Abstract
Category and letter fluency tasks are commonly used clinically to investigate the semantic and phonological processes central to speech production, but the neural correlates of these processes are difficult to establish with functional neuroimaging because of the relatively unconstrained nature of the tasks. This study investigated whether differential performance on semantic (category) and phonemic (letter) fluency in neurologically normal participants was reflected in regional gray matter density. The participants were 59 highly proficient speakers of 2 languages. Our findings corroborate the importance of the left inferior temporal cortex in semantic relative to phonemic fluency and show this effect to be the same in a first language (L1) and second language (L2). Additionally, we show that the pre-supplementary motor area (pre-SMA) and head of caudate bilaterally are associated with phonemic more than semantic fluency, and this effect is stronger for L2 than L1 in the caudate nuclei. To further validate these structural results, we reanalyzed previously reported functional data and found that pre-SMA and left caudate activation was higher for phonemic than semantic fluency. On the basis of our findings, we also predict that lesions to the pre-SMA and caudate nuclei may have a greater impact on phonemic than semantic fluency, particularly in L2 speakers.
Keywords: bilingual, MRI, phonological, semantic, verbal fluency
Introduction
Semantic and phonemic fluency tasks are exemplary clinical ways to tap into semantic and phonological processes central to speech production. In standard clinical versions, subjects are given 1 min to generate object names from a given category (semantic fluency) or words beginning with a specific letter (phonemic fluency). The relatively unconstrained nature of these tasks make them difficult to study with functional imaging because subjects typically produce bursts of responses followed by pauses while they search for other exemplars. Consequently, the number of semantic or phonemic responses per unit of time is difficult to match, and activation differences related to semantic and phonemic search are confounded with differences in the demands on articulatory processes that support both tasks. To overcome this problem, functional imaging studies have typically adapted the task by instructing subjects to generate only one response per cue (Gourovitch et al. 2000; Billingsley et al. 2004), but this approach underestimates activation related to the search process and has yielded only partially consistent results. It is also not representative of clinical use.
To avoid the methodological pitfalls associated with functional imaging studies of fluency, we used structural imaging to examine the neurological correlates of the standard fluency tasks in normal healthy participants. This technique of correlating cognitive abilities with regional gray or white matter density or volume is now well established (Maguire et al. 2000; Mechelli et al. 2004; Golestani and Pallier 2007; Lee et al. 2007). It has the advantage of offering greater anatomical precision than either lesion studies or functional imaging studies especially in the inferior temporal cortices where neurological damage is rare and the functional imaging signal is distorted by susceptibility artifacts (Devlin et al. 2000; Veltman et al. 2000).
A second novel feature in our design was that we tested semantic and phonemic fluency abilities in participants who were proficient in speaking at least 2 different languages. This allowed us to determine whether differential effects of semantic and phonemic fluency were the same or different in a first language (L1) versus second language (L2). It also allowed us to maximize sensitivity to the effects of interest by having repeated measures on the same participants using a sample that had a wide range of abilities in both their L1 and L2. The comparison of the effects in a L1 versus L2 also has clinical relevance because bilinguals are more representative of the human population, who typically speak more than 1 language (Wei 2007).
On the basis of lesion studies (Milner 1964; Rosser and Hodges 1994; Henry and Crawford 2004; Baldo et al. 2006) and a positron emission tomography (PET) study that used similar tasks (Mummery et al. 1996), we predicted that participants with better semantic fluency would have higher gray matter in the inferior temporal lobes, whereas participants with higher phonemic fluency would have higher gray matter in frontal and premotor regions. We also compared our results with other functional imaging studies that investigated brain activation for semantic fluency (Paulesu et al. 1997; Crosson et al. 1999; Pihlajamaki et al. 2000; Crosson et al. 2001), phonemic fluency (Paulesu et al. 1997; Elfgren and Risberg 1998; Schlosser et al. 1998; Perani, Abutalebi, et al. 2003),or both (Mummery et al. 1996; Gourovitch et al. 2000; Perani, Cappa, et al. 2003; Billingsley et al. 2004; Heim et al. 2008), see Table 1 for details.
Table 1.
Summary of coordinates of interest from previous activation studies of semantic and phonemic fluency
| Region | HEM |
Mummery et al. (1996) |
Gourovitch et al. (2000) |
Billingsley et al. (2004) |
Heim et al. (2008) |
||||||||||||
| x | y | z | BA | x | y | z | BA | x | y | z | BA | x | y | z | BA | ||
| a) Semantic relative to phonemic | |||||||||||||||||
| Inferior or middle temporal/hippocampus | L | −44 | −6 | −20 | 20 | −54 | −22 | −20 | 20 | No results reported for this comparison | — | −60 | −10 | −23 | — | ||
| −32 | −30 | −16 | 20/36 | −24 | −14 | −16 | −28 | −38 | −19 | — | |||||||
| −20 | −16 | −16 | — | ||||||||||||||
| Posterior temporal | L | — | — | −36 | −76 | 20 | 19/39 | — | — | ||||||||
| R | — | — | 42 | −64 | 12 | 19/39 | — | — | — | ||||||||
| Cingulate | L | — | — | −2 | 32 | −8 | 32 | — | — | ||||||||
| −8 | 32 | −8 | 32 | — | |||||||||||||
| b) Phonemic relative to semantic | |||||||||||||||||
| Inferior frontal | L | −48 | 0 | 28 | 44/46 | −54 | 2 | 20 | 44 | Greater activity in left hemisphere sources (no coordinates) | −50 | 10 | 21 | 44 | |||
| −36 | −4 | 24 | 44/46 | −36 | 6 | 0 | 47 | ||||||||||
| −58 | −14 | 24 | — | ||||||||||||||
| R | — | — | 42 | 10 | 8 | 44 | — | — | |||||||||
| Prefrontal | L | — | — | — | — | — | — | ||||||||||
| R | — | — | 22 | 32 | 28 | 9 | — | — | |||||||||
| R | — | — | 44 | 6 | −8 | 38 | — | — | |||||||||
Note: Part (a) reports regions that showed more activation for semantic than phonemic fluency. Part (b) reports regions that showed more activation for phonemic than semantic fluency. Coordinates are in Montreal Neurological Institute space.HEM, hemisphere; L, left hemisphere; R, right hemisphere; BA, Brodmann area.
