Abstract
Fungi grown in pure cultures produce DNA- or RNA-containing particles smaller than spore size (<1.5 μm). High exposures to fungi and bacteria are observed at biofuel plants. Airborne cultivable bacteria are often described to be present in clusters or associated with larger particles with an aerodynamic diameter (dae) of 2–8 μm. In this study, we investigate whether airborne fungal components smaller than spore size are present in bioaerosols in working areas at biofuel plants. Furthermore, we measure the exposure to bacteria and fungal components in airborne particulate matter (PM) with a D50 of 1 μm (called PM1 dust). PM1 was sampled using Triplex cyclones at a working area at 14 Danish biofuel plants. Millipore cassettes were used to sample ‘total dust’. The PM1 particles (29 samples) were analysed for content of 11 different components and the total dust was analysed for cultivable fungi, N-acetyl-β-D-glucosaminidase (NAGase), and (1 → 3)-β-D-glucans. In the 29 PM1 samples, cultivable fungi were found in six samples and with a median concentration below detection level. Using microscopy, fungal spores were identified in 22 samples. The components NAGase and (1 → 3)-β-D-glucans, which are mainly associated with fungi, were present in all PM1 samples. Thermophilic actinomycetes were present in 23 of the 29 PM1 samples [average = 739 colony-forming units (CFU) m−3]. Cultivable and ‘total bacteria’ were found in average concentrations of, respectively, 249 CFU m−3 and 1.8 × 105 m−3. DNA- and RNA-containing particles of different lengths were counted by microscopy and revealed a high concentration of particles with a length of 0.5–1.5 μm and only few particles >1.5 μm. The number of cultivable fungi and β-glucan in the total dust correlated significantly with the number of DNA/RNA-containing particles with lengths of between 1.0 and 1.5 μm, with DNA/RNA-containing particles >1.5 μm, and with other fungal components in PM1 dust. Airborne β-glucan and NAGase were found in PM1 samples where no cultivable fungi were present, and β-glucan and NAGase were found in higher concentrations per fungal spore in PM1 dust than in total dust. This indicates that fungal particles smaller than fungal spore size are present in the air at the plants. Furthermore, many bacteria, including actinomycetes, were present in PM1 dust. Only 0.2% of the bacteria in PM1 dust were cultivable.
Keywords: actinomycetes, aerodynamic diameter of fungi, bacteria, bioaerosol, biofuel, exposure, inhalable dust, PM1 dust
INTRODUCTION
Fungi grown in pure cultures produce DNA- or RNA-containing non-cultivable particles smaller than spore size (Madsen et al., 2005). Green et al. (2005) have described the presence of fragmented conidia in dust collected and cultivated on filters. In indoor air, hyphal fragments constituted 6.3% of the numbers of airborne fungal spores (Li and Kendrick, 1995) and in grain farming, farmers were exposed to 5 × 105 hyphal fragments m−3 (Halstensen et al., 2007). When hyphal fragment counts have been incorporated into longitudinal epidemiological studies, associations with asthma severity and peak expiratory flow rates have been identified (Delfino et al., 1997). In these studies with hyphal fragments, the sizes of these hyphal fragments were not described. During autolysis, fungal hyphae are seen to release particles smaller than fungal spores (Madsen et al., 2005). It is not known whether these separate observations are part of the same phenomenon or biological process. Fungal particles smaller than fungal spores are generally of interest; one reason is they add to the fungal bioburden, often measured as fungal spores or cultivable fungi. The large surface area relative to mass and the possible alveolar deposition may be important for possible biological effects of these small particles. Furthermore, they are of interest because they are expected to have the ability to stay in the air for a longer time than spores and to penetrate small cracks e.g. in building constructions, which may influence the exposure level.
Evidence from both epidemiological and experimental studies supports the hypothesis that exposure to fungal spores is causally associated with development of hypersensitivity pneumonitis, organic dust toxic syndrome, decline in lung function, severity of asthma, respiratory symptoms, and airway inflammation. Furthermore, a recent review document on fungal spores suggests an occupational exposure limit of 105 spores for diverse fungal species in non-sensitized populations (Eduard, 2006). In addition, fungal entities, for example (1 → 3)-β-D-glucan and glucose polymers present in the mycelial cell wall and the fungal spore wall of most fungi, have been shown to have a possible negative impact on health (Douwes et al., 2003). Common fungi such as Aspergillus, Alternaria, Penicillium, and Trichoderma secrete the chitinase N-acetyl-β-D-glucosaminidase (NAGase) (Lahoz et al., 1976; Claeyssens and Aerts, 1992; Draborg et al., 1995; Hearn et al., 1998). NAGase is also produced by some other organisms (Shi et al., 2007; Zhang et al., 2007) which are not likely to be present in aerosols at biofuel plants and by some bacteria antagonistic to fungi (Manjula and Podile, 2005). Cellulases are secreted by many common bacterial and fungal species (Eriksson, 1981; Bayer et al., 1998). Some cellulases and chitinases are described to be inflammogene or to cause respiratory effects (Losada et al., 1986; Robinson et al., 1990; Jeffrey et al., 1999; Baur, 2005; Allermann et al., 2006). Significant correlations have been found between number of fungi in airborne or settled dust and NAGase activity (Madsen, 2003; Madsen and Würtz, 2005). Thus, NAGase activity may act as a marker of fungi in dust and it may directly add to the inflammatory potency of dust.
