Abstract
We previously demonstrated that chemokine receptors are expressed by neural progenitors grown as cultured neurospheres. To examine the significance of these findings for neural progenitor function in vivo, we investigated whether chemokine receptors were expressed by cells having the characteristics of neural progenitors in neurogenic regions of the postnatal brain. Using in situ hybridization we demonstrated the expression of CCR1, CCR2, CCR5, CXCR3, and CXCR4 chemokine receptors by cells in the dentate gyrus (DG), subventricular zone of the lateral ventricle, and olfactory bulb. The pattern of expression for all of these receptors was similar, including regions where neural progenitors normally reside. In addition, we attempted to colocalize chemokine receptors with markers for neural progenitors. In order to do this we used nestin-EGFP and TLX-LacZ transgenic mice, as well as labeling for Ki67, a marker for dividing cells. In all three areas of the brain we demonstrated colocalization of chemokine receptors with these three markers in populations of cells. Expression of chemokine receptors by neural progenitors was further confirmed using CXCR4-EGFP BAC transgenic mice. Expression of CXCR4 in the DG included cells that expressed nestin and GFAP as well as cells that appeared to be immature granule neurons expressing PSA-NCAM, calretinin, and Prox-1. CXCR4-expressing cells in the DG were found in close proximity to immature granule neurons that expressed the chemokine SDF-1/CXCL12. Cells expressing CXCR4 frequently coexpressed CCR2 receptors. These data support the hypothesis that chemokine receptors are important in regulating the migration of progenitor cells in postnatal brain.
Indexing terms: stem cells, development, chemotaxis, brain repair
During embryogenesis, neural stem/progenitor cells must migrate long distances from the germinal epithelia where they are born to their final destinations (Hatten, 1999; Alvarez-Buylla et al., 2001). During migration, stem cells may continue to divide and also become restricted in terms of their ultimate phenotypes. Thus, the timing of neuro- and gliogenesis in the developing embryo is precisely controlled (Rallu et al., 2002). The factors that determine the migration, proliferation, and differentiation of embryonic stem cells have been widely investigated and numerous factors have been shown to influence these processes (Lindvall et al., 2004). One group of molecules that has recently been shown to control progenitor cell migration in the nervous system are the chemokines (CHEMO-tactic cytoKINES) (Tran and Miller, 2003). The chemokines are a family of small secreted proteins that are known to be important regulators of leukocyte trafficking under both normal conditions and during inflammatory responses (Moser et al., 2004). Furthermore, it is now known that chemokine signaling has an important role to play in the developing embryo. Deletion of the genes for the CXCR4 chemokine receptor or for its unique ligand, the chemokine Stromal cell-Derived Factor-1 (SDF-1/CXCL12), is lethal at a relatively late stage of embryogenesis (Ma et al., 1998; Zou et al., 1998). CXCR4 or SDF-1 deficient embryos exhibit a large number of phenotypes affecting the cardiovascular, gastrointestinal, and nervous systems, in addition to the immune system (Nagasawa et al., 1996; Ma et al., 1998; Tachibana et al., 1998; Zou et al., 1998; Kawabata et al., 1999). In the nervous system, in particular, the development of the cerebellum, hippocampal dentate gyrus, cortex, and dorsal root ganglia (DRG) are all abnormal (Zou et al., 1998; Bagri et al., 2002; Lu et al., 2002; Stumm et al., 2003; Belmadani et al., 2005) and deficits in the migration and proliferation of oligodendrocyte progenitors have also been observed (Dziembowska et al., 2005). Examination of the expression patterns of SDF-1 and CXCR4 during embryogenesis (McGrath et al., 1999; Stumm et al., 2002; Tissir et al., 2004) indicates that all of these developmental phenotypes can be explained on the basis of deficits in SDF-1 mediated attraction of embryonic neural stem/progenitor cells. In keeping with this possibility, examination of embryonic neural progenitor cells in culture has confirmed that these cells express CXCR4 receptors and that SDF-1 can act as a chemoattractant (Ji et al., 2004; Krathwohl and Kaiser, 2004; Ni et al., 2004; Peng et al., 2004; Tran et al., 2004). In addition to CXCR4, the CXCR2 chemokine receptor has been shown to be important for regulating the migration of oligodendrocyte progenitor cells in the developing spinal cord (Tsai et al., 2002).
It is now well established that neural stem cells continue to contribute to neuro- and gliogenesis in the postnatal brain (Altman and Das, 1965; Mitchell et al., 2004; Abrous et al., 2005). However, this process normally only occurs to a significant extent in the dentate gyrus (DG), the subventricular zone of the lateral ventricle (SVZ), and in the olfactory bulb (OB). Normally, progenitor cells produce mostly granule cells in the DG (Altman and Das, 1965; Seri et al., 2004) and interneurons in the OB (Lledo et al., 2004). However, experiments have demonstrated that in response to trauma, injury, infection, or neurodegneration, progenitor cells in these neurogenic areas, or populations of latent neural progenitors throughout the neuraxis, can migrate toward areas of brain injury, presumably in an attempt at self repair (Nakatomi et al., 2002; Picard-Riera et al., 2002; Taupin and Gage, 2002; Kokaia and Lindvall, 2003; Jin et al., 2004; Zhang et al., 2004a,b; Glass et al., 2005). In a variation of this paradigm, neural progenitor cells that have been propagated in culture can be introduced into the brain and can also be observed to migrate toward damaged areas (Ben-Hur et al., 2003; Pluchino et al., 2003, 2005; Zhang et al., 2003; Imatola et al., 2004; Kelly et al., 2004; Lindvall et al., 2004). An important question is: What guides this directed migration of endogenous or exogenous progenitor cells? It is known that virtually all cases of acute or long-term brain diseases are accompanied by a neuroinflammatory response. This response involves elements of the innate immune response in the brain including the activation of resident microglia and astrocytes (Huang et al., 2000; Cacquevel et al., 2004). Activation of these cells is associated with the upregulated synthesis of a large number of pro- and antiinflammatory cytokines, including numerous chemokines (Huang et al., 2000). We have demonstrated that neural progenitor cells isolated from postnatal mice, and propagated in culture, also express numerous chemokine receptors, as do their embryonic counterparts (Tran et al., 2004). Thus, in principle, it is possible that chemokines synthesized in association with neuroinflammatory responses might act as chemoattractants for neural progenitors in vivo and so explain the targeted migration of these cells to regions of brain injury.
In order for this hypothesis to be correct it is necessary to demonstrate that neural progenitor cells in their normal neurogenic environments in vivo express chemokine receptors, as do their counterparts in cell culture. This is particularly true as cell culture conditions often alter the characteristics of neural progenitor cells (Gabay et al., 2003). In the present study we examined this issue and observed the expression of chemokine receptors by neural progenitor cells identified by several different criteria. The data suggest that chemokines may be important in the control of neurogenesis in the postnatal brain under normal conditions and in the context of brain disease.
MATERIALS AND METHODS
Animals
CD1 mice were bred in the local animal facilities and maintained on a 12-hour dark/light cycle (7 AM / 7 PM) with food and water ad libitum. The following transgenic animals were also used in this study: Nestin-EGFP transgenic mice: the second intron from the rat nestin gene was placed upstream of the thymidine kinase minimal promoter (tk) to drive a modified form of enhanced green fluorescent protein (EGFPmut4) (Okada et al., 1999). DNA was generously provided by the following individuals: nestin/tk, J.M. Hebert (Albert Einstein College of Medicine); EGFPmut4, A. Okada (Stanford). Transgenic mice in the FVB/N strain were generated by transgenic mouse cores at Children’s Hospital in Boston. Animals are genotyped by polymerase chain reaction (PCR) of tail DNA using primers specific to transgene sequences. Two mm of tail is placed into a 1.5 mL microfuge tube with 200 µL 1 × PBND buffer (Jackson Laboratories, Bar Harbor, ME) and 1 µL of 10 mg/mL Proteinase K and incubated at 55°C overnight. Then 1–2 µL processed, lysed tail DNA was used for each PCR reaction. TLX-LacZ transgenic mice (Shi et al., 2004; kindly provided by Drs. Ruth Yu and Ron Evans, Salk Inst.), CCR2 knockout mice (kindly provided by Dr. William Karpus, Northwestern Univ.) (Fife et al., 2000), GAD-65-EGFP transgenic mice (kindly provided by Dr. Enrico Mugnaini, NUIN, Northwestern Univ.), CXCR4-EGFP and SDF-1-EGFP BAC transgenic mice (kindly provided by Dr. Mary Beth Hatten and the Gene Expression Nervous System Atlas (GENSAT) project; NINDS contract N01Nso2331 to Rockefeller University, NY, http://www.gensat.org/index.html). All animal-related procedures were approved by the Northwestern University animal care and use committee.
In situ hybridization probes
cDNA fragments were amplified by PCR from mouse or rat cDNA and subcloned into the PCR II-TOPO vector (Invitrogen, La Jolla, CA) or pGEM-T vector (Promega, Madison, WI). All the PCR fragments were verified by restriction analysis and automated DNA sequencing (Perkin Elmer, Norwalk, CT). The plasmid templates were linearized with restriction enzyme digested and then transcription labeled by digoxygenin (Roche, Nutley, NJ). The following cDNA fragments were used: for CCR1, 1–553 of NM_009912; for CCR2, 489-1336 of U77349; for CCR2 3′ untranslated regions (3′ UTR), 1231–1850 of U56819; for CCR5, 45–803 of AF022990; for CXCR3, 99–579 of NM_009910.1; for CXCR4, 1–774 of AF452185, respectively. Sense and antisense probes were made for each of the receptors.
Probe specificity
Sequence homology as well as their close chromosomal proximity suggests that CCR2 and CCR5 are closely related genes. Since the CCR2 and CCR5 probes used in this study for in situ hybridization were selected from the open reading frame, they exhibited 86% sequence identity at the nucleotide level, suggesting that some cross-hybridization of the probes is likely. In order to examine the specificity of the probes used for the in situ hybridization studies, the same probes were used for Northern blot hybridization. First, HEK293 cells were transfected with a mouse CCR2 expressing vector and subjected to Northern blot analysis (see below). As expected, a strong signal was detected when the CCR2 probe was used (Fig. 1, upper panel). No significant signal was observed when using probes for CCR1, CXCR3, or CXCR4. Ribosomal RNAs were used as an internal control for equal loading (Fig. 1, lower panel) (U: untransfected, C: CCR2-transfected). Each probe showed no significant sequence identity with any other known gene when searched by BLAST, except for CCR2 and CCR5, whose sequence identities are mentioned above. Thus, the CCR5 probe produced an appreciable signal. Therefore, we cannot exclude the possibility that the in situ hybridization signal observed using the CCR2 probe was contaminated by CCR5 mRNA and vice versa. Because of this potential complication we also included experiments in which CCR2 was detected using a specific antibody (Banisadr et al., 2005) as well as in situ hybridization experiments using a second probe for CCR2 selected from 3′-untranslated region.
