Abstract
Twenty-four hour circadian activity rhythms and light-exposure levels of 28 older schizophrenia patients (mean age=58 years) were examined using an Actillume recorder. Sleep and wake were scored using the algorithm of the ACTION3 software which revealed that the patients slept for 67% of the night and napped for 9% of the day. Patients with more disturbed sleep and less robust circadian rhythms performed more poorly on neuropsychological tests. Patients with higher cognitive functioning and fewer extrapyramidal symptoms were more alert during the day. Few patients were exposed to high levels of illumination during the day, and older age was associated with lower levels of light exposure. Duration of antipsychotic use and higher antipsychotic doses were associated with decreased daytime alertness and less robust circadian activity rhythms. Patients taking antipsychotics were more sleepy both during the day and night than patients not taking antipsychotics. The circadian rhythm disturbances found in these patients did not seem to be due solely to low levels of illumination exposure. Life-style factors, behavioral factors, psychiatric symptoms and medications were likely contributors to the disturbed rhythms. The effects of the sleep disturbances did not seem to be benign. There were strong relationships between sleep and circadian rhythms and functioning.
Keywords: Aging, Antipsychotic, Circadian rhythm, Light, Schizophrenia, Sleep
1. Introduction
Patients with schizophrenia often complain of difficulties with sleep (Kempenaers et al., 1988; Sweetwood et al., 1976, 1980). Studies have shown differences in rapid eye movement (REM) sleep and slow wave sleep between patients with schizophrenia and controls (Jus et al., 1973; Ganguli et al., 1987; Hudson et al., 1993; Kempenaers et al., 1988). Sleep disturbances are seen in medicated and unmedicated patients, suggesting that schizophrenia and its symptoms directly affect sleep (Stern et al., 1969; Ganguli et al., 1987; Lauer et al., 1997). Benca et al. (1992) reviewed studies of sleep and psychiatric disorders, and reported that schizophrenia patients had decreased total sleep time, longer sleep-onset latency and reduced sleep efficiency compared with controls. Unfortunately, available data on sleep in schizophrenia come primarily from younger patients. Few studies have included patients over age 35, and almost none have included patients over age 50 (Benca et al., 1992). Some of the studies cited above included only unmedicated patients (Jus et al., 1973; Lauer et al., 1997), while others included both patients taking medications and patients not taking medications. None the less, the consistent finding across studies was that, regardless of medication status, patients have disturbed sleep compared with non-psychiatric individuals.
Sleep architecture generally changes over the lifespan. Older adults often have less robust (i.e. lower amplitude) and more advanced (i.e. earlier) circadian rhythms than younger adults (Dijk et al., 1999; Bliwise, 1993; Weitzman et al., 1982). Secretion of hormones, body temperature, activity and sleep/wake all follow circadian patterns, that is, rhythms with a phase of about 24 h (see Fig. 1) (Batschelet, 1981).
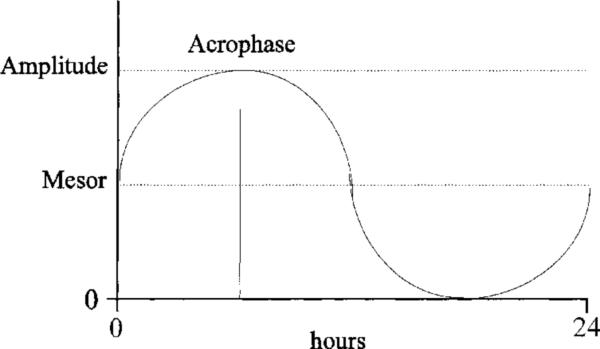
Fig. 1.
Cosine model of circadian rhythms. Mesor represents the midpoint of the curve, amplitude is the distance from the mesor to the peak at its highest point and the acrophase is the time of the rhythm's peak (Batschelet, 1981).
Circadian rhythms are controlled by environmental cues, the strongest of which is light. Reduced light exposure is thought to contribute to circadian rhythm and sleep/wake disturbances in older adults (Campbell et al., 1988; Espiritu et al., 1994). To our knowledge, there have been no reports of illumination exposure in older schizophrenia patients.
