Abstract
Feedback inhibition of the amygdala via medial prefrontal cortex (mPFC) is an important component in the regulation of complex emotional behaviors. The functional dynamics of this corticolimbic circuitry are, in part, modulated by serotonin (5-HT). Serotonin 2A (5-HT2A) receptors within the mPFC represent a potential molecular mechanism through which 5-HT can modulate this corticolimbic circuitry. We employed a multimodal neuroimaging strategy to explore the relationship between threat-related amygdala reactivity, assessed using blood oxygen level–dependent functional magnetic resonance imaging, and mPFC 5-HT2A density, assessed using [18F]altanserin positron emission tomography in 35 healthy adult volunteers. We observed a significant inverse relationship wherein greater mPFC 5-HT2A density was associated with reduced threat-related right amygdala reactivity. Remarkably, 25–37% of the variability in amygdala reactivity was explained by mPFC 5-HT2A density. We also observed a positive correlation between mPFC 5-HT2A density and the magnitude of right amygdala habituation. Furthermore, functional coupling between the amygdala and mPFC was positively correlated with 5-HT2A density suggesting that effective integration of emotionally salient information within this corticolimbic circuitry may be modulated, at least in part, by mPFC 5-HT2A. Collectively, our results indicate that mPFC 5-HT2A is strongly associated with threat-related amygdala reactivity as well as its temporal habituation and functional coupling with prefrontal regulatory regions.
Keywords: 5-HT2A, amygdala, corticolimbic circuitry, habituation, serotonin
Introduction
Regulation of emotional arousal involves coordinated communication between cortical and subcortical structures. Dysfunction within the neural circuitry for emotional arousal and regulation contributes to risk for psychiatric illness, including anxiety disorders and major depression, and represents a pathophysiological marker of these same conditions (Mayberg 2003; Phillips et al. 2003). Among areas of the brain implicated in this regulatory network, the medial prefrontal cortex (mPFC) is thought to play a critical role in regulating amygdala-mediated arousal in response to emotionally salient, especially threat-related, environmental cues (LeDoux 2000; Wood and Grafman 2003). Recent rodent work suggests that homologous prefrontal regions act to inhibit amygdala output via glutamatergic stimulation of inhibitory γ-aminobutyric acidergic (GABAergic) neurons within the amygdala (Quirk et al. 2003). In humans, variation in mPFC–amygdala functional coupling has been associated with individual differences in behavior and risk for psychiatric illness (Drevets et al. 1992; Pezawas et al. 2005).
The central serotonin (5-HT) system has been linked to variation in neural reactivity within the amygdala and PFC to emotionally salient environmental cues (Hariri et al. 2002; Holmes et al. 2003; Pezawas et al. 2005; Fisher et al. 2006; Hariri and Holmes 2006; Harmer et al. 2006; Weisstaub et al. 2006; Bigos et al. 2008). Through negative feedback inhibition, the serotonin 1A (5-HT1A) somatodendritic autoreceptor acts to regulate 5-HT release at corticolimbic targets associated with emotional reactivity. Recently, we reported a significant inverse relationship between 5-HT1A autoreceptor density and threat-related amygdala reactivity in 20 healthy adult volunteers (Fisher et al. 2006). In this sample, 30–44% of the variability in amygdala reactivity was accounted for by 5-HT1A density suggesting that the capacity for regulating 5-HT release is an important modulatory component of the neural circuitry for emotional arousal. More generally, this finding further links increased 5-HT signaling with potentiated amygdala reactivity (Hariri et al. 2002; Forster et al. 2006; Burghardt et al. 2007; Rhodes et al. 2007; Bigos et al. 2008).
In addition to such autoregulatory serotonergic mechanisms impacting amygdala reactivity, postsynaptic receptors are likely instrumental in determining 5-HT modulation of this circuitry. Of these, excitatory serotonin 2A (5-HT2A) receptors localized in the mPFC may be of particular importance. Glutamatergic neurons represent the predominant neuronal population expressing the 5-HT2A receptor within mPFC (Jakab and Goldman-Rakic 1998; Leysen 2004; de Almeida and Mengod 2007). Furthermore, proximal portions of the apical dendrites of these glutamatergic neurons may represent a “hot spot” of 5-HT2A localization coincident with relatively dense 5-HT innervation (Blue et al. 1988; Jakab and Goldman-Rakic 1998). Interestingly, whereas rapid increases in amygdala 5-HT release are associated with the initiation of fear-related behaviors, relatively delayed 5-HT release in mPFC is associated with their attenuation (Forster et al. 2006). Taken together, these data suggest that the 5-HT2A receptor is ideally situated to mediate excitatory effects of 5-HT release on mPFC projection neurons that, in turn, facilitate regulation of amygdala reactivity and associated emotional behaviors.
Previous research in major depression is consistent with this effect of altered prefrontal 5-HT2A receptor density (Stockmeier 2003). One study reported increased 5-HT2A receptor density in frontal cortex within a recovered depressed population (Bhagwagar et al. 2006). In contrast, a second study reported decreased 5-HT2A receptor density in frontal cortex within a currently depressed population (Yatham et al. 2000). A third study reported decreased subgenual prefrontal cortex (sgPFC) 5-HT2A receptor density within a recovered population of women with eating disorders, which often are comorbid with mood disorders including depression (Bailer et al. 2004). These findings further implicate prefrontal 5-HT2A receptors as a potentially important mechanism in the regulation of corticolimbic circuit function supporting emotional behaviors. To date, however, no studies have explored the relationship between 5-HT2A receptor density in the mPFC and amygdala reactivity in humans. In the current study, we explored the relationship between human mPFC 5-HT2A receptor density and threat-related amygdala reactivity using a multimodal neuroimaging strategy in 35 healthy adult volunteers. Blood oxygen level–dependent functional magnetic resonance imaging (BOLD fMRI) was used to measure amygdala reactivity in response to threat-related facial expressions (Brown et al. 2005; Brown et al. 2006; Fisher et al. 2006; Manuck et al. 2007; Zhou et al. 2008). Positron emission tomography (PET) was used to assess 5-HT2A receptor density within 2 subregions of the mPFC, namely the pregenual prefrontal cortex (pgPFC) and sgPFC, using [18F]altanserin, a radioligand with high affinity and specificity for the 5-HT2A receptor. We focused on these 2 mPFC subregions because they are both functionally and structurally interconnected with the amygdala and richly innervated by 5-HT neurons (Pandya et al. 1981; Blue et al. 1988; McDonald 1998; Pezawas et al. 2005).