Fig. 1.
Specificity of probes used for in situ hybridization experiments. HEK293 cells were transfected with a mouse CCR2 expressing vector and subjected to Northern blot analysis. A strong signal was detected when the CCR2 in situ probe was used (upper panel). No significant signal was detected with the CCR1, CXCR3, and CXCR4 probes. The CCR5 probe generated an appreciable signal. Ribosomal RNAs were used as an internal control for equal loading (lower panel). U, untransfected; C, CCR2-transfected.
Northern blot analysis
Total RNA was extracted using the Trizol reagent (Invitrogen). The RNA was dissolved in distilled water and denatured in 50% formamide, 6.2% formaldehyde, 20 mM MOPS (3-[N-morpholino] propanesulfonic acid), 5 mM sodium acetate, and 1 mM EDTA at 60°C for 5 minutes. Electrophoresis was performed at 100 V for 1.5 hours in a 1.2% formaldehyde agarose gel. RNA was transferred to Nytran filter (Amersham, Arlington Heights, IL) for 18 hours by capillary transfer. cDNA probes labeled with 32P-dCTP (Amersham) were prepared by random labeling. Hybridization procedures were performed using ExpressHyb hybridization solution (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions.
In situ hybridization
Adult male mice were killed by transcardial perfusion with 4% paraformaldehyde (PFA) in 1× phosphate-buffered saline (PBS), pH 7.4. Brains were removed and postfixed overnight and then equilibrated in 10% sucrose in PBS. The brains were then frozen by placing them in their cutting mold on dry ice and covered with OCT cryosolution. Then 16-µm sections were collected in the coronal plane on a cryostat and mounted onto Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA). Slides were stored at −70°C. Prior to use, the slides were air-dried for 1 hour at room temperature and then fixed in 4% PFA for 15 minutes and washed in PBS. The slides were then incubated in proteinase K (1 µg/mL in PBS) at 37°C for 30 minutes and postfixed again in 4% PFA for 20 minutes. Then they were acetylated for 10 minutes (2.7 mL Triethanolamine [Fluka, Buchs, Switzerland], 196.5 mL H2O, 0.5 mL acetic anhydride, 375 mL HCl) and washed in PBS. The brain sections were then prehybridized (50% formamide, 5× SSC, 0.1% Tween 20, 500 µg/mL tRNA, 200 µg/mL acetylated BSA, 50 µg/mL heparin) for 1 hour at 65°C, followed by hybridization that was conducted for 20 hours at 65°C using the DIG-labeled probe (100 ng/mL). After hybridization, slides were washed 3 times with the T solution (50% formamide, 2× SSC, 0.1% Tween 20) at 65°C for 20 minutes then washed 3 times with TBST (25 mM Tris-HCl, 136 mM NaCl, 2.68mM KCl, 1% Tween 20). Slides were then incubated with 10% lamb serum in TBST for 1 hour and treated with anti-DIG antibody followed by antibody detection according to the manufacturer’s protocol (Roche Applied Science, Indianapolis, IN).
Fluorescence in situ hybridization (FISH)
This method is similar to the in situ hybridization protocol described above with the exception that the probes used were not DIG-labeled. After hybridization the slides were washed twice with preheated Solution T (50% formamide, 2× SSC, 0.1% Tween 20) and then washed with TBST and blocked with 1% H2O2 in PBS for 30 minutes. The slides were then washed 2 times with PBS and then blocked with blocking solution (100 mM Tris-HCl, 150 mM NaCl, 4% goat serum, 0.1% Triton X100) for 1 hour at room temperature. Horseradish peroxidase (POD)-conjugated sheep anti-digoxigenin (DIG) antibody (1:1,000 dilution) in blocking solution was then added and incubated for 1 hour. Slides were washed 3 times with TBST and fluorescence was developed using a TSA (Tyramide Signal Amplification) fluorescence procedure (Perkin Elmer-NEN, Boston, MA). Sections were incubated in a 1:100 dilution of Cy5 conjugated Tyramide (Perkin Elmer-NEN) for 20 minutes at room temperature. After FISH, slides were incubated with blocking solution for 1 hour and then primary antibody (1:1,000 dilution) was added (anti-β-galactosidase or anti-GFP) and incubated at 4°C overnight. The slides were then washed 3 times with TBST and incubated with the secondary antibody (rabbit conjugated AlexaFluor 488, 1:500 dilution) for 1 hour. Slides were then washed 3 times with TBST and mounted with Vectashield antifade solution. Slides were analyzed by confocal microscopy (Olympus IX70). Image-acquisition software (Fluoview) and “Image J” were used for 3D reconstruction of confocal z-stacks (usually three or four optical sections per micrometer of z-distance) and to assess double labeling in cells depicted in xy-, xz-, and yz-planes. As control for the specificity of in situ hybridization and TSA amplification, additional sections were subjected to hybridization using sense probes for each receptor.
Immunohistochemical studies
All antibodies used in this study are described in Table 1. Astrocytes were identified with mouse anti-glial fibrillary acidic protein (GFAP, 1:300). Progenitor cells were labeled with mouse anti-nestin (1:300). Dividing cells were labeled with mouse anti-Ki67 (1:300). Newly formed migrating neuroblasts were identified with guinea pig anti-doublecortin (1:300). Granule cells were identified by rabbit anti-Prox-1 (1:1,000) and immature granule cells and granule cell progenitors were labeled with mouse anti-polysialic acid NCAM (PSA-NCAM, 1:200). Goat anticalretinin (1:300) and rabbit anti-calbindin D 28k (1:300) were used to label immature and mature granule cells, respectively. CCR2 receptors were labeled using rabbit anti-CCR2 (1:300). We used goat antirabbit biotin conjugated secondary antibody (1:200, Vector Laboratories, Burlingame, CA) for CCR2. The appropriate isotypespecific secondary antibodies for other markers consisted of AlexaFluor 488- and AlexaFluor 633-conjugated preparations (1:300; Molecular Probes, Eugene, OR).
TABLE 1.
Details of Primary Antibodies Used
| Antibody | Species | Company | Catalog No |
Immunizing Antigen |
Characterization and Specificity Controls |
|---|---|---|---|---|---|
| β- Galactosidase |
Rabbit IgG |
Molecular Probes |
A-11132 (lot 71C1- 1) |
E. coli β- Galactosidase |
IB, IP, IHC; does not stain mouse tissues that do not express the Lac Z gene. |
| Calbindin | Rabbit IgG |
Swant | CB-38 (lot 5.5) |
Recombinant rat Calbindin D-28k |
IB, IHC; Fig.16; stains Ca- binding, 28 kDa calbindin-D spot on 2D immunoblot (Manufacturer). |
| Calretinin | Goat IgG |
Chemicon | AB1550 (lot 220/70104) |
Guinea pig Calretinin (whole protein) |
IHC, WB; IF of cortical cells in culture (Manufacturer); Fig. 15. |
| CCR2 | Rabbit IgG |
Gift from Dr. S Melik Parsadaniantz |
C-terminus synthetic peptide1 |
ICC, IHC, WB (stains a single band of 42 kDa molecular weight on WB).2 No staining was seen when the antibody was used to stain tissue from CCR2 knockout mice (Fig. 4). |
|
| Doublecortin | Guinea pig IgG |
Chemicon | AB5910 (lot 23111103) |
Synthetic peptide corresponding to amino acids 350– 365 of mouse and human Doublecortin (DCX) protein |
IF on tissue sections from rat and mouse CNS (Manufacturer). Staining pattern (Fig. 16) was identical to a prior publication using a different antibody.3 |
| GFAP | Mouse IgG1 |
Sigma | G3893 (lot 063K4851) |
Purified GFAP from pig spinal cord4 |
IB, IF, P, Fr, no cross reaction with Vimentin. The GFAP antiserum only stained cells with the classic morphology and distribution of fibrillary astrocytes (Fig. 5, Fig. 12). A single band is detected by WB (Manufacturer).5 |
| GFP | Rabbit IgG |
Molecular Probes |
A11122 (lot 45351A) |
GFP from Aequorea victoria |
IHC, WB, IP; does not stain tissue of wild type mice. |
| Ki67 | Mouse IgG1 |
BD Bioscience |
556003 (lot 67833) |
Immunodominent epitope of the Ki67 protein |
WB, flow cyotometric analysis; staining of sections produced a pattern (Fig. 10) of Ki67 immunoreactivity that was identical to previous descriptions. |
| Nestin | Mouse IgG1, Clone rat 401 |
Pharmingen | 556309(lot 46292) |
Paraformaldehyde- fixed spinal cord from day-15 rat embryos |
WB, IHC, P, Fr, IF; a single band is detected by WB (Manufacturer).6 |
| Prox-1 | Rabbit IgG |
Chemicon | AB5475 (lot 24041815) |
Synthetic peptide from the C terminus of mouse Prox-1 |
P, Fr, IHC; staining pattern (Fig. 14) was identical to prior publication using a different antibody.3 |
| PSA-NCAM | Mouse IgM, Clone 2-2B |
Chemicon | MAB5324 (lot 25050318) |
Viable Meningococcus group B (strain 355) |
WB, ICC, IHC, Fr; the PSA- NCAM antiserum only stained cells with the classic morphology and distribution of immature granule cells and granule cell progenitors (Fig. 17,Fig. 18). Staining pattern was identical to a prior publication.7 |
Fr, frozen sections; P, paraffin sections; IB, immunoblotting; IF, immunofluorescence; IHC, immunohistochemistry; ICC, immunocytochemistry; IP, immunoprecipitation; WB, Western blotting.
Polyclonal anti-CCR2 antiserum was generated in rabbits (Neosystems, Strasbourg, France) using a synthetic peptide, corresponding to the C-terminal segment of the receptor (between amino acids 345 and 359), showing no homology with other known chemokine receptors (Blast submission on NCBI database, GenBank, NIH). This antibody was purified by affinity chromatography (Covalab, Lyon, France) and kindly provided us by Dr. S. Melik Parsadaniantz.
GFAP is expressed by radial glia-like stem cells in SVZ and dentate gyrus (Seri et al., 2001) and astrocytic tube-forming cells in the RMS (Peretto et al., 1007).
A 52-kD band is detected on Western blot with this antibody.
Clone: Rat 401; recognizes a 200-kD band on Western blot. Nestin is expressed by radial glia-like neuronal progenitors in adult mouse SVZ and dentate gyrus (Seri et al., 2001).
Mice were anesthetized and perfused transcardially with cold PBS, followed by a freshly prepared solution of 4% PFA in PBS, pH 7.4. The brains were rapidly removed and postfixed overnight in 4% PFA at 4°C. Forty-micrometer thick coronal sections were cut with a vibratome (Leica VT 1000S; Leica Microsystems, Deerfield, IL) and collected in cold PBS.
