Abstract
Introduction
Hydrogen sulfide (H2S) is produced endogenously in response to myocardial ischemia and thought to be cardioprotective. The mechanism underlying this protection has yet to be fully elucidated, but may be related sulfide’s ability to limit inflammation. This study investigates the cardioprotection provided by exogenous H2S, and its potential anti-inflammatory mechanism of action.
Methods
The mid-LAD coronary artery in 14 Yorkshire swine was acutely occluded for 60 minutes, followed by reperfusion for 120 minutes. Controls(7) received placebo, and treatment animals(7) received sulfide 10 minutes prior to and throughout reperfusion. Hemodynamic and functional measurements were obtained. Evans blue and TTC staining identified the area-at-risk and infarction. Coronary microvascular reactivity was assessed. Tissue was assayed for myeloperoxidase activity and pro-inflammatory cytokines.
Results
Pre-I/R hemodynamics were similar between groups, whereas post-I/R mean arterial pressure (mmHg) was reduced by 28.7±5.0 in controls vs. 6.7±6.2 in treatment animals (p=0.03). +LV dP/dt (mmHg/sec) was reduced by 1325±455 in controls vs. 416±207 in treatment animals (p=0.002). Segmental shortening in the area-at-risk was better in treatment animals. Infarct size (% of area-at-risk) in controls was 41.0±7.8% vs. 21.2±2.5% in the treated group (p=0.036). Tissue levels of IL-6, IL-8, and TNFα and MPO activity decreased in the treatment group. Treated animals demonstrated improved microvascular reactivity.
Conclusions
Therapeutic sulfide provides protection in response to I/R injury, improving myocardial function, reducing infarct size, and improving coronary microvascular reactivity, potentially through its anti-inflammatory properties. Exogenous sulfide may have therapeutic utility in clinical settings in which I/R injury is encountered.
Introduction
Acute myocardial infarction is the leading cause of mortality in the industrialized world, accounting for over 600,000 deaths annually in the United States alone. To salvage ischemic myocardium from inevitable necrosis, re-establishment of blood flow to the ischemic region is essential, and is generally performed utilizing thrombolysis, percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG). Unfortunately, the therapeutic intervention for acute myocardial ischemia results in iatrogenic injury to myocardium, described as “reperfusion injury”1. The combination of ischemia and subsequent reperfusion injury (I/R injury) can result in additional damage to the heart independent of the ischemic insult manifesting as post I/R dysrrythmia2, myocardial stunning3, and continued myocardial ischemia secondary to endothelial damage leading to coronary microvascular dysfunction4.
Research in to the pathogenesis of myocardial I/R injury has identified an endogenous inflammatory response and the release of damaging free radicals and oxidants associated with this response to be key in inducing myocardial damage5. Key to this inflammatory response are neutrophils which have been shown to become activated in response to I/R injury, localizing to the site of ischemia and subsequently releasing damaging free radicals and pro-inflammatory mediators6.
Recently, investigation in to the gaseous signaling molecule hydrogen sulfide (H2S) has demonstrated it may in fact serve as an endogenous mediator to limit inflammation and free radical damage7. Specifically, H2S has been shown to limit neutrophil adhesion and activation in response to inflammatory stimuli, as well as suppress the release of the pro-inflammatory mediator tumor necrosis factor-alpha (TNF-α)8,9. In addition to its effects on neutrophils, H2S, which is a strong reducing agent, is able to react with multiple oxidant stressors including superoxide radical anion10, hydrogen peroxide11, and peroxynitrite7.
H2S, which is synthesized endogenously from L-cysteine via cystathionine-γ-lyase (CSE) in the heart and vasculature, has also become the subject of recent investigation in the context of myocardial protection. These studies, which have all utilized rodent models of myocardial injury, have demonstrated sulfide is able to limit myocardial infarction size and oxidative stress in response to I/R injury11–13. Based on these investigations, we have tested whether administration of exogenous H2S would limit the inflammatory response to acute myocardial I/R injury. We utilized a pre-clinical large animal model of acute myocardial ischemia followed by reperfusion to obtain both functional and molecular data in to the myocardial protection provided by hydrogen sulfide therapy.
Material & Methods
Animals
Animals were housed individually and provided with laboratory chow and water ad libitum. All experiments were approved by the Beth Israel Deaconess Medical Center animal care and use committee and the Harvard Medical Area standing committee on animals (institutional animal care and use committee) and conformed to the US National Institutes of Health guidelines regulating the care and use of laboratory animals (NIH publication 5377-3, 1996).
