Abstract
We conducted a quantitative dermal and inhalation exposure assessment of monomeric and polymeric 1,6-hexamethylene diisocyanates (HDI) in 47 automotive spray painters from North Carolina and Washington State. We report here the use of linear mixed modeling (LMM) to identify the primary determinants of dermal exposure. Dermal concentrations of HDI, uretidone, biuret, and isocyanurate were significantly higher in 15 painters who did not wear coveralls or gloves (N = 51 paint tasks) than in 32 painters who did wear coveralls and gloves (N = 192 paint tasks) during spray painting. Regardless of whether protective clothing was worn, isocyanurate was the predominant species measured in the skin [geometric mean (GM) = 33.8 ng mm−3], with a 95% detection rate. Other polyisocyanates (GM ≤ 0.17 ng mm−3) were detected in skin during <23% of the paint tasks. According to marginal R2 statistics, mixed models generated in this study described no <36% of the variability in dermal concentrations of the different polyisocyanates measured in painters who did not wear protective clothing. These models also described 55% of the variability in dermal concentrations of isocyanurate measured in all painters (N = 288 paint tasks). The product of analyte-specific breathing-zone concentration (BZC) and paint time was the most significant variable in all the models. Through LMM, a better understanding of the exposure pathways governing individual polyisocyanate exposures may be achieved. In particular, we were able to establish a link between BZC and dermal concentration, which may be useful for exposure reconstruction and quantitatively characterizing the protective effect of coveralls and gloves. This information can be used to reduce dermal exposures and better protect automotive spray painters from potential adverse health effects.
Keywords: dermal exposure, exposure determinants, hexamethylene diisocyanate, isocyanate, statistical modeling
INTRODUCTION
Automotive coatings are often based on polyisocyanates of 1,6-hexamethylene diisocyanate (HDI), consisting of trace amounts of HDI monomer and higher amounts of HDI oligomers (e.g. uretidone, biuret, and isocyanurate) (Janko et al., 1992; Sparer et al., 2004; Fent et al., 2009). During spray painting, most of the paint droplets produced by the spray gun land on the surface of the automobile to form a polyurethane coating. However, some of the droplets are captured by the airflow around the surface and become airborne. This ‘overspray’ forms a paint mist that is likely to contain unreacted polyisocyanates. In addition to inhalation exposure to HDI, the potential for dermal exposure exists as polyisocyanate particles and vapor in the overspray may contact the skin via deposition. Even when protective clothing (i.e. coveralls and gloves) is worn, polyisocyanates may break through the protective barriers and contact the skin (Pronk et al., 2006b; Liu et al., 2007; Bello et al., 2008).
Diisocyanates are considered a leading cause of occupational asthma in exposed workers (Chan-Yeung and Malo, 1995; Bernstein, 1996). Although the mechanism of diisocyanate-induced asthma is not well understood, the inhalation route has been considered the primary route of exposure leading to respiratory sensitization. Hence, most investigations have focused on studying inhalation exposure to polyisocyanates (Janko et al., 1992; Lesage et al., 1992; Rudzinski et al., 1995; Sparer et al., 2004; Pronk et al., 2006a). However, the dermal route has received increased attention. Animal studies have shown that topical exposure to diisocyanates can result in respiratory sensitization (Karol et al., 1981; Rattray et al., 1994; Herrick et al., 2002), while case studies and epidemiology studies have shown associations between dermal exposure to diisocyanates (i.e. methylene bisphenyl isocyanate) and occupational asthma (Petsonk et al., 2000; Donnelly et al., 2004; Redlich and Herrick, 2008).
Despite increasing evidence that dermal exposure to diisocyanates may play a role in the development of respiratory sensitization and occupational asthma, very few studies have been conducted to measure dermal exposure to diisocyanates. Of the exposure assessments that have been performed, some used colormetric wipes to determine exposure qualitatively (Liu et al., 2000; Liu et al., 2007) or used gloves as a surrogate measure for exposure loading on the skin (Pronk et al., 2006b). Recently, a few methods have been developed to measure unbound polyisocyanates in the skin. Bello et al. (2008) developed a wipe sampling method for quantifying dermal exposure to total reactive isocyanate groups (TRIGs) and Fent et al. (2008) developed a tape-strip method for quantifying dermal concentrations of individual polyisocyanates (i.e. HDI monomer, uretidone, biuret, and isocyanurate). Although both methods were shown to effectively estimate dermal exposure to polyisocyanates in automotive spray painters, the specificity of the tape-strip method allows investigators to also explore differences among the various polyisocyanates species present in automotive paint formulations. This specificity is important because polyisocyanates may differ in their toxicities (Vandenplas et al., 1992; Vandenplas et al., 1993) and abilities to penetrate biological barriers (Pauluhn and Lewalter, 2002; Bello et al., 2006).
Because of the limited amount of reliable quantitative data, there have been few efforts to model dermal exposure to polyisocyanates. When quantitative data are available, statistical methods can be used to identify the primary determinants of dermal exposure in a given occupational setting. For example, Fent et al. (2008) demonstrated that the product of breathing-zone concentration (BZC) and paint time can be used to describe the variability of dermal concentration in painters who did not wear protective clothing.
The objectives of this study were to (i) quantify dermal concentrations of HDI monomer and oligomers (i.e. uretidone, biuret, and isocyanurate) during automotive spray painting using a tape-strip method (Fent et al., 2009) and (ii) use linear mixed modeling (LMM) (Laird and Ware, 1982) to describe the variability in the dermal concentration estimates. In Part I of this series, we demonstrated the usefulness of LMM for evaluating the fixed effects of covariates on BZCs of individual polyisocyanates (Fent et al., 2009). The LMM models we developed in this study aid in (i) understanding the dermal exposure pathways, (ii) identifying the most effective control interventions for reducing dermal concentrations, and (iii) reconstructing dermal concentrations in unsampled automotive painter populations.
METHODS
Recruitment of painters
The protocol used to recruit automotive painters for study participation in central North Carolina (NC) and the Puget Sound area of Washington (WA) State is described in Part I of this series (Fent et al., 2009). A total of 47 painters (15 from NC and 32 from WA) participated in the study. In order to assess their exposures, painters were visited three times over a 1-year period, with visits at least 1 month apart. Due to attrition, 20 of the 47 painters were visited fewer than three times.
Data collection and analysis
Levels of monomeric and polymeric HDI in the skin were measured after each paint task using a previously described tape-strip sampling method (Fent et al., 2008). The tape-strip samples (4 × 2.5 cm2) were collected from six different 10 cm2 sites of the skin. The sites we sampled (i.e. sampling scheme) depended on whether or not protective clothing was worn (Table 1). This was done to ensure that exposed skin was adequately sampled; however, as a result, the potential for selection bias exists.