Our predictions for any differential effect of semantic and phonemic fluency in a L2 versus L1 are less constrained. Behavioral research on fluency tasks in bilinguals has not typically considered performance in both languages and performance in English as L2 has only been compared with monolingual English controls (Gollan et al. 2002; Portocarrero et al. 2007; Bialystok et al. 2008). Some studies have contrasted L1 and L2 but used identical categories and so potentially confounded differential fluency scores with priming effects (e.g., Rosselli et al. 2000). On the theoretical front, most accounts assume that words in a bilingual's dominant language will compete for selection when bilinguals speak in their L2 (Green 1998; Kroll et al. 2006). We therefore predicted that good performance in L2 may be associated with increased gray matter in regions that control interference from L1. Critically, however, in order to draw this conclusion, we would need to ensure that a differential effect of language was not the consequence of an increased range of ability in the L2 relative to L1.
Materials and Methods
Participants
Our participants were an opportunity sample of 59 speakers of more than 1 language (23 males and 36 females) who volunteered to take part in the study. Only those who were right handed, neurologically normal, magnetic resonance imaging (MRI) compatible, and highly proficient in at least 2 languages were included. In London, most highly proficient bilingual or multilingual speakers use English as their L2. Consequently, we had more volunteers who spoke English as their L2 (n = 49) than English as their L1 (n = 10). The L1s of those who spoke English as their L2 (n = 49) were German (n = 20), Greek (n = 19), Bengali (n = 4), Dutch (n = 1), French (n = 1), Hebrew (n = 2), Hindi (n = 1), or Spanish (n = 1). The L2s of those who spoke English as their L1 were German (n = 5), French (n =2), Hebrew (n = 1), Italian (n = 1), and Spanish (n = 1). The output from each participant was coded by a bilingual speaker who was proficient in both the languages under examination.
The average age of the participants was 34 but included a wide range (18–64, standard deviation [SD] 11.5). As we were unable to manipulate age and proficiency independently, we factored age out of our analysis (see below). We also note that age was not significantly correlated with either total fluency (r = 0.216, P = 0.101, n = 59) or the difference between semantic and phonological fluency (r = 0.096, P = 0.467, n = 59). The average age of L2 acquisition was 9 years (SD 4.5, range 0–26) for 58 participants (the remaining participant did not provide this information).
All participants gave informed consent to participate in the study, which was approved by the Joint Ethics Committee of the Institute of Neurology (University College London, London, UK) and National Hospital for Neurology and Neurosurgery (National Health Service Trust, London, UK).
Task
For the semantic fluency task, participants named as many animals or fruits as possible in 1 min. Although we aimed to achieve an overall balance in the assignment of animals and fruits to L1 and L2, this was not possible because different testers were assigned to participants with different language pairs. Consequently, 23 participants named animals in their L1 and fruits in their L2, whereas 36 participants named fruits in their L1 and animals in their L2. However, this imbalance is not problematic because category and language were modeled independently in the statistical analysis so that we could identify common effects as well as differences between groups who were tested on different categories (see below). For the phonemic fluency task, participants named words beginning with a specific letter (e.g., “s” in English and a different letter in the other language) in 1 min (see Table 2, for details of the behavioral data for each task). This version of the task is commonly used by neurologists as a bedside test of language production abilities. However, it contrasts with the fluency tasks used in functional imaging studies, which typically request the production of 1 word at a time (e.g., a verb in response to a noun) in order to ensure that stimulus and response production are controlled across conditions. In contrast to functional imaging, structural imaging does not require stimulus or performance to be matched across conditions. Therefore, we used the longer bedside version of the task in order to maximize variance in the semantic and phonemic scores. As a measure of general cognitive ability, participants completed set A from the Raven's colored progressive matrices (Raven 1938).
Table 2.
Summary of participants’ performance on Raven's matrices and semantic and phonemic fluency tasks
| Raven’s | Fluency (=average number of items that participants produced in 1 min) |
|||||||||||
| Phonemic |
Semantic |
Total fluency | ||||||||||
| L1 | L2 | Total phonemic | L1 |
L2 |
Total semantic | |||||||
| Animals, n = 23 | Fruits, n = 36 | Total L1, n = 59 | Animals, n= 36 | Fruits, n = 23 | Total L2, n = 59 | |||||||
| Mean | 89.8% | 14.9 | 15.7 | 30.7 | 23.3 | 16.1 | 18.9 | 18.2 | 15.7 | 17.2 | 36.1 | 66.6 |
| SD | 9.3 | 5.6 | 5.8 | 9.0 | 7.3 | 4.3 | 6.6 | 4.9 | 5.4 | 5.2 | 8.4 | 13.2 |
Note: Fluency scores are the number of words generated in 1 min. n = number of participants performing each subtest.
MRI Imaging and Data Preprocessing
Structural MRI was acquired using a 3D T1-weighted sequence (1 × 1 × 1.5 mm voxel size) (Deichmann et al. 2004) on a 1.5-T Magnetom Vision scanner (Siemens Medical Systems, Erlangen, Germany). Within SPM5 (Wellcome Trust Centre of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm) running under Matlab 6.5 (MathWorks, Natick, MA), our images were spatially normalized to Montreal Neurological Institute space and segmented into gray and white matter using the unified segmentation algorithm (Ashburner and Friston 2005) and then spatially smoothed with an isotropic Gaussian kernel of 8 mm at full-width half maximum. In the analyses described below, we opted to use “unmodulated” gray matter images, which means that we did not modulate the signal in each voxel with the volume of the surrounding area. Consequently, our analysis was based on gray matter density (relative to white matter or other tissue types within each voxel) rather than gray matter volume. Our decision to use unmodulated images was based on our previous voxel-based morphometry (VBM) studies of language (Mechelli et al. 2004; Lee et al. 2007) where we found that the most significant effects were observed in unmodulated rather than volume-modulated images.
MRI Statistical Analysis
VBM is a semiautomated technique that gives an unbiased assessment of anatomical differences throughout the whole brain (Ashburner and Friston 2000). Statistical analyses were performed in SPM5 on each voxel in the spatially normalized gray matter images using the behavioral measure as the independent variable and the gray matter density in each voxel as the dependent variable. Global signal intensity differences were removed using proportional scaling. Age and number of languages spoken were included as potentially confounding covariates of no interest (29 of the 59 participants spoke more than 2 languages). We then conducted 2 statistical analyses that identified correlations between the gray matter density and the following:
The effects of overall fluency, summed over fluency type (semantic and phonemic).