Experimental Design
Yorkshire pigs of either sex (35 to 40 kg) were divided randomly into control (n=7) and sulfide treatment (n=7) groups. Animals were subjected to regional left ventricular (LV) ischemia by left anterior descending (LAD) arterial occlusion distal to the second diagonal branch for 60 minutes. The treatment group received sodium sulfide (100μg/kg bolus + 1mg/kg/hr infusion) 10 minutes prior to the onset of reperfusion, whereas the control group received a placebo carrier solution of equal volume. Sodium sulfide was produced and formulated to pH neutrality and iso-osmolarity by Ikaria Inc. (Seattle, WA) using H2S gas (Mattheson, Newark, CA) as the starting material. The myocardium was reperfused for 120 minutes following ischemia. Arterial blood gas (ABG), arterial blood pressure, hematocrit (Hct), LV pressure, heart rate (HR), EKG, O2 saturation, core temperature, and intravenous fluid requirements were measured and recorded. Myocardial segmental shortening in the long axis (parallel to the LAD) and short-axis (perpendicular to the LAD) were recorded at baseline prior to the onset of ischemia, and prior to harvest after 120 minutes of reperfusion. At the completion of the protocol, the heart was excised, and tissue samples from the ischemic-reperfused, distal LAD territory were collected for molecular analyses as described below.
Surgical Protocol
Swine were sedated with ketamine hydrochloride (20 mg/kg, intramuscularly, Abbott Laboratories, North Chicago, IL), and anesthetized with a bolus infusion of thiopental sodium (Baxter Healthcare Corporation, Inc, Deerfield, IL; 5.0 to 7.0 mg/kg intravenously), followed by endotracheal intubation. Ventilation was begun with a volume-cycled ventilator (model Narkomed II-A; North American Drager, Telford, PA; oxygen, 40%; tidal volume, 600cc; ventilation rate, 12 breaths/min; positive end-expiratory pressure, 3 cm H2O; inspiratory to expiratory time, 1:2). General endotracheal anesthesia was established with 3.0% sevoflurane (Ultane; Abbott Laboratories) at the beginning of the surgical preparation, and then maintained with 1.0% throughout the experiment. One liter of Lactated Ringer’s intravenous (IV) fluid was administered after induction of anesthesia and continued thereafter throughout the surgical protocol at 150cc/hour. A right groin dissection was performed and the femoral vein and common femoral artery were isolated and cannulated utilizing 8F sheaths (Cordis Corporation, Miami, FL). The femoral vein was cannulated for intravenous access, drug/placebo delivery, and the right common femoral artery was cannulated for arterial blood sampling and continuous intra-arterial blood pressure monitoring (Millar Instruments, Houston, TX). A median sternotomy was performed exposing the pericardial sac, which was then opened to form a pericardial cradle. A catheter-tipped manometer (Millar Instruments, Houston, TX) was introduced through the apex of the left ventricle to record LV pressure. Segmental shortening in the area-at-risk was assessed utilizing a sonometric digital ultrasonic crystal measurement system (Sonometrics Corp, London, ON, Canada) using four 2-mm digital ultrasonic probes implanted in the subepicardial layer approximately 10 mm apart within the ischemic LV area. Cardiosoft software (Sonometrics Corp, London, ON, Canada) was used for data recording (LV dP/dt, segmental shortening, arterial blood pressure, heart rate) and subsequent data analysis to determine myocardial function. Baseline hemodynamic, functional measurement (global: +LV dP/dt, regional: segemental shortening), arterial blood gas analysis, and hematocrit were obtained. ABG analysis was continued every 15 minutes throughout the protocol and hematocrit was measured every 20 minutes. All animals received 75mg of lidocaine and 20 mEq of potassium chloride as prophylaxis against ventricular dysrhythmia, as well as 60 units/kg of intravenous heparin bolus prior to occlusion of the LAD. The LAD coronary artery was occluded 3mm distal to the origin of the second diagonal branch utilizing a Rommel tourniquet. Myocardial ischemia was confirmed visually by regional cyanosis of the myocardial surface. Fifty minutes after the initiation of regional ischemia (10 minutes prior to the onset of reperfusion), control pigs received a placebo carrier solution infusion intravenously, and treatment animals received exogenous sulfide, generated as sodium sulfide (NaH2S), (100μg/kg bolus + 1mg/kg/hr infusion) until the end of the experimental protocol. The Rommel tourniquet was released 60 minutes after the onset of acute ischemia and the myocardium was reperfused for 120 minutes. At the end of the reperfusion period, hemodynamic and functional measurements were recorded as described above, followed by re-ligation of the LAD and injection of monastryl blue pigment (Engelhard Corp, Louisville, KY) at a 1:150 dilution in PBS into the aortic root after placement of an aortic crossclamp distal to the coronary arterial ostia to demarcate the area-at-risk. The heart was rapidly excised and the entire left ventricle, including the septum, was dissected free. The LV was cut in to 1cm thick slices perpendicular to the axis of the LAD. The area-at-risk was clearly identified by lack of blue pigment staining. Tissue from the area-at-risk of the slice 1cm proximal to the LV apex was isolated and divided for use in molecular and microvascular studies. The remaining slices were weighed utilized for infarct size calculation as described below. Ventricular dysrhythmia (ventricular fibrillation or pulseless ventricular tachycardia) events were recorded and treated with immediate electrical cardioversion (50 J, internal paddles).