Table 1.
Number of sampled paint tasks by Sampling scheme and type of protective clothing worn by the painters
| Sampling schemea | Gloves only | Coveralls only | Both gloves and coveralls | No protective clothing | Total number of paint tasks |
| Volar and dorsal arm, dorsal hand | 23 | 0 | 30 | 40 | 93 |
| Volar arm, dorsal hand, neck | 1 | 4 | 65 | 3 | 73 |
| Volar arm, dorsal wrist, neck | 2 | 0 | 34 | 0 | 36 |
| Volar and dorsal arm, neck | 10 | 0 | 11 | 0 | 22 |
| Volar arm, dorsal hand, dorsal wrist | 0 | 2 | 15 | 2 | 19 |
| Dorsal hand, dorsal wrist, neck | 0 | 3 | 12 | 2 | 17 |
| Volar and dorsal arm, wrist | 0 | 2 | 6 | 2 | 10 |
| Volar arm, dorsal hand, face | 0 | 0 | 9 | 0 | 9 |
| Volar and dorsal arm, face | 1 | 2 | 1 | 1 | 5 |
| Volar and dorsal arm, lower leg | 3 | 0 | 0 | 0 | 3 |
| Dorsal hand, dorsal wrist, face | 0 | 0 | 2 | 0 | 2 |
| Volar arm, dorsal wrist, face | 0 | 0 | 2 | 0 | 2 |
| Right and left volar arm, right and left neck, right dorsal wrist, left dorsal hand | 0 | 0 | 2 | 0 | 2 |
| Volar arm, neck, leg | 0 | 0 | 1 | 0 | 1 |
| Right and left volar arm, right and left dorsal wrist, right dorsal hand, left dorsal arm | 0 | 0 | 1 | 0 | 1 |
| Volar and dorsal wrist, neck | 0 | 0 | 1 | 0 | 1 |
| Total | 40 | 13 | 192 | 50 | 296 |
Unless otherwise indicated, the left and right side of each body part was sampled.
A total of three successive tape-strip samples were collected from each site of the skin to ensure adequate collection of exposed corneocytes. Dermal exposure (ng cm−2) to each sampled site was estimated by summing the polyisocyanate levels measured in three successive tape-strips. However, subsequent tape-strips were excluded if the previous tape-strip collected levels below the limit of detection. For each subject, the regional surface areas were estimated using the Haycock's formula (Haycock et al., 1978) in conjunction with the Berkow chart (Deitch, 2008). The Haycock's formula calculates the total body surface area of skin based on the weight and height of the person, while the Berkow chart provides estimates of the surface area contribution from each body part. Using this approach, we calculated average surface areas for the lower arms, hands, and lower legs of 0.127, 0.105, and 0.296 m2, respectively. These values are within the ranges reported for forearms (0.0945–0.136 m2), hands (0.0596–0.113 m2), and lower legs (0.093–0.296 m2) of adult males in the Exposure Factors Handbook (U.S. EPA, 1997). However, the calculated average surface area of lower legs was in the very upper range of the respective estimates in the Exposure Factors Handbook. It is possible that lower legs are defined differently in the Berkow chart than they are in the Exposure Factors Handbook. Because values were not given in the Berkow chart for the wrist and face, the surface area contribution from these body parts were estimated by measuring the surface areas of the investigators’ wrists and faces and then comparing them to the surface areas of the lower arms (for which the Berkow chart does provide a value). According to this procedure, the wrists and face contribute to ∼1 and 2% of the total surface area of the skin, respectively.
The total mass of exposure (referred to as whole-body dermal concentration hereon) collected from each body part was calculated by multiplying the point measurements (ng cm−2) and regional surface area estimates (cm2) from the sampled body parts. Unsampled regions were assumed to have received no exposure. Measurements taken from the legs were not included in this calculation due to the limited number of measurements. The mass of exposure to each body part was then summed and divided by the total body surface area of the skin (ng cm−2). Lastly, because three successive tape-strip samples are considered to remove a volume of skin ∼1 μm in thickness (Fent et al., 2008), dermal exposure was reported as a concentration (ng mm−3) in the skin, which is consistent with the proposed nomenclature (Zartarian et al., 2005). In addition, the dominant mechanism of chemical transport across the stratum corneum is the diffusion of chemicals driven by a concentration gradient within the skin (Kubota and Ishizaki, 1986; Tojo, 1987; Reddy et al., 1998; George et al., 2004). Thus, this approach provides a biologically relevant measure of exposure and allows comparison between dermal exposure levels measured in experimental animal and human studies as well as occupational field studies. It should be noted that the actual thickness of skin collected with three successive tape-strips is likely to vary somewhat according to region of the skin (Rougier et al., 1987) or other factors, such as the presence of sweat, body hair, or furrows in the skin (van der Molen et al., 1997).
Personal one-stage (N = 98) and two-stage (N = 198) sampling was performed in the breathing zone of spray painters during each paint task as described in Part I of this series (Fent et al., 2009). Greater than one air sample and one set of corresponding tape-strip samples were collected from all but two painters, both of whom painted inside crossdraft booths.
Data were collected from the painters and their work environments for use as potential covariates in LMM. Data on booth type, gun type, airflow, temperature, and humidity are provided in Part I of this series (Fent et al., 2009). Other variables considered in the statistical analysis are described in Table 2. Selection of variables was based on our previous finding that the variability of dermal concentration in painters not wearing coveralls and gloves can be described using the product of BZC and paint time, where both the outcome and predictor are log transformed (Fent et al., 2008). In the following conceptual model, dermal concentration represents the exposure outcome, BZC represents the intensity of exposure surrounding the painter, and paint time represents the duration of exposure:
Table 2.
