Abstract
The medial prefrontal cortex (MPFC) is regarded as a region of the brain that supports self-referential processes, including the integration of sensory information with self-knowledge and the retrieval of autobiographical information. I used functional magnetic resonance imaging and a novel procedure for eliciting autobiographical memories with excerpts of popular music dating to one's extended childhood to test the hypothesis that music and autobiographical memories are integrated in the MPFC. Dorsal regions of the MPFC (Brodmann area 8/9) were shown to respond parametrically to the degree of autobiographical salience experienced over the course of individual 30 s excerpts. Moreover, the dorsal MPFC also responded on a second, faster timescale corresponding to the signature movements of the musical excerpts through tonal space. These results suggest that the dorsal MPFC associates music and memories when we experience emotionally salient episodic memories that are triggered by familiar songs from our personal past. MPFC acted in concert with lateral prefrontal and posterior cortices both in terms of tonality tracking and overall responsiveness to familiar and autobiographically salient songs. These findings extend the results of previous autobiographical memory research by demonstrating the spontaneous activation of an autobiographical memory network in a naturalistic task with low retrieval demands.
Keywords: emotion, episodic memory, fMRI, medial prefrontal cortex, tonality
Introduction
The evocation of autobiographical memories and associated emotions by music counts among the most poignant experiences associated with music; yet, little is known about how these prominent facets of the human experience are bound together in the brain. In this study, I test the hypothesis that the medial prefrontal cortex (MPFC) serves as a hub that associates features of the music with autobiographical memories and emotions. I also describe the broader network of brain areas that is recruited during the reliving of music-evoked autobiographical memories (MEAMs).
There are several reasons to suspect that the MPFC might support the integration of memories, emotions, and music. Meta-analyses of autobiographical memory retrieval tasks indicate MPFC involvement (Gilboa 2004; Svoboda et al. 2006), and more generally, the MPFC is engaged by judgments regarding self-relevance and affect (Ochsner et al. 2004). The reason to suspect that music engages the MPFC is 4-fold. First, the region was observed to track the movement of a melody through the tonal space created by the system of major and minor keys underlying Western tonal music (Janata, Birk, et al. 2002). The trajectory that a piece of music takes in tonal space serves as a signature of that piece of music. Given that excerpts of music serve as potent retrieval cues for memories (Janata et al. 2007), structural descriptors of such pieces (such as their movements in tonal space) constitute probes with which to identify brain regions that are responding to specific ethologically valid memory retrieval cues. These time-varying descriptors may complement simpler static descriptors (such as whether the overall excerpt is familiar to the listener) in identifying brain areas that respond to personally relevant musical stimulation. Second, emotional responses to music and the perceived pleasantness of music modulate activity in the MPFC. Ventral regions in particular were shown to respond more strongly to short consonant musical passages compared with the same passages rendered increasingly more dissonant (Blood et al. 1999), and the same areas were active while listening to unfamiliar but pleasant music (Brown, Martinez, and Parsons 2004). Third, a task involving ancillary familiarity judgments about excerpts of music activated the MPFC broadly (Platel et al. 2003), and judgments about the familiarity of music and odors showed that familiar musical stimuli elicited stronger responses in the MPFC than did unfamiliar stimuli (Plailly et al. 2007). The final impetus for focusing on the MPFC comes from a pair of observations regarding Alzheimer's disease (AD) patients. The first is spared memory for familiar music in AD (Cuddy and Duffin 2005), and the other is the relatively slower rate of cortical atrophy of the MPFC compared with other brain areas during the progression of AD (Thompson et al. 2003). Together, these observations suggest that a neural substrate for associating music, emotions, and at least certain types of memories is preserved in AD and that this neural substrate is encompassed by the MPFC.
Taken together, these findings give rise to the as yet untested hypothesis that the MPFC serves as a hub at which music, memories, and emotions are associated. Two predictions of this hypothesis are tested in this report. The first is that the strength of the blood oxygen level–dependent (BOLD) functional magnetic resonance imaging (fMRI) signal measured in the MPFC will be positively correlated with the degree of familiarity, autobiographical salience, and positive affect evoked by excerpts of music. The second is that portions of the MPFC that either directly overlap or are in close vicinity to those that show such positive correlations will also track the music's movement through tonal space.
Materials and Methods
Subjects
All the data collection procedures used in this study were approved by the University of California at Davis Institutional Review Board. Thirteen UC Davis undergraduates (11 females, 18–22 years; mean age: 20.0 years) participated in the experiment after providing informed consent. These subjects had been invited to participate in the fMRI experiment because they experienced at least 30% MEAMs in an MEAM validation study (Janata et al. 2007).
Stimulus Selection
Selecting the set of stimuli to play to any given subject presented a considerable challenge. The desire to measure the physiological response to the reliving of remote memories without contamination by any prior associations of the specific musical excerpts with the experiment imposed the constraint of not prescreening individuals on a fixed library of song excerpts as hearing a song identified as autobiographically salient a second time in the experiment could simply evoke a memory of having heard that song in the prescreening process. I also did not ask subjects to generate a list of autobiographical memory-evoking music excerpts ahead of time, both to avoid contaminating the experiencing of those pieces with the memory of having added them to a list of personally salient pieces and in recognition of the fact that it is possible to experience strong memories and emotions in response to songs without remembering their titles or the names of the performing artists.
A second constraint, for the purpose of presenting suitable control stimuli, was that the stimulus selection strategy had to result in a distribution of excerpts into 3 stimulus categories: 1) those that were wholly unfamiliar, 2) others that were familiar but not autobiographically salient, and 3) those that were familiar and autobiographically salient. An adequate strategy consisted of selecting excerpts at random from the Billboard Top 100 Pop and R&B charts for the years when the subject was between 7 and 19 years of age. This strategy was validated in a large sample of ∼300 subjects and is described in detail elsewhere (Janata et al. 2007). Subjects from the validation study in whom MEAMs were triggered at least 30% of the time were invited to participate in this fMRI study. Song excerpts that had been heard by the subject in the prescreening phase were excluded from the fMRI phase of the experiment. Across the 13 subjects, 341 unique song excerpts were presented.
Stimulus Presentation and Response Collection
The experiment was controlled by Presentation software (Neurobehavioral Systems, Inc., Albany, CA) in conjunction with a Matlab engine (The Mathworks, Natick, MA) that selected stimuli from and submitted responses to “Ensemble,” an experiment control and database system (http://atonal.ucdavis.edu/ensemble/) (Tomic and Janata 2007). For each subject, all information pertaining to scanner timing pulses, stimulus event codes and responses to questions were written to a text file that was used subsequently to construct the design matrices used in the statistical analyses.
Thirty song excerpts were presented across 2 scanning runs of 15 songs each. Each song excerpt was 30 s in song duration. The excerpts were the samples available from the Apple iTunes Music Store (Apple, Cupertino, CA). Thus, the difficult task of demarcating starting and stopping locations in each song in order to extract a representative and recognizable excerpt was already accomplished. Prior to a scanning run, 15 excerpts were selected at random from a period of time that the subject was between 7 and 19 years of age.
A timing marker from the scanner triggered the Presentation script. One minute of resting state activity was recorded prior to the onset of the first stimulus. Thirty stimuli were presented across 2 scanning runs via electrostatic headphones (MR confon GmbH, Magdeburg, Germany). Subjects also wore foam earplugs. Prior to the scanning runs, the volume of the music was adjusted so that is was as loud as the subject could comfortably stand but without noticeable distortion of the audio quality. Following each excerpt, subjects provided ratings using 5 buttons on a keypad (Lumitouch; Photon Control Inc., Burnaby, Canada) underneath the fingers of their left hand in response to 6 verbal cues for questions pertaining to: 1) affective valence while listening to the music (5-point scale; 1 = very displeasing, 5 = very pleasing), 2) arousal while listening to the music (5-point scale; 1 = not at all aroused, 5 = extremely aroused), 3) familiarity with the song (2-point scale; unfamiliar, familiar), 4) autobiographical associations with the song (3-point scale; 1 = no association, 2 = weak association, 3 = strong association), 5) the orientation of their attention toward the memories (5-point scale; 1 = very unfocused, 5 = very focused), and 6) the orientation of their attention toward the music (5-point scale; 1 = very unfocused, 5 = very focused). When subjects entered a response, they heard the word “Okay,” indicating that their response had been recorded. They were instructed to press the button again in the event that they had made a response but had not heard the confirmation. The next song was loaded and presented 1 s after the confirmation of the response. No time limits were imposed on response latency, so the overall duration of each trial varied somewhat, usually lasting slightly less than 1 min.
Given that subjects had to answer a large number of questions with different response mappings following each stimulus, I provided them with response training during the 10 min in which a high-resolution anatomical MRI scan was obtained. Training trials consisted of hearing 1 of the 6 question cues—pleasantness, aroused, familiarity, autobiographical, memory focus, music focus—followed by a response category cue. Subjects had to press the corresponding category button. If they made an error, they heard 2 warning tones followed by the name of the finger they should have used for their response. Question/category pairs were selected at random until subjects had made 2 correct responses for every pair. Unless subjects responded with minimal errors, the procedure was iterated until they were committing none or extremely few errors and felt comfortable with the response protocol.
