Abstract
Studies in both humans and animals suggest a positive relationship between the intake of ethanol and intake of fat, which may contribute to alcohol abuse. This relationship may be mediated, in part, by hypothalamic orexigenic peptides such as orexin (OX), which stimulate both consumption of ethanol and fat, and circulating triglycerides (TG), which stimulate these peptides and promote consummatory behavior. The present study investigated this vicious cycle between ethanol and fat, to further characterize its relation to TG and to test the effects of lowering TG levels. In Experiment 1, the behavioral relationship between fat intake and ethanol was confirmed. Adult male Sprague-Dawley rats, chronically injected with ethanol (1 g/kg i.p.) and tested in terms of their preference for a high-fat compared to low-fat diet, showed a significant increase in their fat preference, compared to rats injected with saline, in measures of 2 h and 24 h intake. Experiment 2 tested the relationship of circulating TG in this positive association between ethanol and fat, in rats chronically consuming 9% ethanol vs. water and given acute meal tests (25 kcal) of a high-fat vs. low-fat diet. Levels of TG were elevated in response to both chronic drinking of ethanol vs. water and acute eating of a high-fat vs. low-fat meal. Most importantly, ethanol and a high-fat diet showed an interaction effect, whereby their combination produced a considerably larger increase in TG levels (+172%) compared to ethanol with a low-fat diet (+111%). In Experiment 3, a direct manipulation of TG levels was found to affect ethanol intake. After administration of gemfibrozil (50 mg/kg i.g.) compared to vehicle, TG levels were lowered by 37%, and ethanol intake was significantly reduced. In Experiment 4, the TG-lowering drug gemfibrozil also caused a significant reduction in the expression of the orexigenic peptide OX, in the perifornical lateral hypothalamus. These results support the existence of a vicious cycle between ethanol and fat whereby each nutrient stimulates intake of the other. Within this vicious cycle, ethanol and fat act synergistically to increase TG levels, which in turn stimulate peptides that promote further consumption, and these phenomena are reversed by gemfibrozil, which lowers TG levels.
Keywords: Orexin, Hypothalamus, Triglycerides, Ethanol, Fat, Rat
Introduction
Ethanol and dietary fat are closely related at the behavioral level. Clinical evidence shows significantly elevated ethanol intake in subjects consuming fat (Kesse et al., 2001). Conversely, greater fat consumption is seen in ethanol drinkers compared to non-drinkers or in high-ethanol compared to low-ethanol drinkers (Gruchow et al., 1985; Herbeth et al., 1988; Le Marchand et al., 1989; Männistö et al., 1997; Swinburn et al., 1998). Additional studies reveal a co-morbidity of bingeing on fat-rich foods with high rates of alcoholism (Hasunen et al., 1977; Rotily et al., 1990), while carbohydrate intake is inversely related to ethanol consumption (Forsander, 1998; Gruchow et al., 1985). These reports suggest the existence of a positive relationship between ethanol and fat intake in human subjects.
Studies in rats have yielded similar results relating ethanol to fat consumption. Animals that prefer fat exhibit higher ethanol intake than do carbohydrate-preferring rats (Carrillo et al., 2004; Krahn and Gosnell, 1991). Furthermore, rats show greater ethanol intake after acute exposure to a high-fat diet (HFD) compared to a low-fat diet (LFD) rich in carbohydrate, and they also drink more ethanol after injection of the fat-emulsion, Intralipid, compared to saline (Carrillo et al., 2004). In one study, rats exhibited greater ethanol intake when maintained chronically on a HFD (Pekkanen et al., 1978), although this was not confirmed in a subsequent report using a different feeding paradigm and diet composition (Fisher et al., 2002).
Circulating triglycerides (TG) show a strong, positive correlation with both ethanol and fat intake and thus may serve as a common link between these two ingestive behaviors. Triglycerides packaged as chylomicrons invariably rise in proportion to the amount of fat consumed (Bahceci et al., 1999; Schrezenmeir et al., 1997) and can be lowered by fibrate drugs such as gemfibrozil (Lopid) (Donnelly et al., 1994; Frick et al., 1987). These lipids are raised by acute or chronic fat exposure (Chang et al., 2007b; Wortley et al., 2003), which in the short term may have a stimulatory effect on ethanol consumption (Carrillo et al., 2004). Circulating TG are positively correlated with acute and chronic ethanol consumption in animals and humans (Chang et al., 2007a; Contaldo et al., 1989; Goude et al., 2002), perhaps due to a reduced clearance of TG from the blood (Baraona et al., 1983; Siler et al., 1998) and a decrease in fat oxidation (Siler et al., 1998). This stimulatory effect of ethanol on circulating TG may contribute to the enhanced fat consumption seen in ethanol drinkers compared to non-drinkers in previous studies (Herbeth et al., 1988; Le Marchand et al., 1989; Männistö et al., 1997). This is supported by the finding that TG elevated by a high-fat meal are associated with an increase in caloric intake in a subsequent test meal (Gaysinskaya et al., 2007). Moreover, direct manipulations of TG levels reveal their importance in the control of food intake and body weight, as illustrated by the ability of fenofibrate, a TG-lowering drug, to prevent the development of obesity in mice fed a HFD (Jeong et al., 2004). In the clinical literature, ethanol consumed with a high-fat meal has been shown to exacerbate the lipemic effects of the dietary fat (Fielding et al., 2000; Pownall, 1994). This evidence supports the involvement of circulating TG in the positive relationship between ethanol and dietary fat.
