Abstract
It is well established that neuronal circuits can be shaped by experience. Neuronal plasticity can be achieved by synaptic competitive interactions and the addition of new neuronal units in neurogenic regions of the adult brain. Recent data have suggested that neuronal progenitor cells can accommodate somatic LINE-1 (Long Interspersed Nuclear Elements-1 or L1) retrotransposition. Genomic L1 insertions may up- or down-regulate transcriptional control of gene expression. Here, we show that exercise has a positive effect on a L1-EGFP reporter in vivo. We found that neurons from mice that experience voluntary exercise are more likely to activate an EGFP reporter marker, representing L1 insertions in the brain, when compared with sedentary animals. In the hippocampus, a neurogenic region of the adult brain, EGFP expression is mainly found in cells localized in the subgranular layer of the dentate gyrus. This observation implies that neuronal progenitor cells may support de novo retrotransposition upon exposure to a new environment. Such evidence suggests that experience-dependent L1 retrotransposition may contribute to the physiological consequences of neuronal plasticity.
Keywords: L1 retrotransposition, LINE-1, voluntary exercise, new environment, neurogenesis, neuronal diversity
Introduction
Experience in a new environment can change the structure of the adult mammalian brain. Exposing laboratory rodents to complex or “enriched” settings enhances many aspects of brain structure, including the number and size of neurons, the complexity of dendritic trees, and the number of synapses, thereby affecting cognition, such as learning and memory (van Praag et al., 2000). The genetic background of the animal also influences the response of the brain when interacting with the environment (Kempermann et al., 1997; Kempermann and Gage, 2002). Likely, most of these changes are consequences of new settings of gene expression in the affected brain target region. These findings indicate that the adult brain remains highly plastic and responsive to complex experiences. Thus, it is clear that structural processes typically associated with brain development continue to operate in the adult brain on an ongoing basis. To our knowledge, no genetic alteration has ever been reported in the brain after an individual exposure to a new environment.
The dogma that neurons share identical genomes was recently challenged by the finding that adult neural stem cells isolated from rat hippocampus can give rise to neuronal progenitor cells (NPCs) that support LINE-1 (Long Interspersed Nuclear Elements 1 or L1) retrotransposition (Muotri et al., 2005). L1s are abundant retrotransposons that comprise ∼20% of mammalian genomes (Lander et al., 2001; Waterston et al., 2002; Gibbs et al., 2004). Most of the L1s are retrotransposition-defective because they are 5′ truncated, contain internal rearrangements, or harbor mutations within their open reading frames (Grimaldi et al., 1984; Moran and Gilbert, 2002). The mouse genome is estimated to contain at least 3,000 active L1s (DeBerardinis et al., 1998; Goodier et al., 2001). In contrast, the average human genome is estimated to contain 100 retrotransposition-competent L1s, and ∼10% of these elements are classified as highly active or “hot” (Brouha et al., 2003). L1 retrotransposition can affect the genome in a variety of ways, creating insertions, deletions, new splicing sites, etc. (Kazazian, 1998; Muotri et al., 2007). L1 retrotransposition events in or near genes may also behave as “molecular rheostats” to fine-tune gene expression (Perepelitsa-Belancio and Deininger, 2003; Han et al., 2004).
Our previous data showed that, during neuronal differentiation, L1 elements can insert into genes expressed in neurons, changing their expression (Muotri et al., 2005). However, the extent and impact of L1 activity on the neuronal genome, both of inherited and somatic insertions, is unknown (Muotri and Gage, 2006). Whatever the extent of the impact, it can be amplified by epigenetic modifications and their effects as modulators of neighboring gene expression either in cis or in trans (Muotri et al., 2007; Garen and Song, 2008). Here, we present evidence indicating that L1 retrotransposition in NPCs and L1 insertions in mature neurons can be regulated by environmental factors, such as voluntary exercise.