At biofuel plants, large amounts of straw are handled and there is a high exposure to inhalable fungal spores (Madsen, 2006). Studies with instillation of respirable dust from a biofuel plant in mice have demonstrated a strong inflammatory response (Madsen et al., 2008). In this study, we want to investigate whether fungal material smaller than spore size is present in the air at working areas at biofuel plants and in which concentrations these particles are present. According to Levetin (1995), fungal spores are between <2 μm and 100 μm. Eduard et al. (2001) have studied spores from aerosols by microscopy and they have classified fungal spores as spores >1.5 μm. Table 1 presents aerodynamic diameters (dae) of fungi in aerosols and shows that fungi are often present as particles with dae between 2 and 6 μm. The small fungal particles in aerosols from pure fungal cultures have dae of different sizes and are ∼0.4 to 1.1 μm (Table 1). Accordingly, we have tried to sample particles <1 μm to avoid fungal spores and to sample the small fungal particles observed to be released from pure fungal cultures. For this sampling, a Triplex cyclone has been used as it has a sharp penetration curve with a cut point of 1 μm (D50) (Gussman and Kenny, 2000). The Triplex cyclone has previously been used both as a stationary and personal sampler (Davis et al., 2006).
Table 1.
Diameters (d) and aerodynamic diameters (dae) of microbial particles
dae measured by an aerodynamic particle sizer.
Peak number of cultivable organisms measured by an Andersen sampler.
Methods described in Kildesø et al. (2003).
Peak number measured by a particle sizer.
Synonym: Stachybotrys atra.
Synonym: Lecanicilium lecanii.
Synonym: Saccharopolyspora rectivirgula.
Bacteria including actinomycetes are also present in high concentrations in inhalable and ‘total dust’ from biofuel (Madsen et al., 2004; Madsen, 2006), but the concentrations of bacteria in particulate matter (PM) with a D50 of 1 μm (PM1) dust have not been described. Spores of actinomycetes have diameters of ∼1 μm and spores <1.5 μm have been classified as bacteria (Table 1). Therefore, bacteria can be expected to be present in the PM1 dust. On the other hand, bacteria seem often to be aerosolized in clusters or associated with larger particles (Table 1). The aim of this study is to investigate whether and in which concentrations fungal components and bacteria are present in airborne PM1 dust.
MATERIALS AND METHODS
The biofuel plants
The investigation was carried out at 14 Danish biofuel plants. Straw was primarily used to generate energy at the biofuel plants but at one plant wood chips were also used. Measurements were performed for each plant in the winter season in 2004–2006 and each time over two or three successive working days. In the winter time some of the plants converted more straw than in the summer time. The sampling and measurement of numbers of particles were performed in the straw storage room 1.5 m above floor level. In straw storage rooms, people mainly worked with unloading of straw from trucks, measurement of water content of straw, and cleaning of the straw storage room and of the truck body. The employed at the biofuel plants (exclusive people only working in the office) worked on average 56% of their working days in straw storages and 12% of their working days in the boiler rooms. These people did not use any kind of dust protection masks. Earlier measurements show a high personal exposure to both fungi and bacteria in straw storage rooms (Madsen, 2006).
Sampling and extraction of PM1
Airborne particles (29 samples) were sampled using a Triplex cyclone (BGI, MA, USA) with a flow rate of 3.5 l min−1. The Triplex cyclone has a well-defined sharp penetration curve at a flow rate of 3.5 l min−1 with a cut point of 1 μm (D50) and ∼1% of particles with dae 1.7 μm penetrate the cyclone. On average, the Triplex cyclones were sampling for 6 h and 18 min day−1. The Triplex cyclone was fitted with a polycarbonate filter with pore size of 1.0. After sampling, the filters were transported carefully to the laboratory. The polycarbonate filters were carefully removed from the samplers 16 h after sampling. We did not see dust deposited on the walls of the cyclones and we have found no papers describing deposition of bioaerosol components on the walls in Triplex cyclones. Following, the dust was extracted in 5.0 ml sterile 0.05% Tween 80 and 0.85% NaCl aqueous solution by shaking for a 15-min period (500 r.p.m.) at room temperature. Parts of the suspensions were immediately used for cultivation of microorganisms and other parts stored at −80°C.
Sampling and extraction of total dust
The main purpose of this study was to characterize particles of PM1 size and not total dust but to be able to compare PM1 and total dust. Total dust was sampled in 20 of the areas where PM1 was sampled simultaneously. Total dust has been defined as the dust collected by a sampler with an entry velocity of 1.25 m s−1 (Kenny and Ogden, 2000); we sampled total dust using 25-mm closed-face cassettes (Millipore holder; Millipore, Bedford, MA, USA) with a 5.6-mm inlet and a flow of 1.9 l min−1 corresponding to an inlet velocity of 1.25 m s−1. On average, the total dust was sampled for 6 h and 36 min day−1. The samplers were fitted with polycarbonate filters (pore size 0.8 μm) for analysis of cultivable fungi, NAGase, and β-glucan. Polycarbonate filters are known to be non-hygroscopic. The polycarbonate filters were removed from the samplers 16 h after sampling. Following, the dust was extracted in 5.0 ml sterile 0.05% Tween 80 and 0.85 % NaCl aqueous solution by shaking for a 15-min period (500 r.p.m.) at room temperature. Parts of the suspensions were immediately used for cultivation of microorganisms and other parts stored at −80°C.