Based on the work summarized above illustrating 1) mPFC regulation of amygdala reactivity via feedback (i.e., “top–down”) inhibition and 2) localization of 5-HT2A receptors to proximal areas of the dendrites of mPFC glutamatergic projection neurons, we hypothesized that greater 5-HT2A receptor density within mPFC (both pgPFC and sgPFC) would be inversely related to amygdala reactivity reflecting a greater capacity for prefrontal regulation. We sought to further characterize the relationship between serotonergic regulation of mPFC and amygdala reactivity using functional connectivity and hypothesized that greater pgPFC and sgPFC 5-HT2A receptor density would be associated with increased functional coupling between these regions and the amygdala. Additionally, we explored the degree to which mPFC 5-HT2A receptor density was associated with habituation of amygdala reactivity, a commonly observed phenomenon similar to extinction and likely to reflect the capacity for prefrontal regulatory control (Breiter et al. 1996; Büchel et al. 1998; Herry et al. 2007).
Materials and Methods
Subjects
Thirty-five healthy adult volunteers participated after providing written informed consent in accordance with the University of Pittsburgh Institutional Review Board (18 males, age: 37.7 ± 12.8 [mean ± standard deviation {SD}] years). Subjects were recruited through local advertisements, referrals, and ongoing studies. Subjects were generally healthy with exclusion criteria including 1) current or lifetime psychiatric diagnoses assessed by Structured Clinical Interview (Diagnostic and Statistical Manual of Mental Disorders, Version IV), 2) cardiovascular disease or diabetes, 3) history of substance abuse or use of antidepressants, 4) early dementia or mild cognitive impairment according to the Mini-Mental State Examination (scores exceeding 27) (Folstein et al. 1975), and 5) sleep disorders assessed by the Pittsburgh Quality Sleep Index (Buysse et al. 1989). Most subjects (N = 30) completed both fMRI and PET scans on the same day. All subjects completed the fMRI scan in the morning and the PET scan in the afternoon. Those subjects who did not complete both scans on the same day (N = 5) completed the PET and fMRI scans within 1 month. To limit the potential effects of circulating reproductive hormone levels on measures of 5-HT2A density, all premenopausal women (N = 11) were scanned within days 3 and 7 of their menstrual cycle (Moses et al. 2000).
fMRI Protocol
The experimental fMRI paradigm consisted of 4 blocks of a face-processing task interleaved with 5 blocks of a sensorimotor control task as described previously (Fisher et al. 2006). Subject performance (accuracy and reaction time) was monitored during all scans. During the face-processing task, subjects viewed a trio of faces (expressing either anger or fear) and selected 1 of 2 faces (bottom) identical to a target face (top). Angry and fearful facial expressions can represent honest indicators of ecologically valid threat, especially that related to conspecific challengers (Darwin and Ekman 1998). Within this context, we interpret the amygdala activation elicited by our task as being threat related. Each face-processing block consisted of 6 images, balanced for sex and representing one target affect (angry or fearful) all derived from a standard set of pictures of facial affect (Ekman and Friesen 1976). During the sensorimotor control blocks, subjects viewed a trio of simple geometric shapes (circles and vertical and horizontal ellipses) and selected 1 of 2 shapes (bottom) identical to a target shape (top). Each sensorimotor control block consisted of 6 different shape trios. All blocks were preceded by a brief instruction (“Match Faces” or “Match Shapes”) lasting 2 s. In the face-processing blocks, each of the 6 face trios was presented for 4 s with a variable interstimulus interval of 2–6 s (mean = 4 s) for a total block length of 48 s. In the sensorimotor control blocks, each of the 6 shape trios was presented for 4 s with a fixed interstimulus of 2 s for a total block length of 36 s. Total protocol time was 390 s. Task and control blocks were interleaved in a fixed order (CFCACFCAC; C = control block, F = task block with fear expressions, A = task block with anger expressions) with each task block presented twice. As we were not interested in neural networks associated with face-specific processing per se, but rather in eliciting a maximal amygdala response across all subjects that we could then interrogate for genotype effects, we chose not to use neutral faces as control stimuli because neutral faces can be subjectively experienced as affectively laden or ambiguous and thus engage the amygdala (Schwartz, Wright, Shin, Kagan, and Rauch, 2003; Wright et al. 2003).
fMRI Acquisition Parameters
Acquisition parameters have been described previously (Fisher et al. 2006). Briefly, each subject was scanned using a GE Signa 1.5-T head-only scanner (GE Medical Systems, Milwaukee, WI). BOLD functional images were acquired using a reverse spiral sequence covering 28 slices (3.8-mm thick) encompassing the entire cerebrum and the majority of the cerebellum (repetition time = 2000 ms, echo time = 35 ms, field-of-view [FOV] = 24 cm, matrix = 64 × 64). All scanning parameters were selected to optimize BOLD signal while maintaining enough slices to acquire whole-brain data. Prior to the acquisition of fMRI data for each subject, we acquired and visually inspected localizer scans for artifacts (e.g., ghosting) as well as good signal across the entire volume of acquisition, including the medial temporal lobes. Additionally, an autoshimming procedure was conducted before the acquisition of BOLD data in each subject to minimize field inhomogeneities. fMRI data for all 35 subjects included in this study were cleared of such problems.