In addition to their similar effects on TG levels, ethanol and fat are also found to have similar effects on specific hypothalamic, orexigenic peptides (Leibowitz, 2007). Recent studies have focused on opioid peptides (Chang et al., 2007a; Chang et al., 2007b) and on the peptide orexin (OX), which is believed to have a role in processes of arousal and reward (Harris and Aston-Jones, 2006). Chronic and acute consumption of a HFD, along with raising TG, stimulates the expression of OX (Wortley et al., 2003), and similar effects on TG and OX are seen with peripheral injection of the fat emulsion, Intralipid (Chang et al., 2004). These results, suggesting that TG are involved in the effect of dietary fat on OX, are substantiated by evidence showing Intralipid to increase c-Fos-like immunoreactivity in specific neurons of the perifornical lateral hypothalamus (PFLH) that synthesize OX (Chang et al., 2004; Lo et al., 2007). Further investigations reveal a similar relationship of ethanol to OX. Studies in rats chronically consuming ethanol, which increases circulating TG (Chang et al., 2007a), demonstrate an analogous increase in OX in the PFLH (Lawrence et al., 2006). The involvement of OX in this positive relationship between fat and ethanol is also supported by injection studies, which show an increase in ethanol intake after hypothalamic OX injection (Schneider et al., 2007), increased intake of a HFD after ventricular OX injection (Clegg et al., 2002), and a reduction in ethanol craving and self-administration after peripheral administration of an OX 1 receptor antagonist (Lawrence et al., 2006). This evidence suggests that a positive feedback exists between OX and ethanol, similar to the relationship between OX and dietary fat.
The present study was carried out to further elucidate the relationship between ethanol and fat and determine whether TG and OX mediate this connection. Specifically, this investigation sought to determine whether: 1) ethanol intake increases preference for dietary fat, just as fat increases ethanol intake; 2) ethanol and fat synergize in their stimulatory effect on TG levels; 3) lowering TG levels with gemfibrozil reduces ethanol intake; and 4) lowering TG suppresses the orexigenic peptide, OX, which stimulates ethanol as well as fat intake.
Materials and methods
Subjects and home cage conditions
Subjects in all studies were 200-250 g, male Sprague-Dawley rats from Taconic Farms (Germantown, NY). They were individually housed in hanging wire cages and maintained on a 12:12-hr light-dark cycle with ad libitum access to LabDiet rodent chow (St. Louis, MO) unless otherwise stated. Water was always available, either through an automated system or through graduated cylinders attached to the cages. All animal procedures used in these studies were reviewed in advance by the Princeton University Institutional Animal Care and Use Committee and followed the NIH Guide for the Care and Use of Laboratory Animals. Adequate measures were taken to minimize animal pain and discomfort.
Diets
The HFD and LFD used in this report have been described in detail in previous publications (Dourmashkin et al., 2006; Leibowitz et al., 2004). The constituents of the LFD were fat from 10% vegetable oil, carbohydrate from 30% dextrin, 30% cornstarch (ICN Pharmaceuticals), and 40% sucrose (Domino), and protein from casein (Bioserv, Frenchtown, NJ) and 0.03% L-cysteine hydrochloride (ICN Pharmaceuticals). The constituents of the HFD were fat from 75% lard (Armour) and 25% vegetable oil (Crisco), carbohydrate from 30% dextrin, 30% cornstarch (ICN Pharmaceuticals), and 40% sucrose (Domino), and protein from casein (Bioserv) and 0.03% L-cysteine hydrochloride (ICN Pharmaceuticals). These solid diets were supplemented with minerals (USP XIV Salt Mixture Briggs; ICN Pharmaceuticals) and vitamins (Vitamin Diet Fortification Mixture; ICN Pharmaceuticals). The macronutrient composition of these diets was calculated as percentage of total kcal, with the LFD containing 10% fat, 65% carbohydrate, and 25% protein (3.8 kcal/g) and the HFD containing 50% fat, 25% carbohydrate, and 25% protein (5.2 kcal/g).
Acquisition of oral ethanol intake
For experiments using voluntary ethanol intake, subjects were acclimated to ethanol gradually, using a variant of the two-bottle choice paradigm (Martinetti et al., 2000). Animals were exposed to unsweetened ethanol in plastic bottles for 12 h each day, starting 4 h into the dark cycle. Unless otherwise stated, every four days, the concentration of ethanol was increased in the following manner: 1%, 2%, 4%, 7% and, in some cases, 9% (v/v).
Test procedures
Experiment 1 was designed to test the effect of ethanol vs. saline on the rats’ preference for the HFD compared to the LFD. Rats (N = 12) were matched for weight and given daily injections of ethanol 10% v/v (1 g/kg i.p.) or saline for 21 days. During the next 4 days, they were injected with ethanol or saline (n = 6/group) as before but were additionally given a 2-h meal test at dark onset with lab chow removed. For the next 2 days after that, they were given both HFD vs. LFD ad libitum for 24 h and their intake was measured to determine their relative preference for the two diets.
Experiment 2 tested the effect on TG levels of a meal test with HFD vs. LFD in combination with chronic drinking of ethanol vs. water. Rats were trained to consume 9% ethanol with water and chow available or were maintained on water and chow alone. They were then matched for weight and ethanol consumption and divided into 4 groups as follows: 1) HFD plus chronic ethanol and water (n = 6); 2) HFD plus only water (n = 4); 3) LFD plus chronic ethanol and water (n = 5); and 4) LFD plus only water (n = 4). After the rats were acclimated to their assigned diet with 1 h of exposure for each of 6 days, they were tested for 1 h with 25 kcal of the diet, with chow removed, immediately prior to daily ethanol access. Ethanol and water intake were measured 2 h after the end of the test meal. Trunk blood from rats that consumed at least 16 kcal of the meal was then collected for measurements of TG levels. At this time, the ethanol-drinking rats had 26 days of access to ethanol.