Another factor which affects the sleep/wake of schizophrenia patients is antipsychotic medications (Nicholson et al., 1994; Maixner et al., 1998). Antipsychotics are known to affect sleep, but their impact on circadian rhythms is largely unknown. One question being explored is the differential effects of typical vs. atypical antipsychotics. Wirz-Justice and colleagues recorded wrist activity in hospitalized schizophrenia patients. They found that changing the antipsychotic medication of one patient from haloperidol (a typical antipsychotic) to clozapine (an atypical antipsychotic) improved the organization of his rest/activity rhythm (Wirz-Justice et al., 1997). They also found that patients taking atypical antipsychotics had more stable rest/activity cycles than patients taking typical antipsychotics (Wirz-Justice et al., 1996). This suggests that traditional antipsychotics may adversely impact circadian rhythms.
Older schizophrenia patients are at risk for sleep and circadian rhythm disturbances, because of both their age and their psychiatric status. The goal of this study was to describe circadian activity rhythms and sleep/wake patterns of such patients and to examine specific clinical factors which may be related to circadian activity rhythms, sleep and wake. We hypothesized that sleep/wake patterns would be disturbed, circadian activity rhythms would be blunted and antipsychotics would account for much of these disturbances. Although our sample size was small, we also expected that patients taking atypical antipsychotic medications would have less disturbed sleep and circadian rhythms than patients taking typical antipsychotic medications. We expected that clinical symptoms would be associated with more sleep and circadian rhythm disruption, and more disturbed rhythms would be associated with poorer functioning.
2. Methods
2.1. Subjects
The participants were 14 men and 14 women enrolled in a larger study of late-life psychosis. All were diagnosed with schizophrenia using DSM-III-R criteria (American Psychiatric Association, 1987). The Structured Clinical Interview for DSM-III-R (SCID) (Spitzer and Williams, 1986) was administered to patients by geriatric psychiatry or psychology fellows. Diagnosis was confirmed at a research staffing with at least two Board-certified psychiatrists present. These diagnostic procedures have been previously described (Jeste et al., 1995). Patients were excluded if they were clinically unstable, had a history of violence, or lived in a home where there was evidence of violence. The mean age of patients was 58.3 years (S.D. = 9.8; range = 45–76); 82% were Caucasian. All patients were participants in the UCSD Geriatric Psychiatry Clinical Research Center (CRC) and signed consent forms approved by the UCSD Committee on the Investigation of Human Subjects.
At the time of recording, 24 patients were taking antipsychotics. Of these 24 patients, 11 were taking anticholinergic medications, 13 were taking antidepressants, and nine were taking benzodiazepines. Seven patients were taking both antidepressants and benzodiazepines. Two were taking only sedative medications. Of the four patients not taking antipsychotics, one was taking both antidepressants and sedatives. The others (three patients) were psychotropic-medication-free. Patients were on stable doses of their medications for at least several weeks prior to the study. Patients who were undergoing medication changes were not included.
Nine patients were diagnosed with late-onset schizophrenia (LOS; symptom onset after age 45), and 19 were diagnosed with early-onset schizophrenia (EOS). Seventy-eight per cent of patients were employed less than half time. Ten patients were living alone, eight were living with family or friends and 10 were living in board-and-care or assisted living facilities. Table 1 lists the clinical status of patients.
Table 1.
Clinical description of schizophrenia patients (n=28)
| Mean | S.D. | Range | |
|---|---|---|---|
| Age (years) | 58.3 | 9.8 | 45–76 |
| Duration of illness (years) | 23.6 | 12.5 | 3–51 |
| Antipsychotic dose (mg CPZE)a | 328 | 369 | 0–1080 |
| Duration of antipsychotic use (years)b | 4.2 | 7.1 | 0.07–24.2 |
| Anticholinergic dose | 1.7 | 2.7 | 0–10 |
| SAPSc score | 5.5 | 4.2 | 0–16 |
| SANSd score | 8.7 | 4.5 | 1–18 |
| Adjusted QWBe score | .57 | .11 | 0.42–0.81 |
| MMSEf score | 26.0 | 3.0 | 17–30 |
| BPRSg score | 32.3 | 8.8 | 21–62 |
| AIMSh score | 7.3 | 4.3 | 1–19 |
| Simpson–Angus EPSi score | 21.3 | 6.1 | 12–35 |
| Hamilton Depression Rating Scale score | 9.6 | 4.8 | 3–24 |
mg chlorpromazine equivalencies.
For patients taking antipsychotics only.
Scale for the Assessment of Positive Symptoms (range=0–20).