Measurement of Global and Regional Myocardial Function
Global myocardial function was assessed by calculating the maximum positive first derivative of LV pressure over time (+dP/dt). Regional myocardial function was determined by using subepicardial 2-mm ultrasonic probes to calculate the percentage segment shortening (%SS), which was normalized to the baseline. Measurements were taken at baseline prior to the onset of ischemia and at the end of reperfusion. The ventilator was stopped during data acquisition to eliminate the effects of respiration. Measurements were made during at least three cardiac cycles in normal sinus rhythm and then averaged. Digital data were inspected for the correct identification of end-diastole and end-systole. End-diastolic segment length (EDL) was measured at the onset of the positive dP/dt, and the end-systolic segment length (ESL) at the peak negative dP/dt.
Coronary Microvessel Studies
Coronary microvessel studies were performed to examine the effects of sulfide on endothelial and vascular smooth muscle injury after ischemia-reperfusion in the coronary microcirculation. After cardiac harvest, myocardial specimens from the ischemic LAD territory were immersed in 4°C Krebs solution and coronary arterioles (80 to130 μm in diameter and 1 to 2 mm in length) were dissected sharply from the surrounding tissue with a 40x magnification dissecting microscope. Microvessels were mounted and examined in a pressurized isolated organ chamber, as described previously14. The responses to sodium nitroprusside (SNP) (1 nM to 100 μM), an endothelium-independent cGMP-mediated vasodilator, as well as adenosine 5′-diphosphate (ADP) (1 nM to 10 μM), an endothelium-dependent receptor-mediated vasodilator that acts via bioavailable nitric oxide, were studied after pre-contraction to 20–50% of the baseline diameter with the thromboxane A2 analog U46619 (0.1–1 μM). Relaxation responses were defined as the percent relaxation of the pre-contracted diameter.
Quantification of Myocardial Infarct Size
The left ventricle was isolated (including septum) and cut in to 1cm slices and immediately immersed in 1% triphenyl tetrazolium chloride (TTC, Sigma Chemical Co, St Louis, MO) in phosphate buffer (pH 7.4) at 38°C for 30 minutes. The infarct area (characterized by absence of staining), the non-infarcted area-at-risk (characterized by red tissue staining), and the non-ischemic portion of the LV (characterized by purple tissue staining) were sharply dissected from one another and weighed. The percentage area-at-risk was defined as: (Infarct mass + non-infarct area-at-risk mass) / Total LV mass × 100. Infarct size was calculated as a percentage of area at risk to normalize for any variation in AAR size using the following equation: (Infarct mass / total mass AAR) × 100.
Tissue Myeloperoxidase Activity Assay
Myocardial tissue from the distal LAD territory was harvested, and myeloperoxidase (MPO) activity was measured as previously described 15. Assessment of this assay in our laboratory demonstrated a linear relationship (r = 0.92) such that 1 U of MPO activity correlated with 2.9 × 106 porcine neutrophils.
Tissue Inflammatory Marker Quantification
Myocardial tissue (50 mg) from the area at risk was homogenized in RIPA buffer (Boston BioProducts, Worcester, MA) with protease inhibitor added (Complete Tablets, Roche Applied Sciences, Indianapolis, IN) and centrifuged at 12,000g for 10 minutes. Supernatants were aliquoted and a cytokine array was utilized (Allied Biotech Inc., Ijamsville, MD) for detection of interleukin (IL)-6, IL-8, and TNF-α in triplicate as described previously 16. Tissue levels of inflammatory mediators were calculated based on standards provided by the manufacturer.
Immunohistochemistry
Myocardial tissue from the ischemic territory was place in 10% buffered formalin for 24 hours, followed by paraffin mounting and sectioning in to 4μm slices. For the immunohistochemical detection of nitrosative stress, rabbit polyclonal anti-nitrotyrosine (NT) antibody (Upstate Biotechnology, Lake Placid, NY, USA) (1:80, 4 °C, overnight) was used. Secondary labeling was achieved by using biotinylated horse anti-mouse antibody (Vector Laboratories, Burlingame, CA, USA) (30min room temperature). Horseradish peroxidase-conjugated avidin (30min, room temperature) and brown colored diaminobenzidine (6min, room temperature) was used to visualize the labeling (Vector Laboratories, Burlingame, CA, USA). The sections were counterstained with hematoxilin (blue color). The intensity of specific staining of individual sections was determined by a blinded experimenter. For NT labeling a score range between 1–10 was utilized, where 1= no staining and 10=maximal staining.