Summary of variables used to model dermal concentrations of monomeric and polymeric HDI
| Type | Name | Description | Range of values | Mean value | Median value |
| Continuous | BZC (HDI) × paint timea | Product of HDI BZC (μg m−3) and paint time (min) | 0.0038–1480 | 89.6 | 34.5 |
| BZC (uretidone) × paint timea | Product of uretidone BZC (μg m−3) and paint time (min) | 0.0007–9740 | 373 | 7.70 | |
| BZC (biuret) × paint timea | Product of biuret BZC (μg m−3) and paint time (min) | 0.019–26 400 | 1060 | 34.4 | |
| BZC (isocyanurate) × paint timea | Product of isocyanurate BZC (μg m−3) and paint time (min) | 0.56–582 000 | 26 300 | 12 100 | |
| Dichotomous | Coveralls | Were coveralls worn during spray painting? | 1: yes; 0: no | 0.70 | 1 |
| Coveralls old | Were the coveralls used for more than 8 weeks? | 1: yes; 0: no | 0.25 | 0 | |
| Coveralls nylpoly | Was the coverall material a nylon/polyester blend? | 1: yes; 0: no | 0.39 | 0 | |
| Coveralls poly | Was the coverall material polyester? | 1: yes; 0: no | 0.071 | 0 | |
| Coveralls polycot | Was the coverall material a polyester/cotton blend? | 1: yes; 0: no | 0.16 | 0 | |
| Gloves | Were gloves worn during spray painting? | 1: yes; 0: no | 0.78 | 1 | |
| Gloves nitrile | Was the glove material nitrile (as opposed to latex)? | 1: yes; 0: no | 0.38 | 0 | |
| Gloves thick | Were the gloves thick (i.e. > 0.13 mm)? | 1: yes; 0: no | 0.38 | 0 | |
| Hood | Was a hood or neck covering worn during spray painting? | 1: yes; 0: no | 0.28 | 0 |
Selected based on a previous finding relating dermal concentrations of HDI and isocyanurate in painters not wearing protective clothing to the products of respective BZCs and paint time. Note that HDI, uretidone, biuret, and isocyanurate were non-detectable in 9.7, 61, 45, and 1.0% of all air samples, respectively. Multiple imputation (n = 10 imputed data sets) was used to impute BZCs below detection limits.
Using this conceptual model as the framework, protective clothing would provide a barrier to aerosol deposition or vapor absorption. The protective effect of coveralls, gloves, and hood (i.e. protective neck covering) may depend on a number of factors, including material type, age, and thickness. Consequently, statistical modeling may provide a way to estimate the effectiveness of protective clothing commonly used in the automotive refinishing industry.
Statistical analysis
The data were analyzed using SAS 9.1 statistical software (Cary, NC, USA). Due to a relatively high fraction of non-detectable levels of HDI, uretidone, and biuret (>64%) in dermal samples and of uretidone and biuret (>45%) in air samples, multiple imputation (n = 10 imputed data sets) was used to impute exposure data below detection limits. Several authors have considered imputation from truncated normal distributions, including Lubin et al. (2004). Our use of a multivariate version of these methods allowed us to control for correlations among like exposures as well as within-subject correlations. The logarithmic transformation was taken for all the BZCs and dermal exposure estimates. In order to account for correlations in the multivariate exposure data, we imputed from truncated multivariate normal distributions, with truncation at the limit of detection. After imputation, all the data were transformed back to their original scale to allow for additional computations (i.e. calculating whole-body dermal concentrations, etc.).
SAS PROC MIANALYZE was used to combine the results of the analyses carried out on the 10 imputed data sets and to obtain valid estimates and statistical inferences. Averages were computed where PROC MIANALYZE could not be used (i.e. computing W-statistics for Shapiro Wilks tests for normality and marginal R2 statistics). Whole-body dermal concentrations and the products of BZC and paint time for each polyisocyanate (following multiple imputation) were approximately log normally distributed (Shapiro Wilks W ≥ 0.93). The covariates used for LMM were evaluated for potential collinearity by examining the Spearman correlation coefficients among the pairs of covariates. None of the variables exceeded our criterion for high correlation (i.e. r > 0.70).
LMM (PROC MIXED) was used to investigate the relative influences of fixed effects representing booth type and covariates on dermal concentrations of monomeric and polymeric HDI, while accounting for the random effects due to each individual painter and visit day. The general form of the mixed model and assumptions for these data are provided in Part I of this series (Fent et al., 2009).
Candidate covariates were selected by analyzing separate models that considered individual covariates and the products of BZC and paint time. From these models, those variables with P-values of <0.15 were used to obtain final models. As in Part I of this series (Fent et al., 2009), transformed residuals and Mahalanobis distance were examined to assess model fit and a marginal R2 statistic proposed by Vonesh and Chinchilli (1997) was used to estimate explained variation from the fixed effects. The diagnostic measures did not identify excessive outliers or problematic observations.
RESULTS
Summary statistics
Body region estimates (ng cm−2) of dermal exposure to monomeric and polymeric HDI are summarized in Table 3 for each sampled region by use of protective clothing. Because the data are positively skewed and contain a large percentage of non-detects, geometric mean (GM) and geometric standard deviation (GSD) best describe central tendency and scatter, respectively. As expected, higher GM levels were collected from unprotected skin than protected skin. These differences were not significant due to the high variability in measurements. The highest GM levels were collected from the legs for all the analytes except uretidone. This finding may be due to the fact that legs were only sampled if they were uncovered during spraying. For this reason and because of the limited number of measurements, exposure to the legs was not included in the whole-body dermal concentration calculations.
Table 3.
Dermal exposurea to monomeric and polymeric HDI (ng cm−2) by the sampled body region and use of protective clothing
| Sampled regionb | Contribution to total body surface areac (%) | No. measurementsd | HDI |
Uretidone |
Biuret |
Isocyanurate |
||||||||
| No. non-detectse | GM | GSD | No. non-detectse | GM | GSD | No. non-detectse | GM | GSD | No. non-detectse | GM | GSD | |||
| Lower arms | 6 | 820 | 634 | 0.03 | 30.9 | 721 | 0.10 | 65.1 | 780 | 0.08 | 37.8 | 117 | 27.7 | 12.0 |
| Coveralls | 6 | 488 | 447 | 0.01 | 27.3 | 477 | 0.03 | 50.0 | 478 | 0.03 | 26.3 | 104 | 8.76 | 7.95 |
| No coveralls | 6 | 332 | 187 | 0.11 | 14.8 | 244 | 0.51 | 20.1 | 302 | 0.29 | 25.1 | 13 | 150 | 8.11 |
| Hands | 5 | 428 | 374 | 0.01 | 25.3 | 418 | 0.04 | 53.2 | 399 | 0.10 | 23.7 | 95 | 14.9 | 14.7 |
| Gloves | 5 | 314 | 308 | 0.01 | 22.1 | 313 | 0.02 | 52.7 | 307 | 0.05 | 14.0 | 92 | 5.42 | 8.67 |
| No gloves | 5 | 114 | 66 | 0.10 | 10.5 | 105 | 0.18 | 17.4 | 92 | 0.66 | 23.0 | 3 | 240 | 6.86 |
| Neck | 2 | 304 | 257 | 0.01 | 27.3 | 299 | 0.04 | 32.5 | 294 | 0.05 | 38.6 | 56 | 13.1 | 8.07 |
| Hood | 2 | 86 | 77 | 0.01 | 17.5 | 86 | 0.02 | 34.9 | 84 | 0.03 | 12.1 | 25 | 6.11 | 8.98 |
| No hood | 2 | 218 | 180 | 0.02 | 20.5 | 213 | 0.05 | 16.2 | 210 | 0.05 | 41.7 | 31 | 17.6 | 7.82 |
| Wrists | 1 | 180 | 153 | 0.02 | 28.8 | 170 | 0.05 | 28.7 | 164 | 0.08 | 48.7 | 19 | 26.6 | 8.53 |
| Face | 2 | 36 | 31 | 0.01 | 21.2 | 35 | 0.05 | 17.5 | 34 | 0.06 | 11.7 | 5 | 16.0 | 6.99 |
| Lower legs | 14 | 8 | 2 | 0.16 | 4.65 | 6 | 0.35 | 6.29 | 6 | 0.81 | 9.62 | 0 | 281 | 2.77 |
Multiple imputation (n = 10 imputed data sets) was used to impute tape-strip sampling data below detection limits.