Two different immunohistochemical methods were used in this study. CCR2 staining was performed as previously described (Banisadr et al., 2005). Briefly, free-floating sections were pretreated for 20 minutes with 3% hydrogen peroxide in PBS to quench endogenous peroxidase. They were then washed with PBS (3 × 10 minutes), preincubated for 90 minutes at room temperature (25°C) in PBS containing 0.1% Triton X-100 and 6% normal goat serum. Then sections were incubated overnight at 4°C, with the rabbit anti-CCR2 antibody at a final dilution of 1:300. Antibody was diluted in PBS containing 2% normal goat serum and 0.1% Triton X-100. After incubation sections were rinsed extensively (6 times, 10 minutes each) with PBS and incubated for 90 minutes in a 1:100 dilution of biotin conjugated goat antirabbit antibody (Vector) used against the CCR2 antibody. Then sections were washed with PBS (3 times, 10 minutes each). For signal amplification, a TSA fluorescence procedure (Perkin Elmer-NEN) was used. Briefly, sections were incubated for 30 minutes in a 1:100 dilution of streptavidin horseradish peroxidase-conjugated (Amersham Pharmacia Biotech). Then they were washed extensively with PBS and incubated in a 1:50 dilution of Cy5 conjugated Tyramide (Perkin Elmer-NEN) for 5 minutes. After washing, sections were mounted on slides, mounted in Vectashield (Vector) and observed on a confocal microscope (Olympus IX70). In order to identify the phenotype of neural progenitors, the aforementioned markers were used. Free-floating sections were incubated in PBS containing 4% serum and 0.1% Triton for 90 minutes. They were then incubated with antibodies overnight at 4°C. All antibodies were diluted in PBS containing 2% serum and 0.1% Triton. After incubation, sections were washed with PBS and incubated with secondary antibodies for 90 minutes. Sections were then washed again, mounted on slides, and analyzed by confocal microscopy (Olympus IX70). Image-acquisition software (Fluoview) was used. All captured images were exported to Adobe Photoshop 7.0 (Adobe, San Jose, CA) and adjustments were made to the brightness and contrast to reflect true colors as closely as possible.
In both approaches, the absence of cross-reactivity of the secondary antibodies as well as the specificity of TSA staining was verified by omitting one or both primary antibodies during the overnight incubation. Furthermore, in order to assess the performance of CCR2 antibody in tissue sections, staining using this antibody was performed in sections obtained from CCR2 knockout mouse brain (Fife et al., 2000). Wildtype animals were used as controls.
Chemotaxis assay
For measurement of chemotaxis using neural progenitor cells, Dunn chambers were obtained from Hawksley Technology (West Sussex, UK). Dunn chambers allow for the generation of a stable chemotactic gradient and observation of cell migration in the context of this gradient (Zicha et al., 1991). Neural progenitor cells were plated as single cells onto poly-D-lysine-coated 15-mm glass coverslips and allowed to adhere in serum-free medium for 3 hours. Dunn chambers were prepared by adding vacuum grease around the outer edge of the outer chamber. As a control, neurobasal medium was added to the inner and outer chambers. To create a chemokine gradient, neurobasal medium was added to the inner chamber and SDF-1/CXCL12, MCP-1/CCL2 (monocyte chemoattractant protein-1), RANTES/CCL5 (regulated on activation normal T cell-expressed and secreted), MCP-2/CCL8 (monocyte chemoattractant protein-2) and IP-10/CXCL10 (interferon γ-inductible protein) (all 50 nM; R&D Systems, Minneapolis, MN) were added to the outer chamber. After preparation of these chambers, coverslips were washed twice with neurobasal medium, placed into the chambers with cells facing down into the wells, and incubated at 37°C. Using an inverted microscope, the number of cells were counted for eight fields each in the inner and outer chamber at the time points indicated.
RESULTS
Expression of chemokine receptors in neurogenic areas of mouse brain
In order to examine the expression of chemokine receptors by neural stem/progenitor cells in vivo we first performed in situ hybridization studies to localize chemokine receptor mRNA expression in regions of the postnatal brain (5 weeks) where neurogenesis normally occurs. We began with the CXCR4 receptor, which has been the most widely studied chemokine receptor with respect to its role in the nervous system (Tran and Miller, 2003). CXCR4 signaling has been clearly shown to be important for the migration of neural progenitor cells in several parts of the embryo (Zou et al., 1998; Lu et al., 2002; Stumm et al., 2003; Belmadani et al., 2005) and neural progenitors cultured from adult brain express CXCR4 receptors (Tran et al., 2004). We observed expression of CXCR4 mRNA in the dentate gyrus (DG), the subventricular zone of the lateral ventricle (SVZ), and in the olfactory bulb (OB) (Fig. 2). In the DG expression of CXCR4 mRNA was observed in the granule cell layer including the subgranular zone (SGZ) between the granule cell layer and the hilus (Fig. 2C). In the SVZ CXCR4 mRNA expression was observed in many cells surrounding the ventricle and in the dorsolateral extension of the SVZ (Fig. 2B). The vast majority of the labeling was of ependymal cells lining the ventricle. In the OB, CXCR4 mRNA expression was observed in the rostral extent of the olfactory ventricle, which represents the termination of the rostral migratory stream (RMS) along which progenitors migrate to the OB from the SVZ (Fig. 2A). In addition, many cells in the granule cell and periglomerular cell layers also showed CXCR4 expression. Some cells in the mitral cell layer were also stained. The pattern of CXCR4 expression is consistent with the possibility that the receptor is expressed in neural progenitor cells in all of these regions of the brain, although it is also clearly expressed in cells other than progenitors, as described in previous publications (Banisadr et al., 2002a; Stumm et al., 2002), an observation which we shall discuss further below. No labeling was observed in sections incubated with the sense probe (Fig. 2D–F) demonstrating the specificity of the labeling. The procedure used to generate the images shown in Figure 2A–C was identical to that used to generate the images in Figure 2D–F.
Fig. 2.
Expression pattern for CXCR4 mRNA expression in neurogenic regions of mouse brain. In situ hybridization was performed with a probe specific for CXCR4, using digoxygenin (DIG) labeling (A–F) and fluorescence in situ hybridization (FISH) (G–I) methods. CXCR4 mRNA was expressed in the olfactory bulb (OB), subventricular zone (SVZ), and dentate gyrus (DG) of 5-week-old mouse brain. A: In the OB, CXCR4 mRNA was expressed in the rostral extent of the olfactory ventricle, as well as in the granule, periglomerular, and mitral cell layers. B: In the SVZ, CXCR4 mRNA expression was observed in cells surrounding the ventricle and in the dorsolateral extension of the SVZ. C: In the DG, CXCR4 mRNA was expressed in the granule cell layer including the subgranular zone (SGZ). D–F: In situ hybridization performed using a CXCR4 sense probe to test the specificity of the probe. G–I: CXCR4 mRNA expression patterns obtained by FISH. Comparison of the two methods shows that in each case the expression pattern was the same, thereby validating this fluorescent method of detection. Scale bars = 100 µm.
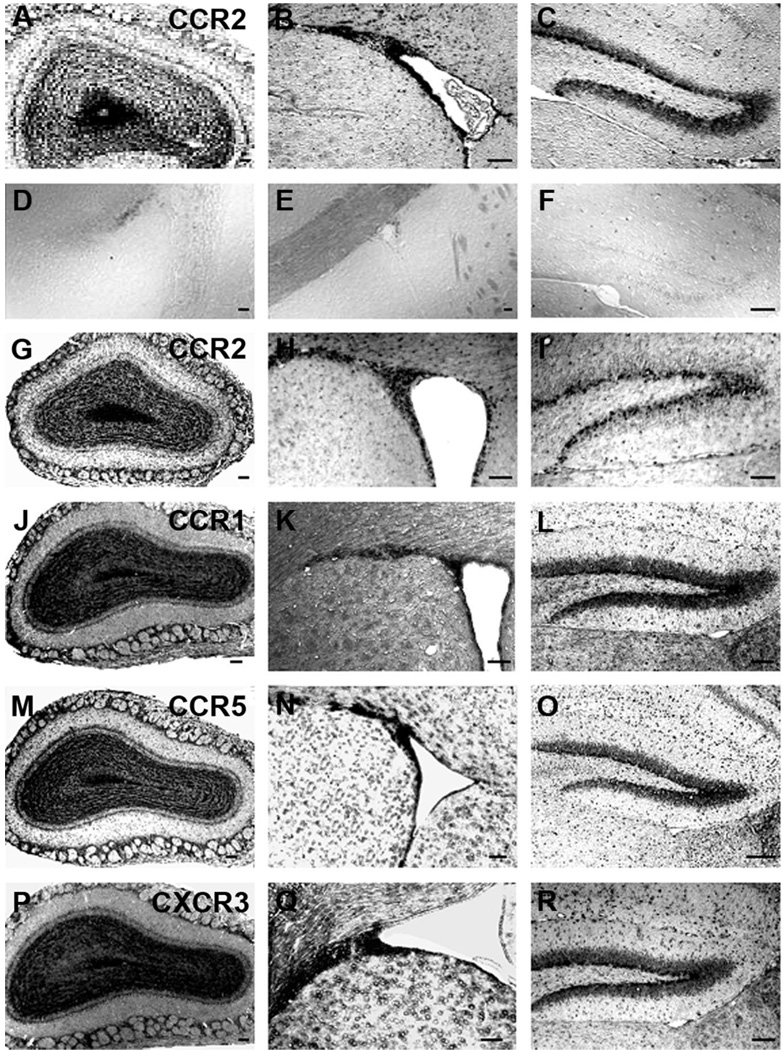
Previous studies have indicated that neural progenitors cultured from postnatal brains expressed numerous chemokine receptors, in addition to CXCR4 (Ji et al., 2004; Krathwohl and Kaiser, 2004; Tran et al., 2004; Widera et al., 2004). This might allow neural progenitors to respond to different chemokines upregulated in the context of neuroinflammation in the brain. We therefore also examined the expression patterns for several other receptors of interest including the CCR1, CCR2, and CCR5 receptors for β chemokines (e.g., MIP-1 α, MCP-1, and RANTES, respectively) and CXCR3 receptors for α chemokines (e.g., IP-10). The chemokines that activate these receptors have been shown to be strongly upregulated in numerous brain disorders (Huang et al., 2000). In each of these cases the staining pattern in all three areas was very similar and was similar to that observed for CXCR4 receptors, consistent with the possibility that they are all expressed in thesame population of cells (Fig. 3), as would be predicted from our cell culture experiments (Tran et al., 2004).
Fig. 3.