Number and size distribution of airborne particles
At each sample station a Grimm (used during 17 of the sampling days) or an aerodynamic particle sizer (used during 12 of the sampling days) measured particles of different sizes. On average, they were measuring for 6 h day−1. Particles with dae between 0.75 and 1.0 μm or between 0.487 and 0.523 μm were revealed.
Quantification of microorganisms and DNA/RNA-containing particles
Microorganisms in PM1 dust and fungi in total dust were quantified using a modified CAMNEA method (Palmgren et al., 1986). The numbers of fungi cultivable on dichloran glycerol agar (DG 18 agar; Oxoid, Basingstoke, UK) at 25 and 45°C were measured. Quantifications were made of the number of bacteria cultivable at 25°C on Nutrient agar (Oxoid) with cycloheximide (50 mg l−1) and of the number of mesophilic actinomycetes (25°C) and thermophilic actinomycetes (55°C) cultivable on, respectively, 10 and 100% nutrient agar with cycloheximide (50 mg l−1). Microorganisms were identified by macroscopic morphology studies and expressed in colony-forming units (CFU) per millilitre of liquid. Actinomycetes were distinguished from bacteria by morphology and by their more firm attachment to the growth medium. The detection limit was 5 CFU ml−1. The airborne concentrations of microorganisms were calculated from the airflow through the filters and were expressed as CFU per cubicmetre. Fungi in total dust were also quantified by cultivation on DG 18 agar. These data were only used to study the correlation between fungi in PM1 dust and total dust.
Acridine orange is a nucleic acid-selective fluorescent, cell-permeable dye which interacts with DNA and RNA. The PM1 dust samples were stained in 20 p.p.m. acridine orange (Difco, 212536, Maryland, MD, USA) in acetate buffer for 30 s, with subsequent filtration through a polycarbonate filter (25 mm, 0.4 μm; Nuclepore, Cambridge, MA, USA). After this particles of different sizes were counted at a magnification of ×1250 using epifluorescence microscopy (Orthoplan; Leitz Wetzlar, GMBH Wetzlar, Germany). Thus, the number of fungi (in the following called ‘total fungal spores’) and bacteria (in the following called ‘total bacteria’) were determined in 40 randomly chosen fields or until at least 400 cells were counted. In addition, particles stained with acridine orange and which were not fungal spores or bacteria were quantified in three different categories of DNA/RNA particle length: (i) between 0.5 and 1.5 μm, (ii) between 1.0 and 1.5 μm, and (iii) >1.5 μm. Bacteria and fungi were identified by shape and structure and by their size. Bacteria have a diameter <1.5 μm and fungal spores a diameter between 1.5 and 10 μm.
Quantification of (1 → 3)-β-D-glucans, NAGase, and β-glucosidase
Dust suspensions from the polycarbonate filters were used to quantify (1 → 3)-β-D-glucan (in the following called β-glucan) in duplicate using the kinetic Fungitic G Test (Seikagaku Co., Tokyo, Japan). The triple-helix structure of the β-glucan was made water soluble using 0.3 M NaOH (Fogelmark et al., 2001). The results were expressed in picogram per millilitre of liquid and the detection limit was 4 pg ml−1.
To quantify the activity of NAGase (EC3.2.1.30) and the cellulase β-glucosidase (EC 3.2.1.21) (at 37°C), release of p-nitrophenol from p-nitrophenol-N-acetyl-β-D-glucosaminide or p-nitrophenol-N-acetyl-β-D-glucopyranoside (Sigma–Aldrich Chemical, Steinheim, Germany) was quantified (Madsen, 2003). Controls were without either the enzyme or the substrate. The results were expressed in picomole per second per millilitre of liquid and the detection limit was 0.02 pmol s−1 ml−1. One unit of enzyme activity is defined as the amount of enzyme, which releases 1.0 μmol of p-nitro-phenol ml−1 enzyme min−1.
The airborne concentrations of these three components were calculated from the airflow through the filters and were expressed as units per cubicmetre.
Treatment of data
For correlation analysis, all data were log transformed, as they were log normally distributed. If the measured component was found in >75% of the samples, the value ‘50% of limit of detection (LOD) value’ was used for measurements below the LOD; if the measured component was found in between 20 and 75% of the samples, only measurements above the detection limit were included; and if the measured component was found in <20% of the samples, the component was not included in the correlation studies.
RESULTS
Airborne PM1
In general, airborne fungal spores have both a diameter as measured by microscopy and a dae >1.5 μm (Table 1), while the Triplex cyclone has a penetration cut point at 1 μm (D50). Cultivable mesophilic fungi and ‘total fungal spores’ were found in, respectively, 6 and 22 of the 29 samples. However, the median concentration of cultivable fungi was below detection level, and no thermotolerant fungi were found. β-Glucan and NAGase were found in all dust samples and thus also in samples with no fungal spores (Table 2). The median concentration of DNA particles >1.5 μm was 4403 m−3 and only a fraction (11%) of the number of these particles were identified as fungal spores. On average, 4 × 104 DNA- or RNA-containing particles between 0.5 and 1.5 μm were present in the air and almost 50% of these particles were bacteria. Hence, up to 6.2 × 105 ‘total bacteria’ m−3 were found in PM1 dust.
Table 2.
