Abstract
Neuroimaging studies examining the effects of aging and neuropsychiatric disorders on the cerebral cortex have largely been based on measures of cortical volume. Given that cortical volume is a product of thickness and surface area, it is plausible that measures of volume capture at least 2 distinct sets of genetic influences. The present study aims to examine the genetic relationships between measures of cortical surface area and thickness. Participants were men in the Vietnam Era Twin Study of Aging (110 monozygotic pairs and 92 dizygotic pairs). Mean age was 55.8 years (range: 51–59). Bivariate twin analyses were utilized in order to estimate the heritability of cortical surface area and thickness, as well as their degree of genetic overlap. Total cortical surface area and average cortical thickness were both highly heritable (0.89 and 0.81, respectively) but were essentially unrelated genetically (genetic correlation = 0.08). This pattern was similar at the lobar and regional levels of analysis. These results demonstrate that cortical volume measures combine at least 2 distinct sources of genetic influences. We conclude that using volume in a genetically informative study, or as an endophenotype for a disorder, may confound the underlying genetic architecture of brain structure.
Keywords: cortical volume, genetic correlation, heritability, magnetic resonance imaging, twin study
Introduction
Over the past decade, the field of neuroimaging has made extraordinary advances, leading to increased precision in how the living brain can be measured. Although researchers were once limited to gross measures of volume or cross-sectional surface areas, contiguous maps of cortical thickness measurements can now be generated, greatly expanding the realm of hypotheses to be tested. Yet, as the approaches to measurement have changed, there has been remarkably little examination of how these different methods of measuring brain structure may capture different aspects of the underlying neural architecture.
Nowhere is this trend more evident than in neuroimaging studies examining the effects of aging and neuropsychiatric disorders on the cerebral cortex. Until relatively recently, these studies have largely been based on measures of volume (for reviews, see Wright et al. 2000; Sheline 2003; Zakzanis et al. 2003; Raz et al. 2005; Ellison-Wright et al. 2008). Aided largely by methods of high-resolution, semiautomated measurement, researchers have begun to examine cortical thickness as a relevant structural measure. However, examination of the literature demonstrates that measures of volume and thickness are often utilized interchangeably as descriptors of cortical structure, whereas few studies have acknowledged or examined how they may be related to or distinguished from one another (Dickerson et al. 2007; Goghari et al. 2007; Wang et al. 2007; Fornito et al. 2008; Venkatasubramanian et al. 2008).
By definition, volume is the product of surface area and thickness; therefore, measures of cortical volume could combine structural properties that are unique to cortical surface area and unique to cortical thickness. The cellular architecture of the cortex itself suggests that this may be the case. It has been well documented that neurons within the cerebral cortex are organized into ontogenetic columns that run perpendicular to the surface of the brain (Mountcastle 1997). The radial unit hypothesis of cortical development argues that the cells within a column share a common origin and migrate to their location within the cortex during development (Rakic 1988, 1995, 2007). It also postulates that the size of the cortical surface area is driven by the number of columns, whereas cortical thickness is influenced by the number of cells within a column (Rakic 1988). A recent extension of the radial unit hypothesis, referred to as the radial amplification hypothesis, highlights the contribution of intermediate progenitor cells to neurogenesis and how their manipulation within animal models results in changes to cortical thickness but not surface area (Pontious et al. 2008). In addition, studies of interindividual variation in adult brain size have found that differences in cortical gray matter volume are driven almost exclusively by differences in the cortical surface area rather than cortical thickness, suggesting little covariation between the 2 measures (Pakkenberg and Gundersen 1997; Im et al. 2008). Such evidence suggests that surface area and thickness are distinct rather than redundant features of cortical structure.
If cortical surface area and cortical thickness are indeed driven by distinct cellular mechanisms, then it is reasonable to propose that they will have distinct genetic etiologies. Twin studies have clearly demonstrated significant genetic contributions to variation in brain structure (for reviews, see Glahn et al. 2007; Peper et al. 2007; Schmitt et al. 2007). The majority of these studies, however, have been based on measures of cortical volume, cortical density, or whole brain/lobar volumes. Although informative as to the overall impact of genes, the use of such measures overlooks fundamental aspects of the brain's architecture and ultimately results in an incomplete picture of the genetic factors at work. Indeed, we are aware of only one sample in which the thickness for specific cortical regions of interest (ROIs) was assessed (Lenroot et al. 2007; Schmitt et al. 2008). Furthermore, we are unaware of any human magnetic resonance imaging (MRI) studies of cortical surface area alone or studies of the potential relationship between surface area and thickness.
Rather than determining the heritability of an individual measure (i.e., the proportion of the phenotypic variance that is accounted for by genetic influences), multivariate applications of the twin design can be utilized to examine the genetic relationships between primary elements of multidimensional measurements and thus determine if multiple genetic factors are at work. For example, if the volume of the cerebral cortex is influenced by one set of genetic factors, then there should be a strong relationship between the latent genetic factors influencing measures of both surface area and thickness. In other words, there should be a highly significant genetic correlation observed. If, however, surface area and thickness are each heritable but do not possess a strong genetic relationship, it would mean that there are different genetic influences that underlie these measures, influences that would be obscured by the use of volume.
The aim of the present study is to examine the genetic relationships between measures of cortical surface area and thickness. As predicted by the radial unit hypothesis, as well as other structural evidence, we hypothesize that the genetic influences responsible for the thickness of the cerebral cortex are distinct from the genetic influences that determine the measurable surface area. If this should prove to be the case, it will be necessary to reconsider the use of cortical volume as a phenotype for genetically informative studies of brain structure.