Expression patterns for chemokine receptors in neurogenic regions of mouse brain. A–C: The expression of CCR2 mRNA in the OB, SVZ, and DG, respectively, obtained by in situ hybridization using a probe for CCR2. D–F: In situ hybridization performed using a CCR2 sense probe to test the specificity of the probe. Because of potential crossreactivity of the CCR2 probe with CCR5 (see Fig. 1), we performed additional in situ hybridization experiments using a probe for CCR2 taken from the 3′ UTR. In situ hybridization using this probe showed the same expression pattern (G–I). In situ hybridization was also performed using antisense probes for CCR1 (J–L), CCR5 (M–O), and CXCR3 (P–R) chemokine receptors. Experiments were carried out on sections obtained from the OB, SVZ, and DG using antisense probes for each chemokine receptor. Scale bars = 100 µm.
Because of the potential cross-reactivity observed between probes for CCR2 and CCR5 receptors we performed additional in situ hybridization experiments using a second probe for CCR2 (3′ UTR) (Fig. 3G–I). This probe was selected from 3′-untranslated region (3′-UTR), because there was considerable sequence identity between coding regions of CCR2 and CCR5. BLAST alignment showed no significant sequence identity of the new probe to any other known genes. In situ hybridization using the two CCR2 probes showed the same expression pattern (Fig. 3). We also confirmed the expression pattern of the CCR2 receptor using immunohistochemistry employing a specific antibody against mouse CCR2 that has been shown not to cross-react appreciably with CCR5 (Fig. 4) (Banisadr et al., 2005). The staining pattern obtained was similar to that observed with the probe for CCR2 using in situ hybridization, expression of the receptor being observed in all three areas of the brain examined. CCR2 staining was not observed in brain tissue of CCR2 KO mouse confirming the specificity of the antibody (Fig. 4D).
Fig. 4.
Expression pattern for CCR2 receptors in neurogenic regions of the mouse brain. Immunohistochemistry was carried out on free-floating sections obtained from adult (5-week-old) mouse brain using a CCR2 antibody. A: In the olfactory bulb (OB), CCR2- immunoreactive cells were detected in the core as well as the glomerular layer (GI), mitral cell layer (Mi), and granular cell layer (Gr). B: In the subventricular zone (SVZ), CCR2 labeling was observed in cells surrounding the lateral ventricle and in the dorsolateral extension of the SVZ. C: In the dentate gyrus (DG), CCR2-immunopositive cells were localized in the granule cell layer (Gr) as well as in the hilus. D: Lack of CCR2 staining in sections from the dentate gyrus of CCR2 KO mice demonstrated the specificity of the CCR2 antibody. Scale bars = 100 µm.
Expression of chemokine receptors by neural progenitor cells
In order to demonstrate that some of the cells expressing chemokine receptors in the areas examined were neural progenitors we investigated the coexpression of chemokine receptors with several neural progenitor markers. We selected the CXCR4 and CCR2 receptors for further study owing to the fact that we envisage specific roles for these receptors in the functions of neural progenitor cells (see Discussion). In order to perform colocalization studies we used FISH for each chemokine receptor combined with fluorescent immunohistochemistry for each progenitor cell marker. First, we compared the distribution patterns of chemokine receptor expression using the FISH method with those obtained above. As can be observed in Figure 2G–I for CXCR4 receptors, in each case the same expression pattern was obtained using either method. We then examined colocalization of chemokine receptor expression with 3 separate markers, nestin, the TLX gene and the Ki67 protein. Nestin is an intermediate filament protein, which is highly expressed in neural progenitor cells (Lendahl et al., 1990). TLX is a transcription factor whose expression has been shown to be important for the proliferation of adult neural progenitor cells (Shi et al., 2004). Ki67 is a protein whose expression is associated with cell division and so is used as a marker for cell proliferation. Staining for Ki67 has been used to identify dividing neural progenitor cells in the brain (Shi et al., 2004).
Nestin/chemokine receptor coexpression
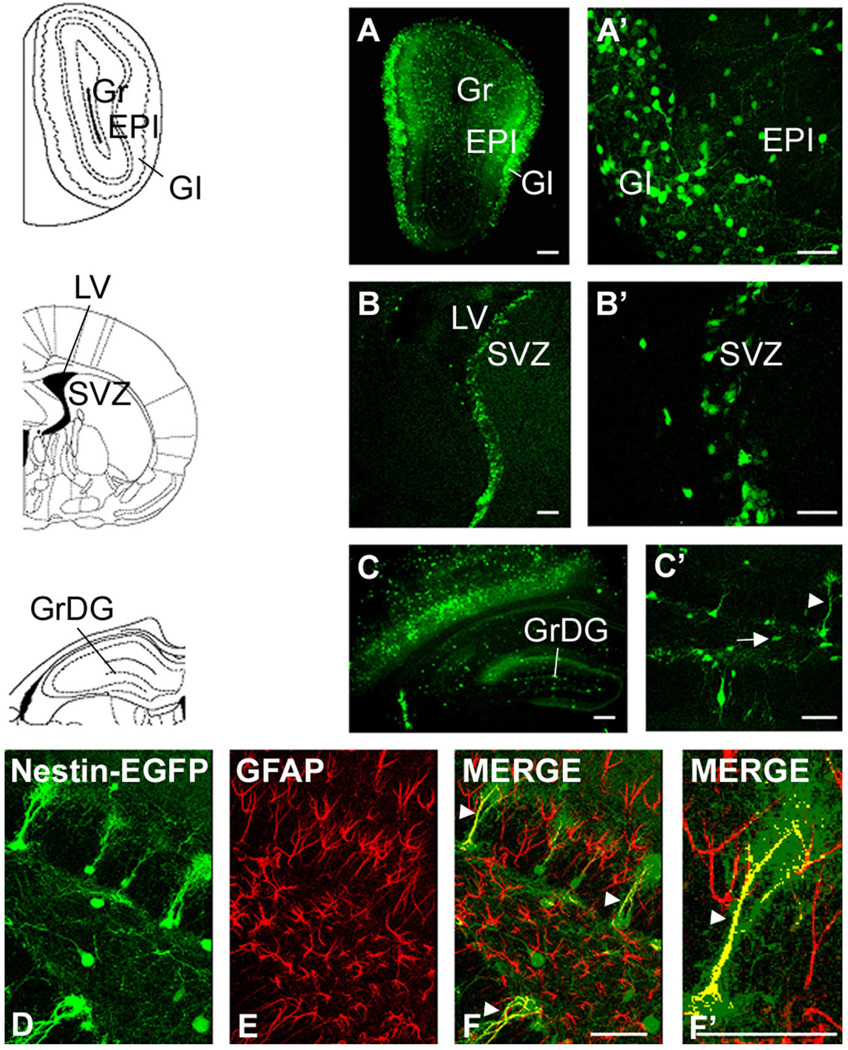
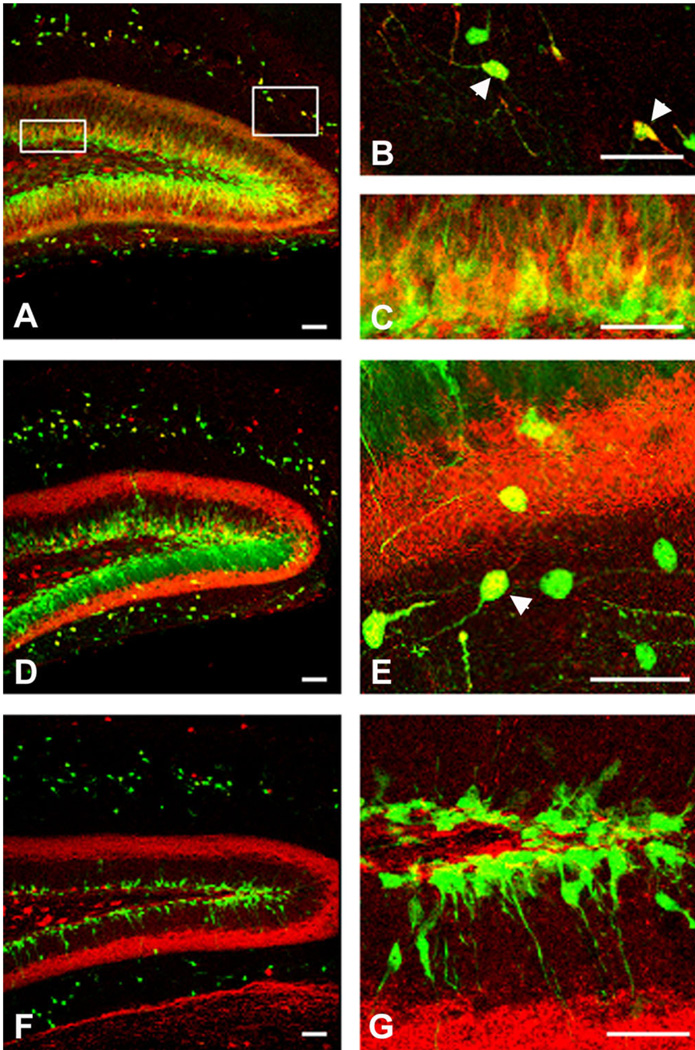
In order to examine the expression of nestin in the brain we used nestin-EGFP transgenic mice, in which the expression of EGFP is driven by the well-characterized enhancer element contained in the second intron of the rat nestin gene, which directs expression in CNS progenitor cells (Yaworsky and Kappen, 1999). Chemokine receptors were localized using FISH and nestin-expressing cells using immunohistochemistry for EGFP. Consistent with other publications we observed EGFP-expressing cells in all three regions of the brain we examined (Fig. 5) (Kronenberg et al., 2003; Mignone et al., 2004). In the DG, the EGFP-labeled cells were of two distinct types (Fig. 5C,C′) (Filippov et al., 2003; Fukuda et al., 2003). One type of cell resembled a radial-astrocyte with its cell body in the SGZ and a major extensively tufted process extending into the granule cell layer (type 1 cells, arrowhead in Fig. 5C′). As shown in Figure 5D–F′, these cells were also immunoreactive for GFAP (Garcia et al., 2004). The second type of cell also had its cell body in the SGZ and sometimes had one or two small processes running perpendicular to the granule cell layer (type 2 cells, arrow in Fig. 5C′). These cells did not stain for GFAP. In the SVZ, EGFP-expressing cells were observed in the subependymal zone around the lateral ventricle and in the dorsolateral extension of the SVZ (Fig. 5B,B′). In the OB some EGFP-labeled cells were observed in the core of the OB, but many EGFP-labeled cells were observed in the granule and periglomerular cells layers (Fig. 5A,A′). Cells expressing EGFP in these layers generally had the morphology of neurons, as previously reported with other lines of nestin-EGFP mice (Beech et al., 2004). When we performed colocalization studies using confocal microscopy, we found that CXCR4 was coexpressed with cells that expressed EGFP in all of the areas examined (Fig. 6). In the DG CXCR4 receptor expression was observed in EGFP-expressing cells with both morphologies (i.e., type 1 and 2 cells). Overall, the extent of CXCR4 expression was clearly greater than that of EGFP, suggesting that the receptor was also expressed in cells other than neural progenitor cells such as granule neurons and hilar interneurons (Banisadr et al., 2002a; Stumm et al., 2002). We shall discuss this further below. In the SVZ we observed colocalization of CXCR4 and nestin in numerous cells in all parts of the area, although here again the extent of CXCR4 expression was greater than that of EGFP. Similarly, in the OB CXCR4 was coexpressed with nestin in all parts but, as in the case of the DG, was also observed in cells that did not express nestin. We were interested to see whether some of the extensive chemokine receptor expression in the OB was neuronal. We demonstrated that there was robust expression of CXCR4 in the periglomerular layer (see also Stumm et al., 2002). Interneurons in this region are known to express a variety of neurotransmitters, including GABA and DA (Kosaka et al., 1998). In order to examine whether chemokine receptor expression occurred in interneurons we used GAD65-EGFP transgenic mice together with FISH for chemokine receptor expression. We observed numerous GAD65-expressing cells in the periglomerular layer, many of which also expressed CXCR4 receptors (Fig. 7). In addition, there were also other periglomerular cells that expressed CXCR4 but did not express GAD65. Thus, we conclude that CXCR4 is expressed by different subtypes of periglomerular interneurons including GAD65 expressing cells, as well as interneurons with other phenotypes.