PET Acquisition Parameters
Technical detail concerning the PET imaging procedures can be found in previously published work and is described below (Price et al. 2001, 2005; Bailer et al. 2004; Bailer et al. 2007). [18F]altanserin is a 5-HT2A receptor antagonist with high affinity and specificity for the 5-HT2A receptor relative to other 5-HT receptor subtypes as well as noradrenergic α1 and dopaminergic D2 receptors (Leysen 1989, 1990). The radiosynthesis of [18F]altanserin was performed using a modification of the original method (Lemaire et al. 1991) that has been applied in several studies in our laboratory (Meltzer et al. 1998; Smith et al. 1998; Price et al. 2001; Bailer et al. 2004, 2007). Catheters were placed in an antecubital vein for radiotracer injection and a radial artery for arterial blood sampling. PET scans were acquired using an ECAT HR+ PET scanner (CTI PET systems, Knoxville, TN) in 3D imaging mode (63 transaxial planes, 2.4-mm thickness, 15.2-cm FOV). Head movement was minimized by use of a softened thermoplastic face mask system. A 10-min transmission scan was acquired using rotation rods of 68 Ge/68 Ga for attenuation correction of emission data. Dynamic PET imaging was initiated at the start of the [18F]altanserin injection (slow bolus over 20 s) for which the mean injected dose was 7.23 ± 0.32 (mean ± SD) mCi [18F]altanserin. The PET data were acquired as 22 time frames over 91 min (6 × 20 s, 2 × 30 s, 1 × 1 min, 2 × 1.5 min, 3 × 3 min, 1 × 5 min, and 7 × 10 min). PET data were corrected for scatter, and image reconstruction was performed using filtered back projection for a final reconstructed image resolution of about 6 mm. Approximately thirty-five 0.5-mL hand-drawn arterial blood samples were collected for each subject over the scanning interval (approximately 20 samples in the initial 2 min of scanning). The total plasma radioactivity concentration was corrected for the presence of radiolabeled [18F]altanserin metabolites, and this “metabolite-corrected” arterial input function was used for data analysis.
Structural MR images (GE Signa 1.5-T scanner) were acquired for each subject using a spoiled-gradient recalled sequence with parameters optimized for contrast between gray matter, white matter, and cerebrospinal fluid. Regions of interest (ROIs) were drawn on the resliced MR images for each subject and applied to their respective, coregistered PET images (ROIs drawn by S.K.Z. and C.B.). ROIs were identified for the sgPFC, pgPFC, and cerebellum, which were used to estimate nondisplaceable radiotracer uptake (ND: free and nonspecific concentrations). The data were analyzed using the Logan graphical method (Logan et al. 1990; Bailer et al. 2007). The Logan analysis was applied over the 12- to 90-min postinjection integrals (10 points) to obtain regional [18F]altanserin distribution volume values (VT). The VT values were used to determine the nondisplaceable binding potential value, BPND, that is a measure of specific receptor binding. The BPND is directly proportional to Bavail/Kd, where Bavail is the concentration of receptors available for radiotracer binding (i.e., not occupied by endogenous 5-HT) and Kd is the equilibrium dissociation rate constant (i.e., inversely related to binding affinity). Partial volume effects due to differences in cerebral volumes were corrected for in calculating BPND values using a previously validated 2-component MR-based atrophy correction algorithm (Meltzer et al. 1990, 1999; Cidis Meltzer et al. 2001).
fMRI Data Analysis
Whole-brain image analysis was completed using the general linear model (GLM) of SPM2 (http://www.fil.ion.ucl.ac.uk/spm). Images for each subject were realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model, and smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter, set at 6-mm full-width at half-maximum. Voxelwise signal intensities were ratio normalized to the whole-brain global mean. These preprocessed data sets were analyzed using second-level random-effects models that account for both scan-to-scan and participant-to-participant variability to determine task-specific regional responses. Following preprocessing, linear contrasts employing canonical hemodynamic response functions were used to estimate task-specific (i.e., task > control) BOLD activation for each individual and scan. These individual contrast images (i.e., weighted sum of the beta images) were then used in second-level random-effects models to determine mean task-specific amygdala reactivity using 1-sample t-tests. Our amygdala ROI was constructed using the Talairach Daemon option of the WFU PickAtlas Tool (v 1.04) (Maldjian et al. 2003, 2004). All analyses were thresholded at a voxel level of P < 0.05, false-discovery rate (FDR) corrected for multiple comparisons, within an inclusive mask of activations of interest and an extent threshold of at least 10 contiguous voxels. Because of our a priori, directionally specific hypotheses and our use of a rigorous random-effects model, these statistical thresholds effectively control for “false positives” arising from multiple comparisons. All neuroimaging data are reported using the coordinate system of Talairach and Tournoux.
Regression Analyses
The relationship between threat-related amygdala reactivity and mPFC 5-HT2A density was determined using linear regression analyses between the single-subject amygdala BOLD and ROI-specific 5-HT2A BPND values. Single subjects with BPND values < 0.0 within an ROI were excluded because BPND < 0.0 indicates signal intensity equivalent to free or nonspecific binding. Previous studies have reported that 5-HT2A BPND is inversely correlated with age (Meltzer et al. 1998; Bailer et al. 2004). Additionally, previous studies suggest that the amygdala reactivity elicited by our task may decrease with age (Tessitore et al. 2005). To account for age-related variability in these 2 measures, age was included as a covariate in all analyses. Considering the broad age range of participants in this study (20–57 years), we report amygdala reactivity and BPND values standardized for age effects. These values are the standardized residuals of the respective measurements after accounting for effects of age. This procedure was adopted to more clearly illustrate the relationship between regional 5-HT2A BPND and amygdala reactivity, independent of age. The statistics reported reflect comparisons between observed fMRI BOLD and BPND values including age as a covariate. As sex was not significantly correlated with any BOLD fMRI (all r‘s ≤ 0.23 and P‘s ≥ 0.18) or 5-HT2A BPND measures (all r‘s ≤ 0.12 and P‘s ≥ 0.50), it was not included in any subsequent analyses exploring the relationship between prefrontal 5-HT2A BPND and amygdala reactivity, habituation, or functional coupling.
Habituation Analyses
To assess the magnitude of amygdala habituation and the degree to which this habituation was related to 5-HT2A BPND, single-subject amygdala reactivity data were extracted and separated by block type (i.e., Fear block 1, Angry block 1, etc.). We used paired t-tests to examine differences in amygdala reactivity between first and second exposure to fearful (experimental task blocks 1 and 3) and angry (experimental task blocks 2 and 4) expressions. Linear regression analyses were used to examine the relationship between mPFC 5-HT2A BPND and the magnitude of amygdala habituation represented as the difference in average signal intensity between first and second presentation blocks for each expression type for each subject.