Materials and Methods
Participants
Data were obtained from participants in the Vietnam Era Twin Study of Aging (VETSA), a longitudinal study of cognition and aging beginning in midlife (Kremen et al. 2006). All participants in the VETSA were drawn from the Vietnam Era Twin (VET) Registry, a nationally distributed sample of male–male twin pairs who served in the US military between 1965 and 1975 (Goldberg et al. 2002). Detailed descriptions of the VET Registry's composition and method of ascertainment have been previously reported (Eisen et al. 1987; Henderson et al. 1990). The VETSA MRI study began in year 3 of the primary VETSA project. Upon initial contact, 6% of the sample declined to participate. Participants were excluded from the study for reasons such as metal in the body (7%), claustrophobia (3%), inability to travel to the testing site (5%), problems with the scanner (8%), the exclusion of their co-twin (9%), or other reasons (3%). Ultimately, 59% of those who were contacted participated in the MRI study. As part of the primary VETSA project, participants traveled to either Boston University or the University of California San Diego (UCSD) for a daylong series of physical, psychosocial, and neurocognitive assessments. Informed consent was obtained from all participants, and the scanning protocol was approved of by both the UCSD and Massachusetts General Hospital (MGH) Institutional Review Boards. On either the day before or the day following these assessments, the participants were scanned at either MGH or the UCSD Medical Center. The present analyses were performed on data from 474 participants (110 monozygotic [MZ] pairs, 92 dizygotic [DZ] pairs, and 68 unpaired individuals) for whom suitable imaging data were available. Despite the more stringent inclusion criterion for the MRI protocol, participants in this study were roughly equivalent to the larger VETSA sample with respect to age, education, ethnicity, employment, and self-reported health status (see Table 1).
Table 1.
Sample demographics
| VETSA (N = 1237) | VETSA MRI (N = 474) | |
| Age (mean, SD) | 55.4 (2.5) | 55.8 (2.6) |
| Education (mean, SD) | 13.8 (2.1) | 13.9 (2.1) |
| Ethnicity (% of sample) | ||
| Caucasian | 89.3% | 88.3% |
| African–American | 4.2% | 5.3% |
| Hispanic | 2.8% | 3.4% |
| Other | 3.7% | 3.0% |
| Employment (% of sample) | ||
| Full-time | 77.0% | 74.9% |
| Part-time | 4.2% | 4.2% |
| Retired | 9.0% | 11.2% |
| Handedness (% right) | 85.9% | 85.2% |
| Self-reported health status (% of sample) | ||
| Excellent | 12.1% | 14.8% |
| Very good | 36.7% | 36.5% |
| Good | 39.4% | 37.4% |
| Fair | 10.5% | 10.4% |
| Poor | 1.3% | 0.9% |
SD, standard deviation.
At the time the manuscript was prepared, zygosity for 56% of the sample had been determined by analysis of 25 satellite markers obtained from blood samples. Due to the fact that DNA results were not yet available for the remaining 44%, zygosity that had been previously determined through a combination of questionnaire and blood group methods was also used (Eisen et al. 1989). Consistent with previous studies, the latter techniques demonstrated approximately 95% accuracy with respect to DNA-based results (Nichols and Bilbro 1966; Eisen et al. 1989; Peeters et al. 1998). For those who had both measures, the results from the DNA analyses were used.
Image Acquisition
Images were acquired on Siemens 1.5-T scanners (241 at UCSD and 233 at MGH). Sagittal T1-weighted magnetization prepared rapid gradient echo sequences were employed with a time to inversion = 1000 ms, time echo = 3.31 ms, time repetition = 2730 ms, flip angle = 7 degrees, slice thickness = 1.33 mm, and voxel size = 1.3 × 1.0 × 1.3 mm. Raw DICOM MRI scans (including 2 T1-weighted volumes per case) were downloaded to the MGH site. These data were reviewed for quality, registered, and averaged to improve signal-to-noise. Of the 493 scans available at the time of these analyses, quality control measures excluded 3 cases due to scanner artifact and 16 cases due to inadequate image processing results (e.g., poor contrast caused removal of nonbrain to fail).
Image Processing
Cortical Surface Area and Thickness Measures
The cortical surface was reconstructed to measure surface area and thickness at each surface location, or vertex, using methods based on the publicly available FreeSurfer software package (Dale et al. 1999; Fischl et al. 1999; Fischl and Dale 2000; Fischl et al. 2004). Intensity variations due to magnetic field inhomogeneities are corrected, a normalized intensity image is created, and the skull (nonbrain) is removed from the normalized image. The preliminary segmentation is partitioned using a connected components algorithm, with connectivity not allowed across the established cutting planes. Any interior holes in the components representing white matter are filled, resulting in a single-filled volume for each cortical hemisphere. The resulting surface is covered with a polygonal tessellation and smoothed to reduce metric distortions. After the initial surface model has been constructed, a refinement procedure is applied to obtain a representation of the gray/white boundary. This surface is subsequently deformed outwards to obtain an explicit representation of the pial surface. Once generated, the cortical surface model is manually reviewed and edited for technical accuracy. Minimal manual editing was performed in alignment with standard, objective editing rules. Studies demonstrate a high correlation of automatic and manual measures in vivo and ex vivo (Fischl and Dale 2000; Walhovd et al. 2005).
The surface was then divided into distinct cortical ROIs (Fischl et al. 2004). Each vertex was assigned a neuroanatomical label based on 1) the probability of each label at each location in a surface-based atlas space, based on a manually parcellated training set; 2) local curvature information; and 3) contextual information, encoding spatial neighborhood relationships between labels (conditional probability distributions derived from the manual training set). The parcellation scheme (Desikan et al. 2006) labels cortical sulci and gyri, and then surface area and thickness values are calculated in the 66 ROIs (33 per hemisphere). Cortical thickness was calculated as the average distance between the gray/white boundary and the pial surface within each ROI. Surface area was calculated as the sum of the areas of each tessellation falling within a given ROI; this is done in each subjects’ native space. Global and lobar measures of cortical thickness and surface area were subsequently calculated as averages or sum totals of these ROIs.
