Abstract
Objective
To evaluate the efficacy, safety and tolerability of ritonavir-boosted tipranavir (TPV/r) in HIV-1-infected pediatric patients.
Design
Open-label randomized pediatric trial (1182.14/PACTG1051) comparing TPV/r at two doses plus optimized background regimen (OBR).
Methods
HIV-1-infected patients (2–18 years) with plasma viral load (VL) ≥1500 copies/mL were randomized to TPV/r 290/115 mg/m2 or TPV/r 375/150 mg/m2 BID oral solution plus OBR. Week 48 efficacy, safety and tolerability results were evaluated.
Results
Children (n=115; 97% treatment experienced) were randomized to low or high dose. 88 remained on-treatment through 48 weeks. Baseline characteristics were similar between dose groups. At study entry, half of the HIV-1 isolates were resistant to all protease inhibitors. At 48 weeks, 39.7% low-dose and 45.6% high-dose TPV/r recipients had VL <400 copies/mL and 34.5% and 35.1%, respectively, achieved VL <50 copies/mL. Vomiting, cough and diarrhea were the most frequent adverse events. Grade 3 alanine aminotransferase (ALT) elevations were observed in 6.3% of patients. No Grade 4 ALT or Grade 3/4 aspartate aminotransferase elevations were reported.
Conclusions
TPV/r achieved a sustained virologic response, showed a good safety profile and was tolerated at either dose. In pediatric patients with high baseline resistance profiles, high-dose TPV/r tended to demonstrate a better sustained response.
Keywords: Tipranavir, ritonavir, protease inhibitor, antiretroviral therapy, pediatric, efficacy, safety
INTRODUCTION
Highly active antiretroviral (ARV) therapy (HAART) can provide sustained viral suppression and beneficial immunological responses in ARV-naïve and-experienced pediatric patients [1–8]. HAART has significantly decreased hospital admissions, progression to AIDS, and mortality in these populations [9, 10]. However, approximately 30–50% of HIV-infected children and adolescents fail to maintain virologic suppression beyond 2 years of initial treatment [7, 11]. Key factors associated with virologic failure include potentially serious adverse events (AEs), drug-resistant virus emergence , and difficulty maintaining long-term adherence [12]. Drug-resistant isolates occur in 70–90% of HIV-infected pediatric patients failing therapy [13, 14]. The FDA has approved 24 ARV drugs for treating adult and adolescent HIV infection in the US [15]; yet, pediatric guidelines recommend only 13 ARVs [16].
Tipranavir (TPV), a next-generation protease inhibitor (PI) approved for use in treatment-experienced adults infected with PI-resistant HIV-1 [17–19], is co-administered with ritonavir (RTV; TPV/r) at low doses to achieve therapeutic concentrations. After 24 and 48 weeks of administration in treatment-experienced adults harboring PI-resistant HIV-1 strains, a TPV/r-based regimen was shown to be superior to a comparator RTV-boosted PI regimen [17–19].
TPV is available as a soft-gelatin capsule and an oral solution is being developed for pediatric patients. The current study, evaluated the safety, tolerability, pharmacokinetics (PK) and efficacy of TPV oral solution and soft-gelatin capsules in combination with low-dose RTV at two dose levels, given with at least two other non-PI ARVs in pediatric patients (2–18 years). Pharmacokinetic results were presented elsewhere [20]. Week 48 efficacy, safety and tolerability results are presented here.
METHODS
Study design
This ongoing, open-label, multicenter, randomized Phase I/IIa trial was conducted at 26 sites in North America, Europe and Latin America. The primary endpoint was establishment of safety and tolerability, using AEs and significant changes graded by the Division of AIDS (DAIDS) standardized Toxicity Table for Grading Severity of Pediatric (>3 months) Adverse Experiences. Key secondary endpoints included establishment of steady-state TPV and RTV PK parameters and Week 48 virologic response (proportion of patients achieving and maintaining viral load [VL] <400 copies/mL, VL <50 copies/mL and VL reduction of ≥1 log10 copies/mL). Changes in CD4+ T cell percentages and T-cell counts from baseline were also evaluated. The relationship between baseline HIV ARV resistance and virologic response was evaluated and study drug adherence monitored.
Study population
HIV-1-infected children and adolescents (2–18 years) with a VL >1500 copies/mL (log10 >3.17 copies/mL), with or without prior exposure to ARV agents, were eligible for study entry. No CD4+ T-cell count or HIV resistance-profile requirements were used for inclusion criteria. Safety screening laboratory values were to be DAIDS Grade <1, although Grade 2 gamma-glutamyl transferase (GGT), cholesterol and triglycerides were acceptable. Exclusion criteria included active hepatitis B or C (confirmed by positive HBsAg or HCV antibody and/or viral load) coupled with alanine aminotransferase (ALT) or aspartate aminotransferase (AST) Grade >2, a positive screening pregnancy test, breast-feeding, or life expectancy <12 months.
Local independent ethics committees or institutional review boards approved the protocol. Each patient and/or the patient′s legal guardian gave written, informed consent according to the regulatory and legal requirements of the participating sites before study participation.
Treatment
Eligible patients were randomized (1:1), post-screening, to low-dose TPV/r (TPV 290 mg/m2 plus RTV 115 mg/m2 BID) or high-dose TPV/r (TPV 375 mg/m2 plus RTV 150 mg/m2 BID), combined with an optimized, non-PI ARV background regimen (OBR). Low-dose TPV/r represents the body surface area (BSA)-equivalent of the adult TPV/r 500/200 mg dose. High-dose TPV/r (approximately 30% higher than low-dose TPV/r) was included in case TPV concentrations in children were lower than those in adults. Genotypic resistance screening was performed for all patients at screening. The OBR was selected by investigator on the basis of history and genotypic resistance. Patients were stratified according to age (2 to <6, 6 to <12 and 12 to 18 years). All patients started treatment with TPV oral solution. BSA was calculated at randomization and each study visit; TPV/r dosing was adjusted according to BSA changes. TPV/r 500/200 mg BID was the maximum dose administered regardless of BSA. Children aged ≥12 years who reached a BSA-adjusted, oral-solution dose equivalent to TPV/r 500/200 mg BID were eligible to switch to TPV capsules after Week 4. TPV was supplied by Boehringer Ingelheim as 100 mg/mL oral solution and 250 mg soft-gel capsules. RTV was obtained commercially as NORVIR® oral solution (80 mg/mL) or 100 mg capsules.
Study procedures
Study visits were scheduled every 2 weeks up to Week 8, and every 4 weeks thereafter until Week 48. Viral load was measured using the Roche Amplicor Standard and Ultrasensitive 1.5 assays and CD4+ T-cell counts using flow cytometry. Genotypic resistance testing was performed using the TruGene® (Version 1.5) method. Adherence was determined by pill counts or oral solution volume measurements at each visit.
Statistical analysis
Descriptive statistics were used to summarize and evaluate efficacy and safety parameters. For analyses of continuous efficacy variables (e.g. changes from baseline over time), last observation was carried forward (LOCF) for patients with missing data. For categorical response data (e.g. data at specific time points), patients with missing data were considered failures (non-completion=failure; NCF).
Relationships between baseline and on-treatment parameters and Week 48 virologic response were explored using logistic regression analyses. Resistance measures included scores based on number of TPV and lopinavir (LPV) baseline mutations (LPV mutations: 10F/I/R/V, 20M/R, 24I, 46I/L, 53L, 54V/T/L, 63P, 71T/V/L, 82F/A/T, 84V or 90M; TPV mutations: L10V, 113V, K20M/R, L33F, E35G, M36I, K43T, M46L, I47V, I54A/M/V, Q58E, H69K, T74P, V82L/T, N83D, I84V or L90M). Genotypic inhibitory quotient (GIQ) was calculated as median TPV trough level divided by TPV mutation score [21, 22]. Genotypic sensitivity score (GSS) represents the sum of the genotypically sensitive background ARVs in the regimen. Each background ARV medication was assigned an algorithm-derived value of 0, 0.25, or 1, based on (i) part of ARV history or baseline determination and (ii) resistance or possible resistance. A zero score indicates full genotypic resistance to OBR ARVs; higher scores indicate susceptibility. Factors associated with virologic response at Week 48 (treatment group, GIQ, TPV mutations, baseline VL, age group, TPV adherence and GSS) were evaluated by multivariate logistic regression analysis.