Methods
Quantification of New Neurons in the Adult Hippocampus
New neurons in the DG of 10-week-old mice were labeled by retrovirus-mediated expression of GFP (Zhao et al., 2006) on day 0 and by intraperitoneal injection of BrdU at 50 mg/kg (two injections, 8 h apart) on day 1. Mice were housed in standard mouse cages until day 8 when one group was transferred to cages with running wheels and the other group to identical cages with wheels removed. Mice were all killed on day 25 by transcardial perfusion with 0.9% w/v NaCl and 4% w/v paraformaldehyde (PFA). The mouse brains were then stored in 4% PFA at 4°C overnight and in 30% w/v sucrose for 48 h. Brain sections of 40 μm thickness were cut with a microtome, and every sixth section was used for cell counting. Cells were directly counted under a Nikon E800 microscope. GFP+ cells were counted from the DG of the right hemisphere. BrdU and Ki67+ cells were counted from the DG of the left hemisphere (contra-lateral to the side of virus injection). The cell number from all sections of every sixth section was multiplied by six and the resulted number was used to represent the number of marker + cells per DG.
Subjects
The L1-EGFP transgenic mice were generated using the standard pronuclear injection protocol as previously described (Muotri et al., 2005). Briefly, the 12.6 kb DNA fragment containing the transgene (L1RP/EGFP) was released from its plasmid by restriction enzyme digestion and was separated from the vector backbone on 1% agarose gel. DNA was purified using Nucleospin extraction kit (BD Bioscience). The purified DNA was diluted to 1 ng/ml in injection buffer (10 mM Tris-HCl, pH7.4, 0.25 mM EDTA) and injected into the pronuclei of zygotes. Zygotes were collected from superovulated B6D2F1 females crossed to B6D2F1 males. The surviving zygotes were transplanted into oviducts of pseudopregnant ICR females. The potential founders were screened by PCR using primers as previously described (Ostertag et al., 2000). L1-EGFP mice, 3–5 months old, were divided into two groups of seven, the controls and the runners. The runners had free access to a running wheel equipped with an electronic counter. During the first 10 days, animals received one 10 mg/ml intraperitoneal injection of BrdU (Sigma), dissolved in 0.9% NaCl, and filtered sterile at 0.2 μm at 50 μg/g of body weight per day to label dividing cells.
Immunofluorescence
Immunofluorescence was performed using standard protocols as previously described (Gage et al., 1995; Muotri et al., 2005). EGFP-positive cells were counted in a one-in-six series of sections (240 μm apart, stained with DAPI in Tris-buffered saline) through a 40× objective (Leitz) throughout the rostro-caudal extent of the granule cell layer.
Results
The Effects of Running on Cell Proliferation and Survival
Neurogenesis in the adult hippocampus can be influenced by many factors, including voluntary exercise, which significantly increases the proliferation of hippocampal progenitors and the total number of newborn neurons. This increase has been shown both by BrdU and retrovirus labeling of newborn cells (van Praag et al., 1999; Zhao et al., 2006). Because running increases the expression of tropic factors in the adult hippocampus, it may also promote the survival of newborn neurons, which could partially contribute to the overall increase in the numbers of newborn neurons (Molteni et al., 2002). To test this hypothesis, we injected 10 mice with the retrovirus CAG-GFP to the right hemisphere (5 × 108/ml, 0.8 μl) on day 0 and with BrdU (50 mg/kg, 2 injections, 8 h apart) on day 1. On day 8, the mice were transferred to 12 special cages that could accommodate running wheels. One group of five mice was allowed unlimited access to a running wheel, and the other group of five mice was housed in the same type of cage but without running wheels. All mice were killed on day 25. We then quantified GFP+ cell numbers in the right hemisphere and BrdU+ cell numbers in the left hemisphere (Fig. 1). The total number of newborn neurons was significantly higher in running mice than in sedentary mice (Fig. 2). These data suggest that running could indeed promote the survival of newborn neurons. We also quantified the number of proliferating cells (Ki67+) and found that these mice continued to display high proliferative activity in the adult hippocampus after 2 weeks of unlimited access to running wheels (Fig. 2).
FIGURE 1.
