Abstract
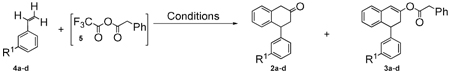
Mixed trifluoroacetyl phenylacetyl anhydride and 3-halostyrenes (fluoro, chloro, and bromo) or vinylcycloalkanes (cyclohexyl, cyclooctyl), undergo cascade Friedel-Crafts cycli-acylalkylation, enolization, and O-acylation to give 4-substituted tetralen-2-ol phenylacetates, without additional solvent in good yields. Base alcoholysis of 4-phenyltetralen-2-ol phenylacetate reveals the tetral-2-one for asymmetric transfer hydrogenation. Bromophenyltetralen-2-ol phenylacetate undergoes Suzuki coupling, and provides a short route to trans-4-phenyl-β-aminotetralin.
Keywords: β-aminotetralin, cascade, halostryene, phenylacetate, tetralen-2-ol
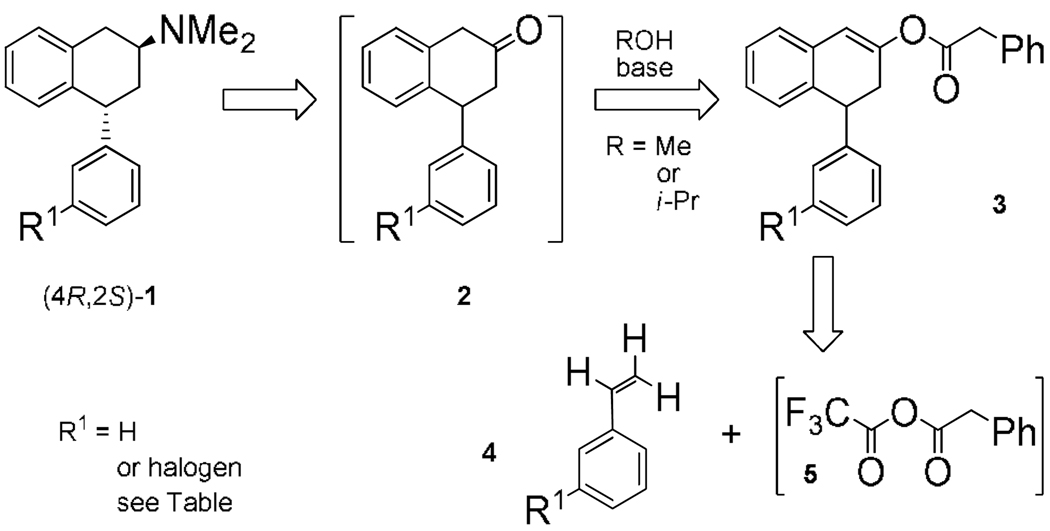
The β-aminotetralin moiety is a pharmacophore element recognized by several classes of aminergic neurotransmitter G protein-coupled receptors (GPCRs). For example, asymmetric (–)-trans-4R-phenyl-2S-dimethylaminotetralin (1, Scheme 1) exhibits anorectic and antipsychotic efficacy after peripheral administration to rodents via actions at brain serotonin (5-hydroxytryptamine, 5-HT) 5-HT2 GPCRs.1 The 4-(3-halophenyl) analogs of 12 are active at 5-HT2 receptors, important drug targets for many human psychological and physiological disorders. Halophenyltetralen-2-ol phenylacetate 3 intermediates, from readily available reagents 4 and [5] (Scheme 1), provide these analogs and avoid the requirement to isolate corresponding 4-(3-halophenyl)tetral-2-ones 2. Versatile aryl halide and enol phenylacetate functionalities on 3 make these molecules useful for diversified organic syntheses, pharmaceuticals, and catalyzed asymmetric transformations.3
Scheme 1.
Retrosynthesis to trans-4-Phenyl-β-aminotetralins.