RESULTS
Baseline patient characteristics
Tables 1 and 2 show baseline characteristics. Most patients (93%, 107/115 patients) acquired HIV-1 perinatally. Median baseline HIV-1 RNA, and CD4+ T-cell counts and percentages were comparable between the two treatment groups overall and within the three age groups. No significant differences in demographic and HIV baseline characteristics were found between groups (p>0.05).
Table 1.
Baseline demographics for randomized study participants
| Characteristic | Low-dose TPV/r group |
High-dose TPV/r group |
Total |
|---|---|---|---|
| Total, n (%) | 58 (100) | 57 (100) | 115 (100) |
| Gender, n (%) | |||
| Male | 32 (55.2) | 33 (57.9) | 65 (56.5) |
| Female | 26 (44.8) | 24 (42.1) | 50 (43.5) |
| Age group, n (%) | |||
| 2 to <6 years | 13 (22.4) | 12 (21.1) | 25 (21.7) |
| 6 to <12 years | 19 (32.8) | 19 (33.3) | 38 (33.0) |
| 12 to 18 years | 26 (44.8) | 26 (45.6) | 52 (45.2) |
| HIV RNA (log10 copies/mL) [median] | |||
| All age groups | 4.94 | 4.60 | 4.70 |
| 2 to <6 years | 5.00 | 4.71 | 4.97 |
| 6 to <12 years | 4.70 | 4.58 | 4.58 |
| 12 to 18 years | 4.73 | 4.67 | 4.69 |
| CD4+ (%) [median] | |||
| All age groups | 20.70 | 18.50 | 20.10 |
| 2 to <6 years | 24.40 | 26.35 | 25.90 |
| 6 to <12 years | 19.55 | 18.70 | 18.70 |
| 12 to 18 years | 20.15 | 16.40 | 17.00 |
| CD4+ count (cells/mm3) [median] | |||
| All age groups | 432.0 | 361.0 | 378.5 |
| 2 to <6 years | 855.0 | 736.5 | 795.0 |
| 6 to <12 years | 532.0 | 376.0 | 389.0 |
| 12 to 18 years | 258.5 | 320.0 | 318.0 |
| Hepatitis B or C co-infected, n (%) | |||
| HBsAg positive | 1 (1.7) | 2 (3.5) | 3 (2.6) |
| HCV positive | 1 (1.7) | 3 (5.3) | 4 (3.5) |
| ARV experienced, n (%) | |||
| No | 1 (1.7) | 2 (3.5) | 3 (2.6) |
| Yes | 57 (98.3) | 55 (96.5) | 112 (97.4) |
Low-dose TPV/r = tipranavir 290 mg/m2 plus ritonavir 115 mg/m2
High-dose TPV/r = tipranavir 375 mg/m2 plus ritonavir 150 mg/m2
ARV = antiretroviral
HbsAg = hepatitis B surface antigen
HCV = hepatitis C virus
Table 2.
Baseline antiretroviral experience and mutation analysis by age group for randomized study participants
| Characteristic | Low-dose TPV/r group |
High-dose TPV/r group |
Total |
|---|---|---|---|
| Total, n (%) | 58 (100) | 57 (100) | 115 (100) |
| Previous ARV medication experience (n)[median] | |||
| All age groups | 8 | 7 | 7 |
| 2 to <6 years | 3 | 4 | 3 |
| 6 to <12 years | 8 | 6 | 8 |
| 12 to 18 years | 10 | 11 | 10 |
| GSS [median (range)] | |||
| All age groups | 0.25 (0–2) | 0.25 (0–2.25) | 0.25 (0–2.25) |
| 2 to <6 years | 1.00 (0–2) | 0.25 (0–2) | 0.25 (0–2) |
| 6 to <12 years | 0.25 (0–2) | 0.25 (0–2.25) | 0.25 (0–2.25) |
| 12 to 18 years | 0.25 (0–2) | 0.13 (0–1.5) | 0.25 (0–2) |
| No. of protease gene mutations [median (range)] | |||
| All age groups | 13 (2–25) | 13 (2–26) | 13 (2–26) |
| 2 to <6 years | 10 (2–25) | 11 (4–16) | 10 (2–25) |
| 6 to <12 years | 14 (2–22) | 12 (3–26) | 13 (2–26) |
| 12 to 18 years | 17 (5–24) | 17 (2–25) | 17 (2–25) |
| TPV mutation score [median (range)] | |||
| All age groups | 3.0 (0–9) | 2.0 (0–9) | 3.0 (0–9) |
| 2 to <6 years | 2.0 (0–6) | 1.0 (0–5) | 1.0 (0–6) |
| 6 to <12 years | 4.0 (0–7) | 1.0 (0–5) | 2.5 (0–7) |
| 12 to 18 years | 3.5 (0–9) | 3.5 (0–9) | 3.5 (0–9) |
| LPV mutation score [median (range)] | |||
| All age groups | 4.0 (0–9) | 4.0 (0–9) | 4.0 (0–9) |
| 2 to <6 years | 2.0 (0–8) | 2.5 (1–6) | 2.0 (0–8) |
| 6 to <12 years | 5.0 (0–8) | 2.0 (0–9) | 4.5 (0–9) |
| 12 to 18 years | 5.5 (0–9) | 6.5 (0–9) | 6.0 (0–9) |
| Resistance to all PIs, n (%) | |||
| All age groups | 29 (50.0) | 28 (49.1) | 57 (49.6) |
| 2 to <6 years | 2 (15.4) | 3 (25.0) | 5 (20.0) |
| 6 to <12 years | 9 (47.4) | 9 (47.4) | 18 (47.4) |
| 12 to 18 years | 18 (69.2) | 16 (61.5) | 34 (65.4) |
Low-dose TPV/r = tipranavir 290 mg/m2 plus ritonavir 115 mg/m2
High-dose TPV/r = tipranavir 375 mg/m2 plus ritonavir 150 mg/m2
ARV = antiretroviral
GSS = genotypic sensitivity score
LPV = lopinavir
PI = protease inhibitor
One patient was ARV-naïve in each age group. ARV exposure prior to study entry increased with age (median of 3, 8, and 10 drugs in the 2 to <6; 6 to <12; and 12 to 18 year age groups, respectively), as well as prior PI exposure (median of 1, 2 and 4 PIs respectively). Consequently, TPV and LPV mutation scores increased with age (Table 2). Resistance to all commercially available PIs was present in >50% of all patient isolates at baseline. There were no significant differences in the number of baseline genotype mutations between dose groups overall, or within each age group. Overall, 54.8% (63/115) of patients had two nucleoside reverse transcriptase inhibitors (NRTIs) in the OBR; 13.9% (16/115) had two NRTIs plus a non-nucleoside reverse transcriptase inhibitor (NNRTI), and 11.3% (13/115) had three NRTIs. The OBR included enfuvirtide (ENF) in 13% (15/115) of patients. Median GSS was 0.25 overall and in both dose groups.