Functional Connectivity Analyses
Using functional connectivity, we examined the degree to which threat-related amygdala reactivity was associated with activity in mPFC. Furthermore, we sought to characterize how functional connectivity between these regions varied as a function of mPFC 5-HT2A receptor density. Connectivity estimates reported reflect functionally relevant correlations between components of neural circuits; however, they do not establish the causal or time-lagged nature of regional neural coupling (Friston 1994). MarsBaR (Brett et al. 2002) was used to extract the mean BOLD time series for each subject from all voxels within a 5-mm radius sphere centered on the voxel exhibiting the maximal main effect of task (right amygdala seed coordinates: 26, −3, −17; left amygdala seed coordinates: −24, −7, −15). Extracted time series were mean centered and drift corrected. Individual values exceeding 3 SDs of the mean of the drift-corrected time series were replaced by the average of the 2 adjacent values. These corrected time series were entered as regressors in individual GLM design matrices also including task condition as an additional regressor. Analyses yielded individual contrast images reflecting the areas wherein BOLD signal changes were temporally coupled with signal changes from each amygdala seed (i.e., left or right). These individual contrast images were then included in second-level analyses including sgPFC or pgPFC 5-HT2A BPND and age as covariates to identify regions of mPFC whose functional coupling with each respective amygdala seed was significantly correlated with 5-HT2A BPND across individuals. Using the WFU PickAtlas Tool described above, we restricted our connectivity analyses to Brodmann areas (BAs) 24, 25, and 32 that overlap with our PET ROIs. Analyses of positive or negative coupling with amygdala reactivity were thresholded at a voxel level of P < 0.05 and FDR corrected for multiple comparisons and an extent of at least 10 contiguous voxels within the regions examined. Analyses of these PFC regions whose coupling with amygdala reactivity was positively or negatively correlated with sgPFC or pgPFC 5-HT2A BPND were thresholded at a voxel level of P < 0.05, uncorrected, and an extent of at least 10 contiguous voxels within PFC regions examined.
Results
Amygdala Reactivity
Consistent with previous reports, the main effect of task comparison (i.e., task > control) was associated with robust, bilateral amygdala reactivity (right amygdala: [26, −3, −17], z = 6.22, 146 voxels, PFDR < 0.05; left amygdala: [−24, −7, −15], z = 5.03, 130 voxels, PFDR < 0.05). The main effect of task activation within the right but not left amygdala was inversely correlated with age (right amygdala: r = −0.414, r2 = 0.171, P = 0.013; left amygdala: r = −0.42, r2 = 0.002, P = 0.809). No clusters within our prefrontal ROIs (i.e., BAs 24, 25, and 32) exhibited a statistically significant main effect of task.
Prefrontal 5-HT2A BPND Analyses
Average [18F]altanserin binding across all individuals revealed specific 5-HT2A binding within the sgPFC (BPND = 1.24 ± 0.45, mean ± SD) and pgPFC (BPND = 1.10 ± 0.37). Across all subjects, 5-HT2A BPND values were highly correlated between these 2 regions (r = 0.761, r2 = 0.579, P < 0.0005). Consistent with previous reports, we found that age was inversely correlated with 5-HT2A BPND in both prefrontal regions examined (sgPFC: r = −0.569, r2 = 0.324, P < 0.0005; pgPFC: r = −0.629, r2 = 0.396, P < 0.0005).
Prefrontal 5-HT2A BPND and Amygdala Reactivity
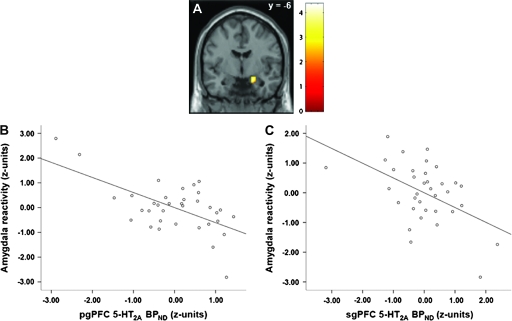
As predicted, 5-HT2A receptor density within both the sgPFC and pgPFC was inversely correlated with amygdala reactivity across all individuals. 5-HT2A BPND in the sgPFC predicted 25% of the variability in right amygdala reactivity (r = −0.497, r2 = 0.247, P = 0.002), whereas pgPFC 5-HT2A BPND predicted 37% of the variability in right amygdala reactivity (r = −0.609, r2 = 0.371, P < 0.0005) (Fig. 1). We observed no activation clusters within the left amygdala exhibiting a statistically significant relationship with prefrontal 5-HT2A BPND.
Figure 1.
Prefrontal 5-HT2A BPND is inversely associated with amygdala reactivity. (A) Statistical parametric map representing the right amygdala cluster inversely correlated with both sgPFC and pgPFC 5-HT2A BPND. (B and C) Plot of inverse correlation between right amygdala reactivity and pgPFC 5-HT2A BPND (r2 = 0.371, P < 0.0005) and sgPFC 5-HT2A BPND (r2 = 0.247, P < 0.002).
Habituation of Amygdala Response
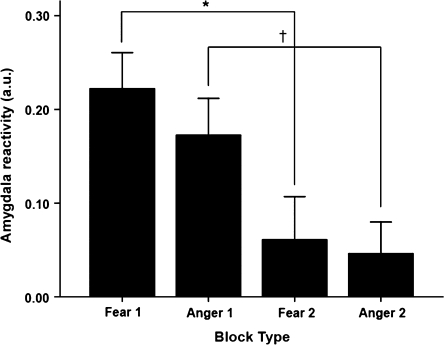
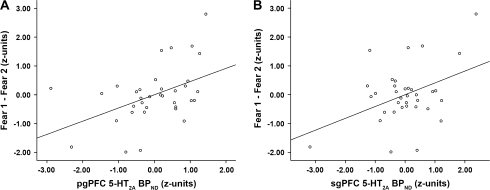
Paired t-tests revealed significant habituation of right amygdala reactivity across blocks of both fearful and angry facial expressions (first vs. second fear blocks: t24 = 3.10, P = 0.005; first vs. second angry blocks: t24 = 2.55, P = 0.018) (Fig. 2). The magnitude of the habituation to fearful expressions (i.e., Fear block 1 > Fear block 2) was positively correlated with mPFC 5-HT2A BPND (pgPFC: r = 0.458, r2 = 0.210, P = 0.006; sgPFC: r = 0.410, r2 = 0.168, P = 0.015) (Fig. 3). However, there was no statistically significant relationship between the magnitude of habituation to angry expressions and mPFC 5-HT2A BPND (pgPFC: r = 0.241, r2 = 0.058, P = 0.164; sgPFC: r = 0.199, r2 = 0.039, P = 0.252). We did not observe significant habituation of left amygdala reactivity across the 2 block types. Though left amygdala reactivity decreased across fearful blocks (first vs. second fear blocks: t24 = 2.77, P = 0.001), we observed a significant increase in left amygdala reactivity across angry blocks (first vs. second angry blocks: t24 = −4.87, P < 0.001). Most importantly, neither pgPFC nor sgPFC 5-HT2A BPND was significantly correlated with the change in left amygdala reactivity across blocks (all t‘s < 1.8, P‘s > 0.08).