Data Analysis
Data are reported as means ± SEM. Microvessel responses are expressed as percent relaxation of the preconstricted diameter and were analyzed using two-way, repeated measures analysis of variance examining the relationship between vessel relaxation, log concentration of the vasoactive agent of interest, and the experimental group (SAS Version 9.1, Cary, NC). Bonferroni corrections were applied to multiple tests and probability values of less than 0.05 were considered statistically significant.
Results
Survival
Twelve of fourteen animals survived until completion of the experimental protocol, with one animal in each group lost to irreversible dysrhythmia. Both animals were lost during the ischemic period prior to administration of placebo or sulfide and were excluded from further data analysis. (Author Note: Functional data from 4 of 6 animals in each group have been utilized in a previous study published by our group utilizing the same experimental protocol 17.)
Arterial Blood Gas, Hematocrit, and Core Temperature
No significant differences were observed between arterial pH, pCO2, pO2, Hct, or core temperature at baseline, or at the end of reperfusion.
Hemodynamic Parameters
Heart rate (HR) and mean arterial blood pressure (MAP) were similar between groups at baseline (HR Placebo 74.0 ± 2.9 vs. Sulfide 69.7 ± 8.5 beats/minute, p=0.64), MAP Placebo 63.2 ± 2.9 vs. Sulfide 56.3 ± 3.9 mmHg, p=0.19). Whereas HR remained similar between groups at the end of reperfusion (Placebo 81.0 ± 3.7 vs. Sulfide 78.0 ± 4.1 beats/min, p=0.60), MAP was significantly lower in the placebo treated group (Placebo 34.5 ± 4.1 vs. Sulfide 49.6 ± 4.8 mmHg, p=0.03*).
Global and Regional Myocardial Function
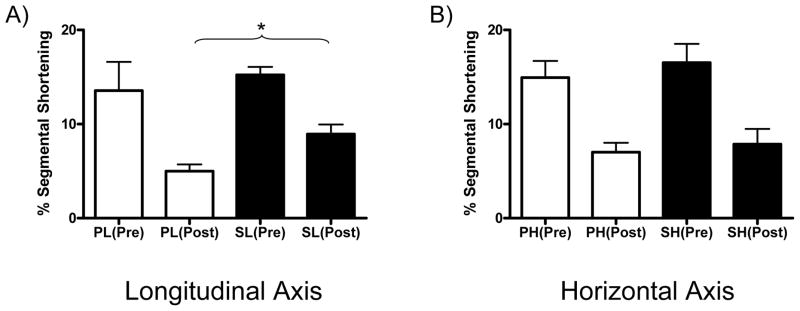
Global LV function as determined from LV +dP/dt was similar between groups prior to the onset of ischemia (Placebo 2585 ± 429 mmHg/sec vs. Sulfide 2517 ± 166 mmHg/sec, p=0.89). At the end of the reperfusion period, LV dP/dt was significantly lowered in the Placebo group (relative to pre-I/R LV dP/dt) compared to the Sulfide treated group (Placebo 1260 ± 152 mmHg/sec vs. Sulfide 2102 ± 123 mm Hg/sec). This reflected a 51.5% reduction in LV dP/dt in the Placebo group and a 16.5% reduction in LV dP/dt in the Sulfide group after I/R injury (p=0.002*). Regional myocardial function in the area at risk was similar between groups in both longitudinal (Placebo 13.57 ± 3.04 vs. Sulfide 15.22 ± 0.86 % segmental shortening(SS), p=0.61) and horizontal axes (Placebo 14.93 ± 1.78 vs. Sulfide 16.53 ± 2.00 %SS, p=0.56) prior to the onset of ischemia. At the end of reperfusion, segmental shortening in the longitudinal axis was impaired significantly in both groups, but was significantly better in the Sulfide treated animals (Placebo 4.98 ± 0.73 vs. Sulfide 8.95 ± 1.01 % SS, p=0.01*) (Figure 1A). Horizontal segmental shortening was also significantly impaired in both groups at the end of reperfusion and no significant differences were seen between groups post I/R (Placebo 7.00 ± 1.01 vs. Sulfide 7.87 ± 1.66 % SS, p=0.66) (Figure 1B).
Figure 1.
Regional LV function (% segmental shortening) was preserved to a greater extent in sulfide treated animals in the (A) longitudinal axis, whereas no differences were observed in the (B) horizontal axis. P: Placebo, S: Sulfide, Pre: pre-I/R, Post: post-I/R. L: Longitudinal axis, H: Horizontal axis. * indicates p<0.05.