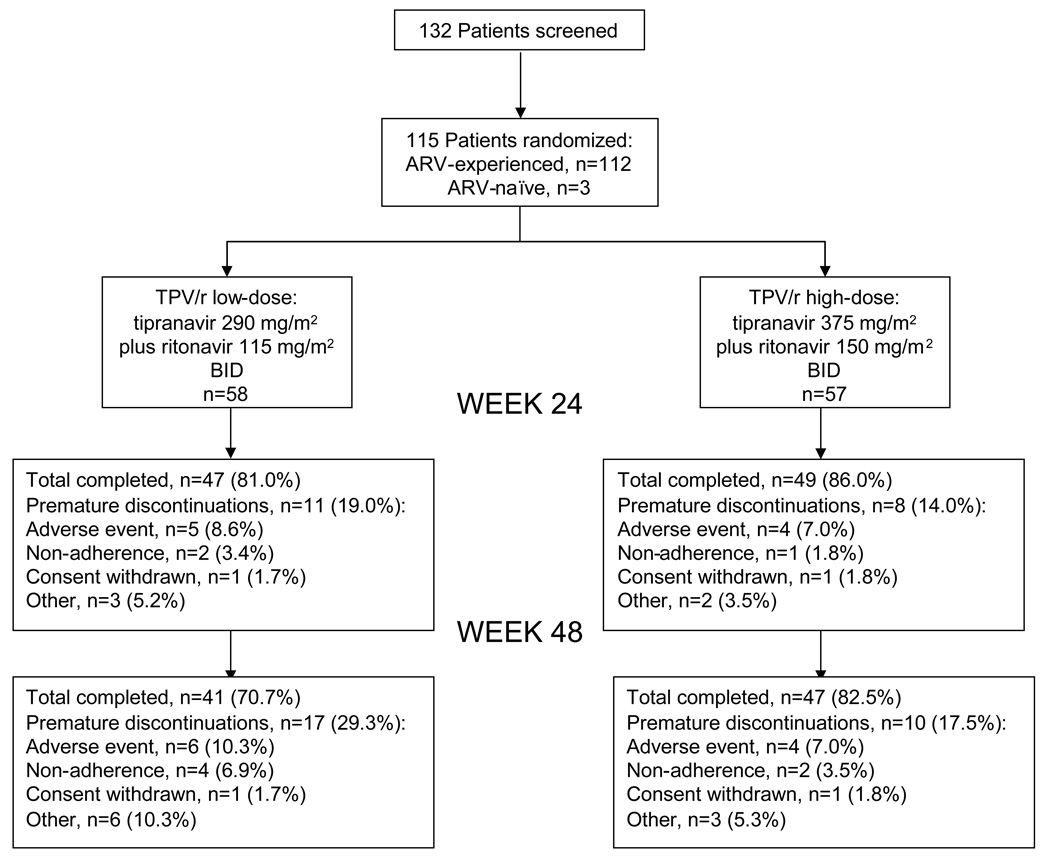
Patient disposition
Overall, 76.5% (88/115) randomized subjects remained on-treatment for 48 weeks (41/58 [70.7%] and 47/57 [82.5%] in low- and high-dose groups, respectively) (Figure 1). Discontinuation frequency due to 'other' reasons (primarily virologic failure) was higher in the low-dose (6/58, 10.3%) than the high-dose group (3/57, 5.3%) but it was not statistically significant (Fischer′s exact 2-sided test; p=0.49).
Fig. 1. Summary of study subject disposition.
ARV, antiretroviral.
Efficacy endpoints: virologic response
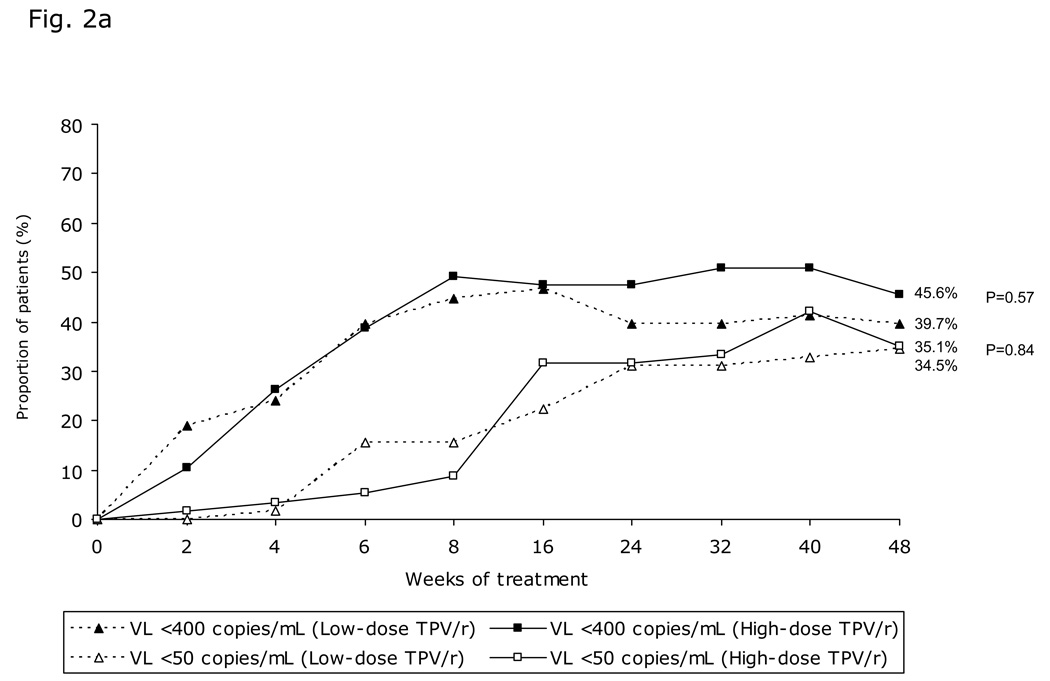
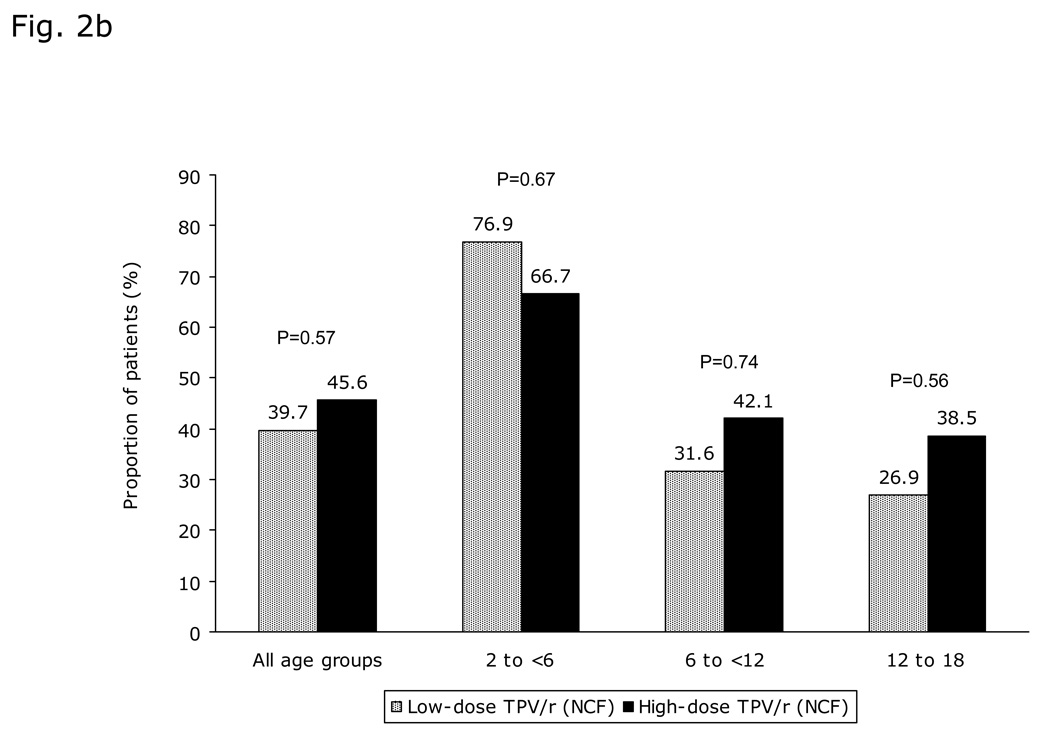
The proportion of patients with VL <400 copies/mL increased from baseline until week 8 then stabilized until Week 48 (Figure 2a). Overall, at 48 weeks, 45.6% (26/57) and 39.7% (23/58) high- and low-dose TPV/r recipients, respectively, had VL <400 copies/mL (intent-to-treat [ITT]-NCF; p=0.57). The proportion of patients with VL <400 copies/mL was greater in the 2 to <6 years age group than in the other two groups (Figure 2b). There was a trend towards better virologic response in the high-dose TPV/r group, even in the 12 to 18 year age group with the highest resistance profile. Similar response pattern was observed for the proportion of patients achieving VL <50 copies/mL (Figure 2a), with better virologic response in the 2 to <6 year age group, followed by those aged 6 to <12 and 12 to 18 years.