Fig. 5.
Distribution pattern of EGFP-expressing cells in neurogenic regions of a 5-week-old nestin-EGFP transgenic mouse brain. EGFP is expressed under a neurally specific enhancer region of the nestin promoter (Yaworsky and Kappen, 1999). A,A’: Expression of nestin-EGFP transgene in the olfactory bulb (OB). Nestin-EGFP is expressed primarily in the rostral extent of the olfactory ventricle as well as in the granule (Gr) and periglomerular (GI) cell layers. B,B’: Expression of nestin-EGFP in the subventricular zone (SVZ). EGFP is expressed by ependymal cells and cells in the dorsal-lateral extension of the SVZ. C,C’: Expression of nestin-EGFP in the dentate gyrus (DG). EGFP is expressed in a class of cells (type 1, arrowhead in C’) whose cell bodies are localized in the subgranular cell layer and they extend their radial projections out toward the molecular layer as well as other cells, which extend one or two processes perpendicular to the granule cell layer (type 2 cells, arrow in C’). D–F: Nestin-EGFP type 1 cells (green) express the astrocytic marker GFAP (red) (arrowheads). F’: The colocalization of nestin-EGFP and GFAP at higher magnification. Scale bars = 100 µm in A–C; 50 µm in A’–C’,F,F’.
Fig. 6.
Colocalization of CXCR4 and EGFP in neurogenic regions of 5-week-old nestin-EGFP transgenic mouse brain. FISH was performed using a CXCR4 antisense probe in conjunction with immunostaining with a GFP antibody. A–C: In the olfactory bulb (OB), most of the EGFP-expressing cells also expressed CXCR4. D–F: In the subventricular zone (SVZ), virtually all the nestin-expressing cells coexpressed CXCR4. G–I: In the dentate gyrus (DG), both populations of nestin-expressing cells (types 1 and 2) expressed CXCR4. Insert in I shows higher magnification of CXCR4 and nestin-EGFP colocalization in the DG. Scale bars = 100 µm.
Fig. 7.
Colocalization of CXCR4 and GAD65 in the olfactory bulb (OB) of 5-week-old GAD65-EGFP transgenic mouse brain. FISH was performed using a CXCR4 antisense probe in conjunction with immunostaining with a GFP antibody. I: An xy-projection of a z-stack, GAD65 (E) in green and CXCR4 (J) in red, in cells of the periglomerular layer of the olfactory bulb. Colocalization of CXCR4 and GAD65 is highlighted by a rectangle and was confirmed by 3D reconstitution (A). The top and left panels display the reconstructed view in the z-dimension. Left panels (F–H) illustrate xz plane and top panels (B–D) show the yz plane. Both xz- and yz-reconstructions are also displayed in split channel mode to allow further assessment of the double labeling. In addition, there were also periglomerular cells that expressed CXCR4 but did not express GAD65. Scale bar = 100 µm.
We conducted a similar set of studies using the probe for the CCR2 receptor, which, as noted above, may also detect expression of CCR5 receptors. In general we observed colocalization of CCR2/CCR5 with nestin-EGFP-expressing cells in the different areas examined, but as with CXCR4, the extent of CCR2/CCR5 expression was wider than that of EGFP (Fig. 8A–I). As with CXCR4 in the DG, colocalization of CCR2/CCR5 with nestin-expressing cells of both morphologies was noted. We also examined CCR2/nestin colocalization using immunohistochemistry for CCR2. Here again, we determined that some cells in the SGZ expressed both nestin and CCR2 (Fig. 8J–M).
Fig. 8.
Colocalization of CCR2 and EGFP in neurogenic regions of 5-week-old nestin-EGFP transgenic mouse brain. A–I: FISH was performed using a CCR2 antisense probe in conjunction with immuno-staining with a GFP antibody. A–C: In the olfactory bulb (OB), most of the EGFP-expressing cells also expressed CCR2. D–F: In the subventricular zone (SVZ), virtually all the EGFP-expressing cells coexpressed CCR2. G–I: In the dentate gyrus (DG), EGFP-expressing cells expressed CCR2, CCR2 expression being more apparent in nonradial astrocyte-like cells. J–M: Immunohistochemistry was carried out on free-floating sections obtained from nestin-EGFP mouse brain using CCR2 antibody. In the DG, CCR2-immunoreactive cells (red) colocalized with type 2 nestin-EGFP cells (green). M: The CCR2 expression by nestin-EGFP cells at higher magnification (arrowhead). Scale bars = 100 µm in A–I; 50 µm in J–M.
In summary, we observed that both CXCR4 and CCR2/ CCR5 receptors were frequently coexpressed with nestin in all three areas of the brain examined, supporting the suggestion that these receptors were expressed by neural progenitor cells in these areas. However, both receptors were also clearly expressed by populations of cells in addition to neural progenitors.
TLX/chemokine receptor coexpression
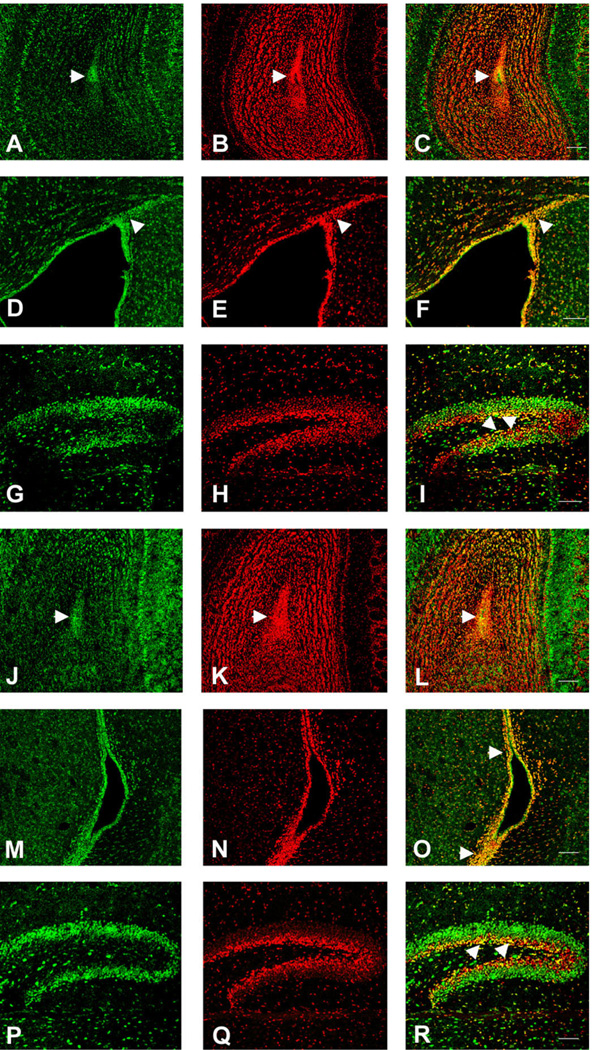
The TLX gene has been reported to be expressed in adult neural progenitor cells, where it plays a role in the maintenance of progenitor proliferation (Roy et al., 2004; Shi et al., 2004). In order to examine the expression of TLX we used TLX-lacZ transgenic mice in which we localized TLX by immunohistochemistry for lacZ (Shi et al., 2004). The expression pattern for TLX revealed it to be present in all three areas examined, with a distribution suggesting its expression in progenitor cells as well as other cell types (Fig. 9). In the DG TLX was expressed in the SGZ and granule cell layers, in the SVZ it was expressed in numerous cells surrounding the lateral ventricle. In the OB the most notable expression of TLX was in the core of the OB where neural progenitor cells are known to reside (Liu and Martin, 2003). When we performed colocalization studies for TLX together with FISH for CXCR4 (Fig. 9A–I) or CCR2/CCR5 (Fig. 9J–R) we clearly observed colocalization in populations of cells in all three areas. This was particularly the case in the SGZ, the SVZ, and the core of the OB. In addition, there were clearly cells in all three areas that expressed TLX without either chemokine receptor or each chemokine receptor in the absence of TLX.
Fig. 9.
Colocalization of CXCR4 or CCR2 with TLX expression in neurogenic regions of TLX-LacZ transgenic mouse brain (16 days). FISH was performed using CXCR4 or CCR2 antisense probes in conjunction with immunostaining with β-gal antibody. Both CXCR4 and CCR2/CCR5 expression colocalized with TLX in the olfactory ventricle (arrowheads in C and L, respectively) of the olfactory bulb (OB) (CXCR4: A–C, CCR2: J–L), in the subventricular zone (SVZ) (arrowheads in F and O) (CXCR4: D–F, CCR2: M–O), and in the subgranular zone (arrowheads in Iand R) of the dentate gyrus (DG) (CXCR4: G–I, CCR2: P–R). Scale bars = 100 µm.