In this analysis, we modeled the effects of fluency in L1 and L2 separately and included the scores from Raven's matrices as an additional regressor to control for general cognitive effects that are known to be correlated with overall fluency ability.
The differential effects of semantic and phonemic fluency.
Here, the behavioral measures were calculated by subtracting the total phonemic from the total semantic fluency score. This was calculated separately for L1 and L2 and for participants who named fruits in their L1 and animals in their L2 and participants who named animals in their L1 and fruits in their L2. Consequently, we were able to test the effect of differential semantic and phonemic fluency ability on gray matter using a fully balanced factorial design that independently manipulated language (L1 vs. L2) and semantic category (animals vs. fruits). This enabled us to compare the effects in different languages and ensure that our results were not confounded by the semantic category tested.
The analyses were conducted on a voxel by voxel basis across the whole brain. We report regions that showed significant effects at P <0.05, corrected for multiple comparisons (using familywise error correction) in either height or extent. In statistical parametric mapping (SPM), the correction for multiple comparisons in extent is based on the Euler characteristic which uses the volume, smoothness, and surface area to calculate the expected extent size, by chance, at any given height threshold. In our case, we used a conservative height threshold of P <0.001 uncorrected when calculating the correction for multiple comparisons in extent across the whole brain.
In addition to the whole-brain analysis, we also used a small volume correction for height in ROIs from previous studies that compared semantic and phonemic fluency. These included the left middle/inferior temporal cortex and the left inferior frontal cortex, using a spherical search volume with a 6-mm radius centered on the coordinates reported in Table 1. In particular, we focused on the coordinates from Mummery et al. (1996) for 2 reasons; first, the study of Mummery et al. (1996) used a version of the fluency task that was closest to ours. Specifically, each of their trials involved generating as many words as possible within 20 s, whereas in the other functional imaging studies, each trial only involved 1 response. Second, Mummery et al. (1996) used PET where there is higher spatial precision in the inferior temporal cortices because, unlike functional magnetic resonance imaging (fMRI), PET is not affected by susceptibility artifacts (Devlin et al. 2000; Veltman et al. 2000; Lipschutz et al. 2001). Nevertheless, for completeness, we also explored our results for each set of the coordinates provided by Gourovitch et al. (2000) and Heim et al. (2008).
Finally, because we found effects in the structural imaging analysis that had not previously been reported in functional imaging analyses, we reexamined the functional imaging data reported by Mummery et al. (1996) using spherical ROIs centered on our structural imaging results to determine whether activation was significantly different for phonemic versus semantic fluency. We repeated this procedure using our standard ROI (6-mm radius = 2 voxels) and a more precise ROI (4-mm radius).
Results
Behavioral
Participants generated more words overall in the semantic fluency task than in the phonemic fluency task: mean = 36.1 and 30.7, respectively, F1,58 = 12.7, P < 0.01 (see Table 2 for details). This result accords with the performance of monolingual English speakers and nonnative speakers of English with a range of L1 backgrounds (Rosselli et al. 2000; Gollan et al. 2002; Portocarrero et al. 2007; Bialystok et al. 2008).
In terms of the effects of language on fluency, there was no effect of L1 versus L2 on phonemic fluency, mean = 14.9 and 15.7, respectively, F < 1. This null result emphasizes that our participants were highly proficient in their L2. In order to examine the effect of language on semantic fluency, we distinguished responses to the category animal and responses to the category fruit. Consistent with previous studies (Martin et al. 1994; Troyer et al. 1997; Baldo and Shimamura 1998; Gollan et al. 2002; Schwartz et al. 2003), participants named more animals than fruits (mean = 20.8 vs. 15.9, respectively, F1,57 = 27.96, P < 0.01). However, this difference was greater for participants naming animals in their L1 compared with their L2, F1,57 = 8.93, P < 0.01. Simple effects showed no difference between L1 and L2 for the category fruit, F < 1, but a significant difference for the category animal, F1,57= 10.51, P < 0.01. As noted above, we therefore included category in our correlational analyses.
Our behavioral indices were the relative scores on the phonemic and semantic fluency tasks. We report the mean differences and their distributional characteristics (skewness and kurtosis) along with the range of their normal variation in brackets (i.e., twice their standard error of mean values). For L1 (N = 23), the animal minus phonemic scores were as follows: mean = 9.91, with skewness = −0.387 (±1.02) and kurtosis = 0.384 (±2.04). For L2 (N = 36), the animal minus phonemic scores were mean = 1.86, with skewness = 0.361 (±0.816) and kurtosis = −1.13 (±1.63). For L1 (N =36), the fruit minus phonemic scores were mean = 0.17, with skewness = −0.361 (±0.816) and kurtosis = −0.270 (±1.63). For L2 (N = 23), the fruit − phonemic scores were mean = 0.96, with skewness = 0.051 (±1.02) and kurtosis = 0.096 (±0.816). In brief, the distributional properties of our difference measures all fell within the normal range of variation.
Correlating Behavioral and Structural Imaging Data
The Main Effect of Fluency
Gray matter density in both the left and right cerebellum, in lobules VIIB and VIIIA, correlated significantly with total fluency scores (semantic plus phonemic) over L1 and L2 (see Table 3). There was no effect of either language (L1 vs. L2) or fluency type (semantic vs. phonemic) in these regions.
Table 3.