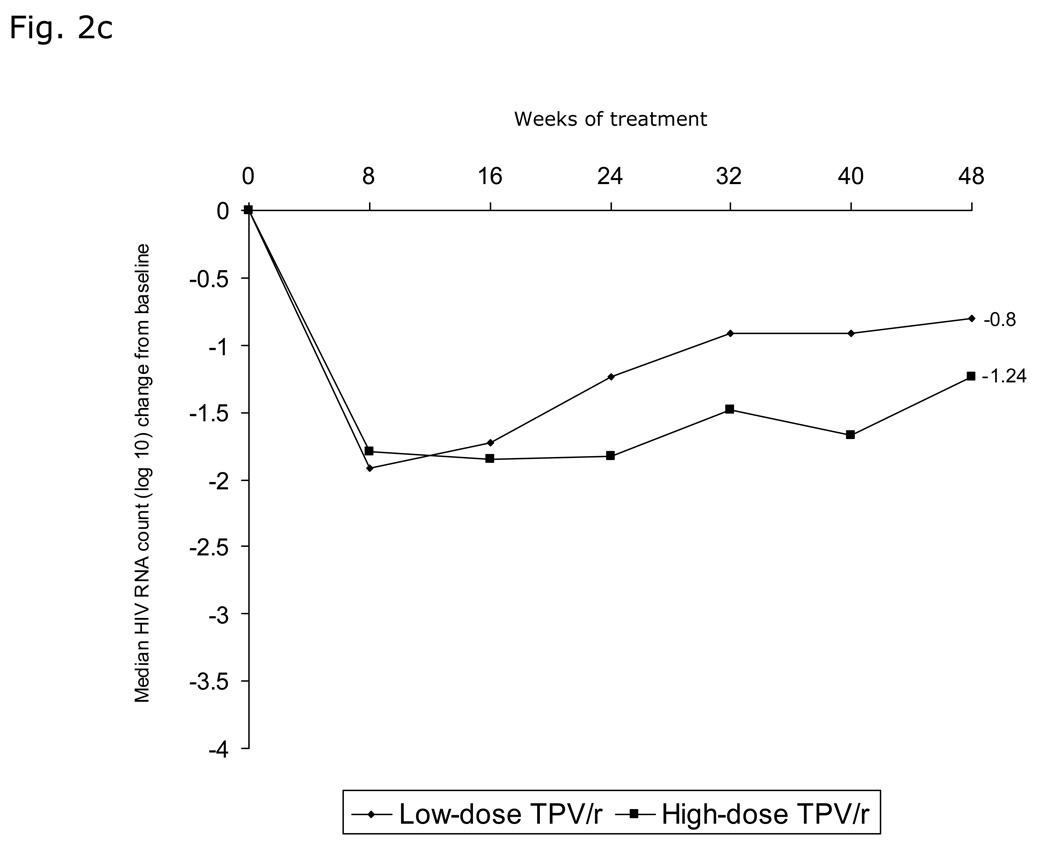
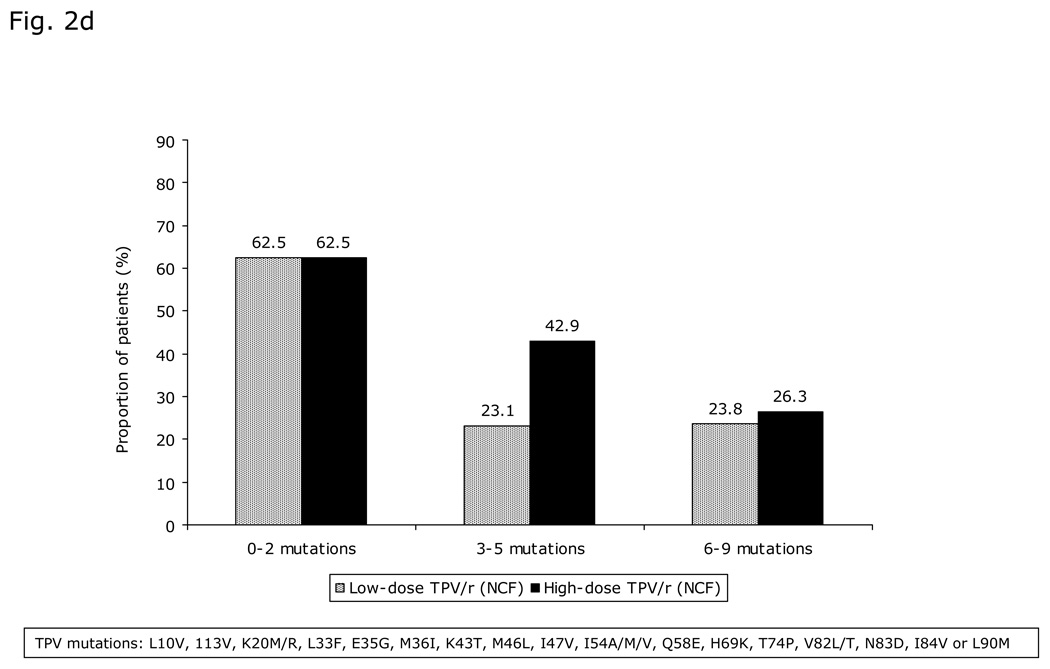
Fig. 2. Virologic responses through 48 weeks.
a) Proportion of patients with viral load (VL) <400 copies/mL and <50 copies/mL over time stratified by TPV/r treatment group; b) Virologic response (viral load [VL] <400 copies/mL) stratified by TPV/r treatment group and age group; c) Median decrease in viral load (VL, log10 copies/mL) over time stratified by TPV/r treatment group; d) Virologic response (viral load [VL] <400 copies/mL) based on LPV mutation score stratified by TPV/r treatment group.
Median VL decrease (log10 copies/mL) was rapid, to nadirs of 1.92 and 1.85 below baseline at Week 8 for the low- and high-dose groups, respectively. Median VL decreased from baseline, by 0.80 and 1.24 log10 copies/mL in the low- and high-dose groups, respectively, at Week 48 (Figure 2c).
Approximately half the patients with available results had >95% adherence at Weeks 24 and 48; overall adherence was similar in both treatment groups.
On the basis of GIQ quartiles, 8.0% (2/25) of patients in the lowest quartile (GIQ range 0.56–7.19) achieved <400 copies/mL at week 48, versus 52% (13/25) in the second quartile (GIQ range 7.23–13.50), 57.7% (15/26) in the third quartile (GIQ range 13.68–38.61) and 68.0% (17/25) in the fourth quartile (GIQ range 39.29–215.38). Patients in the TPV/r high-dose group achieved higher GIQ. Results were similar for VL <50 copies/mL; 4.0% (1/25) in the lowest quartile, 44.0% (11/25) in the second quartile, 50.0% (13/26) in the third quartile and 56.0% (14/25) in the fourth quartile.