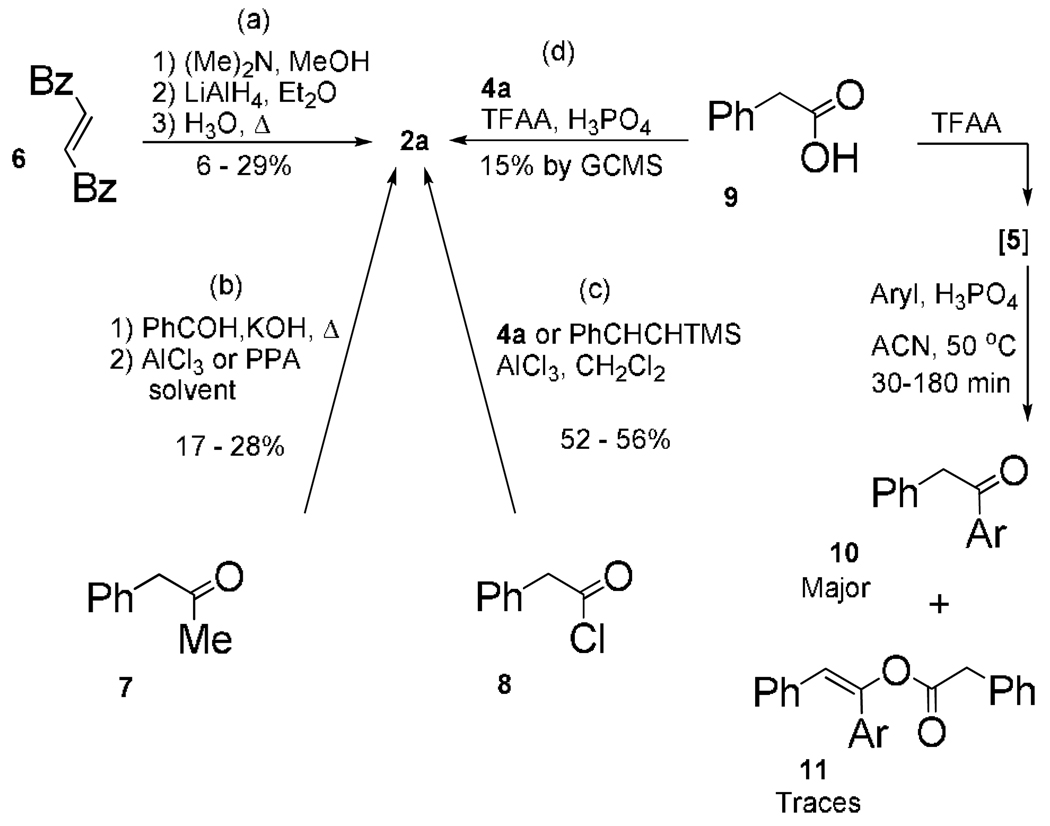
Although 4-phenyltetral-2-ones are of great interest to organic synthesis, methods to synthesize them are low yielding, scarce, difficult to diversify, and require fast, efficient use to avoid decomposition.4 Direct ring-closure reports to non-halogenated 4-phenyltetral-2-one 2a include (Scheme 2): (a) dimethylamine addition to symmetrical dibenzoylethylene 6 gives 2-(N,N-dimethylamino)-1,4-diphenyl-1,4-butanedione, to reduce and then cyclize in refluxing acid;5 (b) enolate addition of phenylacetone 7 to benzaldehyde provides 1,4-diphenylbut-1-en-3-one, to cyclize under Friedel-Crafts (FC) conditions with metal Lewis acid or PPA;6 (c) one-step FC-cycli-acylalkylation (FC-CAA)7 with phenylacetyl chloride 8, styrene 4a (or TMS activated 4a), and metal Lewis acid in dichloromethane.8 Free of many aforementioned drawbacks one-step FC-CAA (d) with phenylacetic acid 9, TFAA, phosphoric acid,9 and 4a, readily dimerizes 4a and furnishes only a trace amount of 2a by GC-MS.10 While, mixed trifluoroacetyl phenylacetyl anhydride [5] can esterify alcohols11 or FC-acylate aryls to give 10, one report includes traces of aryl enolates 11.12 Stable tetralen-2-ol phenylacetates avoid difficulties handling and storing expensive 4-phenyltetral-2-ones and are made directly with one procedure, without additional solvent. We now report a facile cascade reaction to 4-(3-halophenyl)tetralen-2-ol phenylacetates and their utility in asymmetric transfer hydrogenation (ATH), palladium cross-coupling, and palladium hydrodebromination applications.