Components (unit) m−3 air of PM1 samples collected during working days at 14 biofuel plants
| Component | Unit | Average | Median | Interval | Positive samples/total samples |
| Fungi | CFU | 17 | Bd | Bd to 105 | 6/29 |
| Total fungal spores | Number | 639 | 497 | Bd to 2377 | 22/29 |
| Other DNA–RNA, l: >1.5 μm | Number | 5624 | 3906 | 600–18 659 | 29/29 |
| β-Glucan | ng | 5.6 | 3.5 | 0.68–27 | 29/29 |
| NAGase | pmol s−1 | 0.21 | 0.18 | 0.13–0.45 | 29/29 |
| Bacteria | CFU | 249 | 48 | Bd to 2995 | 21/29 |
| Thermophilic actinomycetes | CFU | 739 | 178 | Bd to 4492 | 23/29 |
| Mesophilic actinomycetes | CFU | 274 | 19 | Bd to 3414 | 17/29 |
| Total bacteria | Number | 1.8 × 105 | 0.31 × 105 | 780–6.2 × 105 | 29/29 |
| Other DNA–RNA, l: 0.5–1.5 μm | Number | 2.2 × 105 | 0.23 × 105 | 760–19 × 105 | 29/29 |
| β-Glucosidase | pmol s−1 | 0.16 | 0.15 | 0.099–0.36 | 29/29 |
| Particles dae 0.75–1.0 μm | Number | 164 × 105 | 50 × 105 | 13 × 105–1800 × 105 | 29/29 |
| Particles dae 0.487–0.523 μm | Number | 247 × 105 | 150 × 105 | 23 × 105–850 × 105 | 14/14 |
Bd, below detection level; l, length.
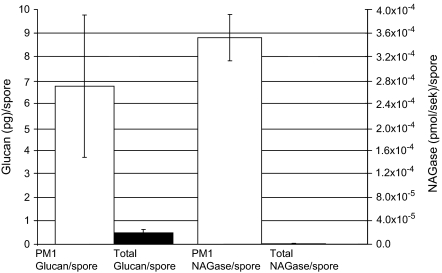
The median amount of β-glucan and NAGase activity per ‘total fungal spore’ was, respectively, 7 pg and 3.5 × 10−4 pmol s−1 (Fig. 1).
Fig. 1.
β-Glucan (pg)/‘total fungal spore’ in PM1 and total dust (1. y-axis) and NAGase (pmol s−1)/total fungal spore in PM1 and total dust (2. y-axis).
Correlations between components in PM1 dust
Significant correlations were found between factors mentioned in Table 3; factors not included in the table did not correlate significantly. The fungal components β-glucan and NAGase correlated significantly and the bacterial components such as CFU of bacteria and ‘total bacteria’ also correlated significantly.
Table 3.
Factors in the PM1 fraction correlating significantly
| β-Glucan | β-Glucosidase | Bacteria (CFU) | Thermophilic actinomycetes | Total bacteria | |
| β-Glucan | — | r = 0.79, P < 0.0001, n = 29 | |||
| NAGase | r = 0.83, P < 0.0001, n = 29 | r = 0.96, P < 0.0001, n = 29 | |||
| Bacteria (CFU) | — | r = 0.72, P = 0.0003, n = 29 | r = 0.73, P = 0.0012, n = 29 | ||
| Mesophilic actinomycetes | r = 0.98, P < 0.0001, n = 17 | r = 0.75, P < 0.0001, n = 17 |
r = Pearson's correlation coefficient, P = level of significance, after the Bonferoni correction only P values <0.05/12 = 0.0042 are significant. CFU of fungi was not included as cultivable fungi were only found in six samples.
Correlations between fungal components in total dust and components in PM1 dust
Significant correlations were found between factors mentioned in Table 4; factors not included in the table did not correlate significantly. Fungi (CFU) and β-glucan in total dust correlated with fungal components and with DNA- or RNA-containing particles in PM1 dust.
Table 4.
Factors in PM1 correlating significantly to CFU of fungi and β-glucan in total dust
| PM1 fraction |
||||||
| β-Glucan | Total fungi | NAGase | DNA–RNA L: 1–1.5 μm | DNA–RNA L: >1.5 μm | β-Glucosidase | |
| CFU of fungi in total dust | r = 0.79, P < 0.0001, n = 20 | r = 0.51, P < 0.004, n = 20 | r = 0.59, P = 0.010, n = 20 | r = 0.50, P = 0.016, n = 20 | r = 0.53, P < 0.0001, n = 20 | r = 0.54, P = 0.020, n = 20 |
| β-Glucan in total dust | r = 0.71, P < 0.0001, n = 20 | r = 0.49, P < 0.003, n = 20 | r = 0.62, P = 0.007, n = 20 | r = 0.56, P = 0.036, n = 20 | r = 0.55, P < 0.0001, n = 20 | r = 0.58, P = 0.008, n = 20 |
L = length, r = Pearson's correlation coefficient, P = level of significance, n = numbers of samples. CFU of fungi in PM1 dust was not included as cultivable fungi were only found in six samples.
The median amount of β-glucan and NAGase per ‘total fungal spore’ in total dust was, respectively, 0.5 pg and 7.2 × 10−7 pmol s−1 (Fig. 1).