GSS score was 0 (indicating resistance to all drugs in the OBR) in almost half the patients (44.3%; 51/115), yet 39.2% (20/51) achieved <400 copies/mL (i.e. virologic response) after 48 weeks of treatment. As GSS increased to between 0.25 and 1 and between 1.25 and 2.25, the proportion of virologic responders increased to 45% (18/40) and 45.8% (11/24), respectively. Virologic response was better in patients with fewer LPV mutations (Figure 2d). However, approximately 25% of patients with 6–9 LPV mutations maintained a virologic response to TPV/r. The proportion of virolgic response at Week 48, based on TPV score, was highest amongst patients with the fewest TPV mutations. For patients with the highest TPV mutation scores, high-dose patients tended strongly towards a better virologic response rate than low-dose patients, although differences remained statistically non-significant between the dose groups in any of these analyses.
Predictors of response in multivariate analyses
Logistic-regression models assessed the effects of treatment group, GIQ, baseline VL, age group, TPV adherence, and GSS on Week 48 outcomes for the virologic endpoints. Table 3 presents results for VL <400 copies/mL.
Table 3.
Logistic regression results for viral load response for HIV-1 RNA <400 at 48 weeks (n=88)
| Factor | Odds ratio (95% CI) | p-value |
|---|---|---|
| Treatment group | ||
| Low-dose TPV/r: High-dose TPV/r | 1.34 (0.49–3.65) | 0.57 |
| GIQ (quartiles) | ||
| Q2: Q1 | 12.35 (2.03–75.01) | 0.01 |
| Q3: Q1 | 17.08 (2.87–101.62) | <0.01 |
| Q4: Q1 | 38.37 (5.14–286.27) | <0.01 |
| Baseline VL (copies/mL) | ||
| ≤1000: >10K-100K | 0.21 (0.01–5.75) | 0.35 |
| >1000–10K: >10K-100K | 1.94 (0.46–8.13) | 0.37 |
| >100K: >10K-100K | 0.30 (0.09–0.94) | 0.04 |
| Age group | ||
| 2-<6: 12-<18 | 2.59 (0.71–9.39) | 0.15 |
| 6-<12: 12-<18 | 0.61 (0.19–1.97) | 0.41 |
| Adherence | ||
| Each improvement of 10% | 1.23 (1.04–1.46) | 0.01 |
| GSS | ||
| 0.25–1.00: 0 | 1.46 (0.49–4.39) | 0.50 |
| 1.25–2.25: 0 | 0.33 (0.08–1.33) | 0.12 |
Low-dose TPV/r = tipranavir 290 mg/m2 plus ritonavir 115 mg/m2
High-dose TPV/r = tipranavir 375 mg/m2 plus ritonavir 150 mg/m2
GIQ = genotypic inhibitory quotient
VL = viral load
GSS = genotypic sensitivity score
Baseline GIQ was the strongest predictor of virologic response, with odds ratios of 12.35–38.37 for second, third and fourth GIQ quartile patients compared to first-quartile patients. Adherence was also a predictor of virologic response; each 10% increase in TPV adherence rate increased the odds of achieving VL <400 copies/mL by 23% at 48 weeks (p=0.01). Baseline VL was a marginally significant predictor of therapeutic success: patients with VL >100,000 copies/mL were less likely to achieve virologic responses (odds ratio=0.30). Age group was not a predictor of virologic response in this model; age group might correlate with drug adherence and especially baseline resistance, which is a determinant of GIQ. Neither dose group, nor GSS value, were significant predictors of VL <400 copies/mL at Week 48. Logistic regression analysis results for achieving ≥1 log10 HIV-1 RNA reduction from baseline were similar for dose group and GSS value. Similar results were obtained when modeling VL <50 copies/mL. However, due to data paucity within some model cells, the final model excluded GSS and GIQ. Final model results indicated adherence was a significant response predictor (p<0.01); likelihood of achieving a VL reduction increased 30% with each 10% adherence increase. Baseline VL also significantly predicted response in this model; patients with VL >100,000 copies/mL were 72% less likely to respond than patients with VL 10,000–100,000 copies/mL.
Logistic regression modeling, using PI mutations instead of GIQ, showed that the likelihood of achieving VL <400 copies/mL decreased by 10% with each additional PI mutation. Patients with PI mutations in the fourth quartile (>19 PI mutations; 19–26) were 82% less likely to achieve VL <400 copies/mL than patients with PI mutations in the first quartile (≤8 PI mutations; 2–8). All other trends were similar to the original model.
Immunologic response
Overall baseline CD4+ T-cell percentages were similar between dose groups; 21% and 19% in the low- versus high-dose TPV/r groups. Baseline CD4+ T cell values were higher in the 2 to <6 year age group (24% and 26% for the two dose groups) compared to the other two age groups. Median increase from baseline over time in CD4+ T-cell percentage was 5% in the low-dose and 3% in the high-dose group at 48 weeks (p=0.11). CD4+ T cell percentage increases were higher in the 2 to <6 year age group (10% and 6% for low- and high-dose groups, respectively) compared to the 6 to <12 year age group (5% and 3% for low- and high-dose groups, respectively) and the 12 to 18 year age group (2% and 0% for low- and high-dose groups). Both dose groups exhibited an overall CD4+ T-cell count increase through Week 48 (+100 and +59 cells/µL for low- versus high-dose TPV/r groups). Median CD4+ T-cell count increase was highly variable among age groups.
AIDS-defining illnesses
Four patients (three in the 6 to <12 and one 12 to 18 year age group) developed a new protocol-defined AIDS-defining illness through 48 weeks, all of whom were in the low-dose group. One patient experienced herpes esophagitis and pharyngeal and esophageal candidiasis, two patients experienced pneumonia and one patient experienced tuberculosis. All these patients recovered. Kaplan-Meier probability of developing an AIDS-defining illness up to 48 weeks was 7.8% versus 0.0% in the low- and high-dose groups, respectively (p=0.04).
Safety and tolerability evaluation
Overall safety and tolerability profiles were similar between dose groups (Table 4). Patients in the low-dose group tended to have more serious AEs (SAEs) than patients in the high-dose group (27.6%,16/58 vs. 22.8%, 13/57, respectively) but the difference was not statistically significant. Infection-related SAEs occurred in 13 and 8 patients in the low- versus high-dose groups. Two patients in each dose group had gastrointestinal SAEs (diarrhea and viral gastroenteritis). Discontinuations due to AEs were no more likely in patients on high-dose than low-dose TPV/r (7.0%, 4/58 vs. 10.3%, 6/57). The main event leading to discontinuation was elevated GGT, occurring in 5.2% (3/58) low-dose patients. In the high-dose group, no common events were associated with discontinuation; each event occurred only once (ALT elevation, abdominal pain, nausea, vomiting, abdominal discomfort, retching and urticaria). Vomiting was the most frequent AE, reported by 37.4% (43/115) of patients, followed by cough (27.0%; 31/115) and diarrhea and pyrexia (24.3%; 28/115). Most AEs were mild (33.9%; 39/115) or moderate (34.8%; 40/115), with 25.2% (29/115) classified as severe. There were 69 patients with gastrointestinal AEs (mild in 54 (78.3%), moderate in 12 (17.4%) and severe in 3 (4.3%). Vomiting (27.0%; 31/115) and nausea (14.8%; 17/115) were the most frequent study drug-related AEs.
Table 4.