The face and lower legs were sampled when protective clothing was not worn. An effort was made to sample the wrists when they were not adequately covered during spray painting by the gloves or coveralls. Thus, these sampled regions are not stratified by protective clothing.
Based on the Berkow chart (Deitch, 2008).
Number of measurements. Summation of levels collected with three successive tape-strip samples corresponds to one measurement.
Number of non-detectable values. Based on whether or not the first successive tape-strip collected detectable levels of the respective polyisocyanates.
Whole-body dermal concentration estimates of the polyisocyanates measured in spray painters from NC and WA are provided in Table 4. According to two-sample t-tests of the log-transformed data, significantly higher dermal exposures were observed in NC than in WA for all analytes (P < 0.0001). These differences may be related to differences in protective clothing use; gloves, coveralls, and hood were worn 47, 40, and 17% of the time in NC versus 94, 88, and 37% of the time in WA. However, the effect of location (i.e. NC versus WA) was significant (P = 0.0011) even after adjustment for other significant fixed effects (i.e. gloves, coveralls, and sampler type) in the multivariate model for predicting dermal concentrations of isocyanurate.
Table 4.
Whole-body dermal concentrationsa (ng mm−3) of monomeric and polymeric HDI measured in 15 painters from NC and 32 painters from WA State
| Analyte | NC (N = 95) |
WA State (N = 201) |
||||
| GM | GSD | Range | GM | GSD | Range | |
| HDI | 0.10 | 10.5 | 0.0002–121 | 0.02 | 9.33 | 0.00003–1.37 |
| Uretidone | 0.44 | 12.9 | 0.001–55.9 | 0.08 | 11.1 | 0.0001–22.5 |
| Biuret | 0.47 | 13.2 | 0.0006–2830 | 0.10 | 9.55 | 0.0001–13.8 |
| Isocyanurate | 143 | 7.99 | 0.17–7880 | 17.1 | 5.83 | 0.01–997 |
In calculating whole-body dermal concentration, multiple imputation (n = 10 imputed data sets) was used to impute tape-strip sampling data below detection limits.
Descriptive statistics are presented in Table 5 for the whole-body dermal concentration estimates, including restricted maximum likelihood estimates of the within- and between-worker variance. The GM dermal concentrations of all polyisocyanates varied among the different booth types, with the lowest levels observed in downdraft booths and highest levels observed in crossdraft booths. Painters who sprayed in crossdraft booths also had the greatest exposure variability for all the analytes except uretidone. It is important to note that, in downdraft, semi-downdraft, and crossdraft booths, coveralls were worn for 72, 77, and 42% of the paint tasks and gloves were worn for 78, 83, and 65% of the paint tasks, respectively. Thus, sporadic use of protective clothing could explain why painters in crossdraft booths had the highest dermal concentrations and exposure variability. Interestingly, after controlling for use of gloves and coveralls, it was found that semi-downdraft booths had a greater influence on dermal concentrations than crossdraft booths (data not shown).
Table 5.
Whole-body dermal concentrationsa (ng mm−3) of monomeric and polymeric HDI measured in painters using different booth types
| Analyte | Booth type | nb | Nc | Non-detectsd | Summary statistics |
REML estimates (logged data) |
|||
| GM | GSD | Range | Within-worker variance | Between-worker variance | |||||
| HDI | Downdraft | 31 | 204 | 154 | 0.03 | 10.0 | 0.00003–3.45 | 2.04 | 1.47 |
| Semi-downdraft | 10 | 61 | 29 | 0.07 | 5.93 | 0.00025–4.57 | 1.88 | 1.13 | |
| Crossdraft | 10 | 31 | 7 | 0.20 | 16.2 | 0.00015–121 | 3.56 | 5.22 | |
| All booths | 47 | 296 | 190 | 0.04 | 11.3 | 0.00003–121 | 2.17 | 1.91 | |
| Uretidone | Downdraft | 31 | 204 | 177 | 0.10 | 14.3 | 0.00012–55.7 | 2.42 | 2.31 |
| Semi-downdraft | 10 | 61 | 52 | 0.21 | 6.83 | 0.00030–22.4 | 1.50 | 1.91 | |
| Crossdraft | 10 | 31 | 22 | 0.49 | 10.5 | 0.00056–35.1 | 3.19 | 3.27 | |
| All booths | 47 | 296 | 251 | 0.14 | 14.1 | 0.00012–55.9 | 2.35 | 2.28 | |
| Biuret | Downdraft | 31 | 204 | 190 | 0.10 | 7.85 | 0.00011–13.7 | 1.72 | 1.56 |
| Semi-downdraft | 10 | 61 | 43 | 0.31 | 8.37 | 0.00064–550 | 3.13 | 0.95 | |
| Crossdraft | 10 | 31 | 20 | 1.12 | 34.9 | 0.00057– 2830 | 3.06 | 11.6 | |
| All booths | 47 | 296 | 253 | 0.17 | 11.9 | 0.00011–2830 | 2.17 | 2.88 | |
| Isocyanurate | Downdraft | 31 | 204 | 11 | 25.2 | 8.23 | 0.014–3980 | 1.71 | 2.62 |
| Semi-downdraft | 10 | 61 | 2 | 50.9 | 5.75 | 0.14–2670 | 1.43 | 1.93 | |
| Crossdraft | 10 | 31 | 2 | 103 | 10.1 | 0.13–7880 | 2.09 | 4.82 | |
| All booths | 47 | 296 | 15 | 33.8 | 8.25 | 0.014–7880 | 1.71 | 2.72 | |
REML, restricted maximum likelihood.
In calculating whole-body dermal concentration, multiple imputation (n = 10 imputed data sets) was used to impute tape-strip sampling data below detection limits.
Number of workers. A total of four workers painted in more than one booth type; two painted in both crossdraft and semi-downdraft booths, one painted in both crossdraft and downdraft booths, and one painted in both semi-downdraft and downdraft booths.
Number of paint tasks.
Number of non-detectable values. Based on whether or not detectable levels of the respective polyisocyanates were recovered with tape-strip samples from any region of the skin following the completion of a paint task.