Data Analysis
In univariate twin analyses, the variance of any trait is decomposed into the proportion attributed to additive genetic (A) influences (also referred to as heritability), shared or common environmental (C) influences (i.e., environmental factors that influence both members of a pair equally), and unique environmental (E) influences, which also include measurement error (Eaves et al. 1978; Neale and Cardon 1992). The resulting model is typically referred to as the “ACE” model. Because MZ twins share 100% of their segregating genes, they correlate perfectly (r = 1.0) in terms of genetic influences. In contrast, DZ twins share on average 50% of their segregating genes, resulting in correlations of 0.50 for additive genetic influences. Shared or common environmental influences, as they are defined as any environmental factor that influences both members of a twin pair equally, correlate 1.0 within a twin pair. Unique or individual-specific environmental influences are assumed to be uncorrelated across twins.
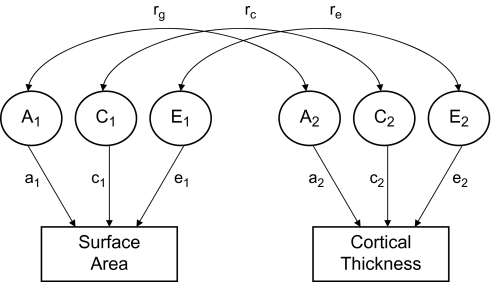
Multivariate analyses extend the ACE model so as to not only decompose the variance of a trait into genetic and environmental components but also decompose the covariance between traits. Thus, one is able to derive genetic and environmental covariance estimates that can then be used to calculate genetic and environment correlations. In statistical terms, the genetic correlation between 2 traits is equal to their genetic covariance, divided by the square root of the product of their separate genetic variances (Neale and Cardon 1992). Common environmental and unique environmental correlations are calculated in a similar fashion utilizing the respective variances and covariances. Conceptually, the genetic correlation provides an indicator of the degree to which 2 traits are genetically unique, evidenced by a genetic correlation near zero, or exhibit genetic overlap. A genetic correlation of 1.0 would indicate that the same set of genes influences both traits. Figure 1 depicts the bivariate application of the multivariate twin model.
Figure 1.
Bivariate correlated factors model. (A) Additive genetic influences. (C) Shared or common environmental influences. (E) Nonshared or unique environmental influences. rg = Genetic correlation; rc = shared environment correlation; re = Unique environment correlation. Arrows from (A1), (C1), and (E1) to surface area represent parameter estimates for the contribution of those components to the variable. The same is true for arrows from (A2), (C2), and (E2) to cortical thickness. Squaring these parameter estimates provides the proportion of variance accounted for by each component.
Analyses were performed using the maximum likelihood–based structural equation modeling software Mx (Neale et al. 2004). Bivariate ACE models were fit to the raw data in order to estimate the genetic and environmental variance components of surface area and thickness, as well as the phenotypic, genetic, and environmental correlations between the measures. The obtained phenotypic correlation is akin to the traditional Pearson's correlation after having adjusted for the nonindependence of the observations. The significance of the genetic correlation in each bivariate relationship was formally tested by constraining the genetic covariance parameter to zero and comparing the subsequent model fit to that of the full, less constrained model. Because genetic correlations may vary in sign and magnitude independent of the phenotypic correlation, all bivariate relationships were examined regardless of the observed phenotypic correlation. Evaluation of relative model fit was performed using the likelihood ratio test (LRT), which is calculated as the difference in the −2 log likelihood (−2LL) of one model relative to another. The LRT is distributed as a chi-square with degrees of freedom (df) equivalent to the difference in the number of parameters between the competing models. Nonsignificant LRT values (P > 0.05) indicate that the model does not result in a significant change in fit relative to the comparison model and can therefore be considered as a comparable representation of the data. If elimination of the genetic correlation results in a significant (P < 0.05) change in model fit, the parameter can be viewed as statistically significant. Although the common environment and unique environment correlations can be tested using the same method, we did not formally test these parameters because they did not pertain to our research objective (i.e., the degree of genetic overlap).
In order to determine if the observed relationships between surface area and thickness were dependent upon the level of analysis (e.g., global vs. regional), data analysis was done in 2 phases. In phase 1, we sought to examine the relationship between surface area and cortical thickness for global measures of brain structure. Therefore, we calculated the total cortical surface area and average cortical thickness for the entire cortex, as well as the 4 lobar regions bilaterally. In phase 2, we examined the relationship between surface area and average thickness for specific ROIs (10 from the left hemisphere and 10 from the right). We intentionally selected large and small regions within each of the major lobes, as well as both lateral and medial structures. Neither the heritability of the ROIs nor their functional properties were considered during the selection process. All measures were standardized to a mean of 0 and a standard deviation of 1 in order to facilitate the estimation of means and variances without changing the inherent relationships between variables and members of a twin pair. With the exception of the global measures of thickness and surface area, no statistical adjustments were made for overall head size or total intracranial volume. Similarly, age was not accounted for in the analyses because it did not correlate with any of the measures utilized.
Results
Global Measures
Table 2 presents the genetic and unique environmental standardized variance components, as well as the phenotypic, genetic, and unique environment correlations for the global measures of surface area and cortical thickness. On average, shared environment effects accounted for a small and nonsignificant proportion of the phenotypic variance (roughly 8% across all measures) and were omitted from the table to simplify presentation. Total cortical surface area and average cortical thickness were both highly heritable, with genetic influences accounting for 89% and 81% of the observed variance, respectively. However, the observed genetic correlation between these 2 measures was small (rg = 0.08) and could be eliminated from the model without resulting in a significant reduction in model fit (P = 0.42), as shown in Table 3.
Table 2.