Summary of patients with adverse events through 48 weeks
| Low-dose TPV/r group n (%) |
High-dose TPV/r group n (%) |
Total N (%) |
||
|---|---|---|---|---|
| Total no. of patients treated | 58 (100) | 57 (100) | 115 (100) | |
| Most frequently occuring AEs in >10% of patients* | ||||
| Vomiting | 19 (32.8) | 24 (42.1) | 43 (37.4) | |
| Cough | 14 (24.1) | 17 (29.8) | 31 (27.0) | |
| Diarrhea | 13 (22.4) | 15 (26.3) | 28 (24.3) | |
| Pyrexia | 16 (27.6) | 12 (21.1) | 28 (24.3) | |
| Nausea | 9 (15.5) | 10 (17.5) | 19 (16.5) | |
| Nasopharyngitis | 8 (13.8) | 7 (12.3) | 15 (13.0) | |
| Headache | 8 (13.8) | 6 (10.5) | 14 (12.2) | |
| Total no. of patients with any AE | 54 (93.1) | 54 (94.7) | 108 (93.9) | |
| Total no. of patients with any study drug–related AE | 28 (48.3) | 34 (59.6) | 62 (53.9) | |
| Total no. of patients with a serious AE | 16 (27.6) | 13 (22.8) | 29 (25.2) | |
| Total no. of patients with AEs leading to discontinuation of study drug | 6 (10.3) | 4 (7.0) | 10 (8.7) | |
Values shown are for numbers of patients, not numbers of AEs
Low-dose TPV/r = tipranavir 290 mg/m2 plus ritonavir 115 mg/m2
High-dose TPV/r = tipranavir 375 mg/m2 plus ritonavir 150 mg/m2
AE = adverse event
GGT = gamma-glutamyl transferase
No Grade 4 ALT or AST elevations occurred through Week 48. DAIDS Grade 3 ALT elevations occurred in 6.3% (7/112) evaluable patients (2/7 patients had baseline Grade 1 ALT; 5/7 patients were aged 12 to 18 years and five received high-dose TPV/r). All these elevations were asymptomatic, returning to normal/Grade 1. Only one patient (15 year old male; high-dose TPV/r) discontinued treatment due to increased ALT. No cases of clinical hepatitis or Grade 3/4 triglyceride increases occurred up to 48 weeks.
Bleeding events occurred in 5.75% and 14.3% of children receiving the oral solution (vitamin E as an excipient) versus capsules. Eight patients (four per dose group) experienced mild bleeding events, with preferred terms of hematochezia, gingival bleeding, epistaxis, hematoma and moderate hemorrhagic diarrhea (one patient in high-dose group). No patient discontinued treatment due to bleeding events up to 48 weeks. One patient, who reported trauma-related bruising, had persistent increases in prothrombin time (PT) and partial thromboplastin time (aPTT) beginning at Week 48. The patient continued with increased PT and PTT but subsequently discontinued study medication due to deteriorating HIV disease status. Another patient discontinued due to thrombotic thrombocytopenic purpura (TTP). One patient died after Week 48 due to gastrointestinal hemorrhage, related to a newly diagnosed gastrointestinal lymphoma and not to study drug.
DISCUSSION
This study shows that TPV/r (oral solution and/or capsules) provided a sustained virologic response in children and adolescents harboring HIV-1-resistant virus and needing alternative therapy to the currently approved ARV treatment options. Recently presented PK data indicated that low-dose TPV/r (290/115 mg/m2), scaled to the 500/200 mg adult dose (BSA 1.73 m2), resulted in TPV exposure similar to that in adult patients [20]. However high-dose TPV/r was associated with better 48-week responses overall, particularly in patients aged 12 to 18, who had more resistant virus, lower GSS, poorer adherence and lower GIQ. The observed differences were not statistically significant; however this study was not powered for efficacy. No new protocol-defined AIDS-defining illnesses were reported in the high-dose group.
Prior ARV exposure resulted in limited options for constructing a background regimen to which the patients′ virus was susceptible. Median baseline GSS was 0.25, confirming limited susceptibility to available ARVs in the OBR, and diminished support for TPV in maintaining a robust treatment response. TPV/r was particularly effective in younger children (approximately 70% in the 2 to <6 years age group achieved VL <400 copies/mL at Week 48), probably due to greater adherence and particularly due to lower baseline resistance levels and in this group. The resistance profiles in 12 to 18 year olds were similar to those observed in adults in the RESIST (Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir) studies [17–19]. Furthermore, similar virologic responses were observed between these two study populations. High-dose TPV/r was more likely to yield a higher GIQ, which is associated with better virologic response [23]. As expected, increased numbers of baseline PI mutations were associated with decreased virologic responses. Nevertheless, patients with numerous protease mutations still achieved a virologic response, indicating that TPV retains significant activity in treatment-experienced patients. This is also true for patients harboring LPV mutations with a sustained virologic response observed in 25% of patients with 6–9 LPV mutations. TPV/r may be beneficial in eliciting a virologic response in pediatric patients with HIV-1 virus that has reduced LPV susceptibility.
The TPV safety and tolerability profile in this study tended to be better than that observed in TPV/r-treated adults [19]. High-dose TPV/r was well tolerated; transaminase elevation rates were lower than in approved TPV/r 500/200 mg adult-dose trials. No new/unexpected toxicities were observed in this trial. TPV/r was safe and well tolerated in the youngest age group (2 to <6 years), with no DAIDS Grade 3 or 4 liver function tests or AE-related discontinuations. Frequencies and types of severe AEs in this study were largely similar to those in other pediatric studies of PIs [24, 25]. Bleeding events were evaluated in this trial because vitamin E, a TPV oral-solution excipient, has been associated with an increased risk of bleeding events in rats [26]. There was no evidence of increased bleeding-event risk in this trial for the oral solution versus the capsules (7.0% vs. 13.8%).
The OBR was investigator-selected, based on history and genotypic resistance testing. ENF and new drug-class use was low in this trial of treatment-experienced patients which was a reflection of the regulatory approval and the availability of these drugs‥ ENF may have been included among older patients but its use was not required, encouraged or discouraged‥ Similarly, at study initiation, the integrase inhibitors and the chemokine receptor (CCR5) blockers lacked regulatory approval for use in children or adolescents and could not be included in the OBR of any study participants.
Virologic and immunologic results from this trial were broadly comparable to results from other pediatric studies, including those which investigated LPV/r [31, 32]. However, differences in both study design and population make direct efficacy comparisons between TPV/r and LPV/r in pediatric patients difficult; interpretation requires caution. Both LPV/r pediatric studies included larger numbers of ARV-naive patients than in this study (LPV/r: 8% and 16% vs. TPV/r: 2.6%) [31, 32]. Furthermore, some TPV/r study participants had previously received and failed LPV/r treatment; many had LPV/r resistance mutations. This TPV/r study was not powered for efficacy but important virologic and immunologic responses were observed, despite large numbers of individuals with evidence of resistance.
Recently published data would suggest that TPV/r can be used in combination with new drug classes in adult patients without requiring dose adjustment [27–30], however, applicability of this finding to the pediatric population is currently not known.
In conclusion, efficacy, safety and tolerability of TPV/r in this study warrant its use in HIV-1-infected children failing PI-based therapy; TPV/r-based therapy effectively reduced VL in HIV-1 infected children at either dose. However, in patients with reduced susceptibility to ARVs, high-dose TPV/r (375/150 mg/m2) tended to give greater, more durable virologic and, possibly, clinical responses compared with low-dose TPV/r (290/115 mg/m2). TPV/r showed a good safety profile and was tolerated at either dose. Therefore, TPV/r suspension should prove to be an advantageous therapeutic option for treating HIV-1 infected children. This is particularly true for heavily pretreated long-term patients whose viral isolates show resistance to other PIs, including LPV/r.
Acknowledgements
Conflicts of interest
J. C. Salazar is a consultant for Boehringer Ingelheim, Merck and Pfizer. He is also a speaker for the Merck vaccine division. Neither Dr Salazar nor any member of his immediate family has any individual stock or financial interest in any pharmaceutical companies.
P. Cahn has no conflict of interests.
R. Yogev has no conflict of interests.
M. Della Negra is not a shareholder of any pharmaceutical industry. She has been a temporary consultant for Abbott and receives funding through clinical research contracts with Boehringer Ingelheim.