Gray matter correlations with semantic and phonemic fluency scores
| Region | HEM | All fluency |
Semantic > phonemic |
Phonemic > semantic |
Statistics (P < 0.001) |
|||||||
| x | y | z | x | y | z | x | y | z | Z score | Vs | ||
| LH | −36 | −60 | −58 | 5.0 | 401 | |||||||
| −48 | −68 | −52 | 3.5 | |||||||||
| RH | +28 | −70 | −60 | 4.4 | 543 | |||||||
| +44 | −76 | −54 | 3.4 | |||||||||
| Inferior temporal | LH | −44 | −10 | −18 | 3.9* | 28 | ||||||
| RH | +54 | −6 | −16 | 3.4 | 9 | |||||||
| Head of caudate | RH | +14 | 0 | +16 | 4.3 | 113 | ||||||
| LH | −14 | +10 | +14 | 3.3 | 11 | |||||||
| Pre-SMA | LH | −4 | +14 | +74 | 4.0 | 184 | ||||||
| LH | −8 | +24 | +70 | 4.0 | ||||||||
| RH | +4 | +12 | +76 | 3.6 | ||||||||
Note: Main effects of fluency and fluency type on gray matter. Coordinates are in Montreal Neurological Institute space. HEM, hemisphere; LH, left hemisphere, RH, right hemisphere; Vs, number of voxels at 0.001 uncorrected. Bold Z score /Vs are significant after familywise correction for height or extent; *corrected in a priori ROI from Mummery et al. (1996) who identified an effect of semantic > phonemic fluency at [x = −44, y = −6, z = −20] and an effect of phonemic > semantic fluency at [x = −48, y = 0, z = 28]. Outside ROI, P values, P < 0.001uncorrected for Z scores >3, P < 0.01 uncorrected for Z scores >2.33, and P < 0.05 uncorrected for Z scores >1.64.
Semantic > Phonemic Fluency
For semantic relative to phonemic fluency, there was nothing significant across the whole brain. However, when the search volume was restricted to our ROI from Mummery et al. (1996), a significant differential effect of semantic relative to phonemic fluency was observed in the left inferior temporal lobe (P < 0.05 corrected) with a corresponding effect in the right inferior temporal lobe (P < 0.001 uncorrected), see Figure 1. These effects were present for both L1 and L2 with no significant interaction (P > 0.05 uncorrected) between language (L1 vs. L2) and fluency type (semantic vs. phonemic), see Table 4 for details. There were no significant effects of semantic versus phonemic fluency in any or the other regions identified by fMRI studies that focused on 1-word responses (as opposed to multiple word responses).
Figure 1.
Gray matter correlations with semantic more than phonemic fluency. (a) Positive correlation between gray matter density, measured as cubic millimeters of gray matter per voxel, and semantic relative to phonemic fluency scores in the left inferior temporal region (x = −44, y = −10, z = −18). (b) The location of this effect in the left inferior temporal lobe on an axial slice of the canonical brain in Montreal Neurological Institute space using a threshold of P <0.01 (to show all effects). A corresponding effect in the right inferior temporal lobe (P < 0.001 uncorrected) can also been seen.
Table 4.
Gray matter correlations with semantic and phonemic fluency scores
| Region | HEM | L2 |
L1 |
L2 versus L1 |
|||||||||
| x | y | z | Z score | x | y | z | Z score | x | y | z | Z score | ||
| Semantic relative to phonemic | |||||||||||||
| Inferior temporal | LH | −44 | −10 | −18 | 3.1 | −44 | −10 | −18 | 4.1 | NS | NS | ||
| RH | +54 | −6 | −16 | 3.0 | +54 | −6 | −16 | 2.6 | NS | NS | |||
| Phonemic relative to semantic | |||||||||||||
| Head of caudate | RH | +16 | +10 | +14 | 4.1 | +12 | −2 | +16 | 2.9 | +16 | +10 | +14 | 3.1 |
| LH | −14 | +14 | +10 | 3.9 | −8 | +4 | +20 | 1.8 | −14 | +12 | +10 | 3.0 | |
| Pre-SMA | LH | −10 | +22 | +70 | 2.1 | −4 | +14 | +74 | 4.5 | NS | NS | ||
Note: The effect of language (L2 vs. L1) on the effect of fluency type. Coordinates are in Montreal Neurological Institute space. HEM, hemisphere; LH, left hemisphere, RH, right hemisphere; Vs, number of voxels at 0.001 uncorrected. Bold Z score/Vs are significant after familywise correction for height or extent; Outside ROI, P values, P < 0.001uncorrected for Z scores >3, P < 0.01 uncorrected for Z scores >2.33, and P < 0.05 uncorrected for Z scores >1.64. NS, not significant at P <0.05 uncorrected.
Phonemic > Semantic Fluency
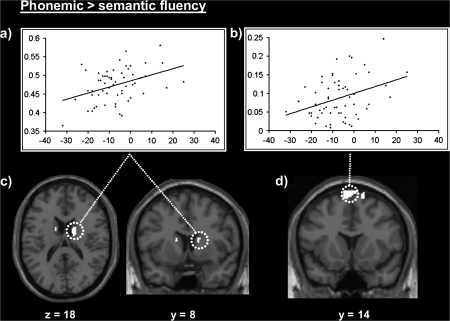
At the whole-brain level, there were 2 significant effects of phonemic relative to semantic fluency on gray matter. First, there was a correlation in the right head of caudate with a corresponding but less significant effect in the left head of caudate. Second, there was a correlation bilaterally in the pre-SMA, see Figure 2. The effect in the caudate nuclei was stronger for L2 than L1, and this was qualified by a group (L2 > L1) by fluency type (phonemic > semantic) interaction (P < 0.001 in the left and right caudate), see Table 4 for details. It is unlikely that the stronger effect of phonemic compared with semantic fluency in L2 compared with L1 is a consequence of a greater range of abilities in L2 because then all effects of phonemic relative to semantic fluency would be stronger for L2 than L1, but this was not the case. To the contrary, in the pre-SMA, there was a nonsignificant trend for phonemic relative to semantic fluency to be stronger in L1 than L2 although this differential effect of language did not reach statistical significance (Z < 1.6; P > 0.05 uncorrected), see Table 4 for details.
Figure 2.
Gray matter correlations with phonemic more than semantic fluency. Above: positive correlation between gray matter density, measured as cubic millimeters of gray matter per voxel, and phonemic relative to semantic fluency scores in the (a) right head of caudate (x = 14, y = 0, z = 16) and (b) the left pre-SMA (x = −4, y = 14, z = 74). Below: the location of the effects in (c) the right and left caudate on axial and coronal slices and (d) bilateral pre-SMA, using a threshold of P <0.01 (to show all effects).