Scheme 2.
Literature Examples for 4-Phenyltetral-2-one.
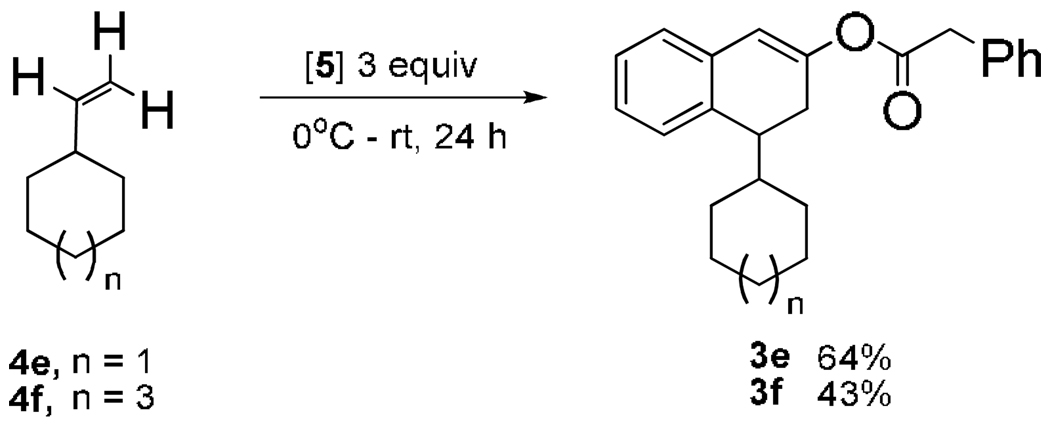
Cascade FC-CAA, enolization, and O-acylation was investigated with TFAA activated phenylacetic acid and 4a, 3-halostyrenes 4b–d (Table), as well as, vinylcycloalkanes 4e,f (Scheme 3). Reactive 4a was heated to 60 °C prior to reaction with [5] in order to accelerate the inherently slow enolization13 of 2a in the reaction media and allow isolation of O-acylated 3a (15%). At rt, or cooling to −78 °C, resulted in loss of reactive 2a in a complex mixture. Additional solvents (ACN, hexanes, dichloromethane) resulted in self-condensed phenylacetyl anhydride with styrene persisting, as did addition of 4a to the activated acid. Surprisingly, moderately reactive 3-halostyrenes 4b–d14 withstood dimerization in the reaction media and resulted in higher conversions to the desirable tetral-2-one. Equimolar 3-fluorostyrene 4b and [5] gave major 2b (42%) and minor 3b (8%). Chlorophenyltetral-2-one 2c (70%) was prepared from 3-chlorostyrene 4c with 3-equiv of [5], and underwent further treatment with equimolar [5] to provide 3c (38%). Warming to rt over 24 h 3-bromostyrene 4d with 3-equiv of [5] gave 3d (50%), over 3-fold increase in yield from non-halogenated 3a. Vinylcyclohexane 4e and vinylcyclooctane 4f provided solids 3e (63%) and 3f (40%), respectively, when reacted separately with [5]. Conformational difference between 4-cycloalkyltetralen-2-ol and 4-phenyltetralen-2-ol cores was indicated by allylic proton coupling in the former. Tetralen-2-ol phenylacetates were isolated with less than 5% of the regioisomer (unlike silyl tetralen-2-ol ethers15), stable to atm, and enantio-resolvable using chiral stationary phase (CSP)-HPLC (e.g., for 3e, tR1 = 15.7 [α]25 D = −79.1, tR2 = 16.8 [α]25 D = +78.8.
Table.
Cascade Reaction with Styrene or 3-Halostyrenes for 2 and 3, Conditions, Yields, and UV Trace.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Yield (%)a | Conditions | UVe Trace of 3 | ||||||
| R1 | 4 | 2 | 3 | [5]:4c | temp (°C) | t (h) | tR1 | tR2 |
| H | a | 0 | 15 | 3:1 | 0–60 | 0.5 | 17.7 | 18.1 |
| F | b | 42 | 8 | 1:1 | 0 | 0.5 | 16.0 | 16.8 |
| Cl | c | 70 | 0(38)b | 3:1 | 0 | 0.5 | 17.9 | 18.9 |
| Br | d | 0 | 50 | 3:1 | 0–rt | 24d | 18.7 | 20.1 |
isolated yield;
from 2c;
equiv;
reaction time not minimized;
220/254 nm, CSP-HPLC.