Isocyanurate was the predominant species measured in skin with GM levels 20 times greater than all other analytes. While isocyanurate was detectable in skin for 95% of the paint tasks, the other polyisocyanates were detectable in skin for <23% of the paint tasks. Exposures were considered non-detectable if none of the tape-strip samples collected detectable levels of polyisocyanates following the completion of a paint task.
Table 6 presents a comparison of polyisocyanate dermal concentrations by whether painters wore protective clothing. According to two-sample t-tests, significant differences were observed between dermal concentrations in painters wearing coveralls and gloves and in painters who did not wear protective clothing for all the measured polyisocyanates (P < 0.0001).
Table 6.
Whole-body dermal concentrationsa (ng mm−3) of monomeric and polymeric HDI measured in 32 painters who wore coveralls and gloves and 15 painters who did not wear coveralls and gloves during spray painting
| Analyte | Coveralls and gloves worn (N = 192 paint tasks) |
Coveralls and gloves not worn (N = 51 paint tasks) |
||||||
| Non-detectsb | GM | GSD | Range | Non-detectsb | GM | GSD | Range | |
| HDI | 149 | 0.02 | 9.16 | 0.00003–1.37 | 20 | 0.21 | 9.75 | 0.00035–121 |
| Uretidone | 185 | 0.06 | 8.90 | 0.00012–2.00 | 41 | 0.52 | 6.97 | 0.00056–34.9 |
| Biuret | 169 | 0.08 | 8.79 | 0.00011–13.7 | 38 | 1.15 | 18.4 | 0.00089–2830 |
| Isocyanurate | 13 | 12.9 | 5.10 | 0.014–539 | 1 | 276 | 6.40 | 0.13–7880 |
In calculating whole-body dermal concentration, multiple imputation (n = 10 imputed data sets) was used to impute tape-strip sampling data below detection limits.
Number of non-detectable values. Based on whether or not detectable levels of the respective polyisocyanates were recovered with tape-strip samples from any region of the skin following the completion of a paint task.
Statistical modeling
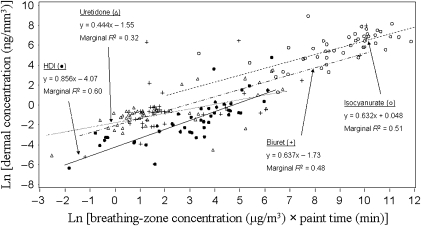
Because the majority of detectable dermal concentrations occurred in painters who did not wear protective clothing, LMM was used to evaluate the fixed effects of the covariates on dermal concentrations of each measured polyisocyanate in painters who did not wear coveralls or gloves. To eliminate sampler type bias, this analysis was further restricted to two-stage air sampling (14 painters, 45 paint tasks). Booth type was not used as a classification variable in these models due to the limited number of measurements from painters in crossdraft booths (i.e. 4 painters, 10 paint tasks) and semi-downdraft booths (i.e. 1 painter, 2 paint tasks). Dermal concentration data from 7 of 14 painters in the subpopulation were collected during a single visit. Therefore, due to a lack of repeated visits for painters in the subpopulation, visit day was not included as a random effect in these mixed models. The products of BZC and paint time were highly significant (P ≤ 0.0011) in all the models. Figure 1 presents the mixed-effects regression of dermal concentration on the product of BZC and paint time for each analyte. For illustration purposes, non-detectable levels in Fig. 1 are represented by the average of the 10 imputations. In addition to the product of analyte-specific BZC and paint time, gun type was a significant variable in the models for HDI (P = 0.0155) and uretidone (P = 0.0773), and airflow was a significant variable in the models for HDI (P = 0.0265) and biuret (P = 0.0066). According to marginal R2 statistics, the full mixed models described 68, 36, 59, and 51% of the variability in dermal concentrations of HDI, uretidone, biuret, and isocyanurate, respectively.
Fig. 1.
Mixed-effect regressions of log-transformed dermal concentrations of HDI, uretidone, biuret, and isocyanurate on the products of respective BZCs and paint times in 15 unprotected painters (no coveralls and gloves worn) performing 45 separate paint tasks.
Because dermal concentrations of isocyanurate were detectable in 95% of the paint tasks, LMM was performed for isocyanurate using the unrestricted data set (i.e. 47 painters, 288 paint tasks). This unrestricted data set was not used for LMM of dermal concentrations of HDI, uretidone, and biuret due to high number of non-detects in painters wearing coveralls and gloves. The mixed model developed for predicting dermal concentrations of isocyanurate is described in Table 7. According to the marginal R2 statistics, significant fixed effects were able to describe 55% of the variability in dermal concentrations of isocyanurate. According to the Akaike information criterion, using visit day as a random effect significantly improved the fit of the final isocyanruate model, suggesting that inter-visit variability was not adequately explained by the significant fixed effects. Although coveralls and gloves were significant predictors in the model, material type, age, and thickness were not significant. The relative effectiveness of coveralls and gloves can be estimated by comparing model predictions calculated with and without the fixed effect of protective clothing. According to this procedure, use of protective clothing was associated with a 95% reduction of isocyanurate concentration in the skin.
Table 7.
Linear mixed effect modela for predicting dermal concentrations of isocyanurateb in automotive spray painters
| Covariatesc | Parameter estimates (downdraft, semi-downdraft, crossdraft)d | P-valuese |
| Intercept | (2.26, 2.78, 2.51) | <0.0001 |
| Isocyanurate BZC × paint time (μg min m−3) | 0.401 | <0.0001 |
| Gloves (1 = yes, 0 = no) | −1.76 | <0.0001 |
| Coveralls (1 = yes, 0 = no) | −1.55 | <0.0001 |
| Sampler type (1 = two stage, 0 = one stage) | −0.322 | 0.0801 |
According to the marginal R2 statistic, the model described 55% of the variability in the dermal concentrations of isocyanurate.
N = 288 (8 of 296 observations were excluded due to missing air sampling data).
Dermal concentrations and the products of BZC and paint time were log transformed prior to statistical analysis.
Separate intercepts were determined for each booth type as specified in the mixed model.
P-values are based on approximate F-tests of fixed effects.
DISCUSSION
In this study, a previously published tape-strip sampling method (Fent et al., 2008) was used to quantify dermal concentrations of individual polyisocyanates in automotive spray painters. Because quantitative dermal concentration and covariate data were obtained, LMM could be used to evaluate the fixed effects of covariates on dermal concentration while estimating within- and between-worker variance components via random effects. However, there were several limitations in this study that were taken into consideration when we evaluated the results.