DISCUSSION
In this study, we found high concentrations of β-glucan in PM1 dust (median 3.5 ng m−3) sampled from straw storage rooms where people were handling straw. We have found no other study measuring β-glucan in PM1 dust. The concentrations were higher than or at the same level as exposure to β-glucan in respirable dust at wood handling plants (0.1–1.76 ng m−3) (Alwis et al., 1999). As expected, the number of ‘total fungal spores’ was low in PM1 dust in comparison with what is earlier found in inhalable dust at biofuel plants (Madsen, 2006). Similarly, the number of both cultivable and ‘total bacteria’ and actinomycetes in PM1 dust was low in comparison with what is earlier found in inhalable dust at biofuel plants (Madsen, 2006). The dust sampling in this study was performed in the winter time where the outdoor exposure to bioaerosol is low. Earlier studies have shown higher exposure to microbial components at biofuel plants in spring than in autumn (Madsen, 2006) and storage of biofuels outdoors over summer can cause an increase in the microbiological dustiness of biofuels (Sebastian et al., 2006).
Airborne β-glucan and NAGase were found in PM1 samples where no cultivable fungi were present, and β-glucan and NAGase were found in higher concentrations per fungal spore in PM1 dust than in total dust. The concentrations of β-glucan, β-glucosidase, and NAGase correlated highly and significantly (Table 3). This indicates that they all mainly origin from the same fungal particles and that β-glucan is not mainly from bacteria or pollen. Together, this suggests that fungal particles smaller than fungal spore size are present in the air at the plants. Pure cultures of fungi have previously been seen to release particles smaller than spore size (Table 1). DNA or RNA has previously been observed in the small fragments released from fungal cultures (Madsen et al., 2005). We found many DNA- or RNA-containing particles of the size described to be released from fungal cultures. Whether these particles also contain the β-glucan measured in the same samples is not possible to conclude. However, it should be noted that CFU of fungi and β-glucan in total dust correlated positively and significantly with β-glucan and DNA–RNA particles in PM1 dust (Table 4). On the other hand, the concentrations of these RNA–DNA particles in PM1 dust did not correlate significantly with the amount of β-glucan in the same samples (Table 3).
Cultivable fungi were found in six of 29 PM1 samples; the cyclone has a sharp penetration curve and ∼1% of the particles with dae 1.7 μm penetrate the cyclone. Similarly, using the Anderson sampler in indoor environments Pastuszka et al. (2000) found between 1 and 100 CFU of fungi m−3 and Rautiala et al. (1996) found between 10 and 2000 CFU fungi m−3 in the size fraction 0.65–1.1 μm. Whether the cultivable fungi found in the six samples in this study and in the two indoor studies are from hyphae, spores, or other fungal components is not known.
β-Glucan has been used as an indicator of fungal exposure, but studies have also shown that it can elicit respiratory inflammation (Rylander, 1999; Thorn et al., 2001; Bonlokke et al., 2006) and systematic inflammation (Sigsgaard et al., 2000). A review concluded that health effects of β-glucans seem plausible, although the underlying inflammatory mechanisms associated with exposure are not clear (Douwes, 2005). Therefore, it is interesting that β-glucan is also present in PM1 dust.
Due to their small size, fungal fragments can stay in the air longer than fungal spores and can also penetrate and be deposited more deeply in the alveolar region when inhaled. By using simulation models, Cho et al. (2005) found that the respiratory deposition of Stachybotrys chartarum fragments was 230 times higher than that of spores for adults and even higher for infants. Additionally, air pollution studies have reported that adverse health outcomes, e.g. respiratory and cardiac responses, are associated with both number and mass of particles (Osunsanya et al., 2001), illustrating the importance of including small fungal fragments when assessing mould exposure.
We have measured a high exposure to ‘total bacteria’ (median = 3×104 m−3) with dae < 1 μm, even though cultivable bacteria are often reported as present in clusters or as associated with other particles (Table 1). Exposure to microorganisms present as particles with different dae has mainly been measured with the Anderson sampler, and using this sampler, only cultivable microorganisms are quantified. Exposure to airborne non-cultivable bacteria present as particles with dae < 1 μm should also be elucidated in other environments.
The median ratio of culturable to ‘total bacteria’ in the PM1 fraction in dust from the biofuel plants is 0.2%. This is much lower than seen in inhalable dust from biofuel plants, where 5% of the bacteria were cultivable (Madsen, 2006). This is probably because bacteria present as single or few cells in general are less protected from irradiation and drying than bacteria associated with larger particles or bacteria present in larger clusters. In support of the theory about protection from irradiation, studies of culturable bacteria in the atmosphere show a preponderance of large-sized culturable bacterial particles during clear days; the phenomenon is explained by a higher sunlight sensitivity of airborne bacteria in small particles (Tong and Lighthart, 1998).
The concentration of airborne cultivable actinomycetes was higher than the concentration of other cultivable bacteria. In contrast ‘total’ and inhalable dust from straw or a straw-converting biofuel plant contains a higher number of cultivable bacteria than cultivable actinomycetes (Madsen et al., 2006). This difference between ‘total’ and inhalable dust on the one hand and PM1 dust on the other hand is probably because a larger fraction of the numbers of spores of actinomycetes than of the bacteria are present as single spores. Furthermore, spores of actinomycetes can be smaller than bacteria, so they are present more often in the PM1 fraction.
CONCLUSIONS
Airborne β-glucan and NAGase were found in PM1 samples where no cultivable fungi were present, and β-glucan and NAGase were found in higher concentrations per fungal spore in PM1 dust than in total dust. Furthermore, the number of cultivable fungi and the amount of β-glucan in the total dust correlated significantly with the number of DNA/RNA-containing particles. A high number of DNA/RNA-containing particles not identified as bacteria or fungal spores were found. Together, this indicates that fungal particles smaller than fungal spore size are present in the air at the biofuel plants. The median exposure to β-glucan (PM1) was 3.5 ng m−3 and may therefore be an important exposure.