Scale for the Assessment of Negative Symptoms (range=0–25).
Quality of Well Being (0=dead, 1.0=optimal health).
Mini-Mental State Examination (range=0–30).
Brief Psychiatric Rating Scale (Overall and Gorham, 1962).
Abnormal Involuntary Movement Scale (Guy, 1976).
Simpson–Angus Extra Pyramidal Symptom Scale (Simpson and Angus, 1970).
2.2. Apparatus
The Actillume recorder (Ambulatory Monitoring, Inc., Ardsley, New York) was used to record activity and light exposure. The Actillume is a wrist-mounted monitor which records minute-by-minute activity and illumination exposure through the use of a linear accelerometer, a light transducer and a microprocessor. The microprocessor has sufficient memory to record activity and illumination data for about 1 week. Since the Actillume is light (85 grams) and small (1 × 3 × 6 cm) and is worn unobtrusively on the wrist, it is a non-invasive method for continuous activity measurement. The reliability of the Actillume for estimating sleep/wake has previously been tested (Cole et al., 1992; Ancoli-Israel et al., 1997).
Procedure
Patients were seen at the CRC every 6 months. Actigraphic recordings were scheduled at one of these visits. At each CRC visit, research assistants administered a battery of tests, including the Mini-Mental State Examination (MMSE; higher scores indicate better cognitive functioning) (Folstein et al., 1975), the Quality of Well Being scale (QWB; higher scores indicate better health-related quality of life) (Kaplan and Anderson, 1990), the Brief Psychiatric Rating Scale (BPRS; higher scores indicate severe more psychiatric symptoms) (Overall and Gorham, 1962), the Scale for the Assessment of Positive Symptoms (SAPS; higher scores indicate more severe positive symptoms) (Andreasen and Olsen, 1982), the Scale for the Assessment of Negative Symptoms (SANS; higher scores indicate more severe negative symptoms) (Andreasen and Olsen, 1982), the Abnormal Involuntary Movement Scale (AIMS; higher scores indicate more dyskinesia) (Guy, 1976), the modified Simpson–Angus Extrapyramidal Symptoms (EPS) scale (clinical rating of Parkinsonism; higher scores indicate greater EPS) (Simpson and Angus, 1970), and a series of neuropsychological examinations (see Heaton et al., 1994 for a complete description). Neuropsychological tests were summarized into an overall standardized t-score controlling for age, gender and education based on normative samples with a mean t-score of 50 (S.D.=10) (Heaton et al., 1991). For all testing, research assistants at the CRC were trained to reach agreement criteria with a gold-standard rater. Neuropsychological testing was performed according to published instructions for the various instruments, by trained psychometrists under the direction and supervision of a board-certified neuropsychologist (R.H.). Specific interrater reliability data have been previously reported (Lindamer et al., 1999; Jeste et al., 1995).
Actillume recorders were placed on the patients’ non-dominant wrist before 0900 h and removed after 0900 h 3 days later. Patients were asked to record their bedtime at night and final awakening time in the morning, and to keep the Actillume uncovered during the day. Times during which they removed the Actillume for bathing, or other activities involving water (e.g., swimming), were also recorded. On average, participants removed the Actillume 50 min per day.
2.4. Data analyses
Maximum activity level per minute was fitted to 24 hour cosine curves from which mesor, amplitude and acrophase were estimated (see Fig. 1) (Batschelet, 1981). Mesor and amplitude are expressed in arbitrary units of activity (higher numbers represent larger movements). The mesor represents the midpoint of the cosine function, or roughly the mean activity level. The amplitude is the distance from the mesor to the peak of the curve at its highest point. The acrophase is the timing of the peak of the rhythm (Batschelet, 1981). Significant cosine fits were found for all patients (P<0.00005).
Bedtime and uptime were determined from patient records and Actillume recordings. For analyses, ‘night’ was defined as the time between bedtime in the evening and uptime in the morning and ‘day’ as the time between uptime and bedtime. Sleep and wake were scored using a validated algorithm in the ACTION3 software package (Ambulatory Monitoring, Inc., Ardsley, NY). The algorithm used for scoring sleep and wake has been validated in a number of populations, including elderly individuals (Webster et al., 1982; Cole et al., 1992; Ancoli-Israel et al., 1997). Although there is not a perfect 1:1 correspondence for sleep/wake as measured by the Actillume and sleep/wake as measured by EEG, the overall correspondence for measures such as total sleep time is comparable to the interrater reliability for two raters scoring the same EEG record. Throughout the remainder of the paper, reference to sleep and wake will indicated such measures as scored from the Actillume data. Total and per cent sleep time at night, total and per cent wake time at night, number of awakenings and length of each awakening, total and per cent sleep time during the day, total and per cent wake time during the day, number of naps and length of time between naps were computed. Mean lux exposure levels and time spent exposed to light>1000 lux during the day were determined from illumination plots. Data were averaged across the 3 days of recording.