Postscan Memory Test and Assay of Memory Content
Immediately following the scanning session, 11 of the 13 subjects were seated at a computer in a quiet room and asked to complete a detailed survey about their episodic memories for the songs that they had flagged as autobiographically salient while in the scanner. This procedure took the form of a recognition memory test in which subjects heard a series of 20 s song fragments that included songs that had been heard and labeled as autobiographical in the scanner as well as 33% foils that had been heard neither in the scanner nor in the prescreening session. For each song, a subject made a yes/no judgment of whether he or she had heard it in the scanner, along with a confidence rating on a 5-point scale (not at all, very little, somewhat, fairly strongly, and very strongly). For songs that were flagged as autobiographically salient and identified correctly as having been heard in the scanner, subjects indicated whether they could recall the content of the memories they experienced in the scanner. If they could, they completed a larger set of questions about the content of the memories, including ratings of image vividness; the degree to which the song and memory evoke and emotion; and memories for lifetime periods, places, people, and events (Janata et al. 2007). Although these postscan memory assays were probably more susceptible to false memories and distortions than if they had been presented immediately following each song in the scanner, I felt they were a reasonable compromise given the technical constraints of the experiment.
fMRI Acquisition Parameters
MRI data were collected on a 3T Siemens Trio (Siemens Medical Solutions, Erlangen, Germany) at the Imaging Research Center on the UC Davis Medical Center campus. Three sets of MR images were acquired for each subject. The first was a high-resolution 3D MPRAGE structural image (field of view = 256 × 256 mm, 192 slices, resolution = 1 × 1 × 1 mm, time repetition [TR] = 2.5 s, time echo [TE] = 4.82 ms, flip angle = 7°). The second was a volume of T1-weighted axial images using the same 34 slice positions and orientations as the subsequently collected echoplanar images (EPI, TR = 600 ms, TE = 8.6 ms, flip angle = 70°, in-plane resolution: 0.86 × 0.86 mm). Slices were positioned obliquely with a superior to inferior tilt along the anterior–posterior direction. Prior to acquisition of the EPI, a point-spread function was obtained to estimate image distortion and was applied to the EPI using the native Siemens distortion correction algorithms. The native motion correction algorithm was also applied during image acquisition. In 2 subjects, EPI volumes consisted of 30 slices (3.5 mm thick; in-plane resolution: 1.54 × 1.54 mm; TR = 2.5). In the remaining subjects, EPI volumes consisted of 34 slices (4 mm thick, no skip; in-plane resolution: 3.4 × 3.4 mm; TR = 2.0 s; TE = 25 ms; flip angle = 90°).
Preprocessing of the MRI Data
The fMRI data were analyzed using SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). I applied the following sequence of preprocessing steps to the series of BOLD image volumes: realignment of the first volume of the second run to the first volume of the first run (the reference volume), realignment of the EPIs to the first volume of each run, coregistration of the reference EPI volume to the “coplanar” T1-weighted axial images, coregistration of the coplanar volume to the subject's high-resolution (hires) structural volume, and spatial normalization of the hires volume to the MNI251 T1 template with propagation of the normalization parameters to the coplanar and EPI volumes. The normalized images were resliced to consist of 2 mm isotropic voxels (the default). Normalized images were smoothed with a 5-mm isotropic kernel. A model was fit for each subject that included the motion parameter estimates from the image realignment stage, linear trends across each run, and the mean offset of each run. The residuals from this model were used as input to the models of interest described below.
Statistical Analyses of Responses during Music Epochs, the Question and Answer Periods, and to Parametric Variation of Familiarity, Autobiographical Salience, and Valence
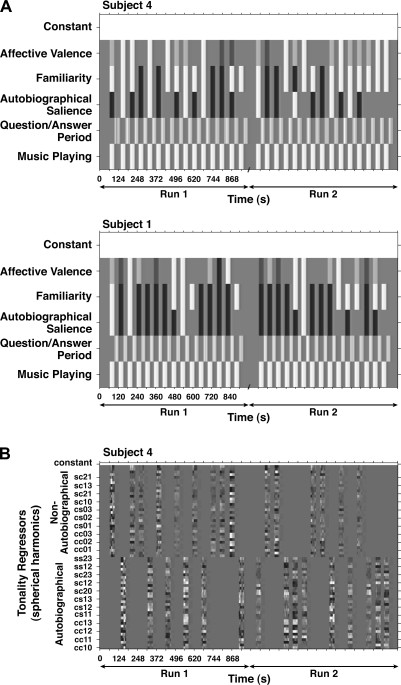
All analyses were performed in Matlab using a combination of third party toolboxes, for example, SPM5, the IPEM Toolbox, and custom code. Four major effects of interest were assessed with 2 models (Fig. 1). The first model (Fig. 1A) consisted of 3 sets of regressors that represented 1) the 30 s periods during which music was playing (MusPlay); 2) the question and answer period (QAP) following each musical excerpt; and 3) a set of parametric regressors that captured the variation in the familiarity, autobiographical salience, and degree of positive affect evoked by each musical excerpt. The MusPlay regressor was modeled as an epoch lasting for the 30 s duration of the excerpt. Note that this regressor indicates only whether music is playing and therefore models a very general response to musical stimulation. It does not provide any information that is specific to the individual pieces of music, such as their tempo, harmonic structure, or genre. Specific information about musical excerpts was implicit in the second model described below. The 6 questions that followed each excerpt and the answers to them were modeled using a single “QAP” regressor with cues and answers modeled as events. The parametric regressors modeling familiarity, autobiographical salience, and affective valence were constructed based on the responses to the questions following each song excerpt. Familiarity was coded with unfamiliar = −1 and familiar = 1. Autobiographical salience was coded with no association = −1, weak association = 0, and strong association = 1. Valence was coded linearly from very displeasing = −2 to very pleasing = 2.
Figure 1.
Design matrices for 2 subjects illustrate the statistical models that were used. The grayscale codes the regressor values, with black representing the most negative values and white the most positive. The ubiquitous gray represents zero. (A) The 30 s music epochs alternated with the question/answer periods during which subjects responded to questions on multipoint scales. Those answers were used to code parametrically varying regressors for autobiographical salience, familiarity, and affective valence. Black bars represent song excerpts that were unfamiliar or not autobiographically salient, whereas white bars represent songs that were familiar and strongly autobiographically salient. Valence ratings range from very displeasing (black) to very pleasing (white) with neither pleasing nor displeasing represented by zero. (B) A design matrix for a single subject with 2 sets of regressors that model the movement of each piece of music through tonal space. A set of 34 spherical harmonics is used to model the movements. One set was used to model those songs marked as autobiographically salient and another to mark songs that were not. Thus, the model tests an interaction of tonality tracking and autobiographical salience.
Two things should be noted about the parametric regressors. First, the weightings were linear weightings. Although it is possible that the degrees of autobiographical salience or valence do not manifest themselves in the BOLD signal in a linear manner, it seemed that linear weightings would at least capture the direction of change accurately and would also reflect the way that the contrasts would be coded had the different levels of each variable been modeled with different regressors. Second, the level of a weak autobiographical association was effectively set to the value that modeled the resting state periods at the beginning and end of a run. It is important to note that each of the parametric regressors model both the attribute of interest and the appropriate levels of control stimuli. For example, familiar contrasted with unfamiliar, or the intermediate levels between the 2 extremes in the case of autobiographical salience and emotional valence.
The model was evaluated using the classical inference approach (traditional general linear model) in SPM5. The parametric effects of familiarity, autobiographical salience, and valence were modeled as parametric regressors belonging to the MusPlay effect, which meant that they were orthogonalized prior to model estimation in order to account for the correlations among them (see Supplementary Fig. 1). For the sake of explanation, these regressors are shown in their nonorthogonal form in Figure 1A.
Several contrasts were evaluated in the individual subject models: MusPlay; QAP; and each of the parametric regressors, familiarity, autobiographical salience, and valence. In addition, a sixth contrast modeled the sum of the familiarity, autobiographical salience, and valence effects (FAV), that is, the contrast weighted the beta-coefficients for each of the parametric regressors equally. Thus, FAV represented the combined effects of hearing pleasing, familiar, and autobiographically salient songs relative to unfamiliar, emotionally neutral, or displeasing songs that elicited no autobiographical association.
The 6 contrasts were evaluated in a second-level model with 1-sample t-tests. All activation clusters reported in the statistical summary tables (Tables 1–4) and shown in Supplementary Figures 2–4 were identified by thresholding the statistical parametric maps with a criterion of P < 0.001 (uncorrected) and a voxel extent threshold of 10 contiguous voxels. Statistical maps shown in Figures 2 and 3 were thresholded with a criterion of P < 0.005 and a cluster size threshold of 40 contiguous voxels. Note that for the figures, the height threshold was not relaxed in order to find activations about which inferences could be made but rather to provide a clearer impression of the spatial extent in which the peak activation clusters identified with the more stringent threshold were embedded.
Table 1.
Summary of significant activations associated with the MusPlay regressor
| Anatomical region | Brodmann area | Left hemisphere locations (mm) |
Right hemisphere locations (mm) |
||||||||
| x | y | z | No. of voxels | Z | x | y | z | No. of voxels | Z | ||
| Frontal | |||||||||||
| IFG/FO | 47 | −40 | 22 | 2 | 12 | 3.61 | |||||
| Temporal | |||||||||||
| STG | 42 | −60 | −28 | 16 | 1899 | 5.14 | 62 | −22 | 6 | 1632 | 5.30 |
| 22/38 | −46 | −2 | −10 | 4.83 | 58 | 0 | −2 | 4.85 | |||
| STS | 22 | −66 | −34 | 4 | 4.81 | ||||||
| Amy | −18 | −14 | −10 | 24 | 4.24 | ||||||
| Subcortical | |||||||||||
| Inferior colliculus | −6 | −36 | −4 | 73 | 4.23 | ||||||
| Cerebellum | −20 | −56 | −36 | 11 | 3.40 | 28 | −56 | −22 | 38 | 3.73 | |
Note: IFG, inferior frontal gyrus; FO, frontal operculum; STG, superior temporal gyrus; STS, superior temporal sulcus; and Amy, amygdala.
Table 2.