Figure 2.
Habituation of amygdala response. Amygdala reactivity is significantly decreased between first and second exposures to both fearful and angry facial expression blocks. *P = 0.005, †P = 0.018, paired t-tests.
Figure 3.
Prefrontal 5-HT2A BPND is positively associated with the magnitude of amygdala habituation in response to fearful facial expression blocks. (A) pgPFC 5-HT2A BPND is positively correlated with magnitude of amygdala habituation to fearful facial expressions (r2 = 0.210, P = 0.006). (B) sgPFC 5-HT2A BPND is positively correlated with magnitude of amygdala habituation to fearful facial expressions (r2 = 0.168, P = 0.015).
Functional Connectivity Analyses
We observed significant positive correlations between right amygdala reactivity and multiple prefrontal ROIs (Table 1). No clusters within our prefrontal ROIs, however, exhibited statistically significant negative correlations with right amygdala reactivity. Both pgPFC and sgPFC 5-HT2A BPND were positively correlated with the magnitude of functional connectivity between right amygdala reactivity and multiple prefrontal regions (Table 1). There were no significant negative correlations between 5-HT2A BPND and amygdala–prefrontal functional connectivity within our prefrontal ROIs. We observed similar patterns of positive correlation between 5-HT2A BPND and functional connectivity between the left amygdala and prefrontal ROIs (data not shown).
Table 1.
Prefrontal regions functionally coupled with the amygdala and their relationship with prefrontal 5-HT2A BPND
| x, y, za | Voxels | z Score | P value | |
| Positively coupled | FDR corrected | |||
| BA 25 | −6, 11, −11 | 54 | 5.85 | <0.0005 |
| BA 25 | 4, 9, −12 | 57 | 5.41 | <0.0005 |
| BA 24/32 | −14, 17, 30 | 934 | 4.78 | <0.0005 |
| BA 24/32 | 18, 45, 7 | 921 | 4.74 | <0.0005 |
| Negatively coupled | ||||
| No regions above threshold | ||||
| Positively correlated with pgPFC 5-HT2A BPND | Uncorrected | |||
| Amygdala–BA 32 | 10, 36, 22 | 88 | 3.47 | <0.0005 |
| Amygdala–BA 24 | −6, 26, 17 | 358 | 3.44 | <0.0005 |
| Amygdala–BA 32 | 4, 6, 44 | 241 | 2.93 | 0.002 |
| Amygdala–BA 24 | 6, −14, 34 | 63 | 2.88 | 0.002 |
| Amygdala–BA 32 | −10, 41, 3 | 30 | 2.77 | 0.003 |
| Amygdala–BA 24 | −16, −15, 41 | 24 | 2.45 | 0.007 |
| Amygdala–BA 24 | 12, −7, 45 | 13 | 2.04 | 0.021 |
| Negatively correlated with pgPFC 5-HT2A BPND | ||||
| No regions above threshold | ||||
| Positively correlated with sgPFC 5-HT2A BPND | ||||
| Amygdala–BA 24 | −4, 4, 33 | 54 | 3.09 | 0.001 |
| Amygdala–BA 24 | −6, 26, 15 | 14 | 2.93 | 0.002 |
| Amygdala–BA 32 | 16, 17, 30 | 30 | 2.92 | 0.002 |
| Amygdala–BA 32 | 12, 36, 24 | 41 | 2.69 | 0.004 |
| Amygdala–BA 32 | 16, 12, 47 | 23 | 2.68 | 0.004 |
| Amygdala–BA 24 | 4, 4, 33 | 56 | 2.47 | 0.007 |
| Amygdala–BA 24 | −10, 15, 27 | 80 | 2.39 | 0.008 |
| Amygdala–BA 32 | 4, 23, 41 | 14 | 2.27 | 0.012 |
| Amygdala–BA 24 | 6, 28, 15 | 20 | 2.21 | 0.013 |
| Negatively correlated with sgPFC 5-HT2A BPND | ||||
| No regions above threshold |
Talairach and Tournoux coordinates.
Discussion
Previous neuroimaging studies have identified important functional relationships wherein prefrontal engagement associated with amygdala reactivity contributes to the shaping of complex emotional behaviors (Ochsner et al. 2002; Johnstone et al. 2007). Complimentary studies suggest that this relationship can be, at least in part, modulated by serotonergic function (Pezawas et al. 2005; Forster et al. 2006; Meyer-Lindenberg et al. 2006; Weisstaub et al. 2006; Buckholtz et al. 2007). The results of our current study suggest that mPFC 5-HT2A receptors play an important role in mediating serotonergic modulation of prefrontal–amygdala circuitry. Specifically, we found that mPFC 5-HT2A density in both sgPFC and pgPFC was inversely correlated with the magnitude of threat-related amygdala reactivity. The density of mPFC 5-HT2A was also correlated with increased amygdala habituation to fear-related expressions, a phenomenon likely dependent on top–down prefrontal regulation (Phelps et al. 2004). Finally, mPFC 5-HT2A density was positively correlated with the magnitude of amygdala–mPFC functional coupling, suggesting that 5-HT plays an important role in facilitating the integration of affective information between these brain regions. Importantly, all the observed relationships between mPFC 5-HT2A and amygdala reactivity, temporal habituation, and functional coupling were independent of age and sex suggesting the general importance and potentially broad impact of mPFC 5-HT2A on the regulation of corticolimbic brain function.