Genetic and unique environmental standardized variance components and correlations (95% CI)
| Surface area |
Cortical thickness |
Correlations |
|||||
| A | E | A | E | Phenotypic | Genetic | Environmental | |
| Total gray matter | 0.89 (0.66, 0.96) | 0.06 (0.04, 0.08) | 0.81 (0.56, 0.87) | 0.17 (0.12, 0.23) | 0.01 (−0.09, 0.12) | 0.08 (−0.11, 0.29) | −0.13 (−0.31, 0.06) |
| Left frontal lobe | 0.88 (0.64, 0.95) | 0.06 (0.05, 0.09) | 0.78 (0.54, 0.84) | 0.21 (0.16, 0.29) | −0.05 (−0.16, 0.05) | −0.02 (−0.19, 0.21) | −0.10 (−0.28, 0.09) |
| Right frontal lobe | 0.81 (0.58, 0.94) | 0.08 (0.06, 0.11) | 0.70 (0.40, 0.80) | 0.26 (0.19, 0.35) | −0.05 (−0.15, 0.06) | 0.08 (−0.18, 0.36) | −0.23 (−0.39, −0.04) |
| Left temporal lobe | 0.87 (0.60, 0.91) | 0.12 (0.09, 0.16) | 0.63 (0.39, 0.73) | 0.36 (0.27, 0.49) | −0.01 (−0.11, 0.09) | 0.05 (−0.14, 0.33) | −0.23 (−0.40, −0.05) |
| Right temporal lobe | 0.85 (0.68, 0.90) | 0.13 (0.10, 0.18) | 0.67 (0.37, 0.82) | 0.24 (0.18, 0.32) | −0.01 (−0.11, 0.10) | 0.01 (−0.09, 0.35) | −0.20 (−0.38, −0.02) |
| Left parietal lobe | 0.87 (0.65, 0.92) | 0.11 (0.08, 0.15) | 0.74 (0.47, 0.83) | 0.23 (0.17, 0.31) | 0.10 (−0.01, 0.20) | 0.17 (−0.01, 0.42) | −0.08 (−0.27, 0.10) |
| Right parietal lobe | 0.77 (0.50, 0.88) | 0.16 (0.12, 0.22) | 0.74 (0.46, 0.80) | 0.26 (0.20, 0.35) | 0.08 (−0.02, 0.18) | 0.14 (−0.11, 0.42) | −0.10 (−0.28, 0.08) |
| Left occipital lobe | 0.64 (0.36, 0.83) | 0.21 (0.16, 0.29) | 0.71 (0.43, 0.79) | 0.27 (0.21, 0.37) | −0.01 (−0.11, 0.09) | 0.09 (−0.22, 0.43) | −0.11 (−0.29, 0.07) |
| Right occipital lobe | 0.31 (0.01, 0.65) | 0.35 (0.26, 0.46) | 0.52 (0.19, 0.77) | 0.29 (0.22, 0.38) | −0.04 (−0.14, 0.07) | 0.10 (−0.58, 0 31) | −0.03 (−0.21, 0.16) |
Note: A, proportion of phenotypic variance due to genetic factors (heritability); E, proportion of phenotypic variance due to unique environmental factors. For simplicity of presentation, shared environment effects (C), although estimated in the model, are omitted.
Table 3.
Test of significance for genetic correlation
| −2LL | df | LRT | Δdf | P value | |
| Total gray matter | 2282.53 | 938 | 0.59 | 1 | 0.44 |
| Left frontal lobe | 2317.63 | 938 | 0.03 | 1 | 0.87 |
| Right frontal lobe | 2358.11 | 938 | 0.32 | 1 | 0.57 |
| Left temporal lobe | 2439.90 | 938 | 0.41 | 1 | 0.52 |
| Right temporal lobe | 2420.25 | 938 | 0.61 | 1 | 0.44 |
| Left parietal lobe | 2386.86 | 938 | 3.50 | 1 | 0.06 |
| Right parietal lobe | 2431.89 | 938 | 1.29 | 1 | 0.26 |
| Left occipital lobe | 2462.36 | 938 | 0.32 | 1 | 0.57 |
| Right occipital lobe | 2501.58 | 938 | 0.11 | 1 | 0.74 |
Note: Δdf, change in df.
Given the potential relationship between these global measures and head size, we performed additional analyses utilizing an estimate of total intracranial volume (TIV). Like the other global measures, TIV was found to be highly heritable, with genetic influences accounting for 78% of the observed variance. At the phenotypic level, TIV correlated 0.15 (P < 0.001) with average cortical thickness and 0.81 (P < 0.0001) with total surface area. At the genetic level, the correlation between TIV and average thickness was 0.23, which translated into a shared genetic variance of 4.3%. With respect to surface area, the genetic correlation with TIV was 0.88, which amounted to a shared genetic variance of 76.8%. Both genetic correlations were significant based on 95% confidence intervals (CIs). After adjusting for TIV, the phenotypic correlation between average cortical thickness and total surface area was −0.15 (P = 0.001). The heritability of TIV-adjusted average cortical thickness fell to 0.77 (95% CI: 0.52–0.86), and the heritability of TIV-adjusted total surface dropped to 0.62 (95% CI: 0.33–0.74). For both measures, the common environment accounted for only 4% of the observed variance. Despite the more pronounced phenotypic correlation between the TIV-adjusted average cortical thickness and total surface area, the genetic correlation between the measures remained nonsignificant, rg = −0.24 (95% CI: −0.45–0.03), and could be eliminated from the model without a significant reduction in fit (LRT = 3.06, Δdf = 1, P = 0.08).
At the lobar level, heritability estimates ranged from 31% for surface area of the right occipital lobe to 88% for surface area of the left frontal lobe. Although heritability estimates for surface area tended to be larger than the estimates for average thickness, these differences were interpreted as nonsignificant based on the overlapping 95% CIs. Like the global measures, the genetic correlations were low (range: −0.02–0.32) and could be eliminated from each bivariate model without a significant reduction in fit (P value range: 0.87–0.06).