Summary of loci at which BOLD signal correlated positively with familiarity
| Anatomical region | Brodmann area | Left hemisphere locations (mm) |
Right hemisphere locations (mm) |
||||||||
| x | y | z | No. of voxels | Z | x | y | z | No. of voxels | Z | ||
| Frontal | |||||||||||
| pre-SMA/SMAa | 6 | 2 | 12 | 54 | 324 | 5.37 | |||||
| IFG | 44 | −44 | 14 | 12 | 408 | 4.81 | |||||
| IFG/IFS | 44/9 | −50 | 12 | 32 | 4.13 | 50 | 12 | 32 | 11 | 3.67 | |
| 56 | 26 | 32 | 11 | 3.93 | |||||||
| MFG | 6 | −32 | 10 | 58 | 56 | 3.98 | |||||
| MFG | 46 | −48 | 42 | 12 | 64 | 4.37 | 56 | 26 | 32 | 11 | 3.93 |
| SFG | 8 | −2 | 34 | 48 | 18 | 4.25 | |||||
| SFG | 9 | −6 | 44 | 36 | 43 | 4.03 | 10 | 54 | 28 | 11 | 3.79 |
| SFG | 10 | −4 | 56 | 2 | 12 | 3.60 | |||||
| Cingulate sulcus | 32 | 0 | 28 | 32 | 46 | 4.14 | |||||
| Anterior insula | −30 | 18 | 8 | 93 | 4.09 | ||||||
| Temporal | |||||||||||
| Posterior STG | 39 | 58 | −62 | 24 | 21 | 4.64 | |||||
| 22 | −50 | −44 | 18 | 56 | 3.75 | ||||||
| −54 | −44 | 26 | 3.66 | ||||||||
| MTG | 21 | −52 | −24 | −12 | 19 | 3.77 | |||||
| Limbic | |||||||||||
| Posterior cingulate | 29 | −4 | −50 | 10 | 28 | 3.53 | |||||
| Subcortical | |||||||||||
| Thalamus | −12 | −12 | 10 | 146 | 4.27 | 16 | −10 | 22 | 78b | 4.13 | |
| 8 | −28 | 2 | 11 | 3.60 | |||||||
| Cerebellum | 20 | −76 | −28 | 150 | 4.45 | ||||||
| −42 | −52 | −26 | 53 | 4.68 | 40 | −52 | −26 | 15 | 3.85 | ||
| 32 | −60 | −20 | 41 | 4.16 | |||||||
| 34 | −72 | −36 | 10 | 3.59 | |||||||
| 4 | −56 | −34 | 10 | 3.70 | |||||||
Note: IFG, inferior frontal gyrus; SMA, supplementary motor area; SFG, superior frontal gyrus; MFG, middle frontal gyrus; IFS, inferior frontal sulcus; STG, superior temporal gyrus; and MTG, middle temporal gyrus.
Bilateral activation.
Primarily activation of the medial dorsal and ventral lateral thalamic nuclei, extending into the caudate nucleus.
Table 3.
Summary of loci in which activity was positively correlated with the degree of autobiographical salience
| Anatomical region | Brodmann area | Left hemisphere locations (mm) |
Right hemisphere locations (mm) |
||||||||
| x | y | z | No. of voxels | Z | x | y | z | No. of voxels | Z | ||
| Frontal | |||||||||||
| SFG | 8 | −16 | 48 | 40 | 42 | 3.83 | 8 | 52 | 30 | 12 | 3.38 |
| −4 | 48 | 40 | 3.26 | ||||||||
| −10 | 32 | 44 | 12 | 3.54 | |||||||
| −12 | 44 | 30 | 10 | 3.46 | |||||||
| MFG | 8 | −32 | 18 | 42 | 16 | 3.66 | |||||
| IFG | 45 | −50 | 20 | 6 | 21 | 3.54 | |||||
| Gyrus rectus and olfactory sulcus | 11 | 8 | 50 | −16 | 25 | 3.51 | |||||
| Temporal | |||||||||||
| ITG/ITS | 20/21 | 62 | −18 | −22 | 15 | 3.99 | |||||
Note: IFG, inferior frontal gyrus; SFG, superior frontal gyrus; MFG, middle frontal gyrus; ITG, inferior temporal gyrus; and ITS, inferior temporal sulcus.
Table 4.
Summary of loci at which changes in BOLD signal were positively correlated with the degree of positive affect (valence)
| Anatomical region | Brodmann area | Left hemisphere locations (mm) |
Right hemisphere locations (mm) |
||||||||
| x | y | z | No. of voxels | Z | x | y | z | No. of voxels | Z | ||
| Frontal | |||||||||||
| SFS | 10 | −22 | 40 | 22 | 17 | 3.35 | |||||
| Temporal | |||||||||||
| Posterior STG | 22 | 56 | −32 | 22 | 16 | 3.43 | |||||
| Limbic | |||||||||||
| Ventral ACC | 33/24 | −2 | 26 | 10 | 16 | 3.85 | |||||
| Ventral ACC | 24 | −6 | 34 | −6 | 36 | 3.80 | |||||
| Subcortical | |||||||||||
| Substantia nigra | 2 | −20 | −6 | 41 | 3.60 | ||||||
| vltn | −14 | −12 | 12 | 18 | 3.36 | ||||||
Note: SFS, superior frontal sulcus; STG, superior temporal gyrus; ACC, anterior cingulate cortex; andvltn, ventral lateral thalamic nucleus.
Figure 2.
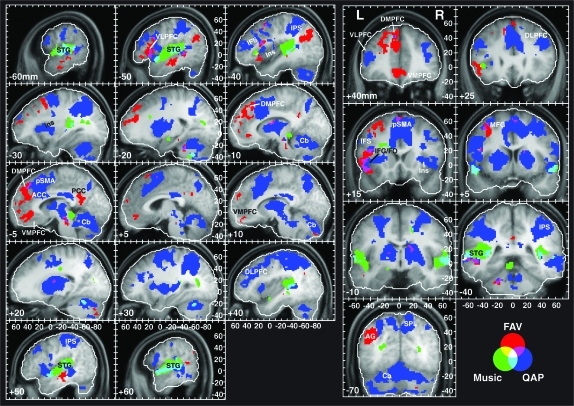
Summary of the activation patterns associated with the MusPlay, QAP, and FAV effects. The color legend for each of the effects of interest and conjunctions of effects of interest is shown at the bottom of the right panel. The statistical maps are thresholded at P < 0.005 (uncorrected) and a 40 voxel extent for all effects. In this and subsequent figures the following apply. The lateral displacement (mm) of the anatomical slice shown in each subpanel is indicated in the bottom left corner. Negative values for the sagittal sections in the left panel denote positions in the left hemisphere. Negative values for the coronal sections in the right panel denote positions posterior to the anterior commissure. The tick values of the scales (in mm) are given relative to the anterior commissure. The white contour lines enclose the volume in which data were available for all subjects. Abbreviations: STG, superior temporal gyrus; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; VMPFC, ventromedial prefrontal cortex; IFG, inferior frontal gyrus; FO, frontal operculum; IPS, intraparietal sulcus; AG, angular gyrus; pSMA, presupplementary motor area; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; IFS, inferior frontal sulcus; MFG, middle frontal gyrus; Ins, insula; SPL, superior parietal lobule; and Cb, cerebellum.
Figure 3.
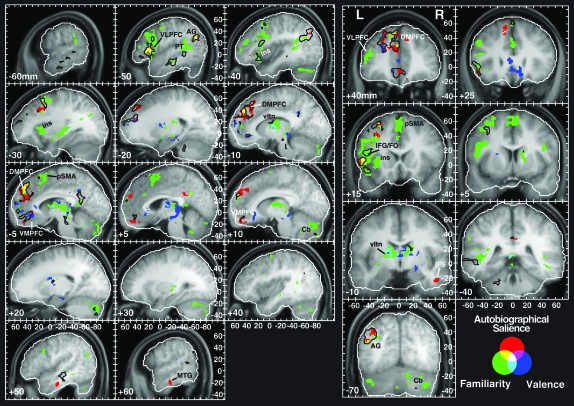
Individual effects of familiarity (green), autobiographical salience (red), and affective (positive) valence (blue). For purposes of comparison, the black outlines are the borders of the FAV contrast shown in Figure 2. The probability thresholds were P < 0.005 (uncorrected) and 40 voxels for all effects. Abbreviations: same as in Figure 2 plus PT, planum temporale; vltn, ventral lateral thalamic nucleus; and MTG, middle temporal gyrus.
A region-of-interest (ROI) analysis was performed for the MPFC for the FAV contrast. The a priori ROI was defined as a region extending 14 mm laterally from the midline in both directions, extending from the ventral to dorsal surfaces of the brain and bounded caudally by a plane tangent to the genu of the corpus callosum at y = +29 mm. Activation foci within this volume all met a false discovery rate corrected significance criterion of P < 0.025.
Statistical Analyses of Tonality Tracking
In order to probe more sensitively how structural aspects of the music interact with the memories and mental images that were called to mind by the music, I performed an analysis based on earlier work that identified brain areas that follow the movements of music through tonal space (Janata, Birk, et al. 2002). The reason for performing this analysis is the assumption that precise descriptors of individual excerpts of music will be more sensitive probes for identifying brain activations associated with those pieces of music than will less specific descriptors. In other words, a model that explicitly captures the temporal dynamics of the melodies and harmonic progressions that define each excerpt is a much better model than one that indicates only generically that some music is playing without regard for the structural properties of the stimulus (as in the case of the MusPlay regressor described above). The objective of this analysis was to identify, primarily within individual subjects, the network of brain areas that was engaged as a person listened attentively to the pieces of music and experienced memories, mental images, and emotions in response to those pieces of music.
It is important to note that a tonality tracking (TT) analysis does not assume that specific parts of a piece of music will always elicit the same memory or emotion. However, it does assume the presence of a broad associative memory network that encompasses implicit memory for structure in Western tonal music, memories of varying strength for specific pieces of music, and episodic memories for events or periods in one's life with which memories of certain musical genres or specific pieces of music became bound. It is therefore expected that as a piece of music unfolds in time, it is providing cues in the form of instrument sounds (timbre), melodies, chord progressions, and lyrics that can trigger a variety of associations. In this way, a model of the piece of music may become a proxy for, or a pointer to, those thoughts, memories, and emotions that are evoked as one follows along with the music in one's mind.