Since sleep/wake variables violated the parametric assumption of normality, non-parametric tests were used. Mann–Whitney tests were used for two-way comparisons. There were no differences between LOS and EOS patients on sleep variables; therefore, data are presented for the combined group. Kruskal–Wallis tests were used to compare patients taking traditional antipsychotics, patients taking atypical antipsychotics and patients not taking antipsychotics; Bonferroni-corrected follow-up tests were performed on all pair-wise comparisons (family-wise error rate=0.05). Spearman rank-order correlations were performed to examine the relationship between clinical variables and sleep/wake and circadian rhythm variables. Since analyses were exploratory in nature, the critical value for alpha was set at 0.05 for two-way comparisons and correlations.
3. Results
3.1. Sleep
Sleep and activity data for all subjects are displayed in Fig. 2. Sleep/wake data are summarized for day and night in Table 2. Daytime and nighttime sleep and wake were correlated. More nighttime awakenings were associated with more daytime naps (r=0.46, P=0.014) and less time awake during the day (r=–0.50, P=0.007).
Fig. 2.
Raw data for all 28 participants are shown with scored sleep in black below maximum per-minute activity data. Each recording began at 0900 h and ended at 0900 h 3 days later. The variability in sleep consolidation and fragmentation can be seen from these plots.
Table 2.
Sleep/wake variables for all subjects (n=28)a
| Mean | S.D. | Range | |
|---|---|---|---|
| Night (time in bed): | |||
| Bed time | 2145 h | 101 min | 1837 h–0050 h |
| Total time in bed (min) | 587 | 96 | 335–771 |
| Per cent sleep | 68% | 12% | 44–85% |
| Per cent wake | 32% | 12% | 15–56% |
| Number of wake bouts | 22 | 5 | 14–33 |
| Mean length of wakes (min) | 7 | 3 | 3–15 |
| Day (time out of bed): | |||
| Final awakening time | 0729 h | 78 min | 0451 h–0939 h |
| Total time out of bed (min) | 796 | 97 | 645–1068 |
| Per cent sleep | 9% | 9% | 0–31% |
| Per cent wake | 90% | 10% | 67–99% |
| Number of naps | 7 | 8 | 0–29 |
| Mean time between naps (min) | 61 | 47 | 0–168 |
Due to periods during which the Actillume was removed for bathing, night and day do not sum to 24 h.
The relationships between sleep/wake and neuropsychological and clinical variables were examined. In general, higher functioning and less symptomatic patients spent more time awake during the day and had fewer awakenings during the night (see Table 3). No other clinical or demographic variables, including age, were related to nighttime or daytime sleep/wake.
Table 3.
Correlations of sleep/wake and clinical variables
| rs | P value | |
|---|---|---|
| Number of nighttime awakenings | ||
| Overall neuropsychological function | –.44 | 0.029 |
| Mean length of nocturnal awakenings | ||
| EPSa | 0.41 | 0.030 |
| Per cent wake during the day | ||
| Overall neuropsychological function | 0.55 | 0.004 |
| Minutes awake during the day | ||
| MMSEb | 0.40 | 0.041 |
| EPSa | –0.38 | 0.046 |
| Antipsychotic dosec | –0.45 | 0.024 |
| Duration of antipsychotic use | –0.60 | 0.001 |
| Minutes napping during the day | ||
| Antipsychotic dose | 0.41 | 0.043 |
| Duration of antipsychotic use | 0.49 | 0.010 |
| Number of daytime naps | ||
| Overall neuropsychological function | –0.49 | 0.013 |
Modified Simpson–Angus EPS Scale (Simpson and Angus, 1970).
Mini Mental State Examination.
mg chlorpromazine equivalencies.