Scheme 3.
Cascade Reaction with Vinylcycloalkanes.
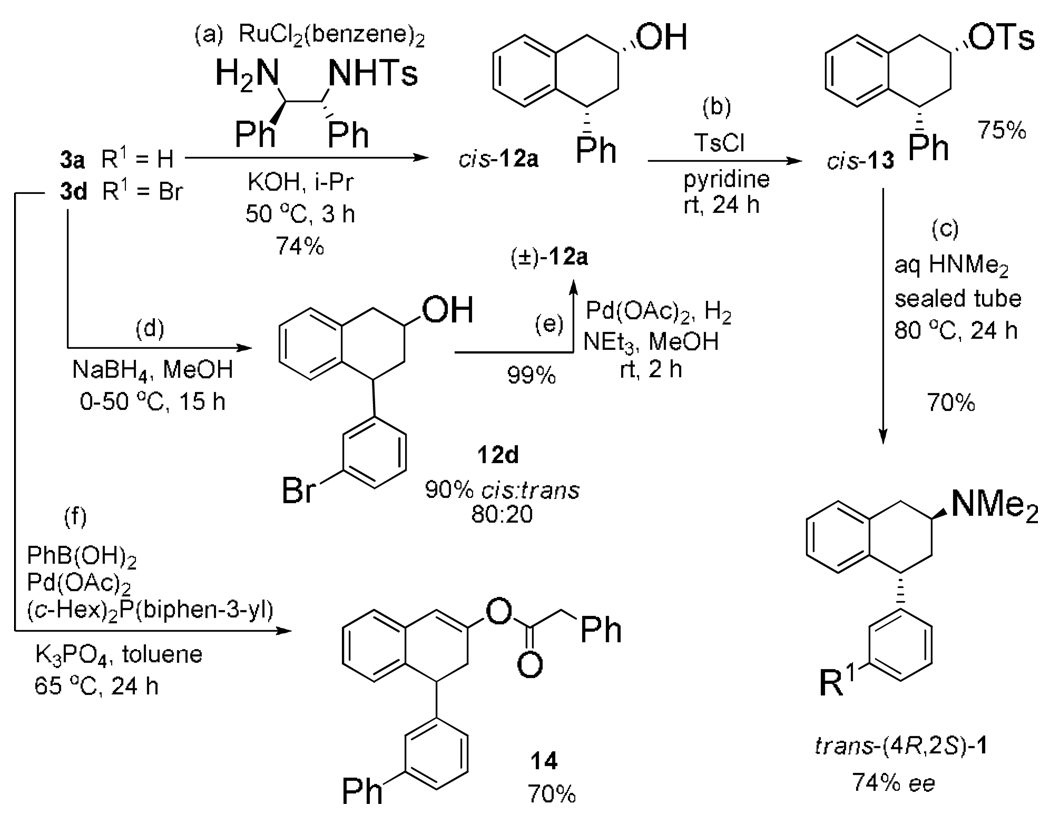
Three steps (Scheme 4), (a) ATH,16 (b) tosylation, and (c) SN2 inversion with aq dimethylamine,17 provided enantioenriched cis-(4R-2R)-12a (74%), cis-(4R-2R)-13 (75%), and trans-(4R-2S)-1 (70%) with β-hydride elimination byproducts.18 Pure trans-4R-2S-1 was obtained by CSP-HPLC (74% ee). Carbonyl reduction of 3d with (d) sodium borohydride gave 12d (90%) and (e) hydrodebromination19 provided (±)-12a (99%). Employing brominated 3d in one additional step gave (±)-12a in 45% yield from reagents, an improvement over the 11% yield using the non-halogenated 3a. Suzuki coupling20 of 3d with (f) phenylboronic acid smoothly provided 4-(biphenyl-3-yl)tetralen-2-ol phenylacetate 14 (70%). Thus, simple palladium insertion modifications to bromophenyl functionality with 3d and 12d were established.