The Structure and Modeling of Tonal Space
Tonal space in Western tonal music comprises the 24 major and minor keys. The shape of tonal space is that of a torus, with different keys dwelling in different regions of the toroidal surface. Their relative locations to each other on the surface are based on their theoretical, psychological, and statistical distances from one another (Krumhansl 1990; Toiviainen and Krumhansl 2003; Janata 2007–2008). Keys are characterized by sets of pitch classes, for example, the notes C, D, E, A-flat, etc. The pitch class sets of adjacent keys have all but one of their members in common, whereas increasingly distant keys share fewer and fewer pitches. Moreover, not all the pitches in a key occur equally often. Thus, any given location on the torus effectively represents a probability distribution across the 12 pitch classes. Similar probability distributions reside next to each other on the surface. Thus, as a piece of music unfolds, the moment-to-moment distributions of pitch classes change as the notes of the melodies and harmonic (chord) progressions change. Consequently, the pattern of activation on the surface of the torus changes in time as a piece of music progresses (for an example, see Supplementary Movies).
In order to correlate the pattern of music's movement through tonal space with the BOLD data, a method is needed for describing the temporal evolution of the changing intensity pattern on the surface of the torus using a reasonable number of degrees of freedom. One set of basis functions that can be used to describe the activation pattern on the surface of a torus consists of spherical harmonics (spatial frequencies on the toroidal surface). The activation pattern on the toroidal surface at each moment in time is described as a weighted sum of spherical harmonics. The time-varying weights of the spherical harmonic components are used as regressors in the models of the BOLD data (Fig. 1B).
The procedure for transforming an audio file of the music into the spherical harmonic regressors is described and illustrated elsewhere (Janata, Birk, et al. 2002; Janata 2005). Briefly, the IPEM Toolbox (http://www.ipem.ugent.be/Toolbox/) was used to process the audio signal. The toolbox models cochlear transduction and auditory nerve firing patterns, which are then used to estimate the pitch distributions in the signal using an autocorrelation method. The time-varying periodicity pitch images are smoothed using leaky integration with a time constant of 2 s in order to capture the pitch distribution information within a sliding window. The pitch distribution at each time frame is then projected to the toroidal surface via a weight matrix that maps a distribution of pitches to a region of the torus. The weight matrix was trained with a self-organizing map algorithm in the Finnish SOM Toolbox (http://www.cis.hut.fi/projects/somtoolbox/) using a melody that systematically moved through all the major and minor keys in Western tonal music (Janata, Birk, et al. 2002; Janata et al. 2003).
The activation pattern at each moment in time was decomposed into a set of spherical harmonics, and the time courses of the spherical harmonic coefficients then served as sets of regressors in the analysis of the BOLD data (Janata, Birk, et al. 2002; Janata 2005). Note that because of the low-pass spatial frequency characteristics of the toroidal activations, it was possible to accurately represent the toroidal activation patterns using fewer degrees of freedom than if one used every spot on the torus as its own regressor.
The TT Regressors
Two groups of 34 spherical harmonic regressors were used to identify TT voxels in each subject. One group modeled those songs that were identified as weakly or strong autobiographically salient and the other group modeled those songs for which the subject had no autobiographical association. Both groups were entered into a single model (Fig. 1B). This model tests an interaction between TT and autobiographical salience. As described below, it allows one to compare the relative amounts of variance explained by autobiographically salient and nonautobiographical songs. This provides the strongest means, given the present data and experimental design, of identifying brain areas that associate music with memories.
Because the spherical harmonic regressors as a group describe the time-varying pattern of activation, assessment of the amount of variance they explain in the BOLD data must be done with an F-test across the group of regressors. Not surprisingly, the F-tests for such large groups of regressors with a multitude of time courses explained considerable amounts of variance in the BOLD data. In order to guard against false positives, it was therefore necessary to determine whether the variance explained for any given voxel was due to the veridical model or whether any model that retained critical time-varying properties of the veridical set of regressors would have explained the data equally well. I used a permutation test for this purpose.
The permutation test was applied to all voxels for which the omnibus F-test of the veridical TT model was significant at P < 0.05 with the SPM5 family-wise error correction. For each subject, 100 models were evaluated in which the order of the songs in the model was shuffled at random. The chosen permutation strategy was a conservative one because it preserved both the correlation structure among the TT regressors and the temporal properties of the music. If all the pieces of music had similar patterns of movement across the tonal surface, the permuted models would tend to be as probable as the original model, rendering the original model nonsignificant. An alternative permutation strategy would have been to randomly shuffle time points. While preserving the correlation structure among the regressors, such permutations would have disrupted smoothly varying trajectories through tonal space, thus presenting a less difficult challenge to the original model.
Statistical significance of the veridical model was tested by comparing the residual mean square error of the veridical model against the distribution of residual mean square errors from the alternative models. The residual errors from the 100 models were always normally distributed, so the mean and standard deviation (SD) of the observed distribution were used to fit a Gaussian function. The observed value for the veridical model was evaluated against the estimated Gaussian function, rather than calculating the exact probability using the number of permutations. Any voxel for which the likelihood of observing the veridical model's residual error given the distribution of permutation model errors was P < 0.05 was considered to be a TT voxel. Clusters of TT voxels were identified using an extent threshold of 40 contiguous voxels.
Identification of Autobiographically Salient TT
The objective of having 2 sets of tonality regressors was to see whether any given TT voxel showed a preference for autobiographically salient songs. This preference was assessed by calculating the ratio of the F-statistics of the autobiographically salient and autobiographically nonsalient sets of tonality regressors:
where n is the number of songs falling into each category. The colormap for the images of individual subject's TT voxels (Fig. 4) reflects this measure.
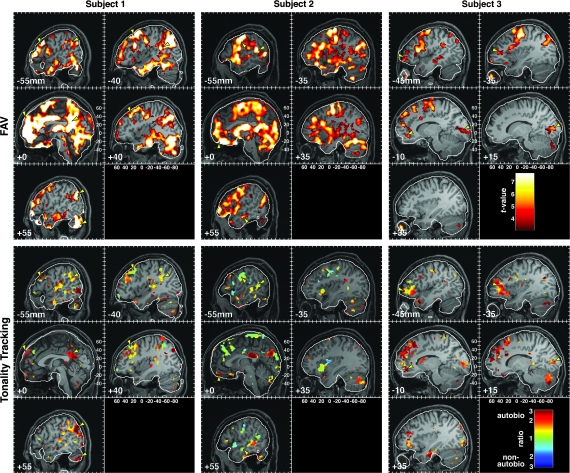
Figure 4.
FAV and TT responses in 3 individual subjects. The TT images show the proportion of variance explained by the TT regressors modeling autobiographically salient pieces of music relative to those modeling pieces of music that elicited no autobiographical association. The color scale represents the ratio of these variances, with each variance corrected by the number of music excerpts contributing to its estimate. Red values indicate a bias toward TT associated with an autobiographical memory, and blue denotes TT without an associated autobiographical memory. Greenish hues denote unbiased TT. The yellow arrowheads mark corresponding locations in the FAV and TT images to indicate regions that showed significant responses to both the parametric variation of familiarity, autobiographical salience, and valence and the dynamic variation captured by the TT model. The color scale for the FAV effect corresponds to the t-statistic for this effect (minimum threshold of P < 0.001, 40 voxels).
Brain areas that showed TT at the group level (across subjects) were identified as follows. For each subject, a binary mask was created from the volume of TT voxels such that any voxel that met the significance criterion from the permutation test and belonged to a cluster of 40 or more voxels in size was assigned a value of 1. These masks were then summed across subjects, resulting in a map showing the count of the number of subjects showing TT activations at each voxel. The statistical significance of voxels in this map was assessed with a Monte Carlo simulation that estimated the likelihood that a voxel would exhibit TT activity in N subjects. For each subject, the number of TT voxels was first counted up and then distributed at random within a brain volume that was defined as the intersection of all brain volume masks from individual subjects. These random TT voxel distributions were then summed across subjects to obtain the number of subjects hypothetically activating each voxel by chance alone. A histogram was constructed from all these voxel values to indicate how many voxels were expected to be activated by N subjects, where N ranges from 0 to 13. The procedure of randomly distributing TT voxels was repeated 500 times, resulting in 500 histograms. These histograms were averaged and normalized to obtain a final histogram showing the expected distribution of numbers of voxels showing TT for N subjects. From this distribution, it was possible to calculate the probability of observing N subjects in any voxel by chance alone. The probabilities were P < 0.088 (N = 3), P < 0.014 (N = 4), P < 0.0015 (N = 5), and P < 0.0001 (N = 6). Thus, the summed TT image was thresholded to retain only those voxels that showed TT for 4 or more subjects and which belonged to clusters of at least 40 contiguous voxels. Each cluster was then examined in more detail to determine the number of unique subjects who showed TT within that cluster and to determine the distribution of TT biases across voxels within that cluster.
Conjunction Analyses
The activation patterns for the different contrasts of interest were compared with a conjunction analysis in order to identify brain areas that responded to multiple effects of interest. The conjunction analysis consists of calculating the intersection of the significance masks created for each contrast (Nichols et al. 2005). The outcomes of these analyses are shown in Figures 2, 3, and 5 through color-coded contrast combinations (Fig. 2) or by showing the contours of one analysis overlaid on the outcomes of other analyses (Figs 3 and 5). The results of the conjunction analyses are not provided in separate tables, but the figures are of sufficient resolution and labeled such that the coordinates of conjunctions can be determined readily. Locations of conjunctions of particular interest are noted in the text.
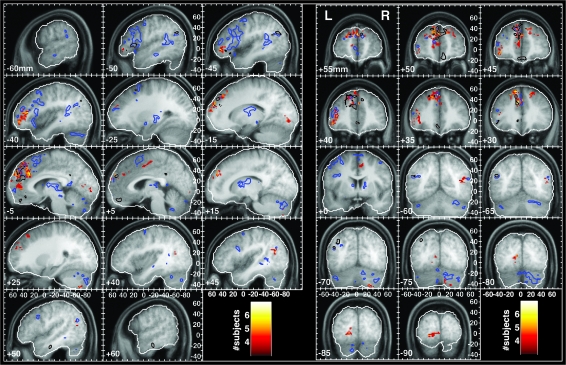
Figure 5.