The predominant localization of excitatory 5-HT2A receptors to glutamatergic projection neurons in the mPFC (Jakab and Goldman-Rakic 1998) supports our observed inverse relationship between mPFC receptor density and amygdala reactivity. The mPFC projects extensively to the amygdala, and these projections are thought to play a key role in regulating amygdala reactivity, specifically in response to emotionally salient environmental cues (Quirk et al. 2003). Given the ubiquitous expression of the 5-HT2A receptor among populations of projection neurons in the mPFC and the extensive connectivity between the mPFC and the amygdala, it is likely that projection neurons targeting the amygdala are characterized by this 5-HT2A distribution pattern, but this has not been specifically confirmed. Consistent with this potential effect, increased 5-HT in the mPFC is associated with decreased fear-related behavior in animals (Forster et al. 2006).
Consistent with previous studies (Breiter et al. 1996; Büchel et al. 1998; Schwartz, Wright, Shin, Kagan, Whalen, et al. 2003; Wright et al. 2003), there was significant habituation of right amygdala reactivity across repeated exposure to both fearful and angry facial expressions. Fear-conditioning studies have indicated that expression of learned extinction involves direct mPFC inhibition of the amygdala (Quirk et al. 2003; Phelps et al. 2004). The similarity in the decreased response of the amygdala during habituation with that documented during fear extinction suggests that capacity for engagement of prefrontal regulatory circuitry may be an important component in more general regulation of amygdala reactivity. Our finding that prefrontal 5-HT2A BPND is positively correlated with the magnitude of right amygdala habituation to fearful facial expressions supports this possibility and further implicates the 5-HT2A receptor as an important modulator of this circuitry. Although 5-HT2A BPND did not significantly predict right amygdala habituation to angry expressions, the direction of the effect was consistent with that of fearful expressions. This difference may simply reflect a bias in our paradigm that always presented fearful before angry expressions thus lessening the overall right amygdala response to angry expressions.
In contrast to the significant relationships observed for the right amygdala, there were no significant relationships between mPFC 5-HT2A BPND and either left amygdala reactivity or temporal habituation. As we are unaware of any data suggesting hemispheric asymmetry in the expression of 5-HT2A or of serotonergic innervation more broadly, we believe these differences likely reflect relative characteristics of the right and left amygdala precipitated by our fMRI challenge paradigm. The right lateralized relationship between 5-HT2A and amygdala reactivity may reflect a bias in the perceptual processing of faces, such as those employed in our paradigm, to right hemisphere structures, including the amygdala (Farah et al. 1998; Fischer et al. 2003). The specificity of the relationship between 5-HT2A and amygdala habituation may reflect the general tendency for greater temporal habituation in the right amygdala, which may be preferentially involved in stimulus novelty and detection, rather than the left amygdala, which may be preferentially involved in sustained stimulus evaluation (Wright et al. 2001; Britton et al. 2008). Thus, the laterality observed in our data likely reflects the relative functional engagement and dynamics of the amygdala and its subsequent modulation by mechanisms regulated by prefrontal 5-HT2A and not any intrinsic properties of 5-HT2A expression or action.
In addition to mapping the relationship between prefrontal 5-HT2A BPND and overall amygdala reactivity as well as amygdala habituation, we used functional connectivity to examine the impact of 5-HT2A receptors on the correlated responses of the amygdala and regions of the mPFC. In general, we observed a strong positive correlation between amygdala reactivity and fMRI BOLD signal in multiple mPFC regions including BAs 24, 25, and 32. The correlations, however, were task independent as none of these mPFC regions exhibited a main effect of task and likely reflect a more general pattern of functional coupling between these regions that is not specific to processing of threat-related stimuli. The task-independent nature of these correlations may explain why, unlike the right lateralized effects observed between 5-HT2A and amygdala reactivity as well as temporal habituation, a bilateral relationship emerged between 5-HT2A and amygdala–prefrontal connectivity. Considering the extensive reciprocal anatomical connectivity between the amygdala and mPFC (Pandya et al. 1981), we believe our observed positive coupling reflects the primarily perceptual processing nature of our simple task and the excitatory drive of the amygdala on these downstream cortical target regions. This is in contrast to paradigms involving active cognitive tasks (e.g., affective labeling or emotion regulation) that explicitly engage prefrontal regulatory inhibitory networks resulting in negative correlations between amygdala and prefrontal activation (Hariri et al. 2000, 2003; Ochsner et al. 2002). However, positive coupling between the amygdala and PFC, especially its medial regions, likely reflects effective integration of amygdala-mediated arousal by prefrontal circuits that subsequently effect complex, adaptive behavioral responses (Pezawas et al. 2005; Heinz et al. 2005). Thus, our results may reflect the importance of 5-HT signaling via mPFC 5-HT2A receptors in facilitating amygdala drive on PFC.
Additional neural circuitry involving direct projections from mPFC to the dorsal raphe nucleus (DRN), which contains the cell bodies of serotonergic neurons, may contribute to the regulation of threat-related amygdala reactivity via an indirect pathway. Rodent studies have indicated that activation of mPFC neurons inhibit 5-HT neurons possibly via action on GABAergic populations within the DRN, the neuronal subpopulation primarily targeted by projections from mPFC (Varga et al. 2001; Jankowski and Sesack 2004). More specifically, the regulation of 5-HT release in the context of stressful and arousing stimuli via mPFC seems to be an important mechanism for effectively dealing with a stressor and is associated with the cessation of fear-related behavior (Amat et al. 2005; Forster et al. 2006). Given our previous neuroimaging findings that a greater capacity for regulation of 5-HT release at downstream targets via 5-HT1A autoreceptor density predicts decreased amygdala reactivity, it may be that greater prefrontal 5-HT2A density reflects, in part, a greater capacity for regulation of 5-HT release via a prefrontal–DRN inhibitory feedback mechanism subsequently resulting in a dampening of amygdala reactivity. Within the current study, however, we are unable to disentangle effects of prefrontal regulation of amygdala reactivity that are the result of direct or indirect feedback.