In the ROI analysis that focused on the left inferior frontal and premotor region reported for phonemic fluency in functional imaging studies (see Table 1), we found no evidence that gray matter was higher for phonemic than semantic fluency (P > 0.05) irrespective of whether we centered our ROI on the coordinates from Mummery et al. (1996), Gourovitch et al. (2000), or Heim et al. (2008). The comparison of the effect of phonemic to semantic fluency therefore appeared to reveal inconsistent effects in our structural imaging study as compared with previously reported functional imaging studies. Our structural imaging analysis showed more gray matter in the caudate nuclei and the pre-SMA, whereas the functional imaging studies showed more activation in the left inferior frontal/premotor cortex.
To explore the consistency/inconsistency in structural and functional imaging modalities further, we reanalyzed the functional imaging study reported by Mummery et al. (1996) using ROIs based on our structural imaging study. These regions were centered on the peak coordinates for the effects in the pre-SMA, left head of caudate, and right head of caudate where gray matter was higher for phonemic than semantic fluency. This yielded 2 significant effects (P < 0.05 corrected for multiple comparisons within the ROI) that were not previously observed in the whole-brain analysis reported by Mummery et al. (1996). Specifically, we found higher activation for phonemic than semantic (animal) fluency in 1) the left head of caudate (Z = 3.0) and 2) the pre-SMA (Z = 2.4) using a spherical ROI with 4-mm radius (i.e., high precision). There was no differential activation (P > 0.05 uncorrected) for phonemic versus semantic fluency (or vice versa) in the right head of caudate.
Discussion
Category (semantic) and letter (phonemic) fluency tasks tap into semantic and phonological processes central to speech production. Both tasks involve processes such as lexical retrieval, search, and speech production but differ in their use of semantic associations, the load they place on search processes, and their use of articulatory processes to identify exemplars (Martin et al. 1994; Schwartz et al. 2003). The aim of this study was to investigate whether differential abilities on semantic versus phonemic fluency tasks are reflected in normal brain structure. Our study provides important confirmation of the role of the left temporal lobe in semantic fluency and identifies the importance of 3 other regions: 2 involved in phonemic fluency (bilateral pre-SMA and head of caudate) and 1 involved in overall fluency (bilateral inferior cerebellum). In addition, we show that these effects were observed irrespective of whether participants were tested in their L1 or L2 with the only differential effect of language observed in bilateral head of caudate where the effect of phonemic versus semantic fluency ability was greater in L2 than L1.
The association of the left inferior temporal cortex with semantic relative to phonemic fluency is consistent with 3 previous functional imaging studies (Mummery et al. 1996; Gourovitch et al. 2000; Heim et al. 2008), see Table 1 for the close correspondence in the location of these effects. It is also consistent with lesion studies (McCarthy and Warrington 1990; Hodges et al. 1992; Chan et al. 1993; Damasio and Tranel 1993; Monsch et al. 1994; Hodges and Patterson 1995; Garrard et al. 2001; Henry and Crawford 2004; Baldo et al. 2006). Our structural imaging data therefore provide further convergent evidence for the importance of the left inferior temporal cortex in semantic access during both L1 and L2 processing. This is important given that neurological damage in this region is rare and the functional imaging signal is distorted by susceptibility artifacts (Devlin et al. 2000; Veltman et al. 2000). Moreover, our observation that right inferior temporal lobe gray matter also correlated with semantic more than phonemic fluency highlights a role for the right hemisphere which needs further investigation.
Turning now to the phonemic fluency results, our analysis identified 2 regions with increased gray matter density in participants with relatively higher phonemic than semantic fluency scores: 1) a large effect in the bilateral pre-SMA and 2) in the right head of caudate with a corresponding but less significant effect in the left head of caudate. Although many previous studies have highlighted the role for both the pre-SMA and caudate nuclei in speech production, our study provides the first direct demonstration that these structures are more closely related to phonemic than semantic fluency, although a study by Perani et al. (2003) lists both regions as being activated during a phonemic fluency task and neither as activated during a semantic fluency task. To investigate activation differences between phonemic and semantic fluency further, we reexamined the data from a previous PET study (Mummery et al. 1996) that provided the ROIs for our temporal lobe finding. Using the coordinates of the caudate and pre-SMA effects in our structural analysis as ROIs, we found greater activation for phonemic than semantic fluency in the pre-SMA and in the left head of caudate. Thus, we further validate our findings by showing that increased gray matter in our structural imaging study corresponds to increased activation in a functional imaging study.
With respect to the specific functions that might be driving these anatomical effects, we predicted that there might be more internal articulatory processing during phonemic, relative to semantic, fluency because participants are more likely to internally generate and test lexical candidates beginning with a certain sound. Consistent with this possibility, the bilateral pre-SMA is a region that has previously been associated with the planning and preparation of movement, initiation of movement on cues, and higher order aspects of speech (e.g., Petrides et al. 1993). More specifically, studies of word production have suggested that the pre-SMA guides the selection of words to be produced during a free generation task (Crosson et al. 2001) with the anterior region particularly involved in word selection and activation in the posterior region reflecting a word's familiarity and length (Alario et al. 2006). Moreover, diffusion tensor imaging studies show that the pre-SMA is well connected to other regions involved in phonological processing, that is, the prefrontal cortices and anterior striatum (Johansen-Berg et al. 2004).
With respect to the functional role of the caudate, activation in this region has been reported in several functional imaging studies of language processing, such as performing a phonological learning task (Tricomi et al. 2006), processing ambiguous words (Ketteler et al. 2008), and during lexical decisions (Abdullaev and Melnichuk 1997). The head of caudate has also been shown to be involved in detecting phonological anomalies (Tettamanti et al. 2005). Ullman (2001) suggested that the basal ganglia, including the caudate nucleus, form part of a procedural system that is involved in the assembly of phonemes into words. A structural imaging study of specific language impairment has also associated bilateral abnormalities in the caudate nuclei with poor nonword repetition, a task that relies on phonology rather than semantics (Watkins et al. 2002). Alternatively, but not exclusively, more candidates may need to be considered in the phonemic task relative to the semantic fluency task (see above) and coupled with the fact that producing words that start with a given letter is not a task that is as regularly practiced as category fluency, more control may be required relative to producing exemplars of a semantic category. The left head of caudate may therefore be activated when the language processing system cannot rely entirely on automatic mechanisms but has to recruit controlled processes as well (Friederici 2006). Consistent with this possibility, previous patient (Abutalebi et al. 2000; Moretti et al. 2001; Gil Robles et al. 2005) and functional imaging studies (Crinion et al. 2006; Abutalebi et al. 2008) implicate the caudate in the selection of competing verbal responses. On the basis of our data, we predict that lesions to the bilateral pre-SMA and caudate nuclei will impair phonemic more than semantic fluency.