Coronary Microvessel Function
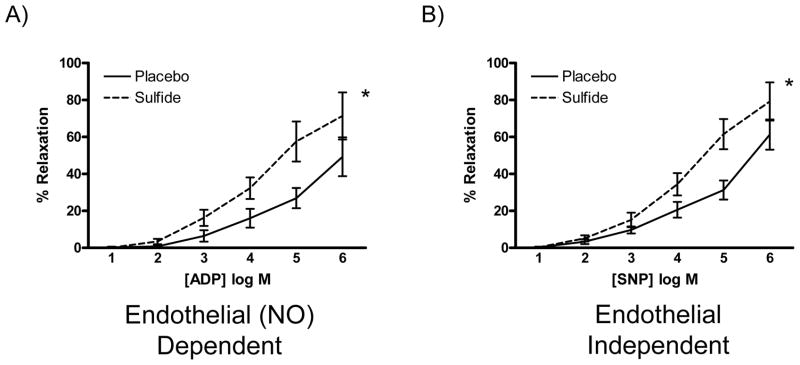
Coronary microvascular relaxation studies were performed to assess endothelial dysfunction and microvascular smooth muscle dysfunction in response to I/R injury utilizing ADP (an endothelial dependent agent) and SNP (an endothelial independent agent), respectively. Both endothelial dependent and independent relaxation was significantly improved in the Sulfide group, when compared to the Placebo group (Figure 2A and B).
Figure 2.
(A) Endothelial dependent and (B) Endothelial independent coronary microvascular relaxation was improved in sulfide treated animals. NO: nitric oxide, * indicates p<0.05.
Incidence of VF/VT
The incidence of ventricular fibrillation or pulseless ventricular tachycardia was similar between groups during the period of ischemia (Placebo 0.17 ± 0.17 vs. Sulfide 0.50 ± 0.22 episodes / animal, n=0.26), and during the period of reperfusion (Placebo 1.50 ± 0.50 vs. Sulfide 0.83 ± 0.31 episodes /animal, n=0.28). One animal in each group was lost to irreversible dysrhythmia during the period of ischemia and were excluded from data analysis as noted above, otherwise all dysrhythmias were successfully terminated with electrical cardioversion.
Myocardial Infarct Size
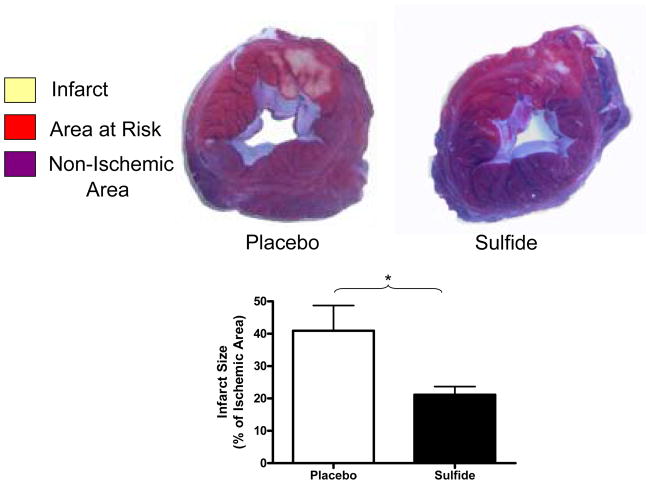
The ischemic area at risk, as a percentage of total LV mass was similar between groups (Placebo 30.9 ± 2.5 vs. Sulfide 32.5 ± 2.5 % of LV mass, p=0.68). Myocardial infarct size was significantly reduced by 1.94-fold in Sulfide treated groups relative to placebo (Placebo 40.95 ± 7.8 vs. Sulfide 21.16 ± 2.5 % of area at risk, p=0.036*) (Figure 3).
Figure 3.
Sulfide treated animals demonstrated significantly smaller infarct sizes relative to placebo treated animals. * indicates p<0.05.
Immunohistochemistry Staining for Free Radical Stress
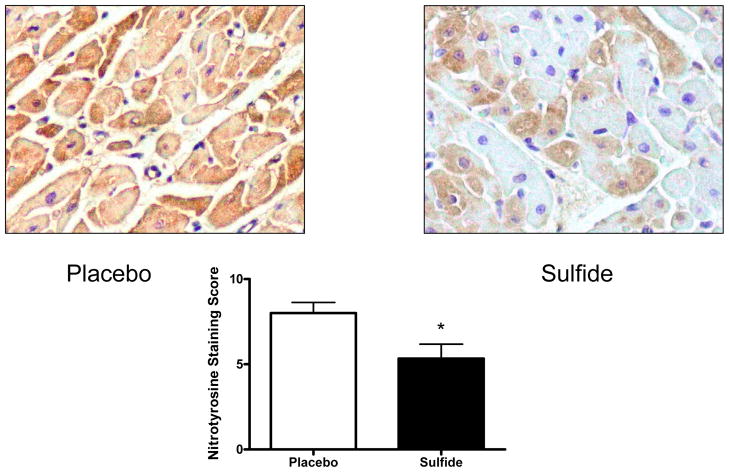
Nitrotyrosine staining scores were significantly lower in sulfide treated animals (Placebo 8.0 ± 0.63 vs. Sulfide 5.3 ± 0.84, p=0.037*) (Figure 4).