Experiment 3 determined the effect of a lipid-lowering drug on ethanol intake. Rats (n = 18) were trained to consume 7% ethanol. Once they had attained a weight of at least 300 g, they were mock-gavaged to acclimate them to the procedure. Following the acclimation, they were tested in a counter-balanced, within-subject design during 2 days, wherein half of the group received gemfibrozil (50 mg/kg i.g.) on one day and vehicle (Tween 20, Sigma-Aldrich Co., St. Louis, MO) on the next day, and vice versa for the other half. Starting 2 h into the dark cycle, food was removed from the home cage, and rats were gavaged with gemfibrozil or vehicle. Two hours later, ethanol and chow were returned to the cage and were measured for 4 h. Tail vein blood was drawn for measurement of TG levels both before and 2 h after gavage, prior to ethanol access.
Experiment 4 tested the effects of gemfibrozil on OX expression. Rats (n = 30) were trained to drink 7% ethanol and were acclimated to the gavage procedure as in Experiment 3. Subjects were tested in a between-subject design, wherein rats were administered gemfibrozil (n = 15) or vehicle (n = 15) for 5 d. On the 5th day, after they had received their last gavage, ethanol and food intake were measured for 1 h, and rats were then sacrificed for analysis of OX mRNA using real-time quantitative PCR. Trunk blood was collected at the time of sacrifice for measurement of TG levels.
Brain dissections
Immediately after sacrifice, the brains examined using real-time quantitative PCR were placed in a matrix with the ventral surface facing up, and three 1.0 mm coronal sections were made, with the middle optic chiasm as the anterior boundary. As previously described (Chang et al., 2004), the sections were placed on a glass slide, and the PFLH (Bregma -2.8 to -3.6 mm) was rapidly microdissected under a microscope, using the fornix and third ventricle as landmarks. The dissection was taken from the area surrounding the fornix, within a range of 0.2 mm medial and ventral to the fornix, 0.3 mm dorsal and 0.1 mm lateral. These dissections were immediately frozen in liquid nitrogen and stored at -80° C until processed.
Real-time quantitative PCR analysis
As previously described (Chang et al., 2008), total RNA from pooled microdissected hypothalamic samples was extracted with TRIzol reagent. RNA was treated with RNase-free DNase I before RT, and cDNA and minus RT were synthesized using an oligo-dT primer with or without SuperScript II reverse transcriptase. The real-time PCR was performed with Applied Biosystems’ system. With Applied Biosystems Primer Express V1.5a software, primers were designed to have a melting temperature of 58-60°C and to produce an amplicon of 50-150 bp. The last five bases on the 3’ end contained no more than 2 G and/or C bases to reduce the possibility of nonspecific product formation. For orexin (OX), the primer pairs (5’-AGATACCATCTCTCCGGATTGC-3’ and 5’ CCAGGGAACCTTTGTAGAAGGA-3’) (GenBank #AF019565) generate a 73 bp amplicon corresponding to the nucleotide 48-121 of the sequence. For the house-keeping genes, the primer pairs for β-actin (5’-GGC CAA CCG TGA AAA GAT GA-3’ and 5’-CAC AGC CTG GAT GGC TAC GT-3’ (GenBank #NM031144) generate a 79 amplicon corresponding to the nucleotide 420-498 of the sequence that crosses exon 2 and exon 3. The SYBR Green PCR core reagents kit (Applied Biosystems, Foster City, CA) was used, with β-actin as endogenous control. PCR was performed in MicroAmp Optic 96-well Reaction Plates (Applied Biosystems, Foster City, CA) on an ABI PRISM 7900 Sequence Detection system (Applied Biosystems, Foster City, CA), with the condition of 2 min at 50°C, 10 min at 95°C, then 40 cycles of 15 s at 95°C and 1 min at 60°C. Each study consisted of 4 independent runs of PCR in triplicate, and each run included a standard curve, non-template control, and negative RT control. The concentrations of primers were 100 to 200 nM, and all reagents, unless indicated, were from Invitrogen (Carlsbad, CA). The levels of target gene expression were quantified relative to the level of β-actin, using standard curve method. While GAPDH and cyclophilin were also tested in some initial experiments and found to yield stable results with no response to a high-fat diet, β-actin was generally the least variable of these 3 house-keeping genes and thus used as the control in the present experiments.
The specificities of RT-PCR products were confirmed by both a single dissociation curve of the product and a single band with corresponding molecular weight revealed by agarose gel electrophoresis. In addition to the non-template control and negative RT control, an anatomical negative control was also performed by using the corpus callosum in the same brain, to verify the specificity of the quantitative PCR. No signal above threshold of OX was detected by quantitative PCR in any of these controls.
Metabolite assessment
Serum from tail vein or trunk blood was assayed for TG using a Triglyceride Assay kit (Sigma-Aldrich Co., St. Louis, MO).
Statistical analysis
All values are expressed as mean ± SEM. Hypotheses were tested using either a one-way or two-way ANOVA, followed by a Bonferroni post-hoc test for multiple comparisons between groups, or an unpaired t-test, where appropriate. For quantitative PCR, the ratio of the relative concentration of peptide to β-actin mRNA in the experimental vs. control samples was analyzed by an unpaired t-test. The criterion for use of the term ‘significant’ in the text is p < 0.05.
Results
Experiment 1: Ethanol stimulates fat intake
Knowing that ingestion of a HFD stimulates ethanol intake (Carrillo et al., 2004), we tested whether ethanol can, in turn, stimulate the ingestion of a HFD, thus creating a positive feedback loop. As shown in Fig. 1, after 21 days of 10% ethanol (1g/kg, i.p.) or saline injection, rats receiving ethanol increased their relative preference for the HFD vs. LFD in a 2-h test, from 39% in the saline group to 60% in the ethanol group [F(1,3) = 5.12, p < 0.05]. An even greater effect was observed in the measure of 24-h intake, which revealed an increase in fat preference from 36% in the saline group to 62% in the ethanol group [F(1,1) = 6.47, p < 0.05]. These results suggest that the relationship between dietary fat and ethanol is bidirectional, with ethanol stimulating fat ingestion in addition to fat potentiating ethanol intake.
Fig. 1.