Specific ROIs
Tables 4 and 5 present the standardized variance components, as well as the phenotypic, genetic, and unique environment correlations for the ROI-based measures of surface area and cortical thickness. Heritability estimates were, on average, lower than for the global- and lobar-based measures, likely reflecting an increase in the degree of measurement error. As observed with the global measures, genetic correlations between surface area and thickness tended to be small, with 95% CIs that overlapped with zero. As shown in Table 6, for each of the bivariate relationships, the genetic correlation could be fixed to zero in the model without a significant change in model fit (P value range: 0.98–0.07).
Table 4.
Genetic and unique environmental standardized variance components and correlations (95% CI) for left hemisphere ROIs
| Surface area |
Cortical thickness |
Correlations |
|||||
| A | E | A | E | Phenotypic | Genetic | Environmental | |
| Lateral orbital frontal cortex | 0.51 (0.24, 0.62) | 0.49 (0.38, 0.63) | 0.52 (0.23, 0.64) | 0.48 (0.36, 0.63) | −0.32 (−0.40, −0.23) | −0.40 (−0.70, 0.06) | −0.24 (−0.39, −0.06) |
| Superior frontal gyrus | 0.69 (0.42, 0.84) | 0.21 (0.16, 0.28) | 0.76 (0.50, 0.83) | 0.23 (0.17, 0.31) | −0.09 (−0.18, 0.01) | −0.18 (−0.47, 0.09) | 0.02 (−0.16, 0.20) |
| Superior parietal cortex | 0.63 (0.33, 0.72) | 0.37 (0.28, 0.49) | 0.64 (0.27, 0.73) | 0.36 (0.27, 0.47) | 0.05 (−0.05, 0.15) | 0.06 (−0.33, 0.25) | 0.04 (−0.14, 0.21) |
| Entorhinal cortex | 0.16 (0.00, 0.34) | 0.83 (0.66, 0.99) | 0.20 (0.00, 0.51) | 0.64 (0.49, 0.82) | 0.05 (−0.04, 0.14) | 0.34 (−1.0, 1.0) | −0.08 (−0.25, 0.10) |
| Parahippocampal gyrus | 0.10 (0.00, 0.46) | 0.67 (0.52, 0.82) | 0.39 (0.01, 0.61) | 0.50 (0.38, 0.66) | 0.29 (0.20, 0.38) | 0.08 (−1.0, 1.0) | 0.21 (0.03, 0.37) |
| Posterior central gyrus | 0.61 (0.30, 0.71) | 0.38 (0.29, 0.52) | 0.59 (0.23, 0.70) | 0.39 (0.30, 0.51) | 0.21 (0.11, 0.30) | 0.19 (−0.36, 0.43) | 0.21 (0.03, 0.38) |
| Posterior cingulate cortex | 0.37 (0.00, 0.58) | 0.54 (0.42, 0.69) | 0.44 (0.06, 0.57) | 0.55 (0.43, 0.70) | −0.01 (−0.10, 0.09) | 0.16 (−1.0, 1.0) | −0.05 (−0.23, 0.12) |
| Precuneus cortex | 0.74 (0.49, 0.80) | 0.26 (0.20, 0.36) | 0.65 (0.47, 0.74) | 0.35 (0.26, 0.46) | −0.02 (−0.12, 0.08) | 0.01 (−0.17, 0.28) | −0.11 (−0.29, 0.08) |
| Middle temporal gyrus | 0.37 (0.00, 0.56) | 0.56 (0.44, 0.71) | 0.37 (0.00, 0.55) | 0.58 (0.45, 0.41) | −0.10 (−0.19, −0.01) | −0.07 (−1.0, 1.0) | −0.02 (−0.19, 0.15) |
| Lateral occipital cortex | 0.33 (0.00, 0.64) | 0.45 (0.34, 0.59) | 0.55 (0.21, 0.68) | 0.42 (0.32, 0.54) | 0.00 (−0.09, 0.10) | 0.35 (−0.26, 1.0) | −0.13 (−0.30, 0.05) |
Note: A, proportion of phenotypic variance due to genetic factors (heritability); E, proportion of phenotypic variance due to unique environmental factors. For simplicity of presentation, shared environment effects (C), although estimated in the model, are omitted.
Table 5.
Genetic and unique environmental standardized variance components and correlations (95% CI) for right hemisphere ROIs
| Surface area |
Cortical thickness |
Correlations |
|||||
| A | E | A | E | Phenotypic | Genetic | Environmental | |
| Lateral orbital frontal cortex | 0.35 (0.00, 0.48) | 0.65 (0.52, 0.79) | 0.55 (0.35, 0.66) | 0.45 (0.34, 0.59) | −0.22 (−0.30, −0.12) | −0.22 (−1.0, 1.0) | −0.22 (−0.38, −0.06) |
| Superior frontal gyrus | 0.67 (0.39, 0.83) | 0.22 (0.16, 0.30) | 0.65 (0.31, 0.77) | 0.30 (0.23, 0.39) | −0.06 (−0.16, 0.04) | −0.14 (−0.46, 0.24) | −0.18 (−0.35, 0.01) |
| Superior parietal cortex | 0.50 (0.14, 0.72) | 0.37 (0.28, 0.50) | 0.67 (0.42, 0.76) | 0.32 (0.24, 0.43) | 0.06 (−0.04, 0.16) | 0.01 (−0.46, 0.39) | 0.06 (−0.13, 0.24) |
| Entorhinal cortex | 0.21 (0.00, 0.40) | 0.60 (0.46, 0.77) | 0.24 (0.00, 0.52) | 0.75 (0.59, 0.93) | −0.14 (−0.23, −0.05) | −0.12 (−1.0, 1.0) | −0.29 (−0.44, −0.12) |
| Parahippocampal gyrus | 0.20 (0.00, 0.37) | 0.80 (0.63, 0.99) | 0.58 (0.26, 0.69) | 0.42 (0.31, 0.55) | 0.07 (−0.02, 0.16) | 0.20 (−1.0, 1.0) | 0.01 (−0.18, 0.19) |
| Posterior central gyrus | 0.08 (0.00, 0.47) | 0.47 (0.36, 0.59) | 0.66 (0.33, 0.74) | 0.33 (0.26, 0.43) | 0.16 (0.07, 0.26) | 0.51 (−1.0, 1.0) | −0.01 (−0.19, 0.17) |
| Posterior cingulate cortex | 0.33 (0.00, 0.56) | 0.52 (0.40, 0.67) | 0.51 (0.24, 0.64) | 0.48 (0.36, 0.63) | −0.06 (−0.16, 0.03) | 0.01 (−1.0, 1.0) | −0.03 (−0.21, 0.14) |
| Precuneus cortex | 0.31 (0.02, 0.64) | 0.45 (0.34, 0.58) | 0.53 (0.24, 0.68) | 0.41 (0.31, 0.53) | −0.12 (−0.22, −0.02) | 0.43 (−0.12, 1.0) | −0.40 (−0.54, −0.24) |
| Middle temporal gyrus | 0.48 (0.15, 0.65) | 0.45 (0.34, 0.60) | 0.41 (0.05, 0.63) | 0.48 (0.37, 0.63) | −0.04 (−0.14, 0.05) | 0.24 (−0.17, 1.0) | −0.14 (−0.32, 0.04) |
| Lateral occipital cortex | 0.03 (0.00, 0.46) | 0.65 (0.49, 0.77) | 0.55 (0.21, 0.67) | 0.43 (0.33, 0.56) | −0.03 (−0.12, 0.07) | 0.88 (−1.0, 1.0) | −0.11 (−0.28, 0.07) |
Note: A, proportion of phenotypic variance due to genetic factors (heritability); E, proportion of phenotypic variance due to unique environmental factors. For simplicity of presentation, shared environment effects (C), although estimated in the model, are omitted.