Our data also intimate that the effect of phonemic relative to semantic fluency in the head of caudate bilaterally was stronger for L2 than L1 processing. This is consistent with our theoretical prediction (see Introduction) that good performance in L2 may be associated with increased gray matter in regions that control interference from the more dominant L1. It is also consistent with the previous studies mentioned above that associate the head of caudate nuclei with language control. Moreover, the fact that this differential effect of L2 relative to L1 was observed for phonemic relative to semantic fluency is consistent with the caudate nuclei being involved in detecting phonological anomalies (Tettamanti et al. 2005). Critically, we are able to exclude an explanation of this effect in terms of greater sensitivity in L2 because, if this had been the case, we would have expected other effects to have been stronger in L2 than L1. Instead, we found a nonsignificant trend for the effect of phonemic versus semantic fluency in the pre-SMA to be stronger in L1 than L2. Future studies are now required to compare the effect of phonemic and semantic fluency in bilingual versus monolingual participants and to determine whether gray matter density in the caudate nuclei provides a differential marker for relative recovery in a L1 or L2.
One further empirical result was that gray matter density in the bilateral inferior cerebellum correlated significantly with overall fluency. Such a finding is consistent with existing literature. It is generally accepted that the cerebellum is involved in language production (Desmond and Fiez 1998), and connectivity studies have shown that the cerebellum has reciprocal connections with regions involved in language processing such as the left inferior frontal gyrus and the left lateral temporal cortex (Booth et al. 2007). Cerebellar lesions can induce aphasia (Marien et al. 1996; Fabbro et al. 2000; Marien et al. 2000), and right cerebellar lesions impair word generation while sparing other cognitive functions (Fiez et al. 1992; Leggio et al. 2000; Richter et al. 2007). Functional imaging studies in neurologically normal controls have also supported the role of the cerebellum in verbal fluency tasks (Fiez et al. 1992; Molinari et al. 1997).
Finally, on the basis of previous patient studies (Baldo et al. 2006) and functional imaging studies (Paulesu et al. 1993; Rueckert et al. 1994; Mummery et al. 1996; Phelps et al. 1997; Elfgren and Risberg 1998; Schlosser et al. 1998; Gourovitch et al. 2000; Perani, Cappa, et al. 2003; Heim et al. 2008), we had predicted that we would find increased gray matter in a left frontal or premotor region for phonemic relative to semantic fluency. We found no such effects. This may be because this structure subserves common processes that are of comparable importance in both semantic and phonological processing (Paulesu et al. 1993; Perani, Cappa, et al. 2003; for a systematic review, see Costafreda et al. 2006). Additionally, studies that have tested both types of fluency in the same patients have reported that both phonemic and semantic fluency are impaired in patients with frontal lesions (Baldo and Shimamura 1998; Schwartz and Baldo 2001). Given the importance of the premotor cortex in all types of speech production, it may also be the case that there is a ceiling effect in our sample. Future studies with younger monolingual participants may find that differential abilities on phonemic and semantic fluency abilities are more consistent with the functional imaging findings.
Conclusion
Structural imaging analyses have allowed us to identify key regions underlying the differential performance on semantic and phonemic fluency tasks and so corroborate the importance of particular structures to speech production and contribute to understanding their distinct contributions. In particular, our study allowed us to circumvent certain problems with functional imaging studies and look at the fundamental components of speech production using tasks that are clinically administered to patients.
On a methodological note, our study indicates the complementary nature of structural and functional imaging studies. We were able to use a ROI from functional imaging data (Mummery et al. 1996) to identify a structural region (the left inferior temporal cortex) as being involved in semantic fluency, and in turn, we used our structural analysis to provide a ROI for the functional results (identifying the left head of caudate and the pre-SMA as being more activated for phonemic than semantic fluency). Thus, the structural imaging of healthy normal controls can be considered a complement to functional imaging techniques in identifying brain regions that support function. Indeed, in this structural imaging study, we identified 2 novel findings, namely, that the pre-SMA and caudate nuclei are more closely associated with phonemic than semantic speech production.
Funding
Wellcome Trust (074735/Z/04/Z).
Acknowledgments
The authors would like to thank the following students for help with data collection: Helen Harth, Marie-Luise Mechias, Sylvie Orabona, Christos Pliatsikas, and Filzah Rahman. Conflict of Interest: None declared.