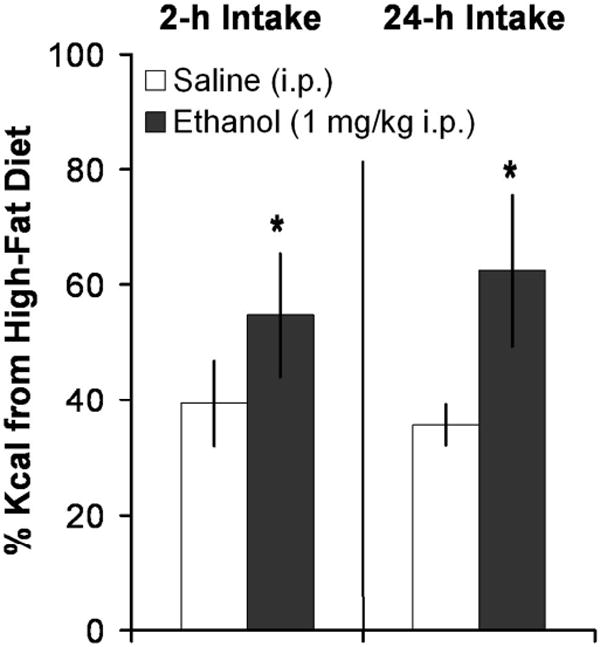
Ethanol preferentially stimulated fat intake over carbohydrate intake. Injection of ethanol (1 g/kg, i.p.) led rats (n = 6/group) to consume more kcal from a high-fat diet compared to a high-carbohydrate diet during a 2-h or 24-h period compared to injection of saline. *p < 0.05 vs. saline.
Experiment 2: Dietary fat synergizes with ethanol in raising TG levels
To identify the physiological link between fat and ethanol, rats trained chronically to drink 9% ethanol or drink only water were given a 1-h meal test with 25 kcal of the HFD or LFD. Measurements of ethanol or water intake were taken 2 h after the end of the meal and, in rats that consumed at least 16 kcal of their respective diet, trunk blood was collected for measurements of circulating TG levels. Focusing on the effect of the meal independent of what the rats were drinking, the HFD meal produced a significant increase in TG compared to the LFD meal in both ethanol- and water-drinking rats (+142%; F(1,16) = 61.89, p < 0.0001) (Fig. 2). Focusing on the effect of ethanol vs. water independent of what the rats had as a meal, the ethanol also produced a significant increase in TG (+30%; F(1, 16) = 4.66, p < 0.05). An interaction effect [F(1,16) = 4.66, p < 0.05] revealed a synergy between ethanol and fat in raising TG. This was evident in the considerably larger increase in TG levels in ethanol-drinking rats after the HFD meal (+172%) compared to the LFD meal (+111%). These groups showed no differences in their measures of caloric intake [F(3.16) = 2.29, ns], daily water intake [F(3.16) = 1.91, ns], or 2-h ethanol intake after the meal (0.22 ± 0.11 g/kg for HFD vs. 0.22 ± 0.08 g/kg for LFD). Thus, the only significant difference between these groups was found in their circulating TG levels.
Fig. 2.
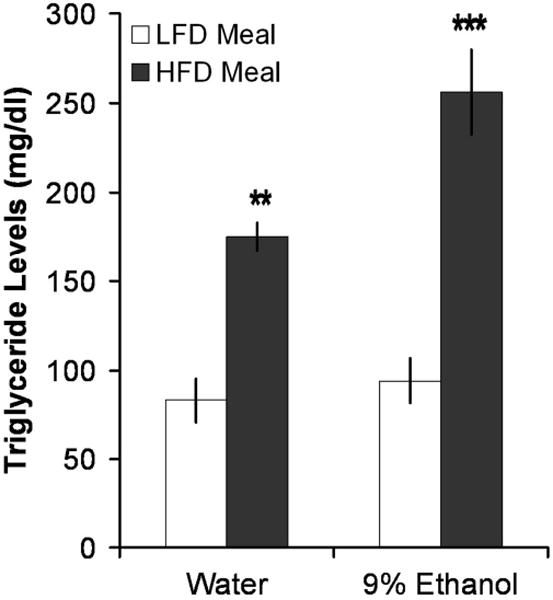
Ethanol and fat synergized to elevate TG levels. A HFD meal raised TG levels more than a LFD meal. Rats given a 25 kcal HFD meal, whether drinking 9% ethanol and water (n = 6) or water only (n = 4), showed a greater elevation in TG levels than did rats given a 25 kcal LFD meal and drinking 9% ethanol and water (n = 5) or water only (n = 4). ***p < 0.001, **p < 0.01 vs. low-fat meal.
Experiment 3: Gemfibrozil reduces TG levels and ethanol intake
Together with published studies (see Introduction), the results of Experiment 2 suggest that dietary fat stimulates the consumption of ethanol and that circulating TG may be involved in this phenomenon. To examine this idea further, the TG-lowering drug gemfibrozil was tested to determine whether an acute reduction in TG can produce a decrease in ethanol intake. Rats consuming at least 2.5 g/kg of 7% ethanol each day were tested in this experiment. Whereas the vehicle (Tween) had no effect on TG levels 2 h after administration, gemfibrozil (50 mg/kg i.g.) produced a significant decrease in circulating TG levels, compared to both pre-drug levels (-28%, p < 0.05) and to levels after the vehicle (Fig. 3A). Subsequent to this decline in TG, gemfibrozil also reduced intake of ethanol, with a statistically significant effect (-22%, p < 0.05) during the first hour of daily access, a smaller, insignificant effect (-14%, ns) during the second hour, and a significant reduction again during the fourth hour (-18%, p < 0.01) (Fig. 3B). In contrast to these changes in TG and ethanol intake, gemfibrozil had no effect on chow intake at any time-point (data not shown). These findings demonstrate that a lipid-lowering drug can reduce voluntary intake of ethanol.