Scheme 4.
Utility of 4-(3-Bromophenyl)tetralen-2-ol Phenylacetate.
Cascade Friedel-Crafts cycli-acylalkylation, enolization, and O-acylation with activated phenylacetic acid and moderately reactive halostyrene or vinylcycloalkanes, provides 4-(3-halophenyl or cycloalkyl)tetralen-2-ol phenylacetate. An electron withdrawing substituted styrene dimerizes less and provides higher yields in the reaction media than unsubstituted styrene. Base alcoholysis on 4-phenyltetralen-2-ol phenylacetate reveals 4-phenyltetral-2-one for use in situ. Simple palladium insertion cross-coupling with 4-(3-bromophenyl)tetralen-2-ol phenyl-acetate is established and a short 5-step sequence provides a 3-times (6% to 18%) more efficient route to trans-1.
Supplementary Material
Acknowledgment
This work was supported by USPHS (NIH) grants MH068655, DA023928, and MH081193.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information Available
General experimental methods, procedures, characterization data, copies of 1H and 13C-NMR spectra for synthesized compounds. This material is available free of charge via the Internet at
References and Notes
- 1.a Rowland NE, Crump EM, Nguyen N, Robertson K, Sun Z, Booth RG. Pharmacol., Biochem., Be. 2008;91:176. doi: 10.1016/j.pbb.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ghoneim OM, Legere JA, Golbraikh A, Tropsha A, Booth RG. Bioorg. Med. Chem. 2006;14:6640. doi: 10.1016/j.bmc.2006.05.077. [DOI] [PubMed] [Google Scholar]; c Bucholtz EC, Brown RL, Tropsha A, Booth RG, Wyrick SD. J. Med. Chem. 1999;42:3041. doi: 10.1021/jm980428x. [DOI] [PubMed] [Google Scholar]; d Youngman MA, Willard NM, Dax SL, McNally JJ. Synth. Commun. 2003;33:2215. [Google Scholar]
- 2.SAR for 4-(3-halophenyl)-β-aminotetralin binding, and function at 5-HT2 GPCRs for will be reported. J. Med. Chem [Google Scholar]
- 3.a Schreiber SL. Nature. 2009;457:153. doi: 10.1038/457153a. [DOI] [PubMed] [Google Scholar]; b Ahn Y, Ko S-B, Kim M-J, Park J. Coord. Chem. Rev. 2008;252:647. [Google Scholar]; c Panella L, Feringa BL, de Vries JG, Minnaard AJ. Org. Lett. 2005;7:4177. doi: 10.1021/ol051559c. [DOI] [PubMed] [Google Scholar]; d Tsuji J, editor. Topics in Organometallic Chemistry. Vol. 14. Berlin: Springer; 2005. Palladium in Organic Synthesis. [Google Scholar]; e Nakagawa H, Okimoto Y, Sakaguchi S, Ishii Y. Tetrahedron Lett. 2003;44:103. [Google Scholar]; f Miyaura N, editor. Topics in Current Chemistry. Vol.219. Berlin: Springer; 2002. Cross-Coupling Reactions. [Google Scholar]
- 4.a Carreño MC, González-López M, Latorre A, Urbano A. J. Org. Chem. 2006;71:4956. doi: 10.1021/jo060688j. [DOI] [PubMed] [Google Scholar]; b Silveira CC, Machado A, Braga AL, Lenardão EJ. Tetrahedron Lett. 2004;45:4077. [Google Scholar]; c Silveira CC, Braga AL, Kaufman TS, Lenardão EJ. Tetrahedron. 2004;60:8295. [Google Scholar]