References
- Abdullaev YG, Melnichuk KV. Cognitive operations in the human caudate nucleus. Neurosci Lett. 1997;1234:151–155. doi: 10.1016/s0304-3940(97)00680-0. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Annoni JM, Zimine I, Pegna AJ, Seghier ML, Lee-Jahnke H, Lazeyras F, Cappa SF, Khateb A. Language control and lexical competition in bilinguals: an event-related fMRI study. Cereb Cortex. 2008;18:1496–1505. doi: 10.1093/cercor/bhm182. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Miozzo A, Cappa SF. Do subcortical structures control ‘language selection’ in polyglots? Evidence from pathological language mixing. Neurocase. 2000;6:51–56. [Google Scholar]
- Alario F-X, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Res. 2006;1076:129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12:896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP. Letter and category fluency in patients with frontal lobe lesions. Neuropsychol. 1998;12:259–267. doi: 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik F, Luk G. Cognitive control and lexical access in younger and older bilinguals. J Exp Psychol Learn Mem Cogn. 2008;34:859–873. doi: 10.1037/0278-7393.34.4.859. [DOI] [PubMed] [Google Scholar]
- Billingsley RL, Simos PG, Castillo EM, Sarkari S, Breier JI, Pataraia E, Papanicolaou AC. Spatio-temporal cortical dynamics of phonemic and semantic fluency. J Clin Exp Neuropsychol. 2004;26:1031–1043. doi: 10.1080/13803390490515333. [DOI] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007;1133:136–144. doi: 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A, Butters N, Paulsen J, Salmon D, Swenson M, Maloney L. An assessment of the semantic network in patients with Alzheimer's disease. J Cogn Neurosci. 1993;5:254–261. doi: 10.1162/jocn.1993.5.2.254. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion J, Turner R, Grogan A, Hanakawa T, Noppeney U, Devlin JT, Aso T, Urayama S, Fukuyama H, Stockton K, et al. Language control in the bilingual brain. Science. 2006;312:1537–1540. doi: 10.1126/science.1127761. [DOI] [PubMed] [Google Scholar]
- Crosson B, Radonovich K, Sadek JR, Gökçay D, Bauer RM, Fischler IS, Cato MA, Maron L, Auerbach EJ, Browd SR, et al. Left-hemisphere processing of emotional connotation during word generation. Neuroreport. 1999;10:2449–2455. doi: 10.1097/00001756-199908200-00003. [DOI] [PubMed] [Google Scholar]
- Crosson B, Sadek JR, Maron L, Gökçay D, Mohr CM, Auerbach EJ, Freeman AJ, Leonard CM, Briggs RW. Relative shift in activity from medial to lateral frontal cortex during internally versus externally guided word generation. J Cogn Neurosci. 2001;13:272–283. doi: 10.1162/089892901564225. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D. Nouns and verbs are retrieved with differently distributed neural systems. Proc Natl Acad Sci USA. 1993;90:4957–4960. doi: 10.1073/pnas.90.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. Neuroimage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: language, learning and memory. Trends Cogn Sci. 1998;2:355–361. doi: 10.1016/s1364-6613(98)01211-x. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, Matthews PM, Tyler LK. Susceptibility-induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- Elfgren CI, Risberg J. Lateralized frontal blood flow increases during fluency tasks: influence of cognitive strategy. Neuropsychologia. 1998;36:505–512. doi: 10.1016/s0028-3932(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Fabbro F, Moretti R, Bava A. Language impairments in patients with cerebellar lesions. J Neurolinguistics. 2000;13:173–188. [Google Scholar]
- Fiez JA, Petersen SE, Cheney MK, Raichle ME. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115:155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- Friederici AD. What's in control of language? Nat Neurosci. 2006;9:991–992. doi: 10.1038/nn0806-991. [DOI] [PubMed] [Google Scholar]
- Garrard P, Lambon Ralph MA, Watson PC, Powis J, Patterson K, Hodges JR. Longitudinal profiles of semantic impairment for living and nonliving concepts in dementia of Alzheimer's type. J Cogn Neurosci. 2001;13:892–909. doi: 10.1162/089892901753165818. [DOI] [PubMed] [Google Scholar]
- Gil Robles S, Gatignol P, Capelle L, Mitchell MC, Duffau H. The role of dominant striatum in language: a study using intraoperative electrical stimulations. J Neurol Neurosurg Psychiatry. 2005;76:940–946. doi: 10.1136/jnnp.2004.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestani N, Pallier C. Anatomical correlates of foreign speech sound production. Cereb Cortex. 2007;17:929–934. doi: 10.1093/cercor/bhl003. [DOI] [PubMed] [Google Scholar]
- Gollan TH, Montoya RI, Werner GA. Semantic and letter fluency in Spanish-English bilinguals. Neuropsychologia. 2002;16:562–576. [PubMed] [Google Scholar]
- Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, Van Horn JD, Berman KF. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology. 2000;14:353–360. doi: 10.1037//0894-4105.14.3.353. [DOI] [PubMed] [Google Scholar]
- Green DW. Mental control of the bilingual lexico-semantic system. Bilingualism Lang Cogn. 1998;1:67–81. [Google Scholar]
- Heim S, Eickhoff SB, Amunts K. Specialisation in Broca's region for semantic, phonological, and syntactic fluency? Neuroimage. 2008;40:1362–1368. doi: 10.1016/j.neuroimage.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 2004;18:284–295. doi: 10.1037/0894-4105.18.2.284. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer's disease? Neuroanatomical and diagnostic implications. Neuropsychologia. 1995;33:441–459. doi: 10.1016/0028-3932(94)00127-b. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA. 2004;101:13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketteler D, Kastrau F, Vohn R, Huber W. The subcortical role of language processing. High level linguistic features such as ambiguity-resolution and the human brain; an fMRI study. Neuroimage. 2008;39:2002–2009. doi: 10.1016/j.neuroimage.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Kroll JF, Bobb SC, Wodniecka Z. Language selectivity is the exception, not the rule: arguments against a fixed locus of language selection in bilingual speech. Bilingualism Lang Cogn. 2006;9:119–135. [Google Scholar]
- Lee H, Devlin JT, Shakeshaft C, Stewart LH, Brennan A, Glensman J, Pitcher K, Crinion J, Mechelli A, Frackowiak RS, et al. Anatomical traces of vocabulary acquisition in the adolescent brain. J Neurosci. 2007;27:1184–1189. doi: 10.1523/JNEUROSCI.4442-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio MG, Silveri MC, Petrosini L, Molinari M. Phonological grouping is specifically affected in cerebellar patients: a verbal fluency study. J Neurol Neurosurg Psychiatry. 2000;69:102–106. doi: 10.1136/jnnp.69.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipschutz B, Friston KJ, Ashburner J, Turner R, Price CJ. Assessing study-specific regional variations in fMRI signal. Neuroimage. 2001;13:392–398. doi: 10.1006/nimg.2000.0687. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien P, Engelborghs S, Pickut BA, De Deyn PP. Aphasia following cerebellar damage: fact or fallacy? J Neurolinguistics. 2000;13:145–171. [Google Scholar]