Ki67/chemokine receptor expression
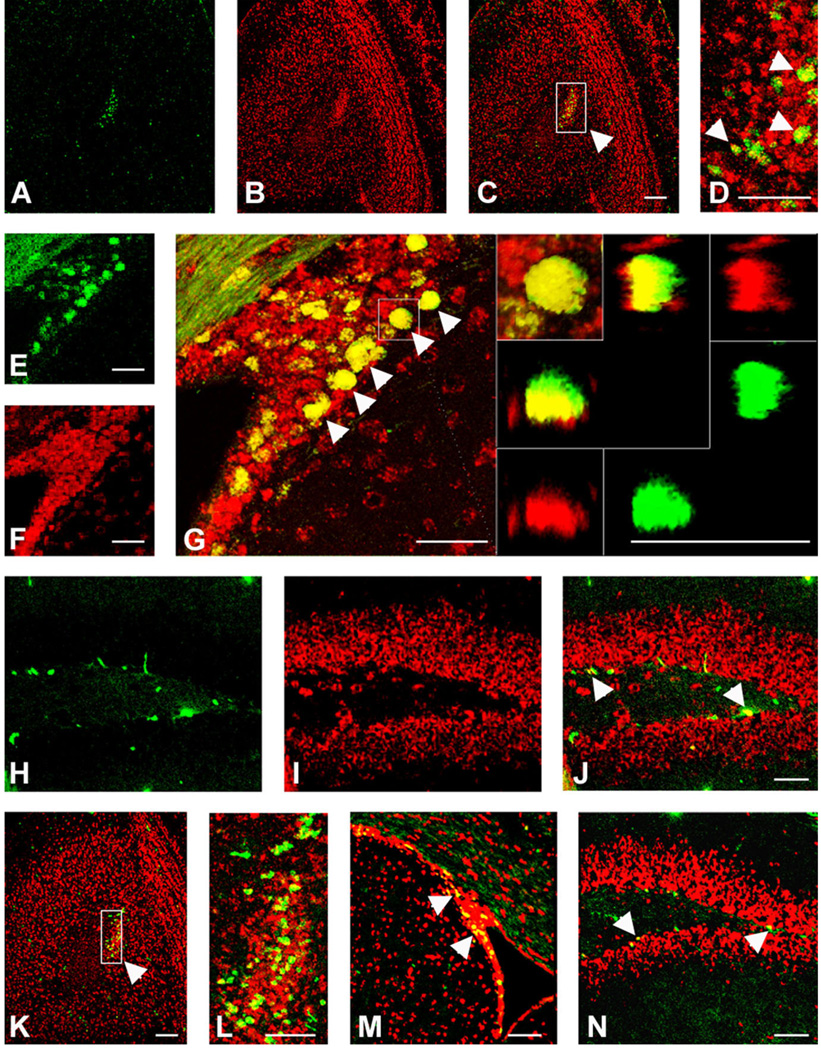
We used Ki67 to label dividing cells in the DG, SVZ, and OB. As anticipated, we observed populations of dividing cells in the SGZ at the interface between the granule cell layer and the hilus, along the border of the SVZ, and in the core of the OB. Ki67 is a protein that is expressed by dividing cells and it has been used to identify dividing progenitor cells in parts of the brain (Shi et al., 2004). Here again, we performed colocalization studies using immunohistochemistry for Ki67 together with FISH for CXCR4 (Fig. 10A–J) and CCR2/CCR5 (Fig. 10K–N) receptors and observed colocalization of both chemokine receptors with populations of Ki67-expressing cells in all three neurogenic regions of the brain.
Fig. 10.
Colocalization of CXCR4 (A–J) or CCR2 (K–N) expression with Ki67 in neurogenic regions of 5-week-old mouse brain. FISH was performed using CXCR4 or CCR2 antisense probes in conjunction with immunostaining with a Ki67 antibody. Both CXCR4 and CCR2/ CCR5 colocalized with Ki67 in the olfactory ventricle (arrowheads in picture obtained at higher magnification, D: CXCR4, L: CCR2) of the olfactory bulb (OB), in the subventricular zone (SVZ) (arrowheads in G: CXCR4 and M: CCR2), and in the subgranular zone (arrowheads in J: CXCR4 and N: CCR2) of the dentate gyrus (DG). The right panels inserted in G shows the colocalization of CXCR4 and Ki67 at higher magnification in the SVZ. The panel at the left corner shows a xy-projection of a z-stack, Ki67 in green and CXCR4 in red. Colocalization of CXCR4 and Ki67 was confirmed by 3D reconstitution. Left and bottom panels illustrate the reconstructed view in xz plane and top and right panels show the yz plane. Both xz- and yz-reconstructions are also displayed in split channel mode to allow further assessment of the double labeling. Scale bars = 100 µm.
CXCR4/SDF-1 expression patterns in the DG of EGFP BAC transgenic mice
In order to further confirm the expression of CXCR4 by neural progenitor cells in the hippocampus, and to further determine the pattern of CXCR4 expression, we also used CXCR4-EGFP BAC transgenic mice. Using these mice we first determined the expression pattern of CXCR4 in the SGZ/DG at different postnatal ages (Fig. 11). As in the studies using in situ hybridization, CXCR4-EGFP was expressed in the DG, especially at its innermost aspect. The strength of EGFP expression tended to decrease in the outward layers of the granule cell layer. Furthermore, the CXCR4-EGFP expression pattern became increasingly restricted with age. Interestingly, CXCR4-EGFP was also highly expressed in a population of cells that had the morphology and position of Cajal-Retzius cells, as previously noted in other studies (Stumm et al., 2003). The cells in the DG that expressed CXCR4 had diverse morphologies. Some of the cells resembled the two types of nestin-expressing cells discussed above. In addition, another type of cell had a long process that extended into the molecular layer. These cells had the morphology of immature granule neurons (Seri et al., 2004). In order to further define the phenotypes of CXCR4-expressing cells in the granule cell layer, we performed immunohistochemistry for nestin and other markers. As discussed above, some progenitor cells in the SGZ can be characterized by their expression of nestin and GFAP. We therefore examined whether any of the CXCR4-expressing cells also expressed these two markers. As shown in Figure 12, we found that some CXCR4-expressing cells also expressed GFAP. This was especially the case at earlier times, but even at 4 or more weeks of age cells with cell bodies in the SGZ that had radial-astrocyte like morphology extending long processes into the granule cell layer expressed both GFAP and CXCR4 (Fig. 12I–L). Similarly, at 2 weeks numerous cells that expressed nestin in their long radial-astrocyte like processes also expressed CXCR4 (Fig. 13D). By 4 weeks or more examples of this colocalization were less common but colocalization was still observed in several cases (Fig. 13I–L).
Fig. 11.
Heterogeneity of EGFP-expressing cells in the dentate gyrus of CXCR4-EGFP BAC transgenic mice during postnatal development. EGFP is expressed in the DG as well as in a population of cells with the position and morphology of Cajal-Retzius cells. At 1 (A) and 2 (B) weeks, CXCR4 is expressed throughout the entire DG, more numerous cells being observed in the inner layer of the granular layer as well as in the SGZ. During development, the concentration of EGFP-expressing cells decreased in the outermost parts of the granule cell layer. At 3–5 weeks (C–F), CXCR4-EGFP-expressing cells are more localized to the SGZ and internal aspects of the granule cell layer. F: The expression pattern of CXCR4-EGFP cells in Cajal- Retzius cells. At 6 weeks (G,H), CXCR4 is expressed mainly in immature granule cells. In addition, it is also expressed in some neural progenitors (type 2 cells, insert in H) and radial astrocyte-like cells (type 1 cells) localized in the SGZ and extending long processes into the granular cell layer, as shown at higher magnification in H. At 3 months (I), the expression of CXCR4 is more restricted. Scale bars = 200 µm in A,B; 100 µm in C,D; 50 µm in E,F,G,I; 20 µm in H.
Fig. 12.
Colocalization of GFAP and CXCR4-EGFP-expressing cells in the dentate gyrus. Immunohistochemistry was carried out on free-floating sections using a GFAP antibody. The overlap of CXCR4-EGFP cells (green) and Alexa 633-labeled GFAP cells (red) shows that at 2 (A–D), 3 (E–H), and 4 (I–L) weeks, some of the CXCR4-EGFP cells expressed the astrocytic marker GFAP (arrowheads in D,H,L). Coexpression was more frequent at earlier times but even at 4 weeks several cells in the SGZ that had radial-astrocyte-like morphology express both CXCR4 (green) and GFAP (red) (L). Arrows illustrate cells that express CXCR4 that do not colocalize with GFAP (L). Scale bars = 50 µm.
Fig. 13.
Colocalization of nestin and CXCR4-EGFP-expressing cells in the dentate gyrus. Immunohistochemistry was carried out on free-floating sections using a nestin antibody. At 2 weeks (A–D), numerous cells that expressed CXCR4 (green, A) also expressed nestin (red, B) in their long radial-astrocyte-like processes (arrowheads in D). The overlap of CXCR4-EGFP cells (green) and nestin-positive cells (red) shows that at 3 (E–H) and 4 (I–L) weeks, the coexpression decreased but is still observed in some cases (arrowheads in H,L). Scale bars = 50 µm.
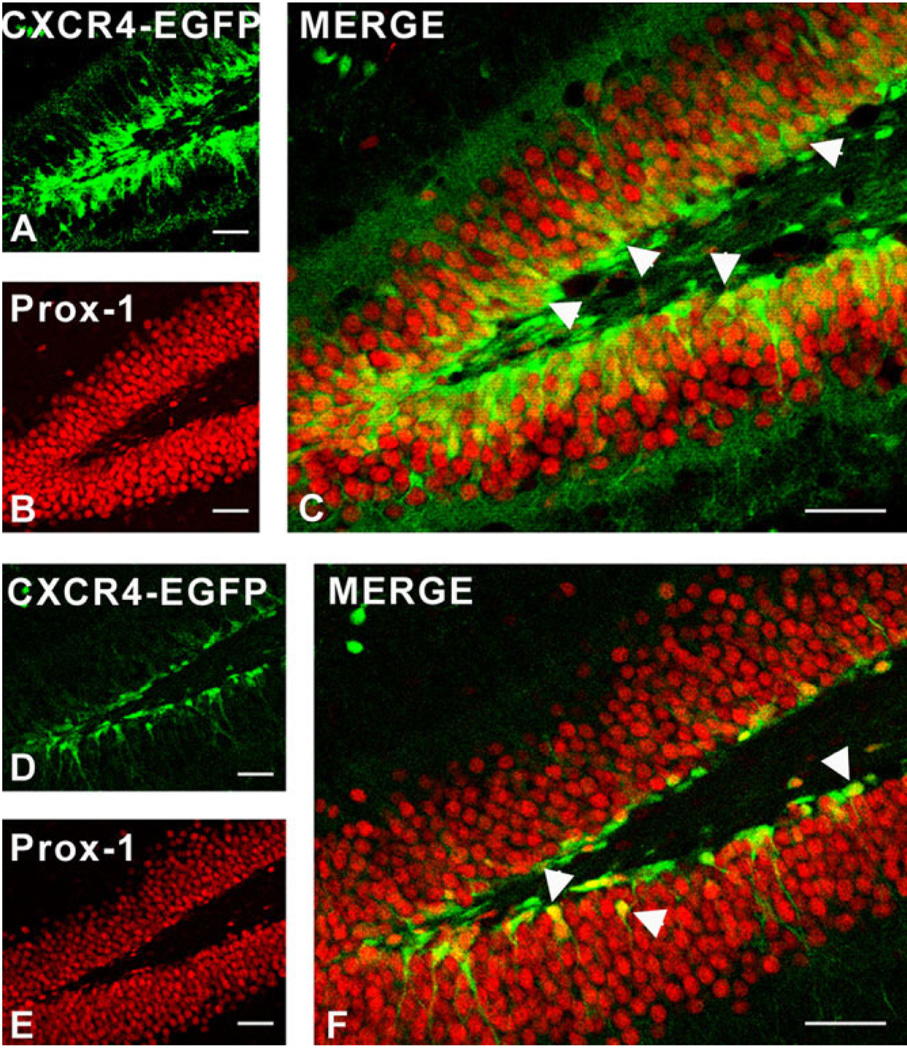
We also examined the time-dependent expression of CXCR4 with other markers that have been used to define the course of granule cell development in the DG. As demonstrated in Figure 14, there was clear colocalization of CXCR4-EGFP with Prox-1, a marker that is expressed early in granule cell development, and which continues to be expressed by these cells (Lu et al., 2002; Seri et al., 2004). At some stages there was also colocalization of CXCR4-EGFP with the Ca binding protein calretinin, which is transiently expressed by young granule cells during their development (Kempermann et al., 2004) (Fig. 15). Calretinin was also clearly expressed by the CXCR4-EGFP-expressing cells that are probably Cajal-Retzius cells (Fig. 15B,E), in keeping with the known expression of this Ca binding protein in this cell population (Berger and Alvarez, 1996). Interestingly, staining of granule cells for calbindin, which is expressed in mature granule neurons following the downregulation of calretinin expression (Kempermann et al., 2004), resulted in no colocalization with CXCR4, the two markers forming separate nonover-lapping bands of expression in the granule cell layer (Fig. 16). Doublecortin is a marker for newly formed migrating neuroblasts and has been localized to cells of this type in the DG (Shapiro et al., 2005). We also observed colocalization of CXCR4 and doublecortin with a population of cells with the morphology of neuroblasts (Fig. 16).