G. Castelli-Gattinara has no conflict of interests.
C. Fortuny is not a shareholder of any company. She has been a consultant to GlaxoSmithKline and Gilead. She also served as speaker for Abbott, Boehringer Ingelheim, Roche, Bristol-Myers-Squibb and Gilead.
P. Flynn has clinical trial agreements with MedImmune and Tibotec Pharmaceuticals.
C. Giaquinto is a consultant and speaker for GlaxoSmithKline, Abbott, Tibotec, Boehringer Ingelheim, Pfizer, Sanofi Pasteur. He has received funding for research from GlaxoSmithKline, Abbott, BMS, Tibotec, Gilead, Boehringer Ingelheim, Pfizer, Sanofi Pasteur.
P. Ruan has no conflict of interests.
J. Mikl and A. Jelaska are employees of Boehringer Ingelheim.
Grant Support: The PACTG/IMPAACT was supported by Grant Number U01AI068632 and 1U01AI068616 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. Juan C. Salazar also had support from the University of Connecticut Health Center′s Clinical Research Center Grant M01RR06192 and R. Yogev from PACTG 2# 5U01AI069512
All authors contributed to the acquisition of data and interpretation, drafting of the manuscript and approval of the version submitted for publication. Statistical analysis was provided by Boehringer Ingelheim.
The authors would like to acknowledge all investigators who participated in the BI 1182.14 trial: Argentina: Pedro Cahn, MD, Fundación Huésped, Buenos Aires; Brazil: Marinella Della Negra, MD, Instituto de Infectologia Hospital Emílio Ribas, Sao Paulo, Maria Zilda de Aquino, MD, PhD, Faculdade de Medicina da Universidade de São Paulo, Sao Paulo; Canada: Normand Lapointe, MD, Hopital Ste-Justine, Montreal, Quebec, Stanley Read, MD, Hospital for Sick Children, Toronto; France: Francoise Mechinaud, MD, Hopital Mere-Enfant, Nantes, Albert Faye, MD, Hopital Robert Debre, Paris, Daniel Floret, MD, Hopital Edouard Herriot, Lyon; Germany: Richard Kreuz, MD, Universitaet Frankfurt, Frankfurt, Gundula Notheis, MD, Klinikum Innenstadt der LMU, Munich, Ilse Grosch-Worner, MD, Cornelia Feiterna-Sperling, MD, Virchow Klinik, Berlin; Italy: Guido Castelli-Gattinara, MD, Ospedale Pediatrica Bambino Gesu, Roma, Carlo Giaquinto, MD, Azienda Ospedaliera di Padova, Padova; Mexico: Luis Xochihua, MD, Instituto Nacional Pediatria, Mexico; Spain: Claudia Fortuny, MD, Hospital San Juan de Dios, Esplugues de Llobregat, Jose-Thomas Ramos, MD, Hospital 12 de Octubre, Madrid; United States: Patricia M. Flynn, MD, St. Jude′s Children′s Research Hospital, Memphis, TN (PACTG/IMPAACT), Katherine Ruiz Luzuriaga, MD, UMass Memorial Medical Center, North Worcester, MA (PACTG/IMPAACT), Ram Yogev, MD, Children′s Memorial Hospital, Chicago, IL (PACTG/IMPAACT), Juan Salazar, MD, Connecticut Children′s Medical Center, Hartford, CT (PACTG/IMPAACT), Eleanor Jimenez, MD, FAAP, San Juan Hospital, San Juan, PR (PACTG/IMPAACT), Grace McComsey, MD, Rainbow Babies and Children′s Hospital, Cleveland, OH, Andrea Kovacs, MD, LaShonda Spencer, MD, LAC + USC Medical Center, Los Angeles, CA (PACTG/IMPAACT), William T. Shearer, MD, Texas Children′s Hospital, Houston, TX (PACTG/IMPAACT), Barbara W. Stechenberg, MD, Baystate Medical Center, Springfield, MA (PACTG/IMPAACT), Joseph Church, MD, Children′s Hospital of Los Angeles, Los Angeles, CA
The PACTG/IMPAACT team would like to thank Jennifer Gardella for her administrative and management support of the study.
Manuscript preparation
Boehringer Ingelheim Pharmaceuticals provided funding for editorial assistance. Editorial assistance was provided by Euro RSCG Life UK.
Footnotes
ClinicalTrials.gov Identifier: NCT00076999
REFERENCES
- 1.Luzuriaga K, McManus M, Mofenson L, Britto P, Graham B, Sullivan JL. A trial of three antiretroviral regimens in HIV-1-infected children. N Engl J Med. 2004;350:2471–2480. doi: 10.1056/NEJMoa032706. [DOI] [PubMed] [Google Scholar]
- 2.Verweel G, Sharland M, Lyall H, Novelli V, Gibb DM, Dumont G, et al. Nevirapine use in HIV-1-infected children. AIDS. 2003;17:1639–1647. doi: 10.1097/00002030-200307250-00008. [DOI] [PubMed] [Google Scholar]
- 3.Puthanakit T, Oberdorfer A, Akarathum N, Kanjanavanit S, Wannarit P, Sirisanthana T, Sirisanthana V. Efficacy of highly active antiretroviral therapy in HIV-infected children participating in Thailand′s National Access to Antiretroviral Program. Clin Infect Dis. 2005;41:100–107. doi: 10.1086/430714. [DOI] [PubMed] [Google Scholar]
- 4.King JR, Nachman S, Yogev R, Hodge J, Aldrovandi G, Hughes MD, et al. Efficacy, tolerability and pharmacokinetics of two nelfinavir-based regimens in human immunodeficiency virus-infected children and adolescents: pediatric AIDS clinical trials group protocol 403. Pediatr Infect Dis J. 2005;24:880–885. doi: 10.1097/01.inf.0000180508.21918.8a. [DOI] [PubMed] [Google Scholar]
- 5.Fraaij PL, Verweel G, van Rossum AM, van Lochem EG, Schutten M, Weemaes CM, et al. Sustained viral suppression and immune recovery in HIV type 1-infected children after 4 years of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:604–608. doi: 10.1086/427696. [DOI] [PubMed] [Google Scholar]
- 6.Resino S, Bellon JM, Munoz-Fernandez MA. Antiretroviral activity and safety of lopinavir/ritonavir in protease inhibitor-experienced HIV-infected children with severe-moderate immunodeficiency. J Antimicrob Chemother. 2006;57:579–582. doi: 10.1093/jac/dki469. [DOI] [PubMed] [Google Scholar]
- 7.Scherpbier HJ, Bekker V, van Leth F, Jurriaans S, Lange JM, Kuijpers TW. Long-term experience with combination antiretroviral therapy that contains nelfinavir for up to 7 years in a pediatric cohort. Pediatrics. 2006;117:e528–e536. doi: 10.1542/peds.2005-1272. [DOI] [PubMed] [Google Scholar]
- 8.Funk MB, Notheis G, Schuster T, Elanjkal Z, von Hentig N, Sturmer M, et al. Effect of first line therapy including efavirenz and two nucleoside reverse transcriptase inhibitors in HIV-infected children. Eur J Med Res. 2005;10:503–508. [PubMed] [Google Scholar]
- 9.Viani RM, Araneta MR, Deville JG, Spector SA. Decrease in hospitalization and mortality rates among children with perinatally acquired HIV type 1 infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39:725–731. doi: 10.1086/423178. [DOI] [PubMed] [Google Scholar]
- 10.Gibb DM, Duong T, Tookey PA, Sharland M, Tudor-Williams G, Novelli V, et al. Decline in mortality, AIDS, and hospital admissions in perinatally HIV-1 infected children in the United Kingdom and Ireland. BMJ. 2003;327:1019. doi: 10.1136/bmj.327.7422.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rossum AM, Geelen SP, Hartwig NG, Wolfs TF, Weemaes CM, Scherpbier HJ, et al. Results of 2 years of treatment with protease-inhibitor--containing antiretroviral therapy in dutch children infected with human immunodeficiency virus type 1. Clin Infect Dis. 2002;34:1008–1016. doi: 10.1086/339443. [DOI] [PubMed] [Google Scholar]