First, the potential for selection bias exists because we used several different sampling schemes throughout the study (Table 1). However, arms and hands were sampled several times whether protective clothing was worn or not. As a result, the significant effect of protective clothing (gloves and coveralls) in the isocyanurate model (Table 7) should be valid. Nevertheless, our exposure estimates may not be entirely accurate or comparable between and/or within painters. Second, >60% of the dermal concentrations were below the limits of detection for HDI, uretidone, and biuret. Consequently, statistical power was reduced for the analysis of this data. We restricted our analysis of the HDI, uretidone, and biuret data to unprotected workers in order to increase our statistical power, but much of this data (≥39%) still fell below the detection limits. As a result, multiple imputation was used for dermal concentrations below the detection limits in an effort to obtain better estimates of the parameters of interest (Lubin et al., 2004). Other limitations are described in Part I of this series (Fent et al., 2009).
Despite these limitations, the mixed models developed in this study described a considerable amount of variability (R2 ≥ 0.36) in dermal concentrations of isocyanurate in all 47 painters as well as dermal concentrations of HDI, uretidone, biuret, and isocyanurate in 15 painters who did not wear coveralls or gloves during spraying.
The product of analyte-specific BZC and paint time was the most significant variable in all the mixed models. The effect of this variable on dermal concentrations of polyisocyanates in painters who did not wear protective clothing can be seen in Fig. 1. Using the same product of analyte-specific BZC and paint time (e.g. 5.0 μg min m−3), the models in Fig. 1 predict ∼2, 10, and 17 times higher dermal concentrations of uretidone, biuret, and isocyanurate than HDI. Because HDI (0.05 mmHg at 25°C) exists partially as vapor in overspray, HDI may supply less exposure to the skin or evaporate off the skin. The oligomers, on the other hand, have relatively low vapor pressures (e.g. biuret 4.7 × 10−7 mmHg at 20°C). Therefore, any differences between predicted dermal concentrations of individual HDI oligomers are likely due to the different rates of skin absorption or chemical reactivity. Further investigation into dermal absorption and reactivity differences among polyisocyanates is warranted.
Although the products of BZC and paint time were able to describe much of the variability (32–60%) in dermal concentrations in painters who did not wear protective clothing, other variables (i.e. gun type and airflow) when included in the mixed models were able to increase the explained variability (36–68%). While these models were developed to describe the variability in unprotected painters, the mixed model described in Table 7 was developed primarily to identify additional determinants of dermal concentration, as well as to evaluate the effectiveness of protective clothing used by painters in this study.
As expected, gloves and coveralls were significant predictors in the mixed model (Table 7). However, wearing a hood was not significant, possibly due to low statistical power, inadequacy of loose-fitting hoods for protection, and/or less intense overspray surrounding the face and neck compared to the arms and hands during painting. The variables related to material type, age, and thickness were not significant in the model, which may suggest that similar protection was achieved for the different types of protective clothing used by painters. However, the effects of material type, age, and thickness are likely to be subtle compared to the major protective effects of wearing coveralls and gloves, and as such, would be difficult to identify with LMM. Therefore, more controlled experiments are needed to fully evaluate the effectiveness of protective materials.
Sampler type (one stage versus two stage) was significant in the isocyanurate model (Table 7). The effect of two-stage sampling was discussed in Part I of this series (Fent et al., 2009). Briefly, two-stage samplers are more likely than one-stage samplers to underestimate the BZC of isocyanurate due to the potential for polymerization on the untreated pre-filter. This finding is important as both one- and two-stage samplers are commonly used to sample atmospheres containing polyisocyanates.
In Part I of this series, we observed significantly higher (P ≤ 0.05) BZCs of HDI, biuret, and isocyanurate in WA than in NC (Fent et al., 2009). However, in this study, we observed that painters in NC had significantly higher dermal concentrations of HDI, uretidone, and isocyanurate than painters in WA (Table 4). The mixed model for predicting dermal concentrations of isocyanurate in all painters (Table 7), which included the protective effect of coveralls and gloves, could not explain this difference (i.e. the effect of location was significant when added to the model). It is possible that climatic differences could be the cause of these differences. However, temperature and humidity were not significant variables in the mixed model (Table 7). In addition, the dermal sampling scheme we used differed depending on whether or not coveralls and gloves were worn. This could have biased the results by location since protective clothing was worn more frequently in WA than in NC. Further investigation is needed to determine the cause of the observed differences in dermal exposures to isocyanurate between painters in NC and WA.
To our knowledge, statistical modeling has not been used to investigate dermal exposure to polyisocyanates in the automotive refinishing industry. However, Brouwer et al. (2001) developed a deterministic model for predicting dermal exposure to overspray in airless spray painters. The primary factors of this model were overspray generation rate, transmission of overspray, and aerosol deposition efficiency. These factors could not be measured directly in our study, but may be estimated by the variables in this study. For example, BZC may be representative of the overspray generation rate, airflow and booth type may be important factors in the transmission of overspray, and gun type, which influences the size of overspray particles, may affect the aerosol deposition efficiency. All these variables were significant in one or more of the models.
Use of high-volume low-pressure (HVLP) guns was associated with lower dermal concentrations, most likely due to the improved transfer efficiency of HVLP guns compared to the conventional guns. Increasing airflow was associated with decreasing dermal concentration, most likely due to the increased capture and removal of overspray from the painters’ personal space at higher airflows. It is important to note that transmission of overspray may be influenced by factors other than the airflow and booth type. These factors may not have been characterized in this study. However, it is possible that BZCs were measured in such close proximity to the painters’ skin that, in effect, transmission of overspray had occurred. Under this scenario, instantaneous BZC would be related to instantaneous dermal concentration by a factor related to aerosol deposition. Consequently, the product of BZC and paint time would be related to cumulative dermal concentration for the paint task, which is essentially what was estimated in this study.
The random effect of visit day was significant in the final isocyanurate model. This suggests that painters’ dermal exposures varied between visits due to factors not evaluated in this study, such as the size and orientation of the objects being painted, the busyness of the shop, and the condition of the work equipment during the sampling day. Further research is needed to identify the primary variables associated with the inter-visit variability. Nevertheless, the inter-visit variability observed in this study emphasizes the importance of sampling personal exposures at various times throughout the year in order to obtain the most representative exposure estimates.
Isocyanurate was the most abundant polyisocyanate collected from the skin whether or not coveralls and gloves were worn (Table 5). The reason for the higher levels and detection rate of isocyanurate in skin may simply be due to the greater abundance of isocyanurate in the atmosphere (GM = 1410 μg m−3) compared to the other analytes (GM ≤ 7.85 μg m−3).