A summary of brain areas exhibiting TT behavior. The color of each voxel indicates the number of subjects in whom that voxel showed significant TT behavior. A Monte Carlo simulation determined the likelihood of a given number of subjects showing TT at any given voxel by chance alone. The probabilities were P < 0.014, P < 0.0015, and P < 0.0001 for 4, 5, and 6 subjects, respectively. The blue and black contours demarcate the borders of the individual familiarity and autobiographical salience activations shown in Figure 3, respectively.
Anatomical Localization of Statistical Effects
Anatomical locations and Brodmann areas (BAs) were assigned through a combination of comparing the locations of activation foci on the average normalized high-resolution T1-weighted image of the group of subjects with images in the Duvernoy atlas (Duvernoy 1999) and finding the activation foci using the SPM5 plugin WFU_PickAtlas (http://www.fmri.wfubmc.edu/cms/software) (Maldjian et al. 2003) which contains masks corresponding to the different BAs.
Results
Triggering MEAMs
For each of 13 subjects, 30 stimuli were selected at random from the Billboard Top 100 Pop and R&B charts for the years when the subject was between 7 and 19 years of age. On average, 56.2% (±18.0% SD) of the stimuli presented to a subject were judged to be familiar (range: 20–80%) and 42.3% (±18.1% SD) were rated as “somewhat” or “strongly” autobiographical (range: 11.1–73.3%). Although the majority (57%) of the excerpts were experienced as “somewhat” or “very” pleasing by subjects and 24.9% were experienced as neutral, there were 6 subjects who experienced between 20% and 40% as somewhat or very displeasing. Overall, the distributions of familiarity, autobiographical salience, and affective response were suitable for examining the 2 hypotheses.
The postscan questionnaires provided information regarding the distinction between weakly and strongly autobiographical remembering episodes. Overall, subjects were very accurate and confident in their identification of songs they had marked as autobiographical while in the scanner (mean correct identification = 93.5%; mean confidence = 4.8/5) and in their correct rejection of foils (mean correct rejection = 93.6%; mean confidence = 4.3/5). Although relatively few correctly identified autobiographically salient songs were marked as not having associated memory content, in 5 of 7 subjects who did have such songs, the majority had been flagged in the scanner as weakly autobiographical.
Images associated with strongly autobiographically songs were rated as more vivid (mean = 2.36 on a 3-point scale where 1 = no clear images, 2 = somewhat vivid, and 3 = very vivid) than for weakly autobiographical songs (mean = 1.79; t(9) = 5.784, P < 0.0003). (In this analysis and following analyses of postscan data, one subject who had no memory content for any weakly salient songs was excluded from the analyses.).
Strongly autobiographical songs elicited stronger agreement (t(9) = 3.442, P < 0.008) with the statement that, “Hearing this song sometimes evokes an emotional reaction that is associated with an event, period, or person in my life,” with a mean rating of 5.62 falling between “agree somewhat” and “agree,” while the mean rating of 4.8 for weakly autobiographical songs fell between “neither agree nor disagree” and “agree somewhat.” The average proportion of memories associated with an event was also significantly higher for strongly autobiographical songs (mean = 0.44) than weakly autobiographical songs (mean = 0.16; t(9) = 3.707, P < 0.005). The average proportion of memories associated with people did not differ significantly between weakly (mean = 0.40) and strongly (mean = 0.52) salient memories (t(9) = 0.906, P < 0.39). The same was true for memories of lifetime periods (weak mean = 0.4; strong mean = 0.31; t(9) = −1.122, P < 0.29). Only the average proportion of memories associated with places was significantly higher for weak (mean = 0.42) than strong (mean = 0.22) memories (t(9) = −3.56, P < 0.006). Overall, greater autobiographical salience was associated with more vivid remembering of more emotion-laden memories of events.
General Task-Related Activations
The first of 2 models examined general task-related activations and the parametric variations in familiarity, autobiographical salience, and valence. Figure 2 illustrates the spatial relationships among the networks identified by the MusPlay, QAP, and FAV regressors. The simple model of whether or not music was playing (MusPlay) showed extensive activation bilaterally in the auditory cortex along the superior temporal gyrus (STG) as expected (Table 1; Fig. 2, regions shown in green, yellow, and cyan). The activation extended rostrally along the STG from the planum temporale to the planum polare. Peak activations were observed in auditory association areas (BA 22) rostral to Heschl's gyrus. One prefrontal activation focus was observed in the ventrolateral prefrontal cortex (VLPFC) in the vicinity of the frontal operculum of the left hemisphere.
The second general task-related regressor modeled activity associated with the QAPs that followed each musical excerpt. From the perspective of mental computations, QAP represents the deployment of attention to service a series of interactions with an external agent. These interactions depend on orienting to, perceiving, and interpreting the auditory retrieval cues, retrieving information prompted by the cue, and preparing and executing a response. The objective of this study was not to dissociate these various cognitive processes as these have been examined in great detail in the cognitive neuroscience literature (Cabeza and Nyberg 2000). Accordingly, I expected this regressor to identify activations of a network consisting of frontal and parietal areas typically engaged in attention tasks and frontal areas involved in decision making, response planning, and response execution along with attendant subcortical activations of the cerebellum, thalamus, and basal ganglia. Aside from the activations of the auditory cortex as modeled by the MusPlay regressor, the QAP activations were the most highly statistically significant effect at the group level. Because the exact loci of the peak activations throughout this network are of secondary importance to this paper, they are not listed in a table. However, Figure 2 provides the interested reader with sufficient information for determining the coordinates of activated nodes in this network.
Parametric Modeling of Familiarity, Autobiographical Salience, and Affective Response
The motivating interest for this study was a test of the hypothesis that the experiencing of MEAMs would evoke activity within the MPFC. Identifying the broader network of areas that supports the experiencing of MEAMs was of comparable interest. The first of 2 tests of the MPFC hypothesis consisted of the 3 parametric regressors that represented the degree of excerpt familiarity (unfamiliar/familiar), autobiographical salience (no association/weak association/strong association), and valence (a 5-point scale ranging from very displeasing to very pleasing).
Tables 2–4 summarize the areas of activation for the independent effects of familiarity, autobiographical salience, and valence regressors, respectively. Activation maps of the 3 effects are illustrated in Figure 3. The most widespread activations were observed for the familiarity regressor, mostly within the left hemisphere (Fig. 3, green blobs; Supplementary Fig. 2) with the largest activations within structures that underlie sequencing and motor planning processes. They included large foci along the inferior frontal gyrus (BA 44) extending into the anterior insula, middle frontal gyrus (BA 46, 6), and in the pre-SMA. Additional notable cortical activations were within the MPFC (BA 9), the posterior STG (BA 22), angular gyrus (BA 39), and middle temporal gyrus (BA 21). Sizable subcortical activations were found in the cerebellum, the caudate nucleus, and the ventral lateral thalamic nucleus.
Several loci in prefrontal cortex, lateralized primarily to the left hemisphere exhibited an independent effect of autobiographical salience (Table 3; Fig. 3, red and yellow blobs; Supplementary Fig. 3). Two of these were found, in accordance with the a priori hypothesis, in the dorsal MPFC along the left superior frontal gyrus (BA 8), right superior frontal gyrus (BA 9), and ventrally along the right gyrus rectus (BA 11). Additional effects of autobiographical salience were found in ventral lateral prefrontal regions of the left hemisphere (BA 45). In posterior regions, activation was observed in the right temporal lobes along the inferior temporal sulcus (BA 20/21). Two of the activation foci—in dorsal MPFC and left VLPFC—overlapped with effects of familiarity (Fig. 3, yellow blobs). It must be emphasized that the regressors were orthogonal to each other, so the effects of autobiographical salience were above and beyond those due to familiarity alone.
A number of cortical and subcortical areas showed a positive correlation between the strength of the BOLD signal and the degree of experienced positive affect mostly in the left hemisphere (Table 4; Fig. 3, blue blobs; Supplementary Fig. 4). The most notable activation was in the ventral anterior cingulate, adjoining and overlapping in part areas that showed increased responses to autobiographically salient songs. An area of overlap between familiarity and valence responses was in the ventral lateral thalamic nucleus. An additional notable effect of valence was found in the substantia nigra.
As noted above in the Materials and Methods, the familiarity, autobiographical salience, and valence regressors were correlated moderately prior to orthogonalization (Supplementary Fig. 1). For this reason, I also evaluated a contrast that summed, at the level of individual subjects, the parameter estimates of these variables and treated them as a single effect, FAV, which reflected the response to familiar, autobiographically salient, and pleasing songs. The FAV effect was then evaluated at the group level in order to identify those brain regions in which BOLD signal increased in response to a combined increase in those factors that typify the experiencing of MEAMs. The activation foci found for this contrast (P < 0.005 uncorrected, >40 voxel extent) are shown alongside the MusPlay and QAP effects in Figure 2 (red, yellow, and magenta areas) and superimposed on the individual parametric regressors in Figure 3 (black outlines). Overall, the pattern of FAV-related activity was lateralized to the left hemisphere, even within the MPFC, with an average of 18.2% more voxels active in the left hemisphere than the right (t(12) = 2.96, P = 0.012). With the exception of an activation focus in the posterior cingulate cortex, areas that correlated positively with FAV overlapped with, or were immediately adjacent to, areas that were significantly correlated with 1 or 2 of the individual regressors (Fig. 3).
As predicted, the most extensive region of FAV-related activation was in frontal areas, primarily in dorsal and ventral regions of the MPFC, and large portions of VLPFC. FAV overlapped with the independent effects of autobiographical salience and familiarity in dorsal MPFC (BA 8). An ROI analysis performed for the MPFC indicated that activation foci within this volume all met a false discovery rate corrected significance criterion of P < 0.025.