- Marien P, Saerens J, Nanhoe R, et al. Cerebellar induced aphasia: case report of cerebellar induced prefrontal aphasic language phenomena supported by SPECT findings. J Neurol Sci. 1996;144:34–43. doi: 10.1016/s0022-510x(96)00059-7. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Lalonde F, Mack C. Word retrieval to letter and semantic cues: a double dissociation in normal subjects using interference tasks. Neuropsychologia. 1994;32:1487–1494. doi: 10.1016/0028-3932(94)90120-1. [DOI] [PubMed] [Google Scholar]
- McCarthy R, Warrington EK. The dissolution of semantics. Nature. 1990;343:599. doi: 10.1038/343599a0. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O'Doherty J, Ashburner J, Frackowiak RS, Price CJ. Neurolinguistics: structural plasticity in the bilingual brain. Nature. 2004;431:757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Milner B. Some effects of frontal lobectomy in man. In: Warren JM, Akert K, editors. The frontal granular cortex and behaviour. New York: McGraw-Hill; 1964. pp. 65–85. [Google Scholar]
- Molinari M, Leggio MG, Solida A, Ciorra R, Misciagna S, Silveri MC, Petrosini L. Cerebellum and procedural learning: evidence from focal cerebellar lesions. Brain. 1997;120:1753–1762. doi: 10.1093/brain/120.10.1753. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi WM, Butters N, Paulsen JS, Salmon DP, Brugger P, Swenson MR. A comparison of category and letter fluency in Alzheimer's disease and Huntington's disease. Neuropsychology. 1994;8:25–30. [Google Scholar]
- Moretti R, Bava A, Torre P, Antonello RM, Zorzon M, Zivadinov R, Cazzato G. Bilingual aphasia and subcortical-cortical lesions. Percept Mot Skills. 2001;92:803–814. doi: 10.2466/pms.2001.92.3.803. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Wise RJ. Generating ‘tiger’ as an animal name or a word beginning with T: differences in brain activation. Proc Biol Sci. 1996;263:989–995. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Goldacre B, Scifo P, Cappa SF, Gilardi MC, Castiglioni I, Perani D, Fazio F. Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport. 1997;8:2011–2017. doi: 10.1097/00001756-199705260-00042. [DOI] [PubMed] [Google Scholar]
- Perani D, Abutalebi J, Paulesu E, Brambati S, Scifo P, Cappa SF, Fazio F. The role of age of acquisition and language usage in early, high-proficient bilinguals: an fMRI study during verbal fluency. Hum Brain Mapp. 2003;19:170–182. doi: 10.1002/hbm.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Tettamanti M, Rosa M, Scifo P, Miozzo A, Basso A, Fazio F. An fMRI study of word retrieval in aphasia. Brain Lang. 2003;85:357–368. doi: 10.1016/s0093-934x(02)00561-8. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Hyder F, Blamire AM, Shulman RG. FMRI of the prefrontal cortex during overt verbal fluency. Neuroreport. 1997;8:561–565. doi: 10.1097/00001756-199701200-00036. [DOI] [PubMed] [Google Scholar]
- Pihlajamäki M, Tanila H, Hänninen T, Könönen M, Laakso M, Partanen K, Soininen H, Aronen HJ. Verbal fluency activates the left medial temporal lobe: a functional magnetic resonance imaging study. Ann Neurol. 2000;47:470–476. [PubMed] [Google Scholar]
- Portocarrero JS, Burright RG, Donovick PJ. Vocabulary and verbal fluency of bilingual and monolingual college students. Arch Clin Neuropsychol. 2007;22:415–422. doi: 10.1016/j.acn.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Raven JC. Progressive matrices: a perceptual test of intelligence. London: H.K. Lewis; 1938. [Google Scholar]
- Richter S, Gerwig M, Aslan B, Wilhelm H, Schoch B, Dimitrova A, Gizewski ER, Ziegler W, Karnath HO, Timmann D. Cognitive functions in patients with MR-defined chronic focal cerebellar lesions. J Neurol. 2007;254:1193–1203. doi: 10.1007/s00415-006-0500-9. [DOI] [PubMed] [Google Scholar]
- Rosselli M, Ardila A, Araujo K, Weekes VA, Caracciolo V, Padilla M, Ostrosky-Solís F. Verbal fluency and repetition skills in healthy older Spanish-English bilinguals. Appl Neuropsychol. 2000;7:17–24. doi: 10.1207/S15324826AN0701_3. [DOI] [PubMed] [Google Scholar]
- Rosser A, Hodges JR. Initial letter and semantic category fluency in Alzheimer's disease, Huntington's disease, and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 1994;57:1389–1394. doi: 10.1136/jnnp.57.11.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert L, Appollonio I, Grafman J, Jezzard P, Johnson R, Jr, Le Bihan D, Turner R. Magnetic resonance imaging functional activation of left frontal cortex during covert word production. J Neuroimaging. 1994;4:67–70. doi: 10.1111/jon19944267. [DOI] [PubMed] [Google Scholar]
- Schlösser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Dewey SL, Brodie JD. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry. 1998;64:492–498. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Baldo J. Distinct patterns of word retrieval in right and left frontal lobe patients: a multidimensional perspective. Neuropsychologia. 2001;39:1209–1217. doi: 10.1016/s0028-3932(01)00053-7. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Baldo J, Graves RE, Brugger P. Pervasive influence of semantics in letter and category fluency: a multidimensional approach. Brain Lang. 2003;87:400–411. doi: 10.1016/s0093-934x(03)00141-x. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Moro A, Messa C, Moresco RM, Rizzo G, Carpinelli A, Matarrese M, Fazio F, Perani D. Basal ganglia and language: phonology modulates dopaminergic release. Neuroreport. 2005;16:397–401. doi: 10.1097/00001756-200503150-00018. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Delgado MR, McCandliss BD, McClelland JL, Fiez JA. Performance feedback drives caudate activation in a phonological learning task. J Cogn Neurosci. 2006;18:1029–1043. doi: 10.1162/jocn.2006.18.6.1029. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- Ullman MT. A neurocognitive perspective on language: the declarative/procedural model. Nat Rev Neurosci. 2001;2:717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Veltman DJ, Friston KJ, Sanders G, Price CJ. Regionally specific sensitivity differences in fMRI and PET: where do they come from? Neuroimage. 2000;11:575–588. doi: 10.1006/nimg.2000.0581. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Vargha-Khadem F, Ashburner J, Passingham RE, Connelly A, Friston KJ, Frackowiak RS, Mishkin M, Gadian DG. MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain. 2002;125:465–478. doi: 10.1093/brain/awf057. [DOI] [PubMed] [Google Scholar]
- Wei L. Dimensions of bilingualism. In: Wei L, editor. The bilingualism reader. New York: Routledge; 2007. pp. 3–25. [Google Scholar]