For all the measured polyisocyanates, the highest dermal concentrations were in painters who sprayed in crossdraft booths. The isocyanurate model generated in this study predicted higher dermal concentrations for workers painting in semi-downdraft and crossdraft booths than for workers painting in downdraft booths. According to the findings in Part I of this series (Fent et al., 2009), painters who sprayed in downdraft booths had lower BZCs than painters who sprayed in the other booths for all the measured polyisocyanates. Flynn et al. (1999) observed that, depending on worker orientation, crossdraft booths may actually draw overspray across the painter's body. It is conceivable that this effect may also occur in semi-downdraft booths. Thus, the higher concentrations of polyisocyanates in the air coupled with the inability of the ventilation system to draw air away from the painters personal space may have led to higher dermal concentrations in painters who used crossdraft and semi-downdraft booths.
The results reported here are consistent with the tape-strip validation measurements previously reported (Fent et al., 2008). Few investigators have quantified and reported exposure to polyisocyanates in human skin. Bello et al. (2008) used wipe sampling to quantify dermal exposure (ng cm−2) to TRIG in painters who did not wear protective clothing (GM = 1.9, GSD = 10.9, n = 49 measurements) and in painters who wore coveralls (GM = 1.0, GSD = 3.2, n = 3) and gloves (GM = 1.0, GSD = 5.2, n = 17). After converting regional dermal exposure estimates (ng cm−2) of individual polyisocyanates into estimates of TRIG for this study, it became clear that we measured higher levels of polyisocyanates in the skin of painters who did not wear protective clothing (GM = 50, GSD = 8.2, n = 306) than in the skin of painters who wore coveralls (GM = 2.3, GSD = 7.4, n = 487) and gloves (GM = 1.5, GSD = 8.0, n = 314). Given the specificity of the analytical method, the polyisocyanates measured and reported here do not necessarily represent all the possible polyisocyanate species in automotive paint. For example, monomeric and polymeric isophorone diisocyanate, which is sometimes present, and polymers of HDI larger than isocyanurate were not quantified. Therefore, the actual TRIG concentrations are most likely to be higher than what we were able to measure with our analyte-specific liquid chromatography-mass spectrometry method. Nevertheless, compared to the wipe sampling method used in Bello et al. (2008), it appears the tape-strip method we describe has superior collection and quantification efficiency. Furthermore, the specificity of the analytical method provides a means to investigate individual monomeric and polymeric HDI concentrations in the skin. Tape-stripping is also the only method available to quantitatively measure polyisocyanate species in the non-viable skin layer, thus, providing an estimate of the absorbed dose.
This study provides a significant contribution to the characterization of the processes governing dermal exposures to individual polyisocyanates (HDI monomer and its oligomers) in automotive spray painters. Through LMM, we were able to identify the primary determinants of dermal exposure to monomeric and polymeric HDI. The mixed models developed related dermal concentration to the product of BZC and paint time. As a result, these models may be particularly useful for exposure reconstruction studies where information on BZC and paint time is readily available or can be estimated. However, further validation is necessary to determine the accuracy of these models. Although this study was able to demonstrate the effectiveness of the use of coveralls and gloves to reduce exposure, isocyanurate was detected in the skin of painters wearing coveralls and gloves for 93% of the paint tasks. This underscores the importance of reducing BZCs in the painting atmosphere. By reducing BZCs, the amount of overspray available for deposition will be reduced, thus providing less loading onto protective clothing and exposed skin. Moreover, this study describes exposure assessment tools to estimate the doses of individual polyisocyanates to the skin and lungs. This information may be used to investigate the roles of monomeric and polymeric HDI, as well as dermal and inhalation routes of exposure, in the development of respiratory sensitization and occupational asthma.
FUNDING
National Institute for Occupational Safety and Health (R01-OH007598, T42/CCT422952, T42 OH008673); National Institute of Environmental Health Sciences (P30ES10126 and T32 ES007018); American Chemistry Council (RSK0015-01).
Acknowledgments
This study was approved by the Institutional Review Board in the Office of Human Research Ethics at the University of North Carolina at Chapel Hill and by the Washington State Institutional Review Board at the Washington State Department of Social and Health Services. The authors are grateful to the automobile-repair shop painters who volunteered to participate in this study and to Diana Ceballos (University of Washington) and Robert Anderson (SHARP program) for their help in collecting the samples in Washington State, to Jean Orelien and Lloyd Edwards for providing SAS code to calculate the marginal R2 statistics, and to Dr Louise M. Ball for the review of this manuscript and her constructive comments.
References
- Bello D, Redlich CA, Stowe MH, et al. Skin exposure to aliphatic polyisocyanates in the auto body repair and refinishing industry: II. A quantitative assessment. Ann Occup Hyg. 2008;52:117–24. doi: 10.1093/annhyg/mem066. [DOI] [PubMed] [Google Scholar]
- Bello D, Smith TJ, Woskie SR, et al. An FTIR investigation of isocyanate skin absorption using in vitro guinea pig skin. J Environ Monit. 2006;8:523–9. doi: 10.1039/b517948c. [DOI] [PubMed] [Google Scholar]
- Bernstein JA. Overview of diisocyanate occupational asthma. Toxicology. 1996;111:181–9. doi: 10.1016/0300-483x(96)03375-6. [DOI] [PubMed] [Google Scholar]
- Brouwer DH, Semple S, Marquart J, et al. A dermal model for spray painters. Part I: subjective exposure modelling of spray paint deposition. Ann Occup Hyg. 2001;45:15–23. [PubMed] [Google Scholar]
- Chan-Yeung M, Malo JL. Occupational asthma. N Engl J Med. 1995;333:107–12. doi: 10.1056/NEJM199507133330207. [DOI] [PubMed] [Google Scholar]
- Deitch EA. Burn management. In: Irwin RS, Rippe JM, editors. Irwin and Rippe's intensive care medicine. 6th edn. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2008. pp. 1930–8. [Google Scholar]
- Donnelly R, Buick JB, Macmahon J. Occupational asthma after exposure to plaster casts containing methylene diphenyl diisocyanate. Occup Med (Lond) 2004;54:432–4. doi: 10.1093/occmed/kqg133. [DOI] [PubMed] [Google Scholar]