Given the common observation that activity in the MPFC decreases during cognitively demanding tasks that do not involve assessments of self-relevance relative to baseline resting states during which more self-referential activity is presumably transpiring (Gusnard and Raichle 2001), it is of some interest whether the variation in MPFC activity that correlated with the parametric variation of the FAV regressor was riding on an overall reduction or increase in MPFC activity as people were listening to the musical stimuli. Overall, the MusPlay regressor revealed neither activation nor deactivation within the MPFC, indicating that, overall, the mean level of activity in the MPFC was close to its resting state. Thus, the FAV-related activity would suggest that MPFC activity was reduced slightly below baseline during songs that elicited no autobiographical association and elevated above baseline during songs that elicited a strong autobiographical association.
Tonality Tracking Responses
One of the prior observations that motivated the hypothesis that the MPFC is an area that binds music and memories together was that the MPFC tracked the movement of a melody through tonal space as it traversed the 24 major and minor keys that underlie Western tonal music. Thus, I also evaluated a model that modeled the patterns of movement through tonal space for the autobiographically salient and nonsalient excerpts of music (Fig. 1B). The model tested an interaction of TT and autobiographical salience. The reason to use the descriptors of how the individual excerpts move through tonal space is that these time-varying descriptors capture the identity of individual excerpts of music and are therefore very specific probes with which to interrogate physiological responses to those excerpts of music. If these pieces are serving as partial retrieval cues for episodic memories or priming other mental images or semantic associations, then the activity within these areas might be expected to exhibit comparable temporal dynamics to those present in the music. Aside from activating memories for autobiographical and semantic knowledge, the music engages memory systems that support memory for the music itself and facilitate attentive engagement with the music. In the case of familiar pieces of music, memories for the lyrics, the melodies, harmonic progressions, rhythms, and timbres may become activated as the person hears the music and sings along in their mind. Thus, the temporal dynamics of activity in brain regions that support the retrieval of this information are expected to follow, in large part, the temporal dynamics of the music.
The bottom panels in Figure 4 illustrate TT responses in 3 representative subjects. The color at each voxel represents the degree to which that part of the brain exhibited relatively more TT during excerpts identified as autobiographically salient (red) than during nonautobiographical excerpts (blue). Green indicates TT without regard for autobiographical salience. The data for each of these subjects show that TT transpired within a network of brain areas distributed throughout the brain and that the details of these networks differed across subjects. However, careful inspection of the activation patterns reveals that certain common brain regions showed TT in these subjects. For example, Ss 1 and 2 showed TT responses along the superior temporal sulcus (STS) and STG. Each of the subjects showed some degree of TT in the MPFC and lateral prefrontal areas. Biases in TT toward autobiographically salient excerpts were observed in the MPFC, in the posterior cingulate cortex (Ss 1 and 2), and in the lateral prefrontal cortex (Ss 1–3). In S3, most TT voxels were biased toward autobiographically salient excerpts, whereas S2 showed a greater mixture of biases.
Figure 4 also illustrates within individual subjects the relationship between the TT and FAV models. Of interest here is the fact that these models reflect different timescales. The FAV model represents sustained responses throughout a 30-s epoch, whereas the TT model reflects the tonal/harmonic dynamics of the pieces that vary on a faster timescale (Fig. 1B). Though some points of overlap exist (small yellow arrowheads), it is clear that TT in one region does not predict a more sustained FAV response at that same location, even in the case of autobiographically salient stimuli. This result indicates that the fluctuations in BOLD signal within a brain area could reflect the movement of the music in tonal space without necessarily showing a change in the overall level of activity over the entire duration of the excerpt. Similarly, brain areas could show overall shifts in the level of activity related to the degree of familiarity, autobiographical salience, and affect, without necessarily following the structural details of the music.
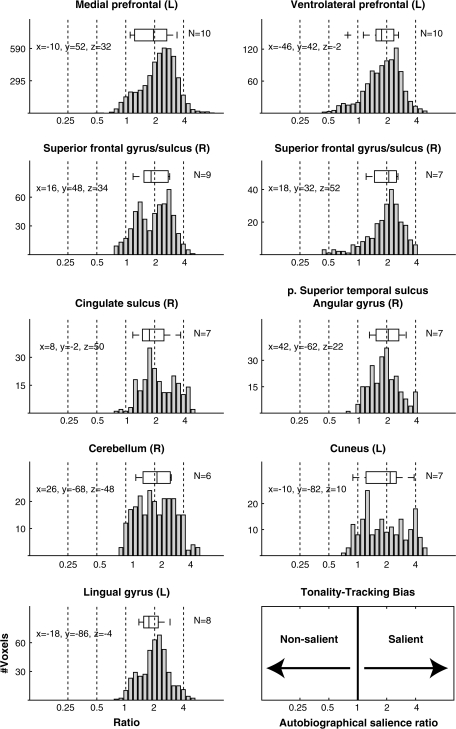
Despite the variability in spatial distributions of FAV and TT foci among individual subjects, several brain areas showed TT in a majority of subjects (Fig. 5; Table 5). Most prominent among these regions was the dorsal aspect of the MPFC encompassing BA 9 and extending both to BA 8 and BA 10. Ten subjects exhibited TT in this region. At a more lenient threshold, which allowed TT clusters to incorporate voxels activated by 3 subjects, the MPFC cluster contained TT voxels for all 13 subjects (Supplementary Fig. 5). Foci of TT in the MPFC overlapped with, or were immediately apposed to, areas that showed FAV responsiveness and more specifically independent effects of familiarity and autobiographical salience (see Fig. 5 sagittal slices at −10 and −5 mm and coronal slices from +35 to +55 mm with blue and black contours for familiarity and autobiographical salience, respectively). These findings provide converging evidence for the role of dorsal MPFC in binding together music and autobiographical memories. The strongest direct evidence, however, comes from the model shown in Figure 1B, which allows one to examine the autobiographical salience biases of those voxels that exhibited TT by comparing the amount of variance explained by the TT regressors for autobiographically salient songs with the amount of variance explained by the TT regressors for nonsalient songs. Figure 6 shows the distributions of biases for all TT voxels in the clusters identified in Table 5, as well as box and whisker plots representing the distribution of mean TT biases within each cluster across subjects. Within the MPFC cluster, the average bias of TT voxels in all subjects was toward autobiographically salient songs.
Table 5.
Summary of tonality tracking activation clusters
| Anatomical location | Brodmann area | No. of voxels in cluster | Location (mm) |
No. of subjects at peak | No. of subjects in cluster | ||
| x | y | z | |||||
| Cluster 1 | 1112 | 10 | |||||
| Superior frontal gyrus | 9 | −10 | 52 | 32 | 7 | ||
| Anterior cingulate cortex | 32 | −4 | 28 | 34 | 6 | ||
| Superior frontal gyrus | 8 | −8 | 32 | 48 | 6 | ||
| Anterior cingulate cortex | 32 | −8 | 42 | 8 | 5 | ||
| Superior frontal gyrus | 10 | −16 | 62 | 22 | 5 | ||
| Superior frontal gyrus | 8 | −4 | 42 | 50 | 5 | ||
| Superior frontal gyrus | 9 | 2 | 40 | 32 | 5 | ||
| Cluster 2 | 219 | 10 | |||||
| Lateral orbital gyrus | 47 | −46 | 42 | −2 | 6 | ||
| Inferior frontal gyrus | 45 | −42 | 38 | 6 | 6 | ||
| Inferior frontal sulcus | 46 | −40 | 32 | 14 | 6 | ||
| Middle frontal gyrus | 46 | −42 | 42 | 18 | 5 | ||
| Cluster 3 | 127 | 9 | |||||
| Superior frontal gyrus | 9/10 | 16 | 48 | 34 | 5 | ||
| Cluster 4 | 104 | 8 | |||||
| Lingual gyrus | 18 | −18 | −86 | −4 | 5 | ||
| Cluster 5 | 43 | 7 | |||||
| Cuneus | 17 | −10 | −82 | 10 | 5 | ||
| Cluster 6 | 55 | 7 | |||||
| Posterior superior temporal sulcus and angular gyrus | 39 | 42 | −62 | 22 | 5 | ||
| Cluster 7 | 60 | 7 | |||||
| Superior frontal sulcus | 8 | 18 | 32 | 52 | 5 | 7 | |
| Cluster 8 | 53 | 7 | |||||
| Cingulate sulcus | 24 | 8 | −2 | 50 | 5 | ||
| Cluster 9 | 61 | 6 | |||||
| Cerebellum | 26 | −68 | −48 | 5 | |||
Figure 6.
Distributions of TT biases in the TT clusters identified in Table 5. The ratios reflect the variance explained by TT of autobiographically salient songs divided by the variance explained TT of nonsalient songs (corrected for the number of songs in each category), thereby providing a measure of TT responsiveness to autobiographically salient versus nonsalient songs. A ratio of 1 indicates that a voxel tracked autobiographically salient and nonsalient songs equally well, whereas larger ratios indicate a bias toward tracking autobiographically salient songs. The box and whisker plots provide information about the distribution of mean TT biases across subjects with TT voxels in that cluster. The number of subjects is indicated in the top right corner of each plot. The whiskers indicate the range of the subject means (outliers indicated with a +), whereas the left, middle, and right lines forming the box represent the lower quartile, median, and upper quartile of the subject means, respectively. The letters in parentheses (R and L) following the anatomical label indicate the brain hemisphere. The x, y, z coordinates (in mm) indicate a location within the cluster that was activated by the greatest number of subjects.
The other prefrontal area in which 10 subjects showed TT was the left VLPFC (BA 45/46/47) along the rostral section of the inferior frontal sulcus and extending to the lateral orbital gyrus (Fig. 5, −40 and −45 mm sagittal slices and +35 through +45 mm coronal slices). As with the MPFC cluster, a more lenient criterion for defining the cluster resulted in the inclusion of TT voxels from all subjects in the cluster (Supplementary Fig. 5). Within the VLPFC cluster, the distribution of TT voxels was also skewed toward autobiographically biased, and all but one subject showed an average TT bias toward autobiographically salient songs.