- 12.Mocroft A, Ledergerber B, Viard JP, Staszewski S, Murphy M, Chiesi A, et al. Time to virological failure of 3 classes of antiretrovirals after initiation of highly active antiretroviral therapy: results from the EuroSIDA study group. J Infect Dis. 2004;190:1947–1956. doi: 10.1086/425424. [DOI] [PubMed] [Google Scholar]
- 13.Chaix ML, Rouet F, Kouakoussui KA, Laguide R, Fassinou P, Montcho C, et al. Genotypic human immunodeficiency virus type 1 drug resistance in highly active antiretroviral therapy-treated children in Abidjan, Cote d′Ivoire. Pediatr Infect Dis J. 2005;24:1072–1076. doi: 10.1097/01.inf.0000190413.88671.92. [DOI] [PubMed] [Google Scholar]
- 14.Mullen J, Leech S, O′Shea S, Chrystie IL, Du Mont G, Ball C, et al. Antiretroviral drug resistance among HIV-1 infected children failing treatment. J Med Virol. 2002;68:299–304. doi: 10.1002/jmv.10203. [DOI] [PubMed] [Google Scholar]
- 15.van Leth F, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet. 2004;363:1253–1263. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 16.Guidelines for the use of antiretroviral agents in pediatric HIV infection. National Institute of Health; 2006. Oct, The working group on antiretroviral therapy and medical management of HIV-infected children convened by the National resource Center at the Francois-Xavier Bagnoud Center UThraS. [Google Scholar]
- 17.Cahn P, Villacian J, Lazzarin A, Katlama C, Grinsztejn B, Arasteh K, et al. Ritonavir-boosted tipranavir demonstrates superior efficacy to ritonavir-boosted protease inhibitors in treatment-experienced HIV-infected patients: 24-week results of the RESIST-2 trial. Clin Infect Dis. 2006;43:1347–1356. doi: 10.1086/508352. [DOI] [PubMed] [Google Scholar]
- 18.Gathe J, Cooper DA, Farthing C, Jayaweera D, Norris D, Pierone G, Jr, et al. Efficacy of the protease inhibitors tipranavir plus ritonavir in treatment-experienced patients: 24-week analysis from the RESIST-1 trial. Clin Infect Dis. 2006;43:1337–1346. doi: 10.1086/508353. [DOI] [PubMed] [Google Scholar]
- 19.Hicks CB, Cahn P, Cooper DA, Walmsley SL, Katlama C, Clotet B, et al. Durable efficacy of tipranavir-ritonavir in combination with an optimised background regimen of antiretroviral drugs for treatment-experienced HIV-1-infected patients at 48 weeks in the Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir (RESIST) studies: an analysis of combined data from two randomised open-label trials. Lancet. 2006;368:466–475. doi: 10.1016/S0140-6736(06)69154-X. [DOI] [PubMed] [Google Scholar]
- 20.Sabo J, Cahn P, Della Negra M, Castelli-Gattinara G, Fortuny C, Yogev R, et al. Population Pharmacokinetic (PK) Assessment of Systemic Steady-State Tipranavir (TPV) Concentrations for HIV+ Pediatric Patients Administered Tipranavir/Ritonavir (TPV/r) 290/115 mg/m2 and 375/150 mg/m2 BID (BI 1182.14 and PACTG 1051 Study Team). 13th Conference on Retroviruses and Opportunistic Infections (CROI); Denver, USA. 2006. [Google Scholar]
- 21.Baxter JD, Schapiro JM, Boucher CA, Kohlbrenner VM, Hall DB, Scherer JR, Mayers DL. Genotypic changes in human immunodeficiency virus type 1 protease associated with reduced susceptibility and virologic response to the protease inhibitor tipranavir. J Virol. 2006;80:10794–10801. doi: 10.1128/JVI.00712-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson VA, Brun-Vezinet F, Clotet B, Kuritzkes DR, Pillay D, Schapiro JM, Richman DD. Update of the drug resistance mutations in HIV-1: Fall 2006. Top HIV Med. 2006;14:125–130. [PubMed] [Google Scholar]
- 23.Marcelin AG, Lamotte C, Delaugerre C, Ktorza N, Ait Mohand H, Cacace R, et al. Genotypic inhibitory quotient as predictor of virological response to ritonavir-amprenavir in human immunodeficiency virus type 1 protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2003;47:594–600. doi: 10.1128/AAC.47.2.594-600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krogstad P, Lee S, Johnson G, Stanley K, McNamara J, Moye J, et al. Nucleoside-analogue reverse-transcriptase inhibitors plus nevirapine, nelfinavir, or ritonavir for pretreated children infected with human immunodeficiency virus type 1. Clin Infect Dis. 2002;34:991–1001. doi: 10.1086/338814. [DOI] [PubMed] [Google Scholar]
- 25.Nachman SA, Stanley K, Yogev R, Pelton S, Wiznia A, Lee S, et al. Nucleoside analogs plus ritonavir in stable antiretroviral therapy-experienced HIV-infected children: a randomized controlled trial. Pediatric AIDS Clinical Trials Group 338 Study Team. JAMA. 2000;283:492–498. doi: 10.1001/jama.283.4.492. [DOI] [PubMed] [Google Scholar]
- 26.Marsh SA, Coombes JS. Vitamin E and alpha-lipoic acid supplementation increase bleeding tendency via an intrinsic coagulation pathway. Clin Appl Thromb Hemost. 2006;12:169–173. doi: 10.1177/107602960601200204. [DOI] [PubMed] [Google Scholar]
- 27.Grinsztejn B, Nguyen BY, Katlama C, Gatell JM, Lazzarin A, Vittecoq D, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369:1261–1269. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 28.Kumar P, Cooper D, Steigbigel R. Efficacy of raltegravir, an HIV integrase inhibitor, in combination with regimens containing enfuvirtide, darunavir, or tipranavir in patients with triple-class resistant virus: combined results from Benchmrk-1 and Benchmrk-2. 11th European AIDS Conference (EACS); Madrid, Spain. 2007. [Google Scholar]
- 29.Mathias A, Hinkle J, Enejosa J, Piliero P, Kearney B. Lack of Pharmacokinetic Interaction Between Ritonavir-Boosted GS-9137 (Elvitegravir) and Tipranavir/r. 4th International AIDS Society Conference (IAS); Sydney, Australia. 2007. [Google Scholar]
- 30.Abel S, Taylor-Worth R, Ridgeway C. Effect of boosted tipranavir on the pharmacokinetics of maraviroc (UK-427, 857) in healthy volunteers. Abstract LBPE4.3/15. 10th European AIDS Conference (EACS); Dublin, Ireland. 2005. [Google Scholar]
- 31.Kline MW, Rugina S, Ilie M, Matusa RF, Schweitzer AM, Calles NR, Schwarzwald HL. Long-term follow-up of 414 HIV-infected Romanian children and adolescents receiving lopinavir/ritonavir-containing highly active antiretroviral therapy. Pediatrics. 2007;119:e1116–e1120. doi: 10.1542/peds.2006-2802. [DOI] [PubMed] [Google Scholar]
- 32.Rudin C, Burri M, Shen Y, Rode R, Nadal D. Long-Term Safety and Effectiveness of Ritonavir, Nelfinavir, and Lopinavir/Ritonavir in Antiretroviral-Experienced HIV-Infected Children. Pediatr Infect Dis J. 2008 doi: 10.1097/INF.0b013e3181646d5a. (Epub, ahead of print). [DOI] [PubMed] [Google Scholar]