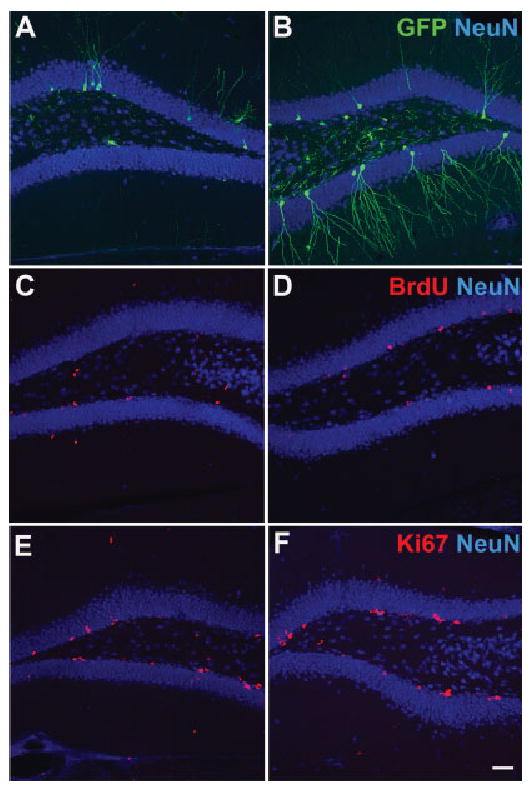
Neurogenesis in the adult hippocampus. Images of new neurons from sedentary (A, C) and running mice (B, D). New neurons were labeled by retrovirus-mediated expression of GFP (A, B) and BrdU incorporation (C, D). The number of Ki67+ cells (E, F) remains elevated in running mice (F) when compared with sedentary mice (E). Green = GFP (A, B), BrdU (C, D) and Ki67 (E, F). Blue = NeuN. Scale bar: 50 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com].
FIGURE 2.
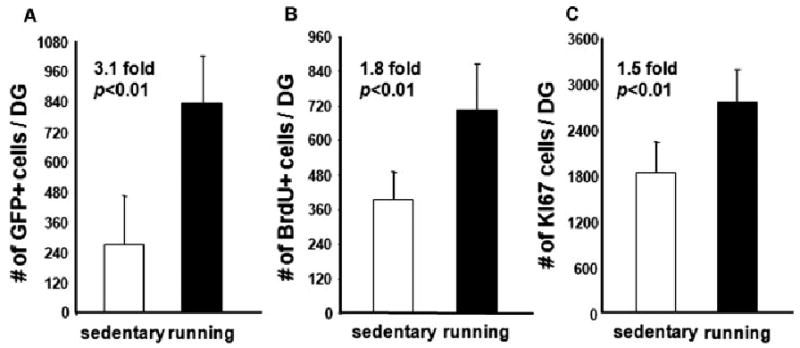
Running promotes the survival of newborn neurons. Running significantly increases the total number of newborn cells labeled by retrovirus (A) and BrdU (B). In addition, the proliferative activity (Ki67+ cells) remains high in mice after more than 2 weeks of continuous access to running wheels (C). Cell numbers shown in the figure are from 1/6 of one hemisphere. GFP cells were counted from the right hemispheres, into which the retrovirus was injected. BrdU+ and Ki67+ cells were counted from the left hemispheres.
Running Increases L1-EGFP Activity in the Brain
L1 somatic retrotransposition may be regulated by environmental signals. Because the environment can influence neurogenesis, and because adult neuroblasts can support L1 transposition, we decided to check the effect of voluntary running in adult L1-EGFP transgenic animals. The L1-EGFP transgenic mice have an L1 indicator cassette that will only activate the expression of the EGFP reporter after somatic retrotransposition (Muotri et al., 2005) (Fig. 3). L1-EGFP transgenic animals were previously shown to support L1 retrotransposition in NPCs but not in other somatic cells in several other types of tissue (Muotri et al., 2005). Mice were assigned to either control nonrunner (n = 7) or runner (n = 7) conditions. Mice in the runner group ran an average distance of 5.1 ± 0.8 km per day. Interestingly, the number of EGFP-positive cells increased about 3-fold in the runners' dentate gyrus (DG) of the hippocampus (Figs. 4 and 5).
FIGURE 3.