TT was also found for the majority of subjects in posterior areas, most notably in the extrastriate visual areas (BA 18), in the temporal lobe, and in the cerebellum. In the right hemisphere, the posterior temporal activations extended to the angular gyrus (BA 39). Supplementary Figure 5 shows the summary TT map that was obtained when clusters could include voxels activated by only 3 subjects. The main consequence of lowering the threshold was an increase in the size of the posterior cluster of TT voxels, such that it encompassed posterior regions of the STG and sulcus and additional extrastriate areas. The greater topographical variability in the posterior TT activations is perhaps best understood in the context of prefrontally guided retrieval of semantic content and representations from posterior cortices, as described below, which would be expected to vary more extensively across memories within and between subjects.
Discussion
Music and Memories in the Prefrontal Cortex
The primary hypothesis, that music and memories are associated in the MPFC, was supported by 2 complementary pieces of evidence. First, dorsal MPFC (BA 8/9) responded parametrically to the degree of autobiographical salience experienced over the course of individual excerpts. Second, the same areas (either the same or adjoining voxels) tracked the movements of the musical excerpts through tonal space, with stronger TT for autobiographically salient songs. The locus of the FAV, Autobio, familiarity, and TT responses in dorsal MPFC was found at the same location at which a familiarity effect has been reported for odors and music (Plailly et al. 2007). The present results demonstrate not only that this area shows a generalized increase to the degree of familiarity and autobiographical salience but also that this region follows the structural aspects of the music. In other words, this region exhibits the properties of a mechanism that associates structural aspects of a retrieval cue with episodic memories. Rostral and ventral aspects of the MPFC also exhibited FAV and TT responses and a main effect of autobiographical salience. Activation foci were in close proximity to a previously reported effect of musical familiarity (Plailly et al. 2007), TT (Janata, Birk, et al. 2002), and positive affect experienced in response to music (Blood et al. 1999; Brown, Martinez, and Parsons 2004). The effect of increased positive valence, in particular, echoed the ventral medial activations reported in the latter studies.
MEAM-related and TT activity were not, however, restricted to the MPFC. One of the unique findings in the present study was the presence of FAV and TT responses in both lateral and medial prefrontal areas. Typically, lateral prefrontal cortex is engaged during tasks that require orienting toward the external environment (Cabeza and Nyberg 2000; Corbetta and Shulman 2002), whereas MPFC is mainly involved in the evaluation of self-relevant information and emotions (Ochsner et al. 2004; Gilbert et al. 2006; Northoff et al. 2006; Schmitz and Johnson 2007). Lateral and medial prefrontal areas are usually associated with “task-positive” and “task-negative” (“default-mode” or “self-referential”) networks, respectively, which exhibit anticorrelated activity (Fox et al. 2005; Fransson 2006). Behaviors and experimental paradigms in which elements of both networks are simultaneously active are, therefore, of considerable interest because they may facilitate our understanding of these networks’ interactions in everyday experiential states.
The most parsimonious explanation for the recruitment of both prefrontal regions during MEAMs is that multiple memory retrieval processes are summoned in order to assemble different bits of semantic and episodic content into a multifaceted recollective experience (sequence of episodic memories) that also has an affective component to it (Miller and Cohen 2001; Wood and Grafman 2003; Ochsner and Gross 2005). These processes may operate on the structural aspects of the stimulus as well as its semantic content. From the perspective of content retrieval, lateral retrieval processes may control the retrieval of semantic or episodic information (Cabeza and Nyberg 2000; Wagner et al. 2001). By contrast, medially situated processes (BA 8/10) serve the retrieval of episodic and affective elements of autobiographical memories (Gilboa 2004; Svoboda et al. 2006). More generally, prefrontal cortex controls the retrieval of representations from posterior brain areas, such as extrastriate visual or temporal lobe areas described below (Miller and Cohen 2001; Gold and Buckner 2002).
In the music domain, VLPFC activity has been reported mainly in studies that manipulate musical structure. Perturbed syntactic relationships such as insertions of unexpected chords into chord progressions elicit robust responses in VLPFC, particularly in the frontal opercular region at the junction with the anterior insula (Tillmann et al. 2003; Koelsch 2005). VLPFC also responds more strongly to extended pieces of real music when they are played normally compared with when they are played in reverse (Levitin and Menon 2003) or simultaneously with a pitch-shifted copy of themselves that renders them unpleasant (Koelsch et al. 2006). These results have suggested that this region helps integrate musical events into syntactically coherent sequences (Levitin and Menon 2003; Tillmann et al. 2003; Koelsch 2005) as part of its domain general role in structuring syntactic relationships (Patel 2003; Fiebach and Schubotz 2006). The TT responses in left VLPFC (BA 45/46/47) in the present study further this set of observations by tying activity in this region to an explicit model of dynamical musical structure.
The observation of TT in both medial and lateral prefrontal regions is also a first step toward understanding the coupling of prefrontal areas during the processing of musical stimuli. In the original study of TT (Janata, Birk, et al. 2002), activation of MPFC was highlighted because the area showed TT consistently across days in individual subjects and across the entire sample of subjects in response to a single melody that modulated through all the major and minor keys. TT was not, however, restricted only to the MPFC, with individual listeners exhibiting consistent TT across recording sessions in other prefrontal and posterior brain areas (see supplementary table S2 in Janata, Birk, et al. 2002). The present study extends the previous TT results by generalizing to a large number of selections of recorded popular music, thereby demonstrating TT responses in a more ethological context.
Music and Autobiographical Memories in a Distributed Network
A distributed-representation view of autobiographical memory (Conway and Pleydell-Pearce 2000) maintains that an autobiographical memory experience is the outcome of a constructive process in which different pieces and forms of autobiographical knowledge—such as specific event or lifetime period knowledge—are dynamically summoned and structured into a particular memory event (Conway and Pleydell-Pearce 2000). The relevant knowledge is distributed in a broad associative network and can vary in the degree of episodic versus semantic content or personal versus nonpersonal salience. Such variations in memory content are, therefore, expected to bias the spatial distributions of activity across a number of cortical areas during remembering episodes (Damasio 1989; Levine et al. 2004; Svoboda et al. 2006).
Overall, the strong left-hemisphere bias in prefrontal and temporal activations for the FAV effect corroborates the results of many neuroimaging studies of autobiographical memory retrieval, though some notable differences exist (Maguire 2001; Svoboda et al. 2006). In relation to previous studies, this study differs substantially in the type of retrieval cue and task instructions and consequently the nature of autobiographical memory retrieval. The degree to which these differences explain the differences in activation patterns remains to be determined by further studies. The most notable difference is in the recruitment of medial temporal lobe (MTL) structures. The analysis of 24 studies by Svoboda et al. (2006) found reports of medial prefrontal and MTL involvement in 62% and 58% of the studies, respectively. Together with posterior cingulate and midlateral temporal areas, these were the most common activation loci. I observed considerable medial prefrontal engagement as a function of familiarity and also autobiographical salience but I did not find any MTL activation. Although the specific details of contrasts between conditions might account for some of the variability in MTL activation sightings across studies (Svoboda et al. 2006), an analysis of the methods in the 24 studies suggests that tasks emphasizing event retrieval, brief, and deliberate retrieval episodes associated with short phrase cues and the need to make decisions about viewed items are all conditions that promote MTL engagement (Maguire and Mummery 1999; Ryan et al. 2001; Niki and Luo 2002; Maguire and Frith 2003; Piefke et al. 2003; Addis et al. 2004; Cabeza et al. 2004; Gilboa et al. 2004; Levine et al. 2004; Svoboda et al. 2006).
Subjects in the present MEAM study were aware that the study was about autobiographical memories and that they would be asked to rate their salience, but subjects were not instructed to attempt to retrieve memories in response to hearing the musical excerpts. A difference in spontaneous versus effortful memory retrieval could, therefore, explain the difference in MTL results. One other study that was designed such that it could elicit spontaneous autobiographical memory recall (in response to photographs of personally familiar and unfamiliar events that had not been viewed prior to the scan) did find activation of the hippocampus for personally relevant events (Gilboa et al. 2004). However, subjects were asked to recall and relive the event depicted in the photographs to the best of their ability and retrieve as much episodic information as possible. Thus, remembering episodes were likely associated with effortful retrieval.
Another commonly reported activation locus in autobiographical memory studies is the posterior cingulate gyrus (Svoboda et al. 2006). In the present study, the most extensive activation of the posterior cingulate was observed in the combined contrast that represented the responses to familiar, autobiographically sensitive, and positively valenced pieces of music (P < 0.005 unc.; Figs 2 and 3). Smaller activation foci in this region were observed for the separate familiarity, autobiographical salience, and valence regressors, with the strictest significance criterion being reached for the effect of familiarity (Table 2). The distinctions among the familiarity and autobiographical salience effects highlight a unique aspect of this study, which is the fact that a subject can have a very vivid experience of the retrieval cue which is associated with their autobiographical past, which leads to a high familiarity rating, yet not necessarily have a strong autobiographical remembering episode that entails retrieval of memories other than the details of the retrieval cue itself.
Finding suitable retrieval cues poses a challenge to autobiographical memory research, and previous neuroimaging studies of autobiographical memory have utilized single words or images as retrieval cues in conditions in which subjects are explicitly required to retrieve memories (Gilboa 2004; Svoboda et al. 2006) or imagine themselves in extended autobiographical episodes (Gilboa et al. 2004; Levine et al. 2004). The present study complements this research by showing that the left-hemispheric bias typically observed in autobiographical memory studies also arises in situations in which 1) the cues are complex, naturally occurring stimuli that evolve over time and 2) the retrieval demands are low. MEAMs occurred spontaneously and presumably with little retrieval effort as there was no aspect of the task design that forced subjects to retrieve memories to any given cue.