- Fent KW, Jayaraj K, Ball LM, et al. Quantitative monitoring of dermal and inhalation exposure to 1,6-hexamethylene diisocyanate monomer and oligomers. J Environ Monit. 2008;10:500–7. doi: 10.1039/b715605g. [DOI] [PubMed] [Google Scholar]
- Fent KW, Trelles Gaines LG, Thomasen J, et al. Quantification and statistical modeling—Part I: breathing-zone concentrations of monomeric and polymeric 1,6-hexamethylene diisocyanate. Ann Occup Hyg. 2009 doi: 10.1093/annhyg/mep046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn MR, Gatano BL, McKernan JL, et al. Modeling breathing-zone concentrations of airborne contaminants generated during compressed air spray painting. Ann Occup Hyg. 1999;43:67–76. [PubMed] [Google Scholar]
- George K, Kubota K, Twizell EH. A two-dimensional mathematical model of percutaneous drug absorption. Biomed Eng Online. 2004;3:18. doi: 10.1186/1475-925X-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–6. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- Herrick CA, Xu L, Wisnewski AV, et al. A novel mouse model of diisocyanate-induced asthma showing allergic-type inflammation in the lung after inhaled antigen challenge. J Allergy Clin Immunol. 2002;109:873–8. doi: 10.1067/mai.2002.123533. [DOI] [PubMed] [Google Scholar]
- Janko M, McCarthy K, Fajer M, et al. Occupational exposure to 1,6-hexamethylene diisocyanate-based polyisocyanates in the state of Oregon, 1980-1990. Am Ind Hyg Assoc J. 1992;53:331–8. doi: 10.1080/15298669291359735. [DOI] [PubMed] [Google Scholar]
- Karol MH, Hauth BA, Riley EJ, et al. Dermal contact with toluene diisocyanate (TDI) produces respiratory tract hypersensitivity in guinea pigs. Toxicol Appl Pharmacol. 1981;58:221–30. doi: 10.1016/0041-008x(81)90426-9. [DOI] [PubMed] [Google Scholar]
- Kubota K, Ishizaki T. A diffusion-diffusion model for percutaneous drug absorption. J Pharmacokinet Biopharm. 1986;14:409–39. doi: 10.1007/BF01059200. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- Lesage J, Goyer N, Desjardins F, et al. Workers’ exposure to isocyanates. Am Ind Hyg Assoc J. 1992;53:146–53. doi: 10.1080/15298669291359410. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bello D, Sparer JA, et al. Skin exposure to aliphatic polyisocyanates in the auto body repair and refinishing industry: a qualitative assessment. Ann Occup Hyg. 2007;51:429–39. doi: 10.1093/annhyg/mem021. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sparer J, Woskie SR, et al. Qualitative assessment of isocyanate skin exposure in auto body shops: a pilot study. Am J Ind Med. 2000;37:265–74. doi: 10.1002/(sici)1097-0274(200003)37:3<265::aid-ajim4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–6. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauluhn J, Lewalter J. Analysis of markers of exposure to polymeric methylene-diphenyl diisocyanate (pMDI) in rats: a comparison of dermal and inhalation routes of exposure. Exp Toxicol Pathol. 2002;54:135–46. doi: 10.1078/0940-2993-00242. [DOI] [PubMed] [Google Scholar]
- Petsonk EL, Wang ML, Lewis DM, et al. Asthma-like symptoms in wood product plant workers exposed to methylene diphenyl diisocyanate. Chest. 2000;118:1183–93. doi: 10.1378/chest.118.4.1183. [DOI] [PubMed] [Google Scholar]
- Pronk A, Tielemans E, Skarping G, et al. Inhalation exposure to isocyanates of car body repair shop workers and industrial spray painters. Ann Occup Hyg. 2006a;50:1–14. doi: 10.1093/annhyg/mei044. [DOI] [PubMed] [Google Scholar]
- Pronk A, Yu F, Vlaanderen J, et al. Dermal, inhalation, and internal exposure to 1,6-HDI and its oligomers in car body repair shop workers and industrial spray painters. Occup Environ Med. 2006b;63:624–31. doi: 10.1136/oem.2005.023226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray NJ, Botham PA, Hext PM, et al. Induction of respiratory hypersensitivity to diphenylmethane-4,4′-diisocyanate (MDI) in guinea pigs. Influence of route of exposure. Toxicology. 1994;88:15–30. doi: 10.1016/0300-483x(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Reddy MB, McCarley KD, Bunge AL. Physiologically relevant one-compartment pharmacokinetic models for skin. 2. Comparison of models when combined with a systemic pharmacokinetic model. J Pharm Sci. 1998;87:482–90. doi: 10.1021/js9702877. [DOI] [PubMed] [Google Scholar]
- Redlich CA, Herrick CA. Lung/skin connections in occupational lung disease. Curr Opin Allergy Clin Immunol. 2008;8:115–9. doi: 10.1097/ACI.0b013e3282f85a31. [DOI] [PubMed] [Google Scholar]
- Rougier A, Lotte C, Maibach HI. In vivo percutaneous penetration of some organic compounds related to anatomic site in humans: predictive assessment by the stripping method. J Pharm Sci. 1987;76:451–4. doi: 10.1002/jps.2600760608. [DOI] [PubMed] [Google Scholar]
- Rudzinski WE, Dahlquist B, Svejda SA, et al. Sampling and analysis of isocyanates in spray-painting operations. Am Ind Hyg Assoc J. 1995;56:284–9. doi: 10.1080/15428119691014422. [DOI] [PubMed] [Google Scholar]
- Sparer J, Stowe MH, Bello D, et al. Isocyanate exposures in autobody shop work: the SPRAY study. J Occup Environ Hyg. 2004;1:570–81. doi: 10.1080/15459620490485909. [DOI] [PubMed] [Google Scholar]
- Tojo K. Random brick model for drug transport across stratum corneum. J Pharm Sci. 1987;76:889–91. doi: 10.1002/jps.2600761209. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Exposure factors handbook. Washington, DC: U.S. Environmental Protection Agency.; 1997. Available at http://cfpub.epa.gov/ncea/index.cfm. Accessed June 2009. [Google Scholar]
- Vandenplas O, Cartier A, Lesage J, et al. Occupational asthma caused by a prepolymer but not the monomer of toluene diisocyanate (TDI) J Allergy Clin Immunol. 1992;89:1183–8. doi: 10.1016/0091-6749(92)90303-j. [DOI] [PubMed] [Google Scholar]
- Vandenplas O, Cartier A, Lesage J, et al. Prepolymers of hexamethylene diisocyanate as a cause of occupational asthma. J Allergy Clin Immunol. 1993;91:850–61. doi: 10.1016/0091-6749(93)90342-d. [DOI] [PubMed] [Google Scholar]
- van der Molen RG, Spies F, van't Noordende JM, et al. Tape stripping of human stratum corneum yields cell layers that originate from various depths because of furrows in the skin. Arch Dermatol Res. 1997;289:514–8. doi: 10.1007/s004030050232. [DOI] [PubMed] [Google Scholar]
- Vonesh EF, Chinchilli VM. Linear and nonlinear models for the analysis of repeated measurements. New York, NY: Marcel Dekker; 1997. [Google Scholar]
- Zartarian V, Bahadori T, McKone T. Adoption of an official ISEA glossary. J Expo Anal Environ Epidemiol. 2005;15:1–5. doi: 10.1038/sj.jea.7500411. [DOI] [PubMed] [Google Scholar]