3.2. Circadian rhythms
On average, the activity rhythm mesor was 22.9 (S.D.=7.7, range=9.8–41.2), the amplitude was 18.0 (S.D.=6.1, range=7.7–35.7), and the acrophase was at 1404 h (S.D.=97 min, range=1006 h–1633 h). Table 4 summarizes correlations between and circadian rhythms and clinical and neuropsychological variables.
Table 4.
Correlations of circadian rhythm and clinical variables
| rs | P value | |
|---|---|---|
| Mesor | ||
| Overall neuropsychological function | 0.42 | 0.035 |
| Antipsychotic dose | –0.44 | 0.030 |
| Amplitude | ||
| Anticholinergic medication dose−.40 | –0.40 | 0.038 |
| Duration of antipsychotic use | –0.44 | 0.020 |
| Acrophase | ||
| Duration of antipsychotic use | 0.45 | 0.018 |
3.3. Light
While out of bed, patients were exposed to a mean of 1263 lux (S.D.=1653, range=71–6190 lux; median=614 lux). Patients spent a mean of 77 mmn per day exposed to bright light>1000 lux (S.D.=67; range=0–222 min median=51.5 min). The average light exposure was <1000 lux for nine patients. Fifty-four per cent of patients were exposed to less than 60 min and 32% were exposed to less than 30 min of bright light (>1000 lux) per day. Light exposure (mean lux level or min of exposure over 1000 lux) was not related to sleep/wake or circadian rhythm variables.
Generally, patients exposed to high levels of illumination were less symptomatic (lower BPRS and lower Hamilton Scale scores), had higher quality of life, and were younger. Significant correlations between light and other variables are summarized in Table 5.
Table 5.
Correlations of daytime light exposure and clinical variables
| rs | P value | |
|---|---|---|
| Mean light exposure | ||
| Age | –0.42 | 0.025 |
| Adjusted QWBa score | 0.59 | 0.002 |
| BPRSb score | –0.45 | 0.022 |
| Hamilton Depression Scale score | –0.51 | 0.008 |
| Minutes exposed to light >1000 lux | ||
| AIMSc total score | –0.41 | 0.048 |
| Hamilton Depression Scale score | –0.45 | 0.020 |
Adjusted Quality of Well Being score.
Brief Psychiatric Rating scale.
Abnormal Involuntary Movements scale.
3.4. Antipsychotic medication
Duration of antipsychotic use and dose of antipsychotic medication were strongly related to sleep/wake variables (see Table 3), and circadian activity rhythms (see Table 4).
Patients taking antipsychotics (n=24) slept more both during the day (77 vs. 15 min, P=0.013) and during the night (414 vs. 261 min, P=0.018) than those not taking antipsychotics (n=4). In addition, patients taking antipsychotics took more naps (8 vs. 1 nap per day, P=0.006) and had lower activity rhythm mesors (34.5 vs. 21.2, P=0.031) than patients not taking antipsychotics. There were no differences in amount of light exposure between these groups.
Patients taking typical antipsychotics (n=18) were compared with patients taking atypical antipsychotics (n=6; risperidol or clozaril) and no antipsychotics (n=4). Results showed a significant difference among the groups in number of daytime naps (P=0.035). Pairwise comparisons indicated that patients taking antipsychotics (typical or atypical) took more naps than patients not taking antipsychotics (P=0.021 and P=0.020, respectively). There were no significant differences between patients taking typical and patients taking atypical antipsychotics in any of the sleep or circadian rhythm variables.
4. Discussion
The circadian rhythms and sleep/wake patterns of these patients were quite disturbed. To place the level of disturbance in context, we compared the circadian rhythm data from these patients to control participants in a study conducted by Youngstedt and Kripke (personal communication). Since control data were available only for individuals over age 60, we selected 12 age- (within 2 years) and gender-matched pairs of patients and controls. In this sub-group, the activity acrophase was not different, (14:08 vs. 14:21, P=0.58), but the mesor (24.0 vs. 132.6, P=0.00007) and amplitude (18.6 vs. 92.9, P=0.0002) were lower in the schizophrenia patients than in the comparison subjects. The peak of the activity rhythm of the schizophrenia patients was about one-fifth that of the control participants.