A median exposure to ‘total bacteria’ in PM1 dust on 3 × 104 m−3 was measured. The concentration of airborne cultivable actinomycetes was higher than the concentration of other cultivable bacteria. Hence, the exposure to total bacteria and actinomycetes in PM1 dust is considerable. The median ratio of culturable to total bacteria in the PM1 fraction was only 0.2%, showing low viability of bacteria in PM1 aerosols.
FUNDING
PSO-ELTRA (5786).
Acknowledgments
Special thanks to Signe H. Nielsen and Pernille Salvarli at the National Research Centre for the Working Environment for specialist technical assistance.
References
- Allermann L, Wilkins CK, Madsen AM. Inflammatory potency of dust from the indoor environment and correlation to content of NAGase and fungi. Toxicol In Vitro. 2006;20:1522–31. doi: 10.1016/j.tiv.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Alwis KU, Mandryk J, Hocking AD. Exposure to biohazards in wood dust: bacteria, fungi, endotoxins, and (1–’3)-β-D-glucans. Appl Occup Environ Hyg. 1999;14:598–608. doi: 10.1080/104732299302404. [DOI] [PubMed] [Google Scholar]
- Baur X. Enzymes as occupational and environmental respiratory sensitisers. Int Arch Occup Environ Health. 2005;78:279–86. doi: 10.1007/s00420-004-0590-6. [DOI] [PubMed] [Google Scholar]
- Bayer EA, Chanzy H, Lamed R, et al. Cellulose, cellulases and cellulosomes. Curr Opin Struct Biol. 1998;8:548–57. doi: 10.1016/s0959-440x(98)80143-7. [DOI] [PubMed] [Google Scholar]
- Bonlokke JH, Stridh G, Sigsgaard T, et al. Upper-airway inflammation in relation to dust spiked with aldehydes or glucan. Scand J Work Environ Health. 2006;32:374–82. doi: 10.5271/sjweh.1033. [DOI] [PubMed] [Google Scholar]
- Bovallius A, Bucht B, Roffey R, et al. Three-year investigation of the natural airborne bacterial flora at four localities in Sweden. Appl Environ Microbiol. 1978;35:847–52. doi: 10.1128/aem.35.5.847-852.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Seo SC, Schmechel D, et al. Aerodynamic characteristics and respiratory deposition of fungal fragments. Atmos Environ. 2005;39:5454–65. [Google Scholar]
- Claeyssens M, Aerts G. Characterisation of cellulolytic activities in commercial Trichoderma reesei preparations: an approach using small, chromogenic substrates. Bioresour Technol. 1992;39:143–6. [Google Scholar]
- Davis ME, Smith TJ, Laden F, et al. Modeling particle exposure in US trucking terminals. Environ Sci Technol. 2006;40:4226–32. doi: 10.1021/es052477m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Zeiger RS, Seltzer JM, et al. The effect of outdoor fungal spore concentrations on daily asthma severity. Environ Health Perspect. 1997;105:622–35. doi: 10.1289/ehp.97105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domsch KH, Gams W, Anderson T-H. Compendium of soil fungi. Regensburg, Germany: IHW-Verlag; 1993. [Google Scholar]
- Douwes J. (1-’3)-β-D-glucans and respiratory health: a review of the scientific evidence. Indoor Air. 2005;15:160–9. doi: 10.1111/j.1600-0668.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- Douwes J, Thorne P, Pearce N, et al. Bioaerosol health effects and exposure assessment: progress and prospects. Ann Occup Hyg. 2003;47:187–200. doi: 10.1093/annhyg/meg032. [DOI] [PubMed] [Google Scholar]
- Draborg H, Kauppinen S, Dalboge H, et al. Molecular cloning and expression in S. cerevisiae of two exochitinases from Trichoderma harzianum. Biochem Mol Biol Int. 1995;36:781–91. [PubMed] [Google Scholar]
- Eduard W. Fungal spores. The Nordic Group for criteria documentation of health risks from chemicals. Oslo, Norway: Arbetslivsinstitutet; 2006. [Google Scholar]
- Eduard W, Blomquist G, Nielsen BH, et al. Recognition errors in the quantification of microorganisms by fluorescence microscopy. Ann Occup Hyg. 2001;45:493–8. [PubMed] [Google Scholar]
- Eduard W, Lacey J, Karlsson K, et al. Evaluation of methods for enumerating microorganisms in filter samples from highly contaminated occupational environments. Am Ind Hyg Assoc J. 1990;51:427–36. doi: 10.1080/15298669091369899. [DOI] [PubMed] [Google Scholar]
- Eriksson KE. Cellulases of fungi. Basic Life Sci. 1981;18:19–32. doi: 10.1007/978-1-4684-3980-9_3. [DOI] [PubMed] [Google Scholar]