Fig. 3.
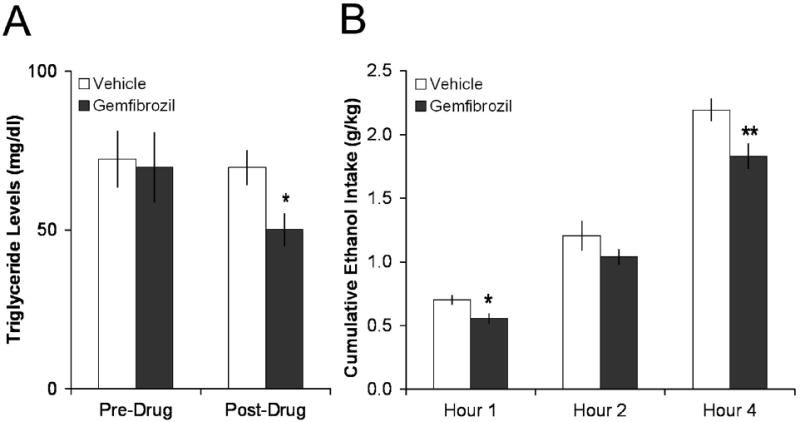
Gemfibrozil lowered TG levels and ethanol intake. A. Administration of gemfibrozil (50 mg/kg i.g.), but not vehicle, lowered TG levels in rats drinking more than 2.5 g/kg/d (n = 6). *p < 0.05 vs. immediately prior to drug administration. B. Gemfibrozil also lowered subsequent 7% ethanol intake (n = 6). **p < 0.01, *p < 0.05 vs. vehicle.
Experiment 4: Gemfibrozil suppresses the expression of OX in the PFLH
This experiment tested whether gemfibrozil, in addition to exerting a physiological effect, alters the brain peptide OX known to stimulate consumption of ethanol, as well as intake of a fat-rich diet (see Introduction). An additional set of rats was examined in the same manner as Experiment 3, except that a between-group design was used. The rats receiving gemfibrozil (50 mg/kg i.g.) compared to vehicle had circulating TG levels that were 35% lower (p < 0.05) (Fig. 4A). They also consumed 52% less ethanol in the first hour of daily access [F(1,1) = 5.99, p < 0.05], thus confirming the results of Experiment 3 (Fig. 4B). To determine the effect of gemfibrozil on OX expression independent of any change in ethanol intake, only rats with similar intakes (n = 5/group) were selected for measurement of peptide mRNA using real-time quantitative PCR. Rats treated with gemfibrozil compared to vehicle exhibited a significant reduction in OX expression in the PFLH (-64%, p < 0.001) (Fig. 4C). Thus, with ethanol intake eliminated as a variable, these results demonstrate that gemfibrozil, while lowering TG, suppresses a peptide known to stimulate ethanol intake, suggesting a mechanism through which this drug may act to reduce ethanol consumption.
Fig. 4.
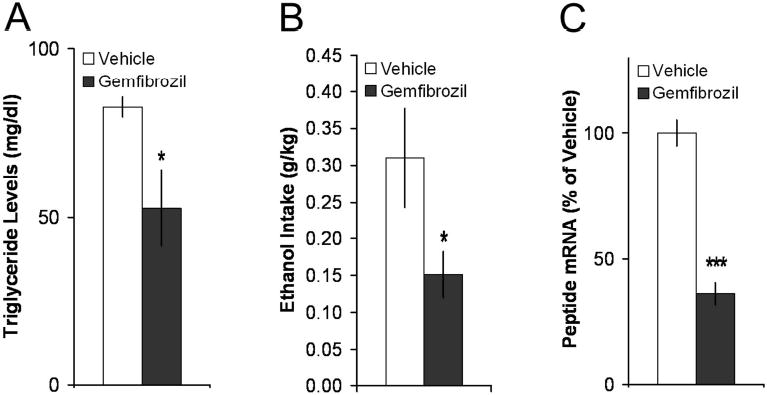
Gemfibrozil decreased OX. A. Gemfibrozil lowered TG levels in rats with chronic access to 7% ethanol (n = 15/group). B. Gemfibrozil (50 mg/kg i.g.) lowered1-h 7% ethanol intake. C. Among rats with similar 7% ethanol intake, gemfibrozil decreased OX mRNA in the PFLH (n = 4/group). *p < 0.05, ***p < 0.001 vs. vehicle.
Discussion
The purpose of this study was to further investigate the interaction between ethanol intake and dietary fat and determine if TG and hypothalamic peptides may contribute to this relationship. To this end, four experiments were conducted which examined: 1) the interaction between fat and ethanol; 2) the association of fat and ethanol with TG levels; 3) a possible pharmacological means for reversing this relationship; and 4) a neural mechanism underlying this reversal.
Ethanol-induced stimulation of fat intake
Given the extensive evidence showing the deleterious effects of ethanol and fat consumption on behavior, physiology, and neurobiology, and the high rate of over-consumption of these two nutrients in our society, it is of interest to determine how they interact. We have previously shown that ingestion of a fat-rich diet or injection of Intralipid, both of which raise TG levels, can stimulate consumption of ethanol (Carrillo et al., 2004) and that fat-preferring rats exhibit a preference for ethanol (Carrillo et al., 2004). From these studies, it was unknown whether ethanol, which also elevates circulating TG (Chang et al., 2007a), can in turn stimulate the ingestion of a fat-rich diet, thus creating a positive feedback. This possibility was suggested by a clinical study showing that acute ethanol consumption stimulates preference for fat-rich foods (Caton et al., 2004). The data presented here demonstrate that ethanol injection preferentially stimulates consumption of a HFD compared to a LFD. This effect was observed with both the 2-h and 24-h access periods, indicating that ethanol has a significant effect on macronutrient selection in the course of a day. The mechanism underlying the effect of ethanol on fat preference is the matter under study. Ethanol injection, with the same paradigm used here, stimulates circulating TG levels 1.5 h after the injection (Chang et al., 2007a). One possibility is that ethanol, in elevating TG levels, works in concert with fat to stimulate the expression and production of the orexigenic peptides (Chang et al., 2007a) that, in turn, promote further consummatory behavior. This is supported by the findings that hypothalamic injection of OX in ethanol-drinking rats preferentially stimulates consumption of ethanol rather than chow (Schneider et al., 2007) and that ventricular injection of OX also stimulates intake of a HFD more than a LFD (Clegg et al., 2002). The possibility that calories themselves from the ethanol injection (approximately 14 kcal/day) are involved in stimulating this orexigenic peptide to promote further consummatory behavior is unlikely, given the finding that food intake, TG levels and OX are stimulated by a small meal (10-15 kcal) high in fat content as compared to an equicaloric meal low in fat content (Gaysinskaya et al., 2007). Together, these data indicate that ethanol has a stimulatory effect on fat consumption, which in turn positively affects ethanol consumption.