- 5.a Lutz RE, Bailey PS, Shearer NH., Jr J. Am. Chem. Soc. 1946;68:2224. [Google Scholar]; b Fine SA, Stern RL. J. Org. Chem. 1967;32:4132. [Google Scholar]; c Fine SA, Stern RL. J. Org. Chem. 1970;35:1857. [Google Scholar]; d Rhee SW, Tanga MJ. J. Labelled Cpd. Radiopharm. 2000;43:925. [Google Scholar]
- 6.a Wyrick SD, Booth RG, Myers AM, Owens CE, Kula NS, Baldessarini RJ, McPhail AT, Mailman RB. J. Med. Chem. 1993;36:2542. doi: 10.1021/jm00069a013. [DOI] [PubMed] [Google Scholar]; b Khalaf AA, El-Khawaga AMA. Rev. Roum. Chim. 1981;26:739. [Google Scholar]; c Bertolini G, Vecchietti V, Mabilia M, Norcini G, Restelli A, Santangelo F, Villa AM, Casagrande C. Eur. J. Med. Chem. 1992;27:663. [Google Scholar]; d Gatti G, Piersanti G, Spadoni G. Farmaco. 2003;58:469. doi: 10.1016/S0014-827X(03)00064-8. [DOI] [PubMed] [Google Scholar]
- 7.Prakash SGK, Yan P, Török B, Olah GA. Catal. Lett. 2003;87:109. [Google Scholar]
- 8.a Brook MA, Henry C, Jefferson E, Jüschke R, Sebastian T, Tomaszewski M, Wenzel S. Bull. Soc. Chim. Fr. 1995;132:559. [Google Scholar]; b Fleming I, Pearce A. J. Chem. Soc. Perkin Trans. 1. 1980;11:2485. [Google Scholar]; c Lucarini S, Bedini A, Spadoni G, Piersanti G. Org. Biomol. Chem. 2008;6:147. doi: 10.1039/b713904g. [DOI] [PubMed] [Google Scholar]
- 9.Galli C. Synthesis. 1979;4:303. [Google Scholar]
- 10.Gray AD, Smyth TP. J. Org. Chem. 2001;66:7113. doi: 10.1021/jo0158074. [DOI] [PubMed] [Google Scholar]
- 11.Smith SM, Reyes SM, Bravo OV, Herrera MAF, Pascual RM, Ramírez JS, Fuente A, Reyes M, Ruiz JA. ARKIVOC. 2005;6:127. [Google Scholar]
- 12.Veeramaneni VR, Pal M, Yeleswarapu KR. Tetrahedron. 2003;59:3283. [Google Scholar]
- 13.a Nevy JB, Hawkinson DC, Blotny G, Yao X, Pollack RM. J. Am. Chem. Soc. 1997;119:12722. [Google Scholar]; b Yao X, Gold MA, Pollack RM. J. Am. Chem. Soc. 1999;121:6220. [Google Scholar]
- 14.Matyjaszewski K, Sawamoto M. In: Cationic Polymerizations Mechanism, Synthesis, and Applications. Matyjaszewski K, editor. New York: Marcel Dekker, Inc.; 1996. p. 323. [Google Scholar]
- 15.Wang X-Z, Yao Z-J, Liu H, Zhang M, Yang D, George C, Burke TR., Jr Tetrahedron. 2003;59:6087. [Google Scholar]
- 16.a Peach P, Cross DJ, Kenny JA, Mann I, Houson I, Campbell L, Walsgrove T, Wills M. Tetrahedron. 2006;62:1864. [Google Scholar]; b Mogi M, Fuji K, Node M. Tetrahedron: Asymmetry. 2004;15:3715. [Google Scholar]; c Alcock NJ, Mann I, Peach P, Wills M. Tetrahedron: Asymmetry. 2002;13:2485. [Google Scholar]
- 17.Bosse K, Marineau J, Nason DM, Fliri AJ, Segelstein BE, Desai K, Volkmann RA. Tetrahedron Lett. 2006;47:7285. [Google Scholar]
- 18.Lamberts JJM, Cuppen TJHM, Laarhoven WH. J. Chem. Soc., Perkin Trans. 1. 1985;9:1819. [Google Scholar]
- 19.Monguchi Y, Kume A, Hattori K, Maegawa T, Sajiki H. Tetrahedron. 2006;62:7926. [Google Scholar]
- 20.a Wolfe JP, Singer RA, Yang BH, Buchwald SL. J. Am. Chem. Soc. 1999;121:9550. [Google Scholar]; b Wolfe JP, Buchwald SL. Angew. Chem. Int. Ed. 1999;38:2413. doi: 10.1002/(sici)1521-3773(19990816)38:16<2413::aid-anie2413>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.