Figure 4.
Nitrotyrosine staining intensity was significantly lower in sulfide treated animals. * indicates p<0.05.
Tissue Myeloperoxidase Activity
Tissue MPO activity, a reflection of neutrophil inflammatory activity was markedly reduced in the Sulfide treated animals. Placebo treated animals demonstrated 0.0397 ± 0.016 MPO activity units/mg protein vs. 0.0025 ± 0.003 MPO activity units/mg protein in the Sulfide treated animals (p=0.04*).
Inflammatory Cytokines
Myocardial IL-6 levels were lower in sulfide treated animals (Placebo 0.76± 0.16 vs. Sulfide 0.23± 0.14 pg/mL, p=0.03*), as were levels of IL-8 (Placebo 0.58± 0.15 vs. Sulfide 0.04± 0.04 pg/mL, p=0.003*). Myocardial levels of TNFα were reduced 1.33-fold in sulfide treated animals (Placebo 0.52± 0.11 vs. Sulfide 0.16± 0.09 pg/mL, p=0.03*).
Discussion
The current study demonstrates that parenteral therapeutic administration of sodium sulfide prior to and during the reperfusion period, provides significant myocardial protection in response to acute I/R injury, limiting infarct size, improving global and regional LV function, improving coronary microvascular function, as well as limiting the inflammatory response to I/R injury in a pre-clinical porcine model of myocardial infarction. Previous work in murine models has demonstrated endogenous sulfide acts as a myocardial protection agent in the setting of acute ischemia13. As an exogenously administered therapeutic agent, Johansen et al, have demonstrated the efficacy of sulfide in an isolated rat heart model to reduce infarct size after I/R injury18, whereas Zhu et al, have demonstrated such findings in a murine model utilizing exteriorized hearts12. Notably, the previously mentioned studies12,18 administered sulfide prior to the onset of ischemia. Recently Elrod et al, have demonstrated that the therapeutic administration of sulfide at the time of reperfusion limits infarct size, inflammation, and preserves myocardial function in mice subjected to I/R injury. This study, which utilized a murine model as well, demonstrated sulfide therapy resulted in decreased levels of IL-1β, but found no differences in TNF-α or IL-10 levels. Our study, which utilizes a pre-clinical large animal model and administers sulfide at a clinically applicable time-point, is consistent with the prior studies, but notably found a significant reduction in TNF- α, as well as IL-6 and IL-8 levels.
H2S is an odorless, water-soluble gas which is produced endogenously in the cardiovascular system. Although it’s mechanism of action has yet to be fully elucidated, three key findings thus far may relate to its ability to protect the myocardium – activation of KATP channels12, limitation of neutrophil endothelial adherence/infiltration19, and its ability to scavenge oxidants and free radicals7. Activation of KATP channels has been shown to be cardioprotective20 and in vivo13 studies have shown that the myocardial protection provided by sulfide in response to myocardial ischemia is limited when KATP channels are pharmacologically inhibited, although it should be noted that inhibition of KATP channels can increase the severity of myocardial injury in control animals as well (independently from the presence or absence of exogenously administered sulfide)21. While there is no evidence to date indicating sulfide directly augments myocardial contractility, its benefits on global and regional LV function may relate to its ability to limit myocardial injury from pro-inflammatory cytokines such as IL-6, IL-8, TNFα and free radicals. The anti-inflammatory effects of sulfide have been demonstrated in multiple studies (non-cardiac) which have shown sulfide can interfere with neutrophil activity7, suppress granulocyte adherence to the endothelium and infiltration, and the expression of TNF-α8,19. IL-6, which is released in response to myocardial I/R injury22 has been shown to depress myocardial function23. IL-8, also released in response to myocardial I/R injury, increases neutrophil adhesion and the inflammatory response24. TNFα may play multiple roles in the pathogenesis of myocardial I/R injury, inducing endothelial cell adhesion molecules allowing for neutrophil infiltration25, amplifying the inflammatory response and increasing the production of reactive oxygen species26, as well as having direct myocardial depressant and apoptotic actions27. Tissue myeloperoxidase activity, a marker of neutrophil infiltration and activity was also significantly reduced in the myocardium of sulfide treated animals. This is likely the result of reduced neutrophil infiltration as has been shown with sulfide administration19, and may protect the myocardium from damaging proteases and free radicals generated by neutrophils.