Posterior and Subcortical Activations
Hearing a familiar song resulted in the activation of several structures that have been implicated in a variety of sequencing tasks and music studies (Janata and Grafton 2003), including the pre-SMA, left IFG, bilateral thalamus, and several cerebellar loci, primarily in the right hemisphere. These activations likely reflect a common response to hearing a piece of music one is familiar with, which is to sing along in one's mind or at least anticipate various melodic, harmonic, lyric, or timbral events in the music. Cerebellar activations, primarily lateralized to the right, are also commonly observed in studies of autobiographical memory (Svoboda et al. 2006). Several of the cerebellar loci that showed an effect of familiarity were within close proximity of activations reported in music studies in which subjects listened to familiar pieces of music that induced chills (Blood and Zatorre 2001), performed target detection tasks on a familiar melody (Janata, Birk, et al. 2002), listened to familiar and unfamiliar excerpts of music (Platel et al. 2003), repeated or harmonized with melodies they had just heard (Brown, Martinez, Hodges, et al. 2004), or completed melodic fragments with spontaneously generated melodies (Brown et al. 2006). Other studies utilizing musical stimuli have also found cerebellar responses, though in those cases the activation loci were quite different from those observed in the present study (Janata, Tillmann, and Bharucha 2002; Levitin and Menon 2003; Tillmann et al. 2003). Further studies will be needed to fully understand the circumstances that give rise to and organize cerebellar responses to familiar stimuli.
Given the absence of visual stimulation in this experiment, the observation of TT in extrastriate visual areas is best interpreted in terms of top-down activations of visual cortex. Top-down activation of extrastriate areas is commonly observed in visual mental imagery paradigms (e.g., Handy et al. 2004), during memory retrieval (Wheeler et al. 2000), and in some autobiographical memory studies (Svoboda et al. 2006). The strong positive correlation between the vividness of visual mental images and salience of MEAMs (Janata et al. 2007) suggests that the activity in extrastriate cortex in this experiment was related to music-evoked visual mental images, and the correlation with TT suggests that memory-related visual images in visual cortex unfold with the same temporal dynamics as the harmonic sequences and phrases in the music.
The posterior portions of the STS exhibited increased activity as a function of responding to questions (QAP), FAV, and TT. This region is an important locus of semantic processing and polymodal binding and is activated in autobiographical memory studies (Svoboda et al. 2006). The N400, an index of brain electrical activity that marks semantic expectancy violations, is often localized to this area (Halgren et al. 2002). N400 evidence indicates that the comparison of semantic concepts primed by music and related and unrelated words occurs in these regions (Koelsch et al. 2004).
In contrast to previous studies of autobiographical memory, the present experiment illustrated the activation of an autobiographical memory network on 2 different timescales. One was on the order of 30 s epochs in which different degrees of familiarity, autobiographical salience, and affective valence were modeled as sustained shifts in BOLD response. The other was on the faster timescale of tonality changes associated with the melodies and chord progressions in the songs, a timescale akin to phrase structure and prosody in language. Although many further experiments remain to be performed, for example, to tease apart contributions of memory processes to the retrieval of lyrics or to relate distributed activation patterns to the content of specific autobiographical episodes, this study establishes the feasibility of studying autobiographical memory in an experimental context that closely resembles the common experience of hearing a song that evokes vivid memories and shows that the MPFC plays a central role in this experience.
Supplementary Material
Supplementary movies and supplementary figures 1–5 can be found at: http://www.cercor.oxfordjournals.org/
Funding
Templeton Advanced Research Program grant from the Metanexus Institute; National Institutes of Health (DC05146). Funding to pay the Open Access charges for this article was provided by a Templeton Advanced Research Program grant from the Metanexus Institute.
Acknowledgments
I am grateful to Susannah Cohen, John Ryan, Michael Buonocore, and Cheryline Mancusi for assistance in data collection and an anonymous reviewer for helpful comments on the manuscript. Conflict of Interest: None declared.
References
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999;2:382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- Brown S, Martinez MJ, Hodges DA, Fox PT, Parsons LM. The song system of the human brain. Cogn Brain Res. 2004;20:363–375. doi: 10.1016/j.cogbrainres.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Brown S, Martinez MJ, Parsons LM. Passive music listening spontaneously engages limbic and paralimbic systems. Neuroreport. 2004;15:2033–2037. doi: 10.1097/00001756-200409150-00008. [DOI] [PubMed] [Google Scholar]
- Brown S, Martinez MJ, Parsons LM. Music and language side by side in the brain: a PET study of the generation of melodies and sentences. Eur J Neurosci. 2006;23:2791–2803. doi: 10.1111/j.1460-9568.2006.04785.x. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC. Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. J Cogn Neurosci. 2004;16:1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cuddy LL, Duffin J. Music, memory, and Alzheimer's disease: is music recognition spared in dementia, and how can it be assessed? Medical Hypotheses. 2005;64:229–235. doi: 10.1016/j.mehy.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation—a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with MRI and blood supply. Vienna (Austria): Springer-Verlag; 1999. [Google Scholar]
- Fiebach CJ, Schubotz RI. Dynamic anticipatory processing of hierarchical sequential events: a common role for Broca's area and ventral premotor cortex across domains? Cortex. 2006;42:499–502. doi: 10.1016/s0010-9452(08)70386-1. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory—one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42:1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, Van Petten C, Marinkovic K, Lewine JD, Dale AM. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. NeuroImage. 2002;17:1101–1116. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- Handy TC, Miller MB, Schott B, Shroff NM, Janata P, Van Horn JD, Inati S, Grafton ST, Gazzaniga MS. Visual imagery and memory—do retrieval strategies affect what the mind's eye sees? Eur J Cogn Psychol. 2004;16:631–652. [Google Scholar]
- Janata P. Brain networks that track musical structure. Ann N Y Acad Sci. 2005;1060:111–124. doi: 10.1196/annals.1360.008. [DOI] [PubMed] [Google Scholar]
- Janata P. Navigating tonal space. In: Hewlett WB, Selfridge-Field E, Correia E, editors. Tonal theory for the digital age. Stanford (CA): Center for Computer Assisted Research in the Humanities; 2007–2008. pp. 39–50. [Google Scholar]
- Janata P, Birk JL, Tillmann B, Bharucha JJ. Online detection of tonal pop-out in modulating contexts. Music Percept. 2003;20:283–305. [Google Scholar]
- Janata P, Birk JL, Van Horn JD, Leman M, Tillmann B, Bharucha JJ. The cortical topography of tonal structures underlying Western music. Science. 2002;298:2167–2170. doi: 10.1126/science.1076262. [DOI] [PubMed] [Google Scholar]
- Janata P, Grafton ST. Swinging in the brain: shared neural substrates for behaviors related to sequencing and music. Nat Neurosci. 2003;6:682–687. doi: 10.1038/nn1081. [DOI] [PubMed] [Google Scholar]
- Janata P, Tillmann B, Bharucha JJ. Listening to polyphonic music recruits domain-general attention and working memory circuits. Cogn Affect Behav Neurosci. 2002;2:121–140. doi: 10.3758/cabn.2.2.121. [DOI] [PubMed] [Google Scholar]
- Janata P, Tomic ST, Rakowski SK. Characterisation of music-evoked autobiographical memories. Memory. 2007;15:845–860. doi: 10.1080/09658210701734593. [DOI] [PubMed] [Google Scholar]
- Koelsch S. Neural substrates of processing syntax and semantics in music. Curr Opin Neurobiol. 2005;15:207–212. doi: 10.1016/j.conb.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Fritz T, von Cramon DY, Muller K, Friederici AD. Investigating emotion with music: an fMRI study. Hum Brain Mapp. 2006;27:239, 250. doi: 10.1002/hbm.20180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S, Kasper E, Gunter TC, Sammler D, Schulze K, Friederici AD. Music, language, and meaning: brain signatures of semantic processing. Nat Neurosci. 2004;7:302–307. doi: 10.1038/nn1197. [DOI] [PubMed] [Google Scholar]
- Krumhansl CL. Cognitive foundations of musical pitch. New York: Oxford University Press; 1990. [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroanatomy of episodic and semantic autobiographical remembering: a prospective functional MRI study. J Cogn Neurosci. 2004;16:1633–1646. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Levitin DJ, Menon V. Musical structure is processed in “language” areas of the brain: a possible role for Brodmann area 47 in temporal coherence. NeuroImage. 2003;20:2142–2152. doi: 10.1016/j.neuroimage.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126:1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9:54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Niki K, Luo J. An fMRI study on the time-limited role of the medial temporal lobe in long-term topographical autobiographic memory. J Cogn Neurosci. 2002;14:500–507. doi: 10.1162/089892902317362010. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, Greck M, Bennpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Patel AD. Language, music, syntax and the brain. Nat Neurosci. 2003;6:674–681. doi: 10.1038/nn1082. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Plailly J, Tillmann B, Royet J-P. The feeling of familiarity of music and odors: the same neural signature? Cereb Cortex. 2007;17:2650–2658. doi: 10.1093/cercor/bhl173. [DOI] [PubMed] [Google Scholar]
- Platel H, Baron JC, Desgranges B, Bernard F, Eustache F. Semantic and episodic memory of music are subserved by distinct neural networks. NeuroImage. 2003;20:244–256. doi: 10.1016/s1053-8119(03)00287-8. [DOI] [PubMed] [Google Scholar]
- Ryan L, Nadel L, Keil K, Putnam K, Schnyer D, Trouard T, Moscovitch M. Hippocampal complex and retrieval of recent and very remote autobiographical memories: evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus. 2001;11:707–714. doi: 10.1002/hipo.1086. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: a brief review and framework of neural systems underlying appraisal. Neurosci Biobehav Rev. 2007;31:585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, et al. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann B, Janata P, Bharucha JJ. Activation of the inferior frontal cortex in musical priming. Cognit Brain Res. 2003;16:145–161. doi: 10.1016/s0926-6410(02)00245-8. [DOI] [PubMed] [Google Scholar]
- Toiviainen P, Krumhansl CL. Measuring and modeling real-time responses to music: the dynamics of tonality induction. Perception. 2003;32:741–766. doi: 10.1068/p3312. [DOI] [PubMed] [Google Scholar]
- Tomic ST, Janata P. Ensemble: a web-based system for psychology survey and experiment management. Behav Res Methods. 2007;39:635–650. doi: 10.3758/bf03193036. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory's echo: vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci USA. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci. 2003;4:139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.