Fig. 14.
Colocalization of CXCR4 and the granule cell marker Prox-1 in the dentate gyrus (DG) of CXCR4-EGFP transgenic mice at different ages. Immunostaining was performed on free-floating sections using an antibody against Prox-1. At 2 weeks (A–C), many cells in the SGZ coexpressed CXCR4 (green, A) and Prox-1 (red, B) (arrowheads in C). At 4 weeks (D–F) a smaller number of CXCR4-EGFP cells also expressed Prox-1 (arrowheads in F). Scale bars = 50 µm.
Fig. 15.
Colocalization of CXCR4 and calretinin in the dentate gyrus (DG) of CXCR4-EGFP transgenic mice at different ages. Immunostaining was carried out on free-floating sections using an antibody against calretinin. At 2 (A–C) and 3 (D,E) weeks, some cells in the granule cell layer as well as many Cajal-Retzius cells (arrowheads in B,E) coexpressed CXCR4 (green) and calretinin (red). Coexpression was mainly observed in the cell bodies and, to a lesser extent, in proximal processes. At 4 weeks there was very little coexpression of CXCR4 and calretinin, as shown by the merged pictures (F,G). Scale bars = 50 µm.
Fig. 16.
Colocalization of CXCR4 and doublecortin (DCX) and lack of colocalization of CXCR4 and calbindin in the dentate gyrus of CXCR4-EGFP transgenic mice at different ages. Immunostaining was carried out on free-floating sections using calbindin and DCX antibodies. Calbindin expression was observed extensively in the granule cell layer, particularly its outer aspect (red), while CXCR4 (green) was expressed by cells in the subgranular zone and primarily in the interior part of the granule cell layer representing immature granule cells. Thus, as shown by the overlap, at 2 (A), 3 (D), and 4 (G) weeks, there was little coexpression of CXCR4 and calbindin at any stage. At 2 (B,C) and 3 (E,F) weeks, some cells resembling neuroblasts coexpressed CXCR4 (green) and DCX (red) (arrowheads in C,F), although at 3 weeks there were more cells that showed this colocalization. At 4 weeks (H,I), fewer cells showed colocalization between CXCR4 and DCX (arrowhead in I). Scale bars = 50 µm.
The question also arises as to the localization of the chemokine SDF-1/CXCL12, the ligand for the CXCR4 receptor in the postnatal DG. We therefore also examined the distribution of SDF-1 using SDF-1-EGFP BAC transgenic mice. Over the same period of time as we had observed CXCR4 expression in the DG, SDF-1-EGFP was also expressed by a population of cells mostly at the inner aspect of the DG (Fig. 17). Many of these cells exhibited the morphology of immature granule cells. Indeed, many were positive for PSA-NCAM or made close contacts with PSA-NCAM expressing cells. PSA-NCAM is known to be expressed by immature granule cells and granule cell progenitors (Kronenberg et al., 2003; Kempermann et al., 2004; Seri et al., 2004). Figure 18 shows that CXCR4-EGFP was similarly colocalized to a population of cells that expressed PSA-NCAM in the same general vicinity, suggesting that the two populations of cells may interact (Seki et al., 2003; Seri et al., 2004; Shapiro et al., 2005).
Fig. 17.
Colocalization of SDF-1 and PSA-NCAM in the dentate gyrus of SDF-1-EGFP transgenic mice at different ages. Immunostaining was carried out on free-floating sections with an antibody against PSA-NCAM. At 2 weeks (A–D), only a few SDF-1-EGFP cells (green) colocalized with the early neuronal marker PSA-NCAM (red) in the dentate gyrus (DG). C: The overlap of SDF-1-EGFP expression and PSA-NCAM labeling. Arrowheads in D show cells that coexpress SDF-1-EGFP and PSA-NCAM. At 3 (E–H) and 4 (I–L) weeks, numerous cells showed coexpression of SDF-1 and PSA-NCAM (arrowheads in H,L). As shown at higher magnification in inserts in H and L, SDF-1-EGFP cells expressed PSA-NCAM at the cell periphery. Scale bars = 50 µm.
Fig. 18.
Colocalization of CXCR4 and PSA-NCAM in the dentate gyrus (DG) of CXCR4-EGFP transgenic mice at different ages. Immunostaining was performed on free-floating sections using an antibody against PSA-NCAM. At 2 weeks (A–D), there was little colocalization of CXCR4 (green) and Alexa 633-labeled PSA-NCAM (red) as shown by the merged picture (C) and picture obtained at higher magnification (D). At 3 weeks (E–H), some cells in the subgranular zone (SGZ) show coexpression of CXCR4 and PSA-NCAM (arrowhead in H). Insert in H shows the cellular localization of CXCR4-EGFP and PSANCAM in cells of the DG. As shown in this picture obtained at higher magnification, PSA-NCAM is expressed at the periphery of CXCR4- EGFP-expressing cell. This coexpression increased over time; thus, at 4 weeks (I–L), more cells coexpressed CXCR4-EGFP and PSA-NCAM (arrowheads in L). As shown at higher magnification in the insert in L, CXCR4-EGFP cells expressed PSA-NCAM at the periphery of the cell. Scale bars = 50 µm.
The results we have obtained using cell culture (Tran et al., 2004) and in situ hybridization suggested that single neural progenitors express multiple chemokine receptors. We therefore also examined the expression of CCR2 receptors by CXCR4-expressing cells in the DG using immunohistochemistry and observed that there were cells with the morphology of neural progenitors that expressed both receptors (Fig. 19).
Fig. 19.
Colocalization of CXCR4 and CCR2 in the subgranular zone (SGZ) of the dentate gyrus. Immunohistochemistry was performed on free-floating sections of CXCR4-EGFP mouse brain using CCR2 antibody. CXCR4-EGFP (A) colocalized with CCR2 (B) in cells with the morphology of neural progenitor cells as shown by the overlap (C). Inserts in C show the colocalization of CXCR4 and CCR2 at higher magnification. Scale bars = 50 µm.
Chemoattractant effects of chemokines on neural progenitor cells
The data described above suggests that chemokines acting at different chemokine receptors should have functional effects on neural progenitor cells. We therefore examined the chemoattractant effects of several chemokines on neural progenitor cells cultured from postnatal subventricular zone and propagated in culture as neurospheres (Tran et al., 2004). We used the chemokines SDF-1/CXCL12 (CXCR4-selective), IP-10/CXCL10 (CXCR3-selective), RANTES/CCL5 (activates CCR5 and other CC receptors), and MCP-1/CCL2 and MCP-2/CCL8 (CCR2-selective). Each of these chemokines was significantly chemottractant for the neural progenitors (Fig. 20).
Fig. 20.
Chemoattractant effects of different chemokines on neural progenitor cells isolated from postnatal mouse brain. Chemotaxis assays were performed using Dunn chambers (see Materials and Methods). A: SDF-1, RANTES, MCP-1, MCP-2, and IP-10 exhibited time-dependent chemoattractant effects on postnatal progenitor cells. B: Graph shows the average responses to the chemokines tested (t-test, *P < 0.05, n = 7).
DISCUSSION
Recent studies from several different laboratories have demonstrated that chemokines (Bajetto et al., 2002; Banisadr et al., 2003; Cowell and Silverstein, 2003; Tran and Miller, 2003) and their receptors (Banisadr et al., 2002a,b;2005; Stumm et al., 2002; Chalasani et al., 2003; Cowell and Silverstein, 2003; Tran and Miller, 2003; Belmadani et al., 2005) exhibit a widespread and previously unappreciated expression pattern in the central and peripheral nervous systems. This suggests a number of roles for these molecules in the regulation of the nervous system beyond any role they may play in the organization of neuroinflammatory responses. Indeed, it is clear that the chemokine SDF-1 and its receptor CXCR4 play an important role in the development of the nervous system and other tissues. In the nervous system, SDF-1/CXCR4 signaling directs the migration of neural stem cells to a number of different parts of the brain (Zou et al., 1998; Bagri et al., 2002; Lu et al., 2002; Stumm et al., 2003) and the DRG (Belmadani et al., 2005) and also plays a role as an axonal guidance cue (Xiang et al., 2002; Arakawa et al., 2003; Chalasani et al., 2003; Lieberam et al., 2005; Pujol et al., 2005). It has also been demonstrated that neurospheres prepared from postnatal brains express CXCR4 as well as other chemokine receptors (Lazarini et al., 2000; Stumm et al., 2003; Ji et al., 2004; Peng et al., 2004; Tran et al., 2004) and that chemokines act as chemoattractants for these cells (Pluchino et al., 2005; Tran et al., 2004, 2005; Widera et al., 2004), suggesting that chemokine-mediated effects may also be important in the regulation of adult progenitor cell migration. However, it is not known whether chemokine receptors are normally expressed by neural progenitor cells in the postnatal brain. Thus, the origins of cells that constitute neurospheres are uncertain. Although neural stem cells have been shown to reside in neurogenic areas of the brain such as the DG (Seaberg and Van der Kooy, 2002), SVZ (Doetsch et al., 1999), and OB (Liu and Martin, 2003), it also appears that further cells with neurogenic potential exist in a dormant state in the parenchyma of the brain throughout the neuraxis (Palmer et al., 1999). Furthermore, the properties of neural stem/progenitor cells grown in cell culture may not be identical to those expressed in vivo (Gabay et al., 2003). Thus, it is not clear what the normal relationship might be between the expression of chemokine receptors by neurosphere cells and their expression pattern by neural progenitors in the brain.