These dramatic differences in circadian activity rhythms are likely the result of poor sleep/wake patterns in the schizophrenia patients. To measure sleep and wake, an automated scoring algorithm based on the wrist activity data was used. Although this algorithm has been validated (Webster et al., 1982; Cole et al., 1992; Ancoli-Israel et al., 1997), there are certainly limitations in using this as a proxy for EEG sleep. None the less, the algorithm is useful for estimation of sleep and wake. Based on actigraphically scored sleep, patients napped an average of over 1 h per day and were awake for over 3 h per night. Daytime napping was associated with nighttime sleep fragmentation. Patients spent nearly 11 h in bed at night, which is substantially longer than is typically needed for sleep. Although these patients were living in the community, nearly all were unemployed. This lack of daytime structure may have contributed to daytime napping which, in turn, upset nighttime sleep.
In these patients, more robust circadian activity rhythms were related to performance on neuropsychological tests. Higher activity mesor was associated with better overall neuropsychological functioning. Although these data are preliminary and the direction of the effect cannot be determined, clinical status and age cannot wholly explain the association between higher levels of activity and better functioning. Sleep/wake measures were also related to neuropsychological functioning. Patients who performed better on neuropsychological tests had less daytime sleep and more consolidated nighttime sleep. Since there is evidence that sleep deprivation impairs some domains of cognitive functioning (Horne, 1993), it is possible that decreased daytime activity causes disturbed sleep, which impairs performance. In this population of patients, who generally have below-average neuropsychological functioning, the negative impact of sleep disruption on functioning should not be discounted.
Patients with higher levels of EPS had more nocturnal awakenings, suggesting that EPS may also adversely impact sleep at night in addition to causing difficulties during the day.
Increased light exposure was related to younger age, higher quality of life and fewer symptoms. As a group, these patients were exposed to more bright light (77 min) compared to what is seen in healthy adults aged 40–64 years old (58 min) from the same geographic region (Espiritu et al., 1994). This finding was surprising given the weak circadian rhythms, poor night sleep and decreased daytime alertness in this patient group. The circadian rhythm disturbance in these patients did not seem to be due to low levels of illumination exposure; rather, life-style factors, psychiatric symptoms and medications were likely contributors. It was interesting to see that lower levels of light exposure were related to poorer quality of life and decreased mood in these patients. The relationship between light exposure and mood has been reported in non-schizophrenic populations as well (Youngstedt et al., 1999).
Higher antipsychotic dose was associated with more sleep during the day and night and less robust activity rhythms. Patients who had been taking antipsychotics longer had more delayed rhythms with lower amplitude. Duration of antipsychotic use was unrelated to age or illness duration. It is impossible to rule out the possibility that initial symptom severity and/or the use of other medications might account for part of the relationship between antipsychotic use and sleep/wake measures.
Reports by Wirz-Justice et al. (1996, 1997) have suggested that typical antipsychotics may be more detrimental to circadian activity rhythms than atypical antipsychotics. Although we did not find differences in circadian rhythms or sleep between patients taking typical vs. atypical antipsychotics, our study is limited by small sample size (six taking atypical antipsychotics) and by a short recording duration (3 days). Additional research is warranted in this area.
Sleep disordered breathing and periodic limb movements (PLNS) commonly impact sleep quality of older adults (Ancoli-Israel et al., 1991a, b). Patients in the current study had their breathing and leg movements recorded for one or two nights. Five patients had a respiratory disturbance index (RDI)>20, 12 had an RDI>10, and two had a myoclonus index (MI)>5. There were no significant relationships between RDI or PLMS and the sleep/wake or circadian rhythm measures used in the current analyses. The relationships found in the current sample do not appear to be wholly due to primary sleep disorders. Ancoli-Israel et al. (1999) have reported that the prevalence of severe sleep disordered breathing (SDB; >20 respiratory disturbances per hour of sleep) was higher and the prevalence of periodic limb movements in sleep was lower in schizophrenia patients compared to what is seen in non-psychiatric older adults. Although severe SDB may contribute to sleep disruption in these patients, it is unlikely to account for all the sleep/wake and circadian rhythm disruption we found.
Additional research on the sleep of older schizophrenia patients is needed. Larger studies need to compare sleep in older patients with that of older controls to determine how much of the sleep disturbance is secondary to age rather than to schizophrenia symptoms and medication use. Further studies should use longer recording periods to confirm the pattern of our findings. In addition, the possibility that atypical antipsychotics are less detrimental to sleep and circadian rhythms should be explored in larger well-controlled studies. Nighttime sleep and daytime alertness should be considered in designing medication regimens for these patients. It may be possible to improve neuropsychological functioning in these patients with treatment strategies targeting daytime alertness and nighttime sleep quality. Such improved functioning could positively impact the patients’ overall level of functioning and quality of life.