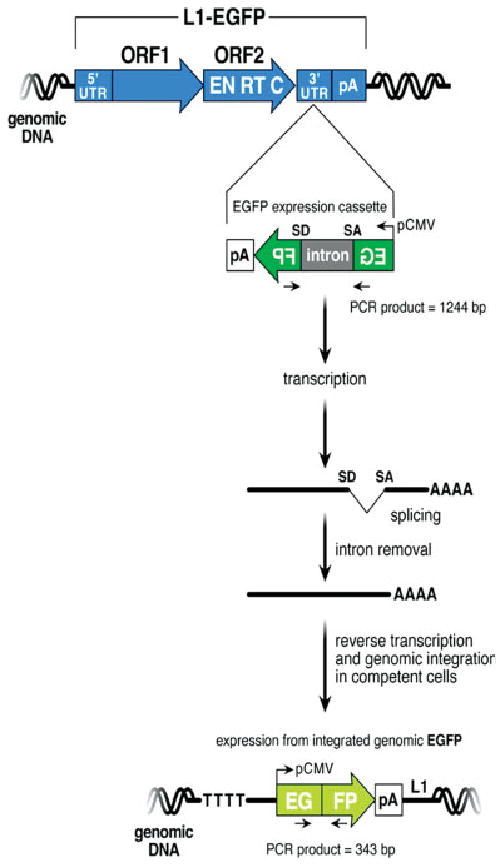
Detection of L1 retrotransposition. The structure of the L1-EGFP transgene is indicated at the top of the figure. The retrotransposition-competent human L1 contains a 5′ untranslated region (UTR) that harbors an internal promoter, two open reading frames (ORF1 and ORF2; not drawn to scale), and a 3′ UTR that ends in a poly (A) tail. The EGFP retrotransposition indicator cassette consists of a backward copy of the EGFP reporter gene whose expression is controlled by the human cytomegalovirus major immediate early promoter (pCMV) and the herpes simplex virus thymidine kinase polyadenylation sequence (pA). This arrangement ensures that EGFP expression will only become activated upon L1 retrotransposition. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com].
FIGURE 4.
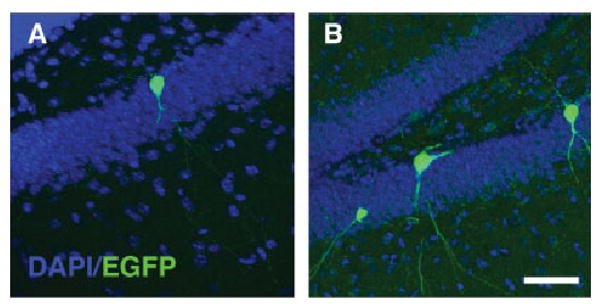
Somatic L1 retrotransposition in the adult hippocampus. EGFP expression in granular cells in the dentate gyrus of the hippocampus is increased in L1-EGFP transgenic mice previously exposed to voluntary exercise (B) when compared with sedentary animals (A). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com].
FIGURE 5.
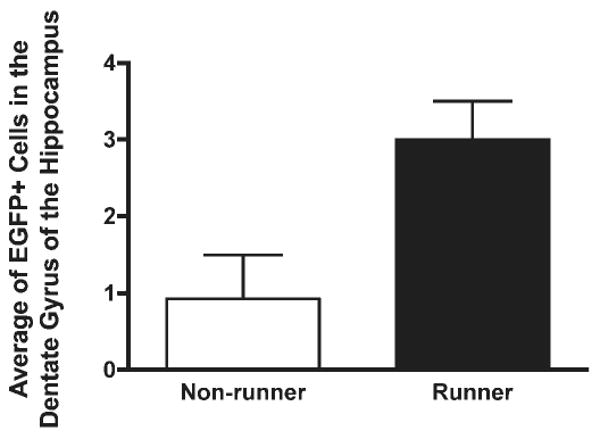
Quantification of EGFP-positive cells in the hippocampus. L1-EGFP transgenic animals housed with running wheels (runners) displayed 3-fold more granular cells expressing EGFP than nonrunners.
It is still unclear whether running can induce direct L1 retrotransposition or promote the survival of L1-inserted cells. Alternatively, other factors, such as hormones, could be acting to stimulate L1 transcription, as previously suggested (Trelogan and Martin, 1995; Morales et al., 2002). Interestingly, the numbers of EGFP-positive cells were increased not only in the adult neurogenic areas but also in nonneurogenic areas, such as the cerebellum (data not shown). Our preliminary data suggest that L1 retrotransposition does not occur in postmitotic neurons (Muotri et al., 2005). This finding indicates that running could not only increase the number of new insertions in the brain but also activate EGFP expression in mature neurons from silenced L1 insertions. In that scenario, it is possible that L1 insertions can work as promoter regions, attracting transcription factors, or influence nearby chromatin structure, modulating the expression of neuronal genes and/or noncoding RNAs (Muotri et al., 2007).