Dietary fat and ethanol synergize in raising TG levels
In identifying physiological links between fat and ethanol, a variety of evidence leads us to focus on TG as a strong, positive correlate of ethanol and fat intake. Circulating TG rise in proportion to the amount of fat consumed (Bahceci et al., 1999; Schrezenmeir et al., 1997). TG also rise from alcohol consumption (Contaldo et al., 1989; Goude et al., 2002), and hypertriglyceridemia is known to be a strong indicator of alcoholism (Baraona et al., 1983). In the clinical literature, ethanol consumed with a high-fat meal has been shown to exacerbate the lipemic effects of the dietary fat (Fielding et al., 2000; Pownall, 1994). This evidence led to the prediction that a high-fat meal and ethanol may interact in their stimulatory effect on TG levels in rats. Experiment 2 confirmed this prediction. Rats drinking ethanol after a HFD meal had markedly elevated levels of circulating TG compared to water-drinking rats after this HFD meal or ethanol-drinking rats after a LFD meal. The failure of ethanol, when combined with a LFD, high-carbohydrate meal, to raise TG levels compared to water alone with this meal may be due to the enhanced metabolism and clearance of ethanol from the blood associated with carbohydrate intake (Rogers et al., 1987; Yonekura et al., 1993). In addition to underscoring the similarity of ethanol and dietary fat in raising circulating TG, this experiment reveals an interaction effect between ethanol and dietary fat on circulating TG.
TG-lowering drug and ethanol intake
In light of the evidence that TG may play a role in the stimulation of ethanol and fat intake, the next step was to manipulate the TG levels directly to see what effect this might have on the consumption of ethanol. For that purpose, a widely used lipid-lowering drug, gemfibrozil (Lopid), was utilized. Gemfibrozil, a fibrate, binds to peroxisome proliferator-activated receptor-alpha (Fruchart and Duriez, 2006) which increases lipoprotein lipase synthesis and thus enhances the clearance of TG (Donnelly et al., 1994; Frick et al., 1987; Fruchart and Duriez, 2006). In the current study, administration of gemfibrozil caused a suppression of both circulating TG levels and ethanol intake. These effects are opposite to the increase in TG and ethanol intake induced by administration of Intralipid (Carrillo et al., 2004; Leibowitz, 2007). This combination of findings demonstrates that ethanol consumption can be directly influenced by circulating TG. Furthermore, it validates the assertion that a high-fat meal, by raising TG, leads to an increase in ethanol intake which in turn increases the intake of a fat-rich diet, thereby driving a vicious cycle. In a society with such a high proportion of fat in its diet (Prevention, 2004), the results of this experiment may have important implications for understanding mechanisms underlying the consumption of ethanol. They may also provide insight into making dietary recommendations for individuals who over-consume ethanol.
TG-lowering drug and hypothalamic peptides
There is a well-established link between ethanol intake and orexigenic peptides known to be related to dietary fat and circulating lipids (see Introduction). In addition to being stimulated by fat intake and Intralipid, the expression of OX in the PFLH is stimulated by ethanol and is closely related to levels of TG (Chang et al., 2004; Lawrence et al., 2006; Wortley et al., 2003). Results from the present study provide further support for this positive relationship between fat intake, ethanol intake, TG and OX. Administration of gemfibrozil, which lowers TG levels, is found to reduce OX mRNA expression in the PFLH. This finding supports the hypothesis that the decrease in ethanol intake produced by gemfibrozil is mediated by a decrease in OX expression in the PFLH, resulting from a decline in TG. This hypothesis is substantiated by previous studies showing that Intralipid, which increases circulating TG and OX expression in the PFLH, has a stimulatory effect on ethanol drinking in rats (Carrillo et al., 2004; Chang et al., 2004). Direct evidence for a positive relationship between OX and ethanol is provided by injection studies, which show a stimulation of ethanol intake after hypothalamic injection of OX and a reduction in ethanol consumption after peripheral injections of an OX receptor antagonist (Lawrence et al., 2006; Schneider et al., 2007). There is evidence that OX neurons of the PFLH regulate both reward processing for food and drugs of abuse as well as arousal and responses to stress (Harris and Aston-Jones, 2006). The present study demonstrates the importance of OX in the control of ethanol intake and its close association with circulating TG. Further studies with opioid peptides, which are known to respond to TG (Chang et al., 2007b), may reveal a similar relationship in the control of ethanol intake. The evidence presented here with OX is the first to suggest that lipid lowering drugs such as gemfibrozil may be of use in the treatment of alcohol abuse.