Table 6.
Test of significance for genetic correlation
| −2LL | df | LRT | Δdf | P value | |
| Left hemisphere | |||||
| Lateral orbital frontal cortex | 2565.31 | 938 | 3.29 | 1 | 0.07 |
| Superior frontal gyrus | 2450.42 | 938 | 1.78 | 1 | 0.18 |
| Superior parietal cortex | 2553.46 | 938 | 0.22 | 1 | 0.64 |
| Entorhinal cortex | 2660.20 | 938 | 0.18 | 1 | 0.67 |
| Parahippocampal gyrus | 2597.17 | 938 | 0.01 | 1 | 0.92 |
| Posterior central gyrus | 2558.52 | 938 | 0.73 | 1 | 0.39 |
| Posterior cingulate cortex | 2630.78 | 938 | 0.25 | 1 | 0.62 |
| Precuneus cortex | 2533.13 | 938 | 0.02 | 1 | 0.88 |
| Middle temporal gyrus | 2625.69 | 938 | 0.03 | 1 | 0.85 |
| Lateral occipital cortex | 2575.92 | 938 | 1.29 | 1 | 0.26 |
| Right hemisphere | |||||
| Lateral orbital frontal cortex | 2603.35 | 938 | 2.64 | 1 | 0.10 |
| Superior frontal gyrus | 2472.72 | 938 | 0.70 | 1 | 0.40 |
| Superior parietal cortex | 2545.01 | 938 | 0.01 | 1 | 0.95 |
| Entorhinal cortex | 2638.93 | 938 | 0.05 | 1 | 0.82 |
| Parahippocampal gyrus | 2636.64 | 938 | 0.99 | 1 | 0.32 |
| Posterior central gyrus | 2533.29 | 938 | 0.85 | 1 | 0.36 |
| Posterior cingulate cortex | 2614.88 | 938 | 0.01 | 1 | 0.98 |
| Precuneus cortex | 2553.60 | 938 | 1.93 | 1 | 0.17 |
| Middle temporal gyrus | 2604.87 | 938 | 0.72 | 1 | 0.40 |
| Lateral occipital cortex | 2612.52 | 938 | 0.65 | 1 | 0.42 |
Note: Δdf, change in df.
Unlike the global and lobar measures, many of the ROI-based surface area heritability estimates (11 out of 20) were nonsignificant, based upon their 95% CIs. In order to determine if the nonsignificant genetic correlations at the ROI level were a product of these nonsignificant heritability estimates, we repeated the analyses with the shared environment effects fixed at zero. By eliminating the shared environment, estimates which themselves were small and not significant, all familial variances are forced to be accounted for by the genetic factor, resulting in an increase of the heritability estimate that is typically to the point of significance. As anticipated, under this new model, all heritability estimates were significant, ranging from 18% to 79% (see Supplementary material Tables 7 and 8). Despite the inflation of the heritability estimates, the majority of the observed genetic correlations remained small and nonsignificant. Of the 20 bivariate relationships examined, only 4 demonstrated significant genetic correlations between surface area and thickness: left lateral orbital frontal cortex, left parahippocampal gyrus, left postcentral gyrus, and right postcentral gyrus (see Supplementary materials Table 9). The absolute value of these correlations ranged from 0.20 to 0.44, indicating that although some degree of genetic overlap exists, there remain substantial unique genetic influences on both measures.
Discussion
To date, multiple twin studies have been published that document the significant heritability of volume-based measures of brain structure. However, the present results clearly demonstrate that for global, lobar, and regional levels of analysis, measures of cortical volume combine at least 2 distinct sources of genetic effects, those influencing surface area and those influencing cortical thickness. We argue that the lack of a genetic correlation between these measures is consistent with the columnar organization of the cerebral cortex and the putative developmental origins of that organization as specified by the radial unit hypothesis (Rakic 1988, 1995, 2007; Mountcastle 1997).