The experiments reported here demonstrate that CXCR4 and other chemokine receptors are expressed in all three areas of the brain examined, consistent with previous studies in the literature (Banisadr et al., 2002a; Lu et al., 2002; Stumm et al., 2002). For example, CXCR4 is expressed in the DG, including the SGZ. Expression of CXCR4 is observed in granule cells to an extent that varies according to their age and time of development. Expression of CXCR4 by hilar neurons and Cajal-Retzius cells has been previously reported (Stumm et al., 2002, 2003) as has its expression in circumventricular regions and in the OB (Banisadr et al., 2002a; Stumm et al., 2002, 2003). It is clear from these studies and the results presented here that although CXCR4 is expressed by neural progenitors in all of these areas its expression is not restricted to these cells. Indeed, it is now clear that the SDF-1/CXCR4 system is widely expressed by neurons throughout the nervous system and possibly by glial elements as well (Banisadr et al., 2002a, 2003; Stumm et al., 2002; Tran and Miller, 2003; Dziembowska et al., 2005). The patterns of expression of the other chemokine receptors in the areas we examined are similar, consistent with previous findings that neural progenitor cells express numerous different chemokine receptor types (Peng et al., 2004; Tran et al., 2004; Pluchino et al., 2005). However, apart from CXCR4 there have been few large-scale anatomical studies on the distribution of chemokine receptors in the brain. In addition to CXCR4, there has been interest in the function of CCR5 in the nervous system owing to the fact that, like CXCR4, it acts as a conduit for the effects of HIV-1. Although it is clear that leukocytes infiltrating into the brain express CCR5, its expression pattern by neurons in nondisease states is unclear at this time (Cowell et al., 2002). One study has demonstrated the expression of CCR1 receptors by different cell types during the development of the cerebellum (Cowell and Silverstein, 2003). In the case of CCR2 receptors, recent data also appears to show the expression of the receptor by different populations of neurons and other cell types throughout the brain (Banisadr et al., 2002b, 2005). In particular, Van Gassen et al. (2005) demonstrated that cerebellar Purkinje neurons expressed both CCR2 and its ligand, the chemokine MCP-1. Additionally, a number of reports from the human neuropathology literature suggest the expression of a number of chemokine receptors by neurons throughout the human brain in the context of diseases such as Alzheimer’s, stroke, and HIV-1 infection (Bajetto et al., 2001, 2002; Kolson, 2002).
Our intention in this study was to demonstrate that neural progenitor cells were among the cell populations that expressed chemokine receptors in vivo. Several studies have defined the properties of neural stem cells in the DG, which continue to provide new granule cells throughout life (Kempermann et al., 2004; Emsley et al., 2005). These cells originate as radial astrocyte-like cells that give rise to progenitors in the SGZ through a process of asymmetric division. These dividing progenitors proceed through diverse morphological stages of development while “migrating” the short distance from the SGZ into the granule cell layer of the DG (Kempermann et al., 2004; Li and Pleasure, 2005). The next stage of progenitor cell development appears to be a cell type that is also labeled in nestin transgenic mice and that can divide more rapidly prior to differentiating into immature granule cells (D cell or “transiently amplifying cell”). The diverse morphologies of nestin-EGFP-expressing cells in the DG in our experiments and those reported elsewhere are consistent with this view, and our results suggest that CXCR4 and CCR2/CCR5 receptor expression is not specifically associated with any well-defined stage of early DG development. CXCR4 seems to be expressed from very early times in radial astrocyte-like cells up until the point that immature granule cells are formed. This is indicated both by the colocalization observed in the in situ hybridization studies and also from the characteristics of the different types of CXCR4-expressing cells observed in CXCR4-EGFP mice. In the latter case we observed colocalization of CXCR4 expression with nestin and GFAP in cells with radial astrocyte morphology. These observations are consistent with the view that CXCR4 expression begins with radial astrocyte cells, the earliest cell type defined in neurogenesis in the mature DG. CXCR4 seems also to be expressed by the “transiently amplifying” cell type that seems to be derived from the radial astrocytes. Subsequently, the populations of CXCR4-EGFP-expressing cells in the DG correspond to Prox-1, PSA-NCAM, and calretinin, but not calbindin-expressing cells. Thus, it seems that CXCR4 expression is downregulated to much lower levels prior to the ultimate maturation of granule cells, the stage at which calbindin expression is apparent (Kempermann et al., 2004). SDF-1, the unique ligand for CXCR4 receptors, is expressed by granule cells postnatally. These cells appear to include the population of young PSA-NCAM-positive granule neurons. Several previous studies have commented on the association of these PSA-NCAM-positive cells with neural progenitor cells and other young granule neurons in the SGZ/DG (Seki, 2002a,b, 2003).Given the chemoattractant role that SDF-1 plays for CXCR4-expressing progenitors during DG development (Bagri et al., 2002; Lu et al., 2002), and its additional role as an axonal guidance cue (Xiang et al., 2002; Arakawa et al., 2003; Chalasani et al., 2003; Pujol et al., 2005), it seems reasonable to suggest that release of SDF-1 from young granule neurons may be involved in guiding the migration and development of CXCR4-expressing progenitors and immature granule cells as they move from the SGZ into the granule cell layer proper.
With respect to the SVZ and OB, we can similarly conclude that CXCR4 receptors are expressed by populations of neural progenitors in these areas. The localization of neural progenitors in the SVZ is also well established and, as in the DG, the pathway by which these cells subsequently develop has been defined in detail (Alvarez-Buylla and Lim, 2004; Merkle et al., 2004). Proliferating progenitors in this area give rise to migrating neuroblasts which, in rodents at any rate, move along the RMS to the OB, where they develop into granule and periglomerular neurons (Luskin, 1998; Sanai et al., 2004). The pattern of chemokine receptor expression in the SVZ suggests that, as in the DG, neural progenitors may express chemokine receptors at several points in their development. The hypothesis that some chemokine receptor-expressing cells in the SVZ are neural progenitors is again supported by the fact that some of these cells express nestin, TLX, and Ki67. Interestingly, in the OB, although it is known that cells in the rostral extent of the RMS in the core of the OB express nestin (Liu and Martin, 2003), it has been observed that only relatively few EGFP-expressing cells are observed there in nestin-EGFP transgenic mice (Beech et al., 2004). This has also been our experience and may be due to the control elements regulating tissue-specific nestin expression used to make these mice. However, there are clearly cells expressing TLX and Ki67 in this region that also express CXCR4 receptors. Furthermore, it has also been demonstrated that in nestin-EGFP transgenic mice levels of EGFP are also high in granule and periglomerular cells, which represent the progeny of neural progenitor cells migrating into the OB along the RMS (Beech et al., 2004). It is clear that many of these cells also express CXCR4 receptors (Stumm et al., 2002). As we also demonstrate, the population of chemokine receptor-expressing cells in the periglomerular layer includes GABAergic neurons, although neurons with other phenotypes seem to be labeled as well (Mugnaini and Oertel, 1985). Our data also indicate that neural progenitor cells in the SVZ/OB express chemokine receptors in addition to CXCR4. First, the expression patterns of many of these receptors in the areas examined are similar. In the case of the CCR2 receptor, which we studied further, both in situ and immunohistochemical data indicate that these receptors are expressed by progenitor cells. As with CXCR4, we observed colocalization of CCR2 with the same progenitor cell markers, nestin, Ki67, and TLX. Furthermore, we also observed that CCR2 receptors were expressed in the same cells as CXCR4 when we examined this question using CXCR4-EGFP mice. In summary, we can say that the expression patterns for different chemokine receptors support the possibility that they are expressed by neural progenitor cells and that the same progenitor cell may express more than one receptor type.
The question arises as to the purpose of chemokine expression by neural progenitors in the postnatal brain. We suggest that there may be two functions. In the case of CXCR4 receptors, it is possible that they play a similar role to that demonstrated during development where they regulate the migration of progenitors during normal adult neurogenesis. The close juxtaposition of SDF-1 and CXCR4 expression in the DG suggests this possibility. Thus, SDF-1/CXCR4 signaling may be important in the early phases of DG neurogenesis in the mature brain, recapitulating its role in the embryo. Much less is known concerning a possible role for CXCR4 or other chemokine receptors in normal neurogenesis in the OB. Although SVZ progenitors seem to express CXCR4 receptors and SDF-1 is expressed in the OB (Stumm et al., 2002; Banisadr and Miller, unpubl. obs.), it is not known whether the chemoattractant effects of SDF-1 are necessary for the migration of progenitors in the RMS. Indeed, the issue as to whether the OB utilizes a diffusible chemoattractant of this type or whether migration in the RMS is all of the “chain migration” type is not settled (Lois et al., 1996; Liu and Rao, 2003; Ng et al., 2005).
It is also possible that neural progenitors may need to respond to chemokines under pathological circumstances. In particular, it is quite possible that chemokines are involved in guiding neural progenitors to sites of brain damage. Such directed migration of progenitors has been a commonly observed phenomenon. It has been shown that endogenous or transplanted progenitors will migrate toward focal or diffuse areas of brain damage or demyelination, although the mechanisms responsible for this directed migration are unknown (Fricker et al., 1999; Aboody et al., 2000; Arvidsson et al., 2002; Ben-Hur et al., 2003; Parent, 2003; Pluchino et al., 2003, 2005; Zhang et al., 2003, 2004a,b; Glass et al., 2005). As chemokine synthesis is greatly upregulated in these areas, it seems reasonable to speculate that chemokines regulate the observed progenitor cell migration. Indeed, it is well established that neuroinflammatory responses that accompany brain infection, trauma, demyelination, or neurodegeneration involve the activation of astrocytes and microglia and the synthesis of many cytokines and chemokines (Huang et al., 2000; Cartier et al., 2005). Indeed, activated microglia have been shown to secrete factors that are attractive to neural progenitor cells (Aarum et al., 2003), although the identity of these factors has not been established. Furthermore, three recent studies have demonstrated that neural progenitors that express CXCR4 receptors migrate toward localized brain lesions and that interference with CXCR4 signaling inhibits this response (Imitola et al., 2004; Kelly et al., 2004; Robin et al., 2005). Thus, just as in the regulation of neurogenesis, SDF-1 may act as a chemoattractant in these cases. In general, however, SDF-1 is not the chemokine that exhibits the greatest degree of upregulation under these circumstances. More frequently, chemokines such as MCP-1, IP-10, RANTES, and MIP-1