Conclusions
The studies conducted here demonstrate that: 1) injection of ethanol stimulates consumption of a high-fat diet in preference to a low-fat carbohydrate-rich diet; 2) intake of ethanol with fat synergistically raises circulating TG levels; 3) lowering TG levels leads to a reduction in voluntary ethanol intake; and 4) lowering TG levels decreases PFLH OX expression in ethanol-drinking animals. These results lead to the conclusion that manipulation of TG levels, by ingestion of a high-fat meal or administration of a lipid-lowering drug, can impact ethanol consumption via its effect on a hypothalamic peptide that controls ethanol intake. Given the increases in obesity and hypertriglyceridemia in many of the world’s populations in recent years (Ford et al., 2004; Lilja et al., 2008), these findings have potentially important implications, both for the treatment of alcohol addiction as well as for nutritional choices that have medical consequences.
Acknowledgments
This research was supported by USPHS Grant AA12882 (B.G.H. and S.F.L.) and to funds from the E.H. Lane Foundation (B.G.H.). Gratitude is expressed to Yu-Wei Chen and Hilary Lambert at Princeton University for their assistance.
References
- Bahceci M, Tuzcu A, Akkus M, Yaldiz M, Ozbay A. The effect of high-fat diet on the development of obesity and serum leptin level in rats. Eat Weight Disord. 1999;4:128–132. doi: 10.1007/BF03339728. [DOI] [PubMed] [Google Scholar]
- Baraona E, Savolainen M, Karsenty C, Leo M, Lieber C. Pathogenesis of alcoholic hypertriglyceridemia and hypercholesterolemia. Trans Assoc Am Physicians. 1983;96:306–315. [PubMed] [Google Scholar]
- Carrillo CA, Leibowitz SF, Karatayev O, Hoebel BG. A high-fat meal or injection of lipids stimulates ethanol intake. Alcohol. 2004;34:197–202. doi: 10.1016/j.alcohol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Caton S, Ball M, Ahern A, Hetherington M. Dose-dependent effects of alcohol on appetite and food intake. Physiol Behav. 2004;81:51–58. doi: 10.1016/j.physbeh.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Chang G-Q, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145:3904–3912. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, Hoebel BG, Leibowitz SF. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007a;31:249–259. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Gaysinskaya V, Marwil Z, Leibowitz SF. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. Am J Physiol Endocrinol Metab. 2007b;292:E561–570. doi: 10.1152/ajpendo.00087.2006. [DOI] [PubMed] [Google Scholar]
- Clegg D, Air E, Woods S, Seeley R. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- Contaldo F, D’Arrigo E, Carandente V, Cortese C, Coltorti A, Mancini M, Taskinen M, Nikkilä E. Short-term effects of moderate alcohol consumption on lipid metabolism and energy balance in normal men. Metabolism. 1989;38:166–171. doi: 10.1016/0026-0495(89)90257-6. [DOI] [PubMed] [Google Scholar]
- Donnelly R, Plato P, Chang H, Reaven G. Effects of gemfibrozil on triglyceride metabolism in Dahl salt-sensitive rats. J Pharmacol Exp Ther. 1994;270:809–813. [PubMed] [Google Scholar]
- Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, Leibowitz SF. Model for predicting and phenotyping at normal weight the long-term propensity for obesity in Sprague-Dawley rats. Physiol Behav. 2006;87:666–678. doi: 10.1016/j.physbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Fielding B, Reid G, Grady M, Humphreys S, Evans K, Frayn K. Ethanol with a mixed meal increases postprandial triacylglycerol but decreases postprandial non-esterified fatty acid concentrations. Br J Nutr. 2000;83:597–604. doi: 10.1017/s0007114500000763. [DOI] [PubMed] [Google Scholar]
- Fisher H, Halladay A, Ramasubramaniam N, Petrucci J, Dagounis D, Sekowski A, Martin J, Wagner G. Liver fat and plasma ethanol are sharply lower in rats fed ethanol in conjunction with high carbohydrate compared with high fat diets. J Nutr. 2002;132:2732–2736. doi: 10.1093/jn/132.9.2732. [DOI] [PubMed] [Google Scholar]
- Ford E, Giles W, Mokdad A. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- Forsander O. Dietary influences on alcohol intake: a review. J Stud Alcohol. 1998;59:26–31. doi: 10.15288/jsa.1998.59.26. [DOI] [PubMed] [Google Scholar]
- Frick M, Elo O, Haapa K, Heinonen O, Heinsalmi P, Helo P, Huttunen J, Kaitaniemi P, Koskinen P, Manninen V. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- Fruchart J, Duriez P. Mode of action of fibrates in the regulation of triglyceride and HDL-cholesterol metabolism. Drugs Today (Barc) 2006;42:39–64. doi: 10.1358/dot.2006.42.1.963528. [DOI] [PubMed] [Google Scholar]
- Gaysinskaya V, Karatayev O, Chang G, Leibowitz S. Increased caloric intake after a high-fat preload: relation to circulating triglycerides and orexigenic peptides. Physiol Behav. 2007;91:142–153. doi: 10.1016/j.physbeh.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Goude D, Fagerberg B, Hulthe J. Alcohol consumption, the metabolic syndrome and insulin resistance in 58-year-old clinically healthy men (AIR study) Clin Sci (Lond) 2002;102:345–352. doi: 10.1042/cs1020345. [DOI] [PubMed] [Google Scholar]