Acknowledgements
We would like to acknowledge the contribution of Dr M. Jacqueline Harris to the Geriatric Psychiatry Clinical Research Center, and to thank Denise M.W. Jones for her assistance with data collection and preliminary data analysis. This research was supported by NIMH 49671, NIA AG02711, NIA AG08415, NHLBI HL44915, NIMH MH45131, NIMH MH43693, the Research Service of the Veterans Affairs San Diego Healthcare System, Department of Veterans Affairs VISN22 MIRECC and the Sam and Rose Stein Institute for Research on Aging.
Portions of these data were presented at the annual meetings of the Association of Professional Sleep Societies, 1995 in Nashville and 1996 in San Francisco, and at the annual meeting of the Society for Light Treatment and Biological Rhythms, 1997 in Vancouver, Canada.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. third ed., revised American Psychiatric S., Association; Washington, DC: 1987. [Google Scholar]
- Ancoli-Israel S, Clopton P, Klauber MR, Fell R, Mason WJ. Use of wrist activity for monitoring sleep/wake in demented nursing home patients. Sleep. 1997;20:24–27. doi: 10.1093/sleep/20.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991a;14(6):496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep disordered breathing in community-dwelling elderly. Sleep. 1991b;14(6):486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Martin J, Jones DW, Caligiuri M, Patterson T, Harris MJ, Jeste DV. Sleep disordered breathing and periodic movements in sleep in older patients with schizophrenia. Biol. Psychiatry. 1999;45:1426–1432. doi: 10.1016/s0006-3223(98)00166-8. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Olsen S. Negative v. positive schizophrenia: definition and validation. Arch. Gen. Psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- Batschelet E. Circular Statistics in Biology. Academic Press; London: 1981. [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch. Gen. Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia review. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Kripke DF, Gillin JC, Hrubovcak JC. Exposure to light in healthy elderly subjects and Alzheimer's patients. Physiol. Behav. 1988;42:141–144. doi: 10.1016/0031-9384(88)90289-2. [DOI] [PubMed] [Google Scholar]
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J. Physiol. 1999;516:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espiritu RC, Kripke DF, Ancoli-Israel S, Mowen MA, Mason WJ, Fell RL, Klauber MR, Kaplan OJ. Low illumination by San Diego adults: association with atypical depressive symptoms. Biol. Psychiatry. 1994;35:403–407. doi: 10.1016/0006-3223(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Minimental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ganguli R, Reynolds CFI, Kupfer DJ. Electroencephalographic sleep in young, never-medicated schizophrenics. Arch. Gen. Psychiatry. 1987;44:36–44. doi: 10.1001/archpsyc.1987.01800130038006. [DOI] [PubMed] [Google Scholar]
- Guy W. Abnormal Involuntary Movement Scale (AIMS). In: N. Psychopharmacology Research Branch, editor. ECDEU Assessment Manual for Psychopharmacology, revised. National Institute of Mental Health; Rockville: 1976. pp. 534–537. [Google Scholar]
- Heaton R, Paulsen JS, McAdams LA, Kuck J, Zisook S, Braff D, Harris MJ, Jeste DV. Neuropsychological deficits in schizophrenics: relationship to age, chronicity and dementia. Arch. Gen. Psychiatry. 1994;51:469–476. doi: 10.1001/archpsyc.1994.03950060033003. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive Norms for an Expanded Halstead–Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- Horne JA. Human sleep. sleep loss and behavior. Implications for the prefrontal cortex and psychiatric disorder. Br. J. Psychiatry. 1993;162:413–419. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Lipinski JF, Keck PE, Jr., Aizley HG, Vuckovic A, Zierk KC, Pope HG., Jr. Polysomnographic characteristics of schizophrenia in comparison with mania and depression. Biol. Psychiatry. 1993;34(3):191–193. doi: 10.1016/0006-3223(93)90391-p. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Harris MJ, Krull A, Kuck J, McAdams LA, Heaton R. Clinical and neuropsychological characteristics of patients with late-onset schizophrenia. Am. J. Psychiatry. 1995;152:722–730. doi: 10.1176/ajp.152.5.722. [DOI] [PubMed] [Google Scholar]