Given these findings, it would appear that careful consideration needs to be made before utilizing any multidimensional measurement of the brain, especially in genetically informative studies. Measurements of gray matter volume conflate the contributions of thickness and surface area and therefore may not capture the basic structural elements of the cerebral cortex. The same is true for measures of gray matter density, defined as the proportion of gray matter per 3 dimensional imaging voxel (Wright et al. 1995; Thompson et al. 2001). Despite this, multidimensional measures have been proposed as possible endophenotypes for numerous neuropsychiatric disorders (Glahn et al. 2007). An endophenotype is defined as a trait that is along the causal pathway from genetic predisposition to clinical outcome (Gottesman and Gould 2003). Historically, the primary criterion for an endophenotype has been that it must be heritable, as has been repeatedly demonstrated for cortical volume (Glahn et al. 2007; Peper et al. 2007; Schmitt et al. 2007). However, when a heritable trait has underlying components that are genetically distinct, it will be a less informative phenotype than the underlying components. Therefore, elucidating these distinct sets of genetic influences, when they do exist, will be a crucial step in identifying the key genetic factors that influence normal and pathological brain structure.
That surface area and thickness are genetically distinct from one another has numerous implications for continued investigations into the genetic influences of brain structure. Perhaps, the most significant of these is the need to explore the genetics of surface area to a greater degree. Like thickness, surface area is a highly heritable construct; yet, it has been largely overlooked in human imaging genetics research. Specific mutations in humans have been linked to excessive gyrification of the cortex as well as an increase in the cortical surface area (Piao et al. 2004; Jansen and Andermann 2005). Animal studies have also demonstrated that manipulation of specific genes can result in dramatic changes in arealization and expansion of select areas of the cerebral cortex like the primary visual area and the primary somatosensory area (Bishop et al. 2000; Mallamaci et al. 2000). Findings such as these would appear to suggest that the genes that influence surface area are critical to the early growth and development of the brain. The observed genetic relationship between total surface area and intracranial volume lends support to this conclusion. If this is the case, then a more focused examination of the genetics of surface area may produce new insights into disorders believed to have early developmental origins, such as schizophrenia.
In this same light, recent findings pertaining to the genetics of cortical thickness and cortical volume may have to be reconsidered. For instance, multivariate twin analyses of cortical thickness have demonstrated genetically mediated networks across the cortex in both pediatric (Schmitt et al. 2008) and adult samples (Rimol LM, Panizzon MS, Fennema-Notestine C, Fischl B, Franz CE, Lyons MJ, Makris N, Neale MC, Pacheco J, Perry ME, Schmitt JE, Seidman L, Thermenos HW, Tsuang MT, Kremen WS, Dale AM, unpublished data). It remains to be seen if similar patterns of results will be obtained using measures of surface area. The genetic relationship between cognition and brain has been examined using measures of brain volume, cortical volume, or cortical density (Carmelli et al. 2002; Posthuma et al. 2002, 2003; Hulshoff Pol et al. 2006), raising the question of which genetic influences are actually associated with cognition. Finally, the distinction between surface area and thickness will have to be addressed in genetic association studies. Defining structural phenotypes more precisely, taking into consideration the multiple latent genetic factors involved, will likely improve the ability of researchers to identify specific genes that are associated with structural differences.
Although the radial unit hypothesis suggests that the cortical surface area is influenced by the number of columns, whereas cortical thickness is influenced by the number of cells within a column (Rakic 1988), it may be the case that the measures of surface area and thickness examined in this study reflect structural aspects other than the columnar organization of the cortex. For example, variations in cortical thickness could be due to differences in myelination of gray matter or the underlying white matter, rather than the number of cells within the column. Other explanations are also possible. Given that MRI measures do not have the resolution to examine brain structure at the cellular level, we cannot tell whether variation in thickness is due to different numbers of cells or the size of the cells.
We must acknowledge several potential limitations of this study. First, the all-male, relatively homogenous nature of this sample limits the degree to which results can be generalized to other populations. We cannot be certain that the genetic relationship between surface area and thickness would be similar in a sample of greater ethnic diversity or within a female twin cohort. Similarly, we must recognize that given the high degree of developmental change in gray and white matter during childhood and adolescence, these results may not apply to younger age cohorts (Giedd et al. 1999; Jernigan et al. 2001; Sowell et al. 2004; Jernigan and Gamst 2005). It remains an issue for future research to determine if the lack of genetic relationship observed in this middle-aged sample may be unique to this age period. The goal of the VETSA projects is to follow participants longitudinally, which may enable us to address this issue in the future.
It is also the case that while the VETSA represents a large MRI twin sample, our power to detect additive genetic effects and genetic correlations may be limited for some of the bivariate relationships. Although the power to detect genetic effects is substantially increased in multivariate twin designs, sufficient power to detect significant genetic correlations, in samples of approximately 200 twin pairs, is obtained when heritability estimates are approximately 40% (Sullivan and Eaves 2002). Although we achieve this criterion for the majority of the bivariate analyses, there were select cases in which heritability estimates were lower than the ideal. Nevertheless, in these few cases, we were still able to demonstrate a lack of complete genetic overlap between surface area and thickness.
Conclusion
As researchers continue to pursue genetic and environmental processes influencing brain structure, it is critical to recognize that the measures selected, such as gray matter volume, may be influenced by multiple underlying genetic factors. The absence of a genetic correlation between cortical surface area and cortical thickness clearly demonstrates this phenomenon. The expansion of current methods to include examination of both surface area and thickness may enhance the ability of future studies to elucidate the genetic influences on neuropsychiatric disorders, as well as normal age-related structural brain changes.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Institutes of Health/National Institute on Aging Grants U24 RR021382, R01 AG18386, RO1 AG18384, RO1 AG22381, RO1 AG 22982; US Department of Veterans Affairs; VA Cooperative Studies Program; Department of Defense; National Personnel Records Center, National Archives, and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; Institute for Survey Research, Temple University; Schulman, Ronca, and Bucuvalas, Inc.
Acknowledgments
We gratefully acknowledge the cooperation and participation of the members of the VET Registry and their families. Without their contribution, this research would not have been possible. Conflict of Interest: None declared.