- Fogelmark B, Thorn J, Rylander R. Inhalation of (1–>3)-beta-D-glucan causes airway eosinophilia. Mediat Inflamm. 2001;10:13–9. doi: 10.1080/09629350123707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny RL, Reponen T, Willeke K, et al. Fungal fragments as indoor air biocontaminants. Appl Environ Microbiol. 2002;68:3522–31. doi: 10.1128/AEM.68.7.3522-3531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BJ, Sercombe JK, Tovey ER. Fungal fragments and undocumented conidia function as new aeroallergen source. J Allergy Clin Immunol. 2005;115:1043–8. doi: 10.1016/j.jaci.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Gussman RA, Kenny LC. Design and calibration of a cyclone for PM-1 ambient air sampling. J Aerosol Sci. 2000;31:194–5. [Google Scholar]
- Halstensen AS, Nordby K-C, Wouters IM, et al. Determinants of microbial exposure in grain farming. Ann Occup Hyg. 2007;51:581–92. doi: 10.1093/annhyg/mem038. [DOI] [PubMed] [Google Scholar]
- Hearn VM, Escott GM, Glynn E, et al. Complex chitinolytic systems of Aspergillus fumigatus. Microbios. 1998;93:85–104. [PubMed] [Google Scholar]
- Jeffrey P, Griffin P, Gibson M, et al. Small bakeries—a cross-sectional study of respiratory symptoms, sensitization and dust exposure. Occup Med (Lond) 1999;49:237–41. doi: 10.1093/occmed/49.4.237. [DOI] [PubMed] [Google Scholar]
- Kenny LC, Ogden TL. Twenty-five years of inhalable dust. Ann Occup Hyg. 2000;44:561–3. doi: 10.1093/annhyg/44.8.561. [DOI] [PubMed] [Google Scholar]
- Kildesø J, Wurtz H, Nielsen KF, et al. Determination of fungal spore release from wet building materials. Indoor Air. 2003;13:148–55. doi: 10.1034/j.1600-0668.2003.00172.x. [DOI] [PubMed] [Google Scholar]
- Lacey J. Genus Micropolyspora Lechevalier, Solotorovsky and McDurmont 1961. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG, editors. Bergey’s manual of systematic bacteriology. Williams & Wilkins; 1986. pp. 1496–501. [Google Scholar]
- Lacey J. Thermoactinomyces. In: Williams ST, Sharpe ME, Holt JG, editors. Bergey’s manual of systematic bacteriology. Williams & Wilkins; 1989. pp. 2573–85. [Google Scholar]
- Lacey J, Dutkiewicz J. Isolation of actinomycetes and fungi from mouldy hay using a sedimentation chamber. J Appl Bacteriol. 1976;41:315–9. doi: 10.1111/j.1365-2672.1976.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Lahoz R, Reyes F, Perez Leblic MI. Lytic enzymes in the autolysis of filamentous fungi. Mycopathologia. 1976;60:45–9. doi: 10.1007/BF00442547. [DOI] [PubMed] [Google Scholar]
- Laitinen S, Kangas J, Kotimaa M, et al. Workers’ exposure to airborne bacteria and endotoxins at industrial wastewater treatment plants. Am Ind Hyg Assoc J. 1994;55:1055–60. doi: 10.1080/15428119491018330. [DOI] [PubMed] [Google Scholar]
- Levetin E. Fungi. In: Burge HA, editor. Bioaerosols. Lewis Publishers; 1995. pp. 87–120. [Google Scholar]
- Li D-W, Kendrick B. A year-round comparison of fungal spores in indoor and outdoor air. Mycologia. 1995;87:190–5. [Google Scholar]
- Lighthart B, Shaffer BT, Marthi B, et al. Artificial wind-gust liberation of microbial bioaerosols previously deposited on plants. Aerobiologia. 1993;9:189–96. [Google Scholar]
- Lin WH, Li CS. Size characteristics of fungus allergens in the subtropical climate. Aerosol Sci Technol. 1996;25:93–100. [Google Scholar]
- Losada E, Hinojosa M, Moneo I, et al. Occupational asthma caused by cellulase. J Allergy Clin Immunol. 1986;77:635–9. doi: 10.1016/0091-6749(86)90358-1. [DOI] [PubMed] [Google Scholar]
- Lundholm IM. Comparison of methods for quantitative determination of airborne bacteria and evaluation of total viable counts. Appl Environ Microbiol. 1982;44:179–83. doi: 10.1128/aem.44.1.179-183.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm M, Rylander R. Work related symptoms among sewage workers. Br J Ind Med. 1983;40:325–9. doi: 10.1136/oem.40.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelin TM, Johnson HE. Fungal and actinomycete spore aerosols measured at different humidities with an aerodynamic particle sizer. J Appl Bacteriol. 1992;72:400–9. doi: 10.1111/j.1365-2672.1992.tb01853.x. [DOI] [PubMed] [Google Scholar]
- Madsen AM. NAGase activity in airborne biomass dust and relationship between NAGase concentrations and fungal spores. Aerobiologia. 2003;19:97–105. [Google Scholar]
- Madsen AM. Exposure to airborne microbial components in autumn and spring during work at Danish biofuel plants. Ann Occup Hyg. 2006;50:821–31. doi: 10.1093/annhyg/mel052. [DOI] [PubMed] [Google Scholar]
- Madsen AM, Kruse P, Schneider T. Characterization of microbial particle release from biomass and building material surfaces for inhalation exposure risk assessment. Ann Occup Hyg. 2006;50:175–87. doi: 10.1093/annhyg/mei057. [DOI] [PubMed] [Google Scholar]
- Madsen AM, Mårtensson L, Schneider T, et al. Microbial dustiness and particle release of different biofuels. Ann Occup Hyg. 2004;48:327–38. doi: 10.1093/annhyg/meh016. [DOI] [PubMed] [Google Scholar]