- Jus K, Bouchard M, Jus AK, Villeneuve A, Lachance R. Sleep EEG studies in untreated, long-term schizophrenic patients. Arch. Gen. Psychiatry. 1973;29:386–390. doi: 10.1001/archpsyc.1973.04200030074011. [DOI] [PubMed] [Google Scholar]
- Kaplan RM, Anderson J. The general health policy model: an integrated approach. In: Spilker B, editor. Quality of Life Assessments in Clinical Trials. Raven Press; New York: 1990. pp. 131–149. [Google Scholar]
- Kempenaers C, Kerkhofs M, Linkowski P, Mendlewicz J. Sleep EEG variables in young schizophrenic and depressive patients. Biol. Psychiatry. 1988;24:833–838. doi: 10.1016/0006-3223(88)90262-4. [DOI] [PubMed] [Google Scholar]
- Lauer CJ, Schreiber W, Pollmacher T, Holsboer F, Krieg JC. Sleep in schizophrenia: a polysomnographic study on drug-naive patients. Neuropsychopharmacology. 1997;16:51–60. doi: 10.1016/S0893-133X(96)00159-5. [DOI] [PubMed] [Google Scholar]
- Lindamer LA, Lohr JB, Harris MJ, McAdams LA, Jeste DV. Gender-related clinical differences in older patients with schizophrenia. J. Clin. Psychiatry. 1999;60:61–67. doi: 10.4088/jcp.v60n0114. [DOI] [PubMed] [Google Scholar]
- Maixner S, Tandon R, Eiser A, Taylor S, DeQuardo J, Shipley J. Effects of antipsychotic treatment on polysomnographic measures in schizophrenia: a replication and extension. Am. J. Psychiatry. 1998;155:1600–1602. doi: 10.1176/ajp.155.11.1600. [DOI] [PubMed] [Google Scholar]
- Nicholson AN, Bradley CM, Pascoe PA. Medications: effects on sleep and wakefulness. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. W.B. Saunders; Philadelphia: 1994. pp. 364–372. [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol. Rep. 1962;10:799–812. [Google Scholar]
- Simpson GW, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr. Scand. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-III-R — Patient Version (SCID-P) New York State Psychiatric Institute, Biometrics Research; New York: 1986. [Google Scholar]
- Stern M, Fram DH, Wyatt R, Grinspoon L, Tursky B. All-night sleep studies of acute schizophrenics. Arch. Gen. Psychiatry. 1969;20:470–477. doi: 10.1001/archpsyc.1969.01740160086012. [DOI] [PubMed] [Google Scholar]
- Sweetwood H, Grant I, Kripke DF, Gerst MS, Yager J. Sleep disorders over time: psychiatric correlates among males. Br. J. Psychiatry. 1980;136:456–462. doi: 10.1192/bjp.136.5.456. [DOI] [PubMed] [Google Scholar]
- Sweetwood HL, Kripke DF, Grant I, Yager J, Gerst MS. Sleep disorder and psychobiological symptomatology in male psychiatric outpatients and male nonpatients. Psychosom. Med. 1976;38:373–378. doi: 10.1097/00006842-197611000-00002. [DOI] [PubMed] [Google Scholar]
- Webster JB, Kripke DF, Messin S, Mullaney DJ, Wyborney G. An activity-based sleep monitor system for ambulatory use. Sleep. 1982;5:389–399. doi: 10.1093/sleep/5.4.389. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC. Chronobiology of aging: temperature, sleep–wake rhythms and entrainment. Neurobiol. Aging. 1982;3:299–309. doi: 10.1016/0197-4580(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Cajochen C, Nussbaum P. A schizophrenic with an arrhythmic circadian rest–activity cycle. Psychiatry Res. 1997;73:83–90. doi: 10.1016/s0165-1781(97)00117-0. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Haug HJ, Cajochen C, Reichert U, Nussbaum P. Differential neuroleptic drug modification of the circadian rest-activity cycle in schizophrenia. Soc. Res. Biol. Rhythms. 1996;5:34. [Google Scholar]
- Youngstedt SD, Kripke DF, Elliott JA, Baehr EK, Sepulveda RS. Light exposure sleep quality and depression in older adults. In: Holick MF, Jung EG, editors. Biologic Effects of Light 1998. Kluwer Academic; Boston: 1999. pp. 427–431. [Google Scholar]