As our data suggest that individual differences in mPFC 5-HT2A density are correlated with behaviorally relevant amygdala function, identifying factors that contribute to the emergence of such interindividual variability may inform ongoing efforts to establish biological markers of disease liability. The emerging field of imaging genetics has begun to demonstrate that common functional genetic polymorphisms have the potential to influence variability in behaviorally relevant brain function by affecting the expression and function of specific molecular signaling cascades (Hariri et al. 2006; Hariri 2009). A recent PET study in momozygotic twins suggests that approximately 40–50% of interindividual variability in cortical 5-HT2A density is genetically driven (Pinborg et al. 2008). Against this general background of likely heritable variation in 5-HT2A density, in vitro studies have identified specific functional polymorphisms in the human 5-HT2A gene (HTR2A) that affect transcriptional regulation and expression (Veenstra-VanderWeele et al. 2002; Parsons et al. 2004). Such genetically driven variability in HTR2A gene expression could explain some of the interindividual variabilities in mPFC 5-HT2A density observed in our current study. Future studies with larger sample sizes that can more effectively control for nongenotype effects (e.g., age, sex, ethnicity, multiple functional polymorphisms) are necessary to explore potential specific genetic contributions to variability in 5-HT2A density and related brain function.
Extrapolation of our current findings to the understanding of serotonergic regulation of corticolimbic circuit function and related emotional behaviors should be done with caution and attention to several study limitations. First, immunolabeling studies have indicated that the 5-HT2A receptor is localized within the amygdala (McDonald and Mascagni 2007). We were interested in exploring the degree to which threat-related amygdala reactivity is associated with 5-HT2A BPND within the amygdala itself, but consistent BPND values in the amygdala near or below zero (data not shown) did not allow for receptor binding measurements in this region. Future studies employing radiotracers with greater signal-to-noise ratios in the amygdala are needed to evaluate the relationship between amygdala 5-HT2A density and reactivity. Second, our paradigm is best suited to the examination of amygdala reactivity and interconnected cortical activation associated with behavioral and physiological arousal to salient stimuli. Although we believe that our results strongly support mPFC 5-HT2A receptors as an important component in a putative corticolimbic regulatory network, it may be beneficial to examine relationships between prefrontal 5-HT2A BPND in the context of a task specifically designed to engage top–down emotional regulatory circuitry. Third, our data only indirectly suggest that mPFC 5-HT2A density regulates the impact of 5-HT on prefrontal circuitry related to amygdala reactivity. The addition of a pharmacological challenge of the 5-HT system (e.g., acute administration of a selective 5-HT reuptake inhibitor; Bigos et al. 2008) to our multimodal neuroimaging design would allow for the direct determination of how individual differences in mPFC 5-HT2A density affect the ability of 5-HT to drive this circuitry. On a related note, our findings are correlational in nature and do not imply causality. Though we believe that the underlying biology of this circuitry supports our interpretation, both our metric of amygdala reactivity (BOLD fMRI) and receptor binding (PET BPND) are indirect, and thus, our findings should be interpreted with caution. Fourth, our findings are within a healthy adult population with no history of psychiatric illness. Although altered prefrontal 5-HT2A density has been reported in patients with mood and anxiety disorders (Yatham et al. 2000), the patterns reported herein may not be related to the pathophysiology of these and related disorders, and comparable multimodal studies in patient populations are needed. Finally, it is highly unlikely that the 5-HT2A receptor represents the exclusive mechanism by which 5-HT signaling in the PFC can modulate this circuitry. Multiple pre- and postsynaptic 5-HT receptors, both excitatory and inhibitory, are likely important in orchestrating signaling patterns that modulate prefrontal circuitry related to regulating arousal (Sharp et al. 2007). Future studies employing multiple PET radiotracers targeting different 5-HT receptor subtypes are needed to better understand these complex signaling pathways.
Funding
National Institute of Mental Health (MH067602 and MH064625 to C.C.M., MH072837 to A.R.H.); National Alliance for Research on Schizophrenia and Depression (Young Investigator Award to A.R.H.); National Institute of Drug Abuse (DA023420 to P.M.F.).
Acknowledgments
We would like to thank S. Hulland, M. Lightfoot, and A. Saul for technical assistance. We would also like to thank the University of Pittsburgh Medical Center (UPMC) PET facility and the UPMC Magnetic Resonance Research Center for imaging resources. Conflict of Interest: None declared.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Mathis CA, Wagner A, Thornton L, Hoge J, Ziolko SK, et al. Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biol Psychiatry. 2007;61:1090. doi: 10.1016/j.biopsych.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Price JC, Meltzer CC, Mathis CA, Frank GK, Weissfeld L, McConaha CW, Henry SE, Brooks-Achenbach S, Barbarich NC, et al. Altered 5-HT(2A) receptor binding after recovery from bulimia-type anorexia nervosa: relationships to harm avoidance and drive for thinness. Neuropsychopharmacology. 2004;29:1143–1155. doi: 10.1038/sj.npp.1300430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P. Increased 5-HT2A receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [11C]MDL 100,907. Am J Psychiatry. 2006;163:1580–1587. doi: 10.1176/ajp.2006.163.9.1580. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33:3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue ME, Yagaloff KA, Mamounas LA, Hartig PR, Molliver ME. Correspondence between 5-HT2 receptors and serotonergic axons in rat neocortex. Brain Res. 1988;453:315–328. doi: 10.1016/0006-8993(88)90172-2. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16:S497. [Google Scholar]
- Britton JC, Shin LM, Barrett LF, Rauch SL, Wright CI. Amygdala and fusiform gyrus temporal dynamics: responses to negative facial expressions. BMC Neurosci. 2008;9:44. doi: 10.1186/1471-2202-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Manuck SB, Flory JD, Hariri AR. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE, Hariri AR. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol Psychiatry. 2005;10:884–888. doi: 10.1038/sj.mp.4001716. [DOI] [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, Egan MF, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol Psychiatry. 2007;13:313. doi: 10.1038/sj.mp.4002020. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Bush DEA, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT2C receptor antagonist. Biol Psychiatry. 2007;62:1111. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cidis Meltzer C, Drevets WC, Price JC, Mathis CA, Lopresti B, Greer PJ, Villemagne VL, Holt D, Mason NS, Houck PR, et al. Gender-specific aging effects on the serotonin 1A receptor. Brain Res. 2001;895:9–17. doi: 10.1016/s0006-8993(00)03211-x. [DOI] [PubMed] [Google Scholar]