The mechanism behind this activation is unknown; however, such re-expression is probably taking place by chromatin remodeling factors. A similar phenomenon was observed in neural clones that harbor L1-EGFP insertions (Muotri et al., 2005). After cloning, EGFP expression is silenced, generating EGFP-variegated clones in which EGFP expression is completely attenuated after a prolonged period in culture. Curiously, EGFP expression can be restored during neuronal differentiation, suggesting that the L1-inserted loci are activated in the neuronal but not in the glial lineage. Treating clones with either 5-Azacytidine (a DNA demethylating agent) or Trichostatin A (an inhibitor of histone deacetylation activity) caused an increase in EGFP expression (Muotri et al., 2005). Moreover, immunostaining revealed that, after both treatments, most of the EGFP-positive cells colocalized with neuronal markers, consistent with recent reports demonstrating that epigenetic modifications accompany neuronal differentiation of neural stem cells (Hsieh and Gage, 2004; Hsieh et al., 2004).
Discussion
The apparent lack of obvious function of retroelements in the genome has long puzzled scientists and inspired the concept of “junk DNA” to illustrate the idea that such sequences are mere evolutionary remnants. However, the recognition that retrotransposons can actively reshape the genome is slowly challenging this terminology. The relationship between retrotransposons and their hosts is probably not entirely antagonistic, as several host genes have a high degree of homology to one or more transposable elements. Evidence in the literature points to a somatic function for L1 transcripts including cell proliferation (Kuo et al., 1998), differentiation (Mangiacasale et al., 2003), and early embryo development (Pittoggi et al., 2003), although it is unclear how these different retrotransposons may be acting. Moreover, it is difficult to reconcile why the genome would need so many copies of the retrotransposon and whether this expansion has any correlation with retrotransposition itself.
Barbara McClintock first proposed the “genomic shocks” hypothesis, stating that environmental factors could induce transposition of “controlling elements” (McClintock, 1984). In fact, the copy number of the plant BARE-1 retrotransposon correlates with a sharp microclimate divergence, suggesting stress-induced mobilization of retroelements in response to environmental stimuli (Kalendar et al., 2000). The “running” experiment described here illustrates how neuronal genomes in the adult brain might modify themselves when confronted with a new or unfamiliar environment. Putative, new L1 genomic integrated elements in the hippocampus could provide a mechanism by which an environmental exposure early in life would be manifested in the generation of a biological effect at a later time in development, such as in several neurodevelopmental and psychotic disorders. Some of these changes are irreversible (L1 insertions in newborn neurons), but others may be reversible (EGFP expression in mature neurons), resetting specific modifications of the genome to their early state.
The observation that genetic modifications caused by L1 elements specifically happen in neurons is another feature contributing to the enormous plasticity observed in the nervous system. Such plasticity predicts that the brain and its neuronal network will never be the same after a new experience. Moreover, individuals lacking factors involved in chromatin remodeling might have abnormal genome resetting that could give rise to a wide range of altered phenotypes.
Acknowledgments
Authors would like to thank M.L. Gage for editorial comments and L.J. Wolff for technical help.
Grant sponsors: The Rett Syndrome Research Foundation, The McDonnell Foundation, The George E. Hewitt Foundation for Medical Research, The Lookout Fund, The Picower Foundation, The National Institutes of Health (National Institute on Aging and National Institute of Neurological Disease and Stroke).