- Madsen AM, Saber AT, Nordly P, et al. Inflammation but no DNA (deoxyribonucleic acid) damage in mice exposed to airborne dust from a biofuel plant. Scand J Work Environ Health. 2008;34:278–87. doi: 10.5271/sjweh.1272. [DOI] [PubMed] [Google Scholar]
- Madsen AM, Wilkins CK, Poulsen OM. Micro-particles from fungi. In: Johanning Eckardt., editor. Bioaerosols, fungi, bacteria, mycotoxins and human health: patho-physiology, clinical effects, exposure assessment, prevention and control in indoor environments and work. New York: 2005. Albany, NY: Fungal Research Group Foundation, Inc; 2005. pp. 276–91. [Google Scholar]
- Madsen AM, Würtz H. Fungal enzymes in indoor dust. In: Johanning Eckardt., editor. Bioaerosols, fungi, bacteria, mycotoxins and human health. New York: Fungal Research Group Foundation, Inc; 2005. pp. 384–91. [Google Scholar]
- Manjula K, Podile AR. Production of fungal cell wall degrading enzymes by a biocontrol strain of Bacillus subtilis AF 1. Indian J Exp Biol. 2005;43:892–6. [PubMed] [Google Scholar]
- Meklin T, Reponen T, Toivola M, et al. Size distributions of airborne microbes in moisture-damaged and reference school buildings of two construction types. Atmos Environ. 2002;36:6031–9. [Google Scholar]
- Osunsanya T, Prescott G, Seaton A. Acute respiratory effects of particles: mass or number? Occup Environ Med. 2001;58:154–9. doi: 10.1136/oem.58.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren U, Ström G, Blomquist G, et al. Collection of airborne micro-organisms on Nuclepore filters, estimation and analysis-CAMNEA method. J Appl Bacteriol. 1986;61:401–6. doi: 10.1111/j.1365-2672.1986.tb04303.x. [DOI] [PubMed] [Google Scholar]
- Pastuszka JS, Paw UKT, Lis DO, et al. Bacterial and fungal aerosol in indoor environment in Upper Silesia, Poland. Atmos Environ. 2000;34:3833–42. [Google Scholar]
- Rautiala S, Reponen T, Hyvärinen A, et al. Exposure to airborne microbes during the repair of moldy buildings. Am Ind Hyg Assoc J. 1996;57:279–84. doi: 10.1080/15428119691015016. [DOI] [PubMed] [Google Scholar]
- Robinson BW, Venaille TJ, Mendis AH, et al. Allergens as proteases: an Aspergillus fumigatus proteinase directly induces human epithelial cell detachment. J Allergy Clin Immunol. 1990;86:726–31. doi: 10.1016/s0091-6749(05)80176-9. [DOI] [PubMed] [Google Scholar]
- Rylander R. Indoor air-related effects and airborne (1 –> 3)-beta-D-glucan. Environ Health Perspect. 1999;107(Suppl 3):501–3. doi: 10.1289/ehp.99107s3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian A, Madsen AM, Mårtensson L, et al. Assessment of microbial exposure risks from handling of biofuel wood chips and straw—effect of outdoor storage. Ann Agric Environ Med. 2006;13:139–45. [PubMed] [Google Scholar]
- Shaffer BT, Lighthart B. Survey of culturable airborne bacteria at four diverse locations in Oregon: urban, forest, and coastal. Microb Ecol. 1997;34:167–77. doi: 10.1007/s002489900046. [DOI] [PubMed] [Google Scholar]
- Shi Y, Jiang Z, Han P, et al. Purification and some properties of beta-N-acetyl-D-glucosaminidase from the cabbage butterfly (Pieris rapae) Biochimie. 2007;89:347–54. doi: 10.1016/j.biochi.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Sigsgaard T, Bonefeld-Jorgensen EC, Kjaergaard SK, et al. Cytokine release from the nasal mucosa and whole blood after experimental exposures to organic dusts. Eur Respir J. 2000;16:140–5. doi: 10.1034/j.1399-3003.2000.16a25.x. [DOI] [PubMed] [Google Scholar]
- Sorenson WG, Frazer DG, Jarvis BB, et al. Trichothecene mycotoxins in aerosolized conidia of Stachybotrys atra. Appl Environ Microbiol. 1987;53:1370–5. doi: 10.1128/aem.53.6.1370-1375.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn J, Beijer L, Rylander R. Effects after inhalation of (1–>3)-beta-D-glucan in healthy humans. Mediat Inflamm. 2001;10:173–8. doi: 10.1080/09629350124119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong YY, Lighthart B. Effect of simulated solar radiation on mixed outdoor atmospheric bacterial populations. FEMS Microbiol Ecol. 1998;26:311–6. [Google Scholar]
- Yeo HG, Kim JH. SPM and fungal spores in the ambient air of west Korea during the Asian dust (Yellow sand) period. Atmos Environ. 2002;36:5437–42. [Google Scholar]
- Zhang JP, Yan YW, Xie JJ, et al. Inhibitory kinetics of phenol on the enzyme activity of beta-N-acetyl-D-glucosaminidase from green crab (Scylla serrata) Int J Biol Macromol. 2007;40:139–43. doi: 10.1016/j.ijbiomac.2006.06.022. [DOI] [PubMed] [Google Scholar]