The coronary microvascular circulation, consisting of arterioles < 175μm in diameter, is the principle site of resistance in the coronary circulation and responds to metabolic stimuli to govern myocardial perfusion. Dysfunction in this vascular bed, which is known to occur after myocardial I/R injury28, is thought to be responsible for the impairments in myocardial perfusion observed after re-establishment of flow in the target vessels of thrombolysis, PCI, and CABG – the large epicardial coronary arteries29. This microcirculatory dysfunction is thought to manifest from diminished bioavailability of nitric oxide, which can be scavenged by free radicals released in the setting of I/R injury30, as well from increased levels of pro-inflammatory mediators such as TNFα which can impair endothelial dependent microvascular vasorelaxation and increase circulating levels of free radicals31. The improvement in endothelium-dependent vasorelaxation in our study with sulfide therapy is consistent with the reduction in TNFα observed, as well as with the ability of H2S to act as an anti-oxidant. Interestingly, we also observed improvements in endothelial-independent vasorelaxation in the coronary microvessels of sulfide treated animals. This vasorelaxation in response to sodium nitroprusside, an agent active on the vascular smooth muscle, is consistent with the ability of sulfide to activate vascular smooth muscle KATP channels, allowing for enhanced vasorelaxation. The improvements in coronary microvascular relaxation may have significant clinical relevance as it is suggested up to 40% of patients may fail to regain appropriate myocardial perfusion despite achieving TIMI Grade 3 epicardial flow after intervention, attributed partly to coronary microvascular dysfunction29,30,32.
Limitations
While providing functional and molecular data in to the effects of sulfide therapy in myocardial I/R injury, this study has several limitations. Our time course for tissue harvest (3 hours after the onset of ischemia) is not able to account for long-term effects of sulfide on myocardial function and infarct extension. Notably, recent research has demonstrated in a murine model, sulfide therapy provides significant preservation of myocardial function in response to acute I/R injury at 72 hours after the initial ischemic insult33. The focus on functional data and inflammatory markers did not include a mechanistic investigation in to sulfide’s method of action in providing myocardial protection. Such studies would be more suitable in rodent studies where multiple dosing groups can be established and genetically modified animals are also available. The porcine model utilized in this study is somewhat electrically unstable given the lack of pre-formed collaterals in the porcine myocardium. This allows for creation of an acute area of ischemia with limited perfusion from collateral vessels from adjoining myocardium. While beneficial in allowing for clear delineation of an ischemic zone, the model is subject to significant ventricular dysrhythmias from the severity of the ischemia – reperfusion injury. The incidence of VF/VT observed may lead to myocardial preconditioning which can subsequently affect infarct size and is an inherent limitation to the porcine model utilized.
Conclusions
Therapeutic administration of H2S, generated as sodium sulfide, prior to the onset of reperfusion markedly attenuates myocardial ischemia-reperfusion injury, limiting inflammation and preserving myocardial function. H2S may have a valuable therapeutic role in the clinical setting when administered concomitantly with the coronary revascularization process.
Acknowledgments
We thank Dr. James D. McCully for his valuable assistance with the surgical protocol and the staff of the Animal Research Facility at the Beth Israel Deaconess Medical Center for their efforts. The sodium sulfide solution used in the current studies was produced and formulated by Paul Hill (Ikaria Inc., Seattle, WA).
Supported by NHLBI grant HL69024-02. Drs. Sodha and Clements are supported in part by NIH grant T-32HL076130-02 and the Irving Bard Memorial Fellowship. Partial funding for this project was provided by Ikaria, Inc., Seattle, Washington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179–90. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Manning AS, Hearse DJ. Reperfusion-induced arrhythmias: mechanisms and prevention. J Mol Cell Cardiol. 1984;16:497–518. doi: 10.1016/s0022-2828(84)80638-0. [DOI] [PubMed] [Google Scholar]
- 3.Ambrosio G, Tritto I. Clinical manifestations of myocardial stunning. Coron Artery Dis. 2001;12:357–61. doi: 10.1097/00019501-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lefer AM, Lefer DJ. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia-reperfusion. Cardiovasc Res. 1996;32:743–51. [PubMed] [Google Scholar]
- 5.Roberts MJ, Young IS, Trouton TG, Trimble ER, Khan MM, Webb SW, Wilson CM, Patterson GC, Adgey AA. Transient release of lipid peroxides after coronary artery balloon angioplasty. Lancet. 1990;336:143–5. doi: 10.1016/0140-6736(90)91661-s. [DOI] [PubMed] [Google Scholar]
- 6.Kloner RA, Giacomelli F, Alker KJ, Hale SL, Matthews R, Bellows S. Influx of neutrophils into the walls of large epicardial coronary arteries in response to ischemia/reperfusion. Circulation. 1991;84:1758–72. doi: 10.1161/01.cir.84.4.1758. [DOI] [PubMed] [Google Scholar]