References
- Bishop KM, Goudreau G, O'Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, DeCarli C, Reed T. Quantitative genetic modeling of regional brain volumes and cognitive performance in older male twins. Biol Psychol. 2002;61:139–155. doi: 10.1016/s0301-0511(02)00056-x. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I: segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, Buckner RL. Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2007;30:432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LJ, Last KA, Young PA, Martin NG. Model-fitting approaches to the analysis of human behavior. Heredity. 1978;41:249–320. doi: 10.1038/hdy.1978.101. [DOI] [PubMed] [Google Scholar]
- Eisen SA, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin Genet. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Eisen SA, True WR, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta Genet Med Gemellol. 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fornito A, Malhi GS, Lagopoulos J, Ivanovski B, Wood SJ, Saling MM, Pantelis C, Yucel M. Anatomical abnormalities of the anterior cingulate and paracingulate cortex in patients with bipolar I disorder. Psychiatry Res. 2008;162:123–132. doi: 10.1016/j.pscychresns.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Thompson PM, Blangero J. Neuroimaging endophenotypes: strategies for finding genes influencing brain structure and function. Hum Brain Mapp. 2007;28:488–501. doi: 10.1002/hbm.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW., 3rd Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cereb Cortex. 2007;17:415–424. doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Res. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Henderson WG, Eisen SE, Goldberg J, True WR, Barnes JE, Vitek M. The Vietnam Era Twin Registry: a resource for medical research. Public Health Rep. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RC, Baare WF, van Oel C, van Haren NE, Collins DL, Evans AC, Amunts K, et al. Genetic contributions to human brain morphology and intelligence. J Neurosci. 2006;26:10235–10242. doi: 10.1523/JNEUROSCI.1312-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18:2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Jansen A, Andermann E. Genetics of the polymicrogyria syndromes. J Med Genet. 2005;42:369–378. doi: 10.1136/jmg.2004.023952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC. Changes in volume with age—consistency and interpretation of observed effects. Neurobiol Aging. 2005;26:1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YJ, Grant MD, Franz CE, Eisen SA, Jacobson KC, Boake C, Lyons MJ. Genes, environment, and time: the Vietnam Era Twin Study of Aging (VETSA) Twin Res Hum Genet. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp. 2007;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallamaci A, Muzio L, Chan CH, Parnavelas J, Boncinelli E. Area identity shifts in the early cerebral cortex of Emx2-/- mutant mice. Nat Neurosci. 2000;3:679–686. doi: 10.1038/76630. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120(Pt 4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: statistical modeling. Richmond (VA): Department of Psychiatry, Medical College of Virginia; 2004. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht (The Netherlands): Kluwer Academic Publishers; 1992. [Google Scholar]
- Nichols RC, Bilbro WCJ. The diagnosis of twin zygosity. Acta Genet Stat Med. 1966;16:265–275. doi: 10.1159/000151973. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen H. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Peeters H, Van Gestel S, Vlietinck R, Derom C, Derom R. Validation of a telephone zygosity questionnaire in twins of known zygosity. Behav Genet. 1998;28:159–163. doi: 10.1023/a:1021416112215. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. Genetic influences on human brain structure: a review of brain imaging studies in twins. Hum Brain Mapp. 2007;28:464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, Straussberg R, Dobyns WB, Qasrawi B, Winter RM, Innes AM, et al. G protein-coupled receptor-dependent development of human frontal cortex. Science. 2004;303:2033–2036. doi: 10.1126/science.1092780. [DOI] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI, De Geus EJ. Genetic correlations between brain volumes and the WAIS-III dimensions of verbal comprehension, working memory, perceptual organization, and processing speed. Twin Res. 2003;6:131–139. doi: 10.1375/136905203321536254. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJ, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nat Neurosci. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Rakic P. The radial edifice of cortical architecture: from neuronal silhouettes to genetic engineering. Brain Res Rev. 2007;55:204–219. doi: 10.1016/j.brainresrev.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Eyler LT, Giedd JN, Kremen WS, Kendler KS, Neale MC. Review of twin and family studies on neuroanatomic phenotypes and typical neurodevelopment. Twin Res Hum Genet. 2007;10:683–694. doi: 10.1375/twin.10.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Lenroot RK, Wallace GL, Ordaz S, Taylor KN, Kabani N, Greenstein D, Lerch JP, Kendler KS, Neale MC, et al. Identification of genetically mediated cortical networks: a multivariate study of pediatric twins and siblings. Cereb Cortex. 2008;18:1737–1747. doi: 10.1093/cercor/bhm211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Eaves LJ. Evaluation of analyses of univariate discrete twin data. Behav Genet. 2002;32:221–227. doi: 10.1023/a:1016025229858. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Kaprio J, Khaledy M, et al. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS. Automated MRI parcellation study of regional volume and thickness of prefrontal cortex (PFC) in antipsychotic-naive schizophrenia. Acta Psychiatr Scand. 2008;117:420–431. doi: 10.1111/j.1600-0447.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Fischl B, Salat D, Quinn BT, Makris N, Dale AM. Cortical volume and speed-of-processing are complementary in prediction of performance intelligence. Neuropsychologia. 2005;43:704–713. doi: 10.1016/j.neuropsychologia.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wang L, Hosakere M, Trein JC, Miller A, Ratnanather JT, Barch DM, Thompson PA, Qiu A, Gado MH, Miller MI, et al. Abnormalities of cingulate gyrus neuroanatomy in schizophrenia. Schizophr Res. 2007;93:66–78. doi: 10.1016/j.schres.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IC, McGuire PK, Poline JB, Travere JM, Murray RM, Frith CD, Frackowiak RS, Friston KJ. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage. 1995;2:244–252. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Graham SJ, Campbell Z. A meta-analysis of structural and functional brain imaging in dementia of the Alzheimer's type: a neuroimaging profile. Neuropsychol Rev. 2003;13:1–18. doi: 10.1023/a:1022318921994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.