531. [DOI] [PubMed] [Google Scholar]
- 10.Wyller VB, Thaulow E, Amlie JP. Treatment of chronic fatigue and orthostatic intolerance with propranolol. J Pediatr. 2007;150:654–655. doi: 10.1016/j.jpeds.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Nogues M, Delorme R, Saadia D, Heidel K, Benarroch E. Postural tachycardia syndrome in syringomyelia: response to fludrocortisone and beta-blockers. Clin Auton Res. 2001;11:265–267. doi: 10.1007/BF02298959. [DOI] [PubMed] [Google Scholar]
- 12.Freitas J, Santos R, Azevedo E, Costa O, Carvalho M, de Freitas AF. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin Auton Res. 2000;10:293–299. doi: 10.1007/BF02281112. [DOI] [PubMed] [Google Scholar]
- 13.Friesinger GC, Biern RO, Likar I, Mason RE. Exercise electrocardiography and vasoregulatory abnormalities. Am J Cardiol. 1972;30:733–740. doi: 10.1016/0002-9149(72)90147-6. [DOI] [PubMed] [Google Scholar]
- 14.da Silva EP, Pedro MM, Varela MG, Cortez-Dias N, Bicho MP, Madeira HC, Lopes MG. Heart rate and blood pressure in mitral valve prolapse patients: divergent effects of long-term propranolol therapy and correlations with catecholamines. Anadolu Kardiyol Derg. 2007;7 Suppl 1:107–109. [PubMed] [Google Scholar]
- 15.Fulco CS, Cymerman A, Reeves JT, Rock PB, Trad LA, Young PM. Propranolol and the compensatory circulatory responses to orthostasis at high altitude. Aviat Space Environ Med. 1989;60:1049–1055. [PubMed] [Google Scholar]
- 16.Convertino VA, Sather TM. Effects of cholinergic and beta-adrenergic blockade on orthostatic tolerance in healthy subjects. Clin Auton Res. 2000;10:327–336. doi: 10.1007/BF02322256. [DOI] [PubMed] [Google Scholar]
- 17.Stewart JM, Munoz J, Weldon A. Clinical and physiological effects of an acute alpha-1 adrenergic agonist and a beta-1 adrenergic antagonist in chronic orthostatic intolerance. Circulation. 2002;106:2946–2954. doi: 10.1161/01.cir.0000040999.00692.f3. [DOI] [PubMed] [Google Scholar]
- 18.Masuki S, Eisenach JH, Schrage WG, Johnson CP, Dietz NM, Wilkins BW, Sandroni P, Low PA, Joyner MJ. Reduced stroke volume during exercise in postural tachycardia syndrome. J Appl Physiol. 2007;103:1128–1135. doi: 10.1152/japplphysiol.00175.2007. [DOI] [PubMed] [Google Scholar]
- 19.Raj SR, Biaggioni I, Yamhure PY, Black BK, Paranjape SY, Byrne DW, Robertson D. The renin-aldosterone paradox and perturbed blood volume regulation underlying the postural tachycardia syndrome. Circulation. 2005;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 20.Fludrocortisone Acetate: DrugPoints Systems. 1-5-2009. Thomson Heathcare. 2009 Jan 23; Ref Type: Online Source. [Google Scholar]
- 21.Jacob G, Robertson D, Mosqueda-Garcia R, Ertl AC, Robertson RM, Biaggioni I. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med. 1997;103:128–133. doi: 10.1016/s0002-9343(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 22.Propranolol: AFHS Drug Information(R) 2008. STAT!Ref Online Electronic Medical Library. 2008 November 17; Available at URL: http://online.statref.com/document.aspx?fxid=1&docid=494.
- 23.Tank J, Diedrich A, Schroeder C, Stoffels M, Franke G, Sharma AM, Luft FC, Jordan J. Limited effect of systemic beta-blockade on sympathetic outflow. Hypertension. 2001;38:1377–1381. doi: 10.1161/hy1201.096120. [DOI] [PubMed] [Google Scholar]
- 24.Schrezenmaier C, Gehrking JA, Hines SM, Low PA, Benrud-Larson LM, Sandroni P. Evaluation of orthostatic hypotension: relationship of a new self-report instrument to laboratory-based measures. Mayo Clin Proc. 2005;80:330–334. doi: 10.4065/80.3.330. [DOI] [PubMed] [Google Scholar]
- 25.Keller SD, Majkut TC, Kosinski M, Ware JE., Jr Monitoring health outcomes among patients with arthritis using the SF-36 Health Survey: overview. Med Care. 1999;37:MS1–MS9. doi: 10.1097/00005650-199905001-00001. [DOI] [PubMed] [Google Scholar]
- 26.Coons SJ, Rao S, Keininger DL, Hays RD. A comparative review of generic quality-of-life instruments. Pharmacoeconomics. 2000;17:13–35. doi: 10.2165/00019053-200017010-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.