- Darwin C, Ekman P. The expression of the emotions in man and animals. New York: Oxford University Press; 1998. [Google Scholar]
- de Almeida J, Mengod G. Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT2A receptors in human and monkey prefrontal cortex. J Neurochem. 2007;103:475–486. doi: 10.1111/j.1471-4159.2007.04768.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto (CA): Consulting Psychologists Press; 1976. [Google Scholar]
- Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychol Rev. 1998;105:482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Res Bull. 2003;59:387. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, Price JC, Hariri AR. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci. 2006;9:1362–1363. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuorimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci. 2009 doi: 10.1146/annurev.neuro.051508.135335. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59:888. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Luthi A, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and γ-aminobutyric acid neurons. J Comp Neurol. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lemaire C, Cantineau R, Guillaume M, Plenevaux A, Christiaens L. Fluorine-18-altanserin: a radioligand for the study of serotonin receptors with PET: radiolabeling and in vivo biologic behavior in rats. J Nucl Med. 1991;32:2266–2272. [PubMed] [Google Scholar]
- Leysen JE. Use of 5-HT2 receptor agonists and antagonists for the characterization of their respective receptor sites. Neuromethods. 1989:299–350. [Google Scholar]
- Leysen JE. Gaps and peculiarities in 5-HT2 receptor studies. Neuropsychopharmacology. 1990;3:361–369. [PubMed] [Google Scholar]
- Leysen JE. 5-HT2 receptors. Curr Drug Targets. 2004;3:11–26. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal stability of individual differences in amygdala reactivity. Am J Psychiatry. 2007;164:1613–1614. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience. 2007;146:306. doi: 10.1016/j.neuroscience.2007.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer CC, Kinahan PE, Greer PJ, Nichols TE, Comtat C, Cantwell MN, Lin MP, Price JC. Comparative evaluation of MR-based partial-volume correction schemes for PET. J Nucl Med. 1999;40:2053–2065. [PubMed] [Google Scholar]
- Meltzer CC, Leal JP, Mayberg HS, Wagner HN, Jr, Frost JJ. Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. J Comput Assist Tomogr. 1990;14:561–570. doi: 10.1097/00004728-199007000-00011. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Smith G, Price JC, Reynolds CF, Mathis CA, Greer P, Lopresti B, Mintun MA, Pollock BG, Ben-Eliezer D, et al. Reduced binding of altanserin to serotonin type 2A receptors in aging: persistence of effect after partial volume correction. Brain Res. 1998;813:167. doi: 10.1016/s0006-8993(98)00909-3. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses EL, Drevets WC, Smith G, Mathis CA, Kalro BN, Butters MA, Leondires MP, Greer PJ, Lopresti B, Loucks TL, et al. Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: a PET study. Biol Psychiatry. 2000;48:854–860. doi: 10.1016/s0006-3223(00)00967-7. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Van Hoesen GW, Mesulam M-M. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp Brain Res. 1981;42:319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, D'Souza UM, Arranz M-J, Kerwin RW, Makoff AJ. The -1438A/G polymorphism in the 5-hydroxytryptamine type 2A receptor gene affects promoter activity. Biol Psychiatry. 2004;56:406. doi: 10.1016/j.biopsych.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Pinborg LH, Arfan H, Haugbol S, Kyvik KO, Hjelmborg JvB, Svarer C, Frokjaer VG, Paulson OB, Holm S, Knudsen GM. The 5-HT2A receptor binding pattern in the human brain is strongly genetically determined. NeuroImage. 2008;40:1175. doi: 10.1016/j.neuroimage.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Price JC, Lopresti BJ, Meltzer CC, Smith GS, Mason NS, Huang Y, Holt DP, Gunn RN, Mathis CA. Analyses of [18F]altanserin bolus injection PET data. II: Consideration of radiolabeled metabolites in humans. Synapse. 2001;41:11–21. doi: 10.1002/syn.1055. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes RA, Murthy NV, Dresner MA, Selvaraj S, Stavrakakis N, Babar S, Cowen PJ, Grasby PM. Human 5-HT transporter availability predicts amygdala reactivity in vivo. J Neurosci. 2007;27:9233–9237. doi: 10.1523/JNEUROSCI.1175-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, Rauch SL. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biol Psychiatry. 2003;53:854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Queree P. Important messages in the `post’: recent discoveries in 5-HT neurone feedback control. Trends in Pharmacological Sciences. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Smith GS, Price JC, Lopresti BJ, Huang Y, Simpson N, Holt D, Mason NS, Meltzer CC, Sweet RA, Nichols T, et al. Test-retest variability of serotonin 5-HT2A receptor binding measured with positron emission tomography and [18F]altanserin in the human brain. Synapse. 1998;30:380–392. doi: 10.1002/(SICI)1098-2396(199812)30:4<380::AID-SYN5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003;37:357. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, Mattay VS. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res. 2005;139:9. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Varga V, Szekely AD, Csillag A, Sharp T, Hajos M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Kim SJ, Lord C, Courchesne R, Akshoomoff N, Leventhal BL, Courchesne E, Cook EH., Jr Transmission disequilibrium studies of the serotonin 5-HT2A receptor gene (HTR2A) in autism. Am J Med Genet. 2002;114:277–283. doi: 10.1002/ajmg.10192. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci. 2003;4:139. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, Shin LM, Fischer HH, McMullin K, Rauch SL. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. NeuroImage. 2003;18:660–669. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Shiah IS, Scarrow G, Lam RW, Adam MJ, Zis AP, Ruth TJ. Brain serotonin2 receptors in major depression: a positron emission tomography study. Arch Gen Psychiatry. 2000;57:850–858. doi: 10.1001/archpsyc.57.9.850. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch M-A, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu X-Z, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]