References
- Brouha B, Schustak J, Badge RM, Lutz-Prigge S, Farley AH, Moran JV, Kazazian HH., Jr Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci USA. 2003;100:5280–5285. doi: 10.1073/pnas.0831042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Goodier JL, Ostertag EM, Kazazian HH., Jr Rapid amplification of a retrotransposon subfamily is evolving the mouse genome. Nat Genet. 1998;20:288–290. doi: 10.1038/3104. [DOI] [PubMed] [Google Scholar]
- Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garen A, Song X. Regulatory roles of tumor-suppressor proteins and noncoding RNA in cancer and normal cell functions. Int J Cancer. 2008;122:1687–1689. doi: 10.1002/ijc.23285. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, Scherer S, Scott G, Steffen D, Worley KC, Burch PE, Okwuonu G, Hines S, Lewis L, DeRamo C, Delgado O, Dugan-Rocha S, Miner G, Morgan M, Hawes A, Gill R, Celera, Holt RA, Adams MD, Amanatides PG, Baden-Tillson H, Barnstead M, Chin S, Evans CA, Ferriera S, Fosler C, Glodek A, Gu Z, Jennings D, Kraft CL, Nguyen T, Pfannkoch CM, Sitter C, Sutton GG, Venter JC, Woodage T, Smith D, Lee HM, Gustafson E, Cahill P, Kana A, Doucette-Stamm L, Weinstock K, Fechtel K, Weiss RB, Dunn DM, Green ED, Blakesley RW, Bouffard GG, De Jong PJ, Osoegawa K, Zhu B, Marra M, Schein J, Bosdet I, Fjell C, Jones S, Krzywinski M, Mathewson C, Siddiqui A, Wye N, McPherson J, Zhao S, Fraser CM, Shetty J, Shatsman S, Geer K, Chen Y, Abramzon S, Nierman WC, Havlak PH, Chen R, Durbin KJ, Egan A, Ren Y, Song XZ, Li B, Liu Y, Qin X, Cawley S, Cooney AJ, D'Souza LM, Martin K, Wu JQ, Gonzalez-Garay ML, Jackson AR, Kalafus KJ, McLeod MP, Milosavljevic A, Virk D, Volkov A, Wheeler DA, Zhang Z, Bailey JA, Eichler EE, Tuzun E, Birney E, Mongin E, Ureta-Vidal A, Woodwark C, Zdobnov E, Bork P, Suyama M, Torrents D, Alexandersson M, Trask BJ, Young JM, Huang H, Wang H, Xing H, Daniels S, Gietzen D, Schmidt J, Stevens K, Vitt U, Wingrove J, Camara F, Mar Alba M, Abril JF, Guigo R, Smit A, Dubchak I, Rubin EM, Couronne O, Poliakov A, Hubner N, Ganten D, Goesele C, Hummel O, Kreitler T, Lee YA, Monti J, Schulz H, Zimdahl H, Himmelbauer H, Lehrach H, Jacob HJ, Bromberg S, Gullings-Handley J, Jensen-Seaman MI, Kwitek AE, Lazar J, Pasko D, Tonellato PJ, Twigger S, Ponting CP, Duarte JM, Rice S, Goodstadt L, Beatson SA, Emes RD, Winter EE, Webber C, Brandt P, Nyakatura G, Adetobi M, Chiaromonte F, Elnitski L, Eswara P, Hardison RC, Hou M, Kolbe D, Makova K, Miller W, Nekrutenko A, Riemer C, Schwartz S, Taylor J, Yang S, Zhang Y, Lindpaintner K, Andrews TD, Caccamo M, Clamp M, Clarke L, Curwen V, Durbin R, Eyras E, Searle SM, Cooper GM, Batzoglou S, Brudno M, Sidow A, Stone EA, Payseur BA, Bourque G, Lopez-Otin C, Puente XS, Chakrabarti K, Chatterji S, Dewey C, Pachter L, Bray N, Yap VB, Caspi A, Tesler G, Pevzner PA, Haussler D, Roskin KM, Baertsch R, Clawson H, Furey TS, Hinrichs AS, Karolchik D, Kent WJ, Rosenbloom KR, Trumbower H, Weirauch M, Cooper DN, Stenson PD, Ma B, Brent M, Arumugam M, Shteynberg D, Copley RR, Taylor MS, Riethman H, Mudunuri U, Peterson J, Guyer M, Felsenfeld A, Old S, Mockrin S, Collins F. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- Goodier JL, Ostertag EM, Du K, Kazazian HH., Jr A novel active L1 retrotransposon subfamily in the mouse. Genome Res. 2001;11:1677–1685. doi: 10.1101/gr.198301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G, Skowronski J, Singer MF. Defining the beginning and end of KpnI family segments. EMBO J. 1984;3:1753–1759. doi: 10.1002/j.1460-2075.1984.tb02042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Szak ST, Boeke JD. Transcriptional disruption by the L1 retrotransposon and implications for mammalian transcriptomes. Nature. 2004;429:268–274. doi: 10.1038/nature02536. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Gage FH. Epigenetic control of neural stem cell fate. Curr Opin Genet Dev. 2004;14:461–469. doi: 10.1016/j.gde.