- Gruchow H, Sobocinski K, Barboriak J, Scheller J. Alcohol consumption, nutrient intake and relative body weight among US adults. Am J Clin Nutr. 1985;42:289–295. doi: 10.1093/ajcn/42.2.289. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Hasunen K, Pekkarinen M, Nuutinen O. Alcohol consumption and dietary intake of Finnish men. Nutr Metab. 1977;21(Suppl 1):132–133. doi: 10.1159/000176136. [DOI] [PubMed] [Google Scholar]
- Herbeth B, Didelot-Barthelemy L, Lemoine A, Le Devehat C. Dietary behavior of French men according to alcohol drinking pattern. J Stud Alcohol. 1988;49:268–272. doi: 10.15288/jsa.1988.49.268. [DOI] [PubMed] [Google Scholar]
- Jeong S, Kim M, Han M, Lee H, Ahn J, Song Y, Shin C, Nam K, Kim T, Oh G, et al. Fenofibrate prevents obesity and hypertriglyceridemia in low-density lipoprotein receptor-null mice. Metabolism. 2004;53:607–613. doi: 10.1016/j.metabol.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Kesse E, Clavel-Chapelon F, Slimani N, van Liere M. Do eating habits differ according to alcohol consumption? Results of a study of the French cohort of the European Prospective Investigation into Cancer and Nutrition (E3N-EPIC) Am J Clin Nutr. 2001;74:322–327. doi: 10.1093/ajcn/74.3.322. [DOI] [PubMed] [Google Scholar]
- Krahn D, Gosnell B. Fat-preferring rats consume more alcohol than carbohydrate-preferring rats. Alcohol. 1991;8:313–316. doi: 10.1016/0741-8329(91)90465-9. [DOI] [PubMed] [Google Scholar]
- Lawrence A, Cowen M, Yang H, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand L, Kolonel L, Hankin J, Yoshizawa C. Relationship of alcohol consumption to diet: a population-based study in Hawaii. Am J Clin Nutr. 1989;49:567–572. doi: 10.1093/ajcn/49.3.567. [DOI] [PubMed] [Google Scholar]
- Leibowitz S. Overconsumption of dietary fat and alcohol: mechanisms involving lipids and hypothalamic peptides. Physiol Behav. 2007;91:513–521. doi: 10.1016/j.physbeh.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz S, Dourmashkin J, Chang G, Hill J, Gayles E, Fried S, Wang J. Acute high-fat diet paradigms link galanin to triglycerides and their transport and metabolism in muscle. Brain Res. 2004;1008:168–178. doi: 10.1016/j.brainres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Lilja M, Eliasson M, Stegmayr B, Olsson T, Soderberg S. Trends in obesity and its distribution: data from the Northern Sweden MONICA Survey, 1986-2004. Obesity (Silver Spring) 2008;16:1120–1128. doi: 10.1038/oby.2008.230. [DOI] [PubMed] [Google Scholar]
- Lo CM, Ma L, Zhang DM, Lee R, Qin A, Liu M, Woods SC, Sakai RR, Raybould HE, Tso P. Mechanism of the induction of brain c-Fos-positive neurons by lipid absorption. Am J Physiol Regul Integr Comp Physiol. 2007;292:R268–273. doi: 10.1152/ajpregu.00334.2006. [DOI] [PubMed] [Google Scholar]
- Männistö S, Uusitalo K, Roos E, Fogelholm M, Pietinen P. Alcohol beverage drinking, diet and body mass index in a cross-sectional survey. Eur J Clin Nutr. 1997;51:326–332. doi: 10.1038/sj.ejcn.1600406. [DOI] [PubMed] [Google Scholar]
- Martinetti M, Andrzejewski M, Hineline P, Lewis M. Ethanol consumption and the matching law: a choice analysis using a limited-access paradigm. Exp Clin Psychopharmacol. 2000;8:395–403. doi: 10.1037//1064-1297.8.3.395. [DOI] [PubMed] [Google Scholar]
- Pekkanen L, Eriksson K, Sihvonen M. Dietarily-induced changes in voluntary ethanol consumption and ethanol metabolism in the rat. Br J Nutr. 1978;40:103–113. doi: 10.1079/bjn19780100. [DOI] [PubMed] [Google Scholar]
- Pownall H. Dietary ethanol is associated with reduced lipolysis of intestinally derived lipoproteins. J Lipid Res. 1994;35:2105–2113. [PubMed] [Google Scholar]
- Prevention C.f.D.C.a. Trends in intake of energy and macronutrients--United States, 1971-2000. MMWR Morb Mortal Wkly Rep. 2004;53:80–82. [PubMed] [Google Scholar]
- Rogers J, Smith J, Starmer GA, Whitfield JB. Differing effects of carbohydrate, fat and protein on the rate of ethanol metabolism. Alcohol Alcohol. 1987;22:345–353. [PubMed] [Google Scholar]
- Rotily M, Durbec J, Berthézène P, Sarles H. Diet and alcohol in liver cirrhosis: a case-control study. Eur J Clin Nutr. 1990;44:595–603. [PubMed] [Google Scholar]
- Schneider E, Rada P, Darby R, Leibowitz S, Hoebel B. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Schrezenmeir J, Fenselau S, Keppler I, Abel J, Orth B, Laue C, Stürmer W, Fauth U, Halmagyi M, März W. Postprandial triglyceride high response and the metabolic syndrome. Ann N Y Acad Sci. 1997;827:353–368. doi: 10.1111/j.1749-6632.1997.tb51847.x. [DOI] [PubMed] [Google Scholar]
- Siler S, Neese R, Parks E, Hellerstein M. VLDL-triglyceride production after alcohol ingestion, studied using [2-13C1] glycerol. J Lipid Res. 1998;39:2319–2328. [PubMed] [Google Scholar]
- Swinburn B, Walter L, Ricketts H, Whitlock G, Law B, Norton R, Jackson R, MacMahon S. The determinants of fat intake in a multi-ethnic New Zealand population. Fletcher Challenge--University of Auckland Heart and Health Study Management Committee. Int J Epidemiol. 1998;27:416–421. doi: 10.1093/ije/27.3.416. [DOI] [PubMed] [Google Scholar]
- Wortley K, Chang G, Davydova Z, Leibowitz S. Peptides that regulate food intake: orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1454–1465. doi: 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- Yonekura I, Nakano M, Sato A. Effects of carbohydrate intake on the blood ethanol level and alcoholic fatty liver damage in rats. J Hepatol. 1993;17:97–101. doi: 10.1016/s0168-8278(05)80528-8. [DOI] [PubMed] [Google Scholar]