- 7.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem. 2004;90:765–8. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 8.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di Sante M, Morelli A, Cirino G, Wallace JL. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–24. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 9.Hu LF, Wong PT, Moore PK, Bian JS. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem. 2007;100:1121–8. doi: 10.1111/j.1471-4159.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- 10.Mitsuhashi H, Yamashita S, Ikeuchi H, Kuroiwa T, Kaneko Y, Hiromura K, Ueki K, Nojima Y. Oxidative stress-dependent conversion of hydrogen sulfide to sulfite by activated neutrophils. Shock. 2005;24:529–34. doi: 10.1097/01.shk.0000183393.83272.de. [DOI] [PubMed] [Google Scholar]
- 11.Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, Du J, Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–63. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 12.Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, Tan CS, Whiteman M, Lu J, Moore PK. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol. 2007;102:261–8. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- 13.Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26:154–61. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- 14.Tofukuji M, Metais C, Li J, Hariawala MD, Franklin A, Vassileva C, Simons M, Sellke FW. Effects of ischemic preconditioning on myocardial perfusion, function, and microvascular regulation. Circulation. 1998;98:II197–204. discussion II204–5. [PubMed] [Google Scholar]
- 15.Amsterdam EA, Stahl GL, Pan HL, Rendig SV, Fletcher MP, Longhurst JC. Limitation of reperfusion injury by a monoclonal antibody to C5a during myocardial infarction in pigs. Am J Physiol. 1995;268:H448–57. doi: 10.1152/ajpheart.1995.268.1.H448. [DOI] [PubMed] [Google Scholar]
- 16.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–5. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 17.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, Szabo C, Sellke FW. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2008;33:906–13. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansen D, Ytrehus K, Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury--Evidence for a role of K ATP channels. Basic Res Cardiol. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- 19.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. Faseb J. 2006;20:2118–20. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 20.Lawton JS, Sepic JD, Allen CT, Hsia PW, Damiano RJ., Jr Myocardial protection with potassium-channel openers is as effective as St. Thomas’ solution in the rabbit heart. Ann Thorac Surg. 1996;62:31–8. doi: 10.1016/0003-4975(96)00260-3. discussion 38–9. [DOI] [PubMed] [Google Scholar]
- 21.Miura T, Miki T. ATP-sensitive K+ channel openers: old drugs with new clinical benefits for the heart. Curr Vasc Pharmacol. 2003;1:251–8. doi: 10.2174/1570161033476646. [DOI] [PubMed] [Google Scholar]
- 22.Kukielka GL, Smith CW, Manning AM, Youker KA, Michael LH, Entman ML. Induction of interleukin-6 synthesis in the myocardium. Potential role in postreperfusion inflammatory injury. Circulation. 1995;92:1866–75. doi: 10.1161/01.cir.92.7.1866. [DOI] [PubMed] [Google Scholar]
- 23.Hennein HA, Ebba H, Rodriguez JL, Merrick SH, Keith FM, Bronstein MH, Leung JM, Mangano DT, Greenfield LJ, Rankin JS. Relationship of the proinflammatory cytokines to myocardial ischemia and dysfunction after uncomplicated coronary revascularization. J Thorac Cardiovasc Surg. 1994;108:626–35. [PubMed] [Google Scholar]
- 24.Kukielka GL, Smith CW, LaRosa GJ, Manning AM, Mendoza LH, Daly TJ, Hughes BJ, Youker KA, Hawkins HK, Michael LH, et al. Interleukin-8 gene induction in the myocardium after ischemia and reperfusion in vivo. J Clin Invest. 1995;95:89–103. doi: 10.1172/JCI117680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–8. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 26.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–53. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 27.Herrera-Garza EH, Stetson SJ, Cubillos-Garzon A, Vooletich MT, Farmer JA, Torre-Amione G. Tumor necrosis factor-alpha: a mediator of disease progression in the failing human heart. Chest. 1999;115:1170–4. doi: 10.1378/chest.115.4.1170. [DOI] [PubMed] [Google Scholar]
- 28.Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. Faseb J. 2003;17:2328–30. doi: 10.1096/fj.03-0115fje. [DOI] [PubMed] [Google Scholar]
- 29.Ito H, Tomooka T, Sakai N, Yu H, Higashino Y, Fujii K, Masuyama T, Kitabatake A, Minamino T. Lack of myocardial perfusion immediately after successful thrombolysis. A predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation. 1992;85:1699–705. doi: 10.1161/01.cir.85.5.1699. [DOI] [PubMed] [Google Scholar]
- 30.Prasad A, Gersh BJ. Management of microvascular dysfunction and reperfusion injury. Heart. 2005;91:1530–2. doi: 10.1136/hrt.2005.064485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, Bagby GJ, Chilian WM. TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:475–80. doi: 10.1161/01.ATV.0000201932.32678.7e. [DOI] [PubMed] [Google Scholar]
- 32.Prasad A, Stone GW, Aymong E, Zimetbaum PJ, McLaughlin M, Mehran R, Garcia E, Tcheng JE, Cox DA, Grines CL, Gersh BJ. Impact of ST-segment resolution after primary angioplasty on outcomes after myocardial infarction in elderly patients: an analysis from the CADILLAC trial. Am Heart J. 2004;147:669–75. doi: 10.1016/j.ahj.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–5. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]