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci USA. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalendar R, Tanskanen J, Immonen S, Nevo E, Schulman AH. Genome evolution of wild barley (Hordeum spontaneum) by BARE-1 retrotransposon dynamics in response to sharp microclimatic divergence. Proc Natl Acad Sci USA. 2000;97:6603–6607. doi: 10.1073/pnas.110587497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian HH., Jr Mobile elements and disease. Curr Opin Genet Dev. 1998;8:343–350. doi: 10.1016/s0959-437x(98)80092-0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Genetic influence on phenotypic differentiation in adult hippocampal neurogenesis. Brain Res Dev Brain Res. 2002;134:1–12. doi: 10.1016/s0165-3806(01)00224-3. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo KW, Sheu HM, Huang YS, Leung WC. Expression of transposon LINE-1 is relatively human-specific and function of the transcripts may be proliferation-essential. Biochem Biophys Res Commun. 1998;253:566–570. doi: 10.1006/bbrc.1998.9811. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, Mc-Ewan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, Szustakowki J, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Mangiacasale R, Pittoggi C, Sciamanna I, Careddu A, Mattei E, Lorenzini R, Travaglini L, Landriscina M, Barone C, Nervi C, Lavia P, Spadafora C. Exposure of normal and transformed cells to nevirapine, a reverse transcriptase inhibitor, reduces cell growth and promotes differentiation. Oncogene. 2003;22:2750–2761. doi: 10.1038/sj.onc.1206354. [DOI] [PubMed] [Google Scholar]
- McClintock B. The significance of responses of the genome to challenge. Science. 1984;226:792–801. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Morales JF, Snow ET, Murnane JP. Environmental factors affecting transcription of the human L1 retrotransposon. I. Steroid hormone-like agents. Mutagenesis. 2002;17:193–200. doi: 10.1093/mutage/17.3.193. [DOI] [PubMed] [Google Scholar]
- Moran JV, Gilbert N. Mammalian LINE-1 retrotransposons and related elements. In: Craig N, Craggie R, Gellert M, Lambowitz A, editors. Mobile DNA II. Washington, DC: ASM Press; 2002. pp. 836–869. [Google Scholar]
- Muotri AR, Gage FH. Generation of neuronal variability and complexity. Nature. 2006;441:1087–1093. doi: 10.1038/nature04959. [DOI] [PubMed] [Google Scholar]
- Muotri AR, Chu VT, Marchetto MC, Deng W, Moran JV, Gage FH. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature. 2005;435:903–910. doi: 10.1038/nature03663. [DOI] [PubMed] [Google Scholar]
- Muotri AR, Marchetto MC, Coufal NG, Gage FH. The necessary junk: New functions for transposable elements. Hum Mol Genet 16 Spec No. 2007;2:R159–R167. doi: 10.1093/hmg/ddm196. [DOI] [PubMed] [Google Scholar]
- Ostertag EM, Prak ET, DeBerardinis RJ, Moran JV, Kazazian HH., Jr Determination of L1 retrotransposition kinetics in cultured cells. Nucleic Acids Res. 2000;28:1418–1423. doi: 10.1093/nar/28.6.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perepelitsa-Belancio V, Deininger P. RNA truncation by premature polyadenylation attenuates human mobile element activity. Nat Genet. 2003;35:363–366. doi: 10.1038/ng1269. [DOI] [PubMed] [Google Scholar]
- Pittoggi C, Sciamanna I, Mattei E, Beraldi R, Lobascio AM, Mai A, Quaglia MG, Lorenzini R, Spadafora C. Role of endogenous reverse transcriptase in murine early embryo development. Mol Reprod Dev. 2003;66:225–236. doi: 10.1002/mrd.10349. [DOI] [PubMed] [Google Scholar]
- Trelogan SA, Martin SL. Tightly regulated, developmentally specific expression of the first open reading frame from LINE-1 during mouse embryogenesis. Proc Natl Acad Sci USA. 1995;92:1520–1524. doi: 10.1073/pnas.92.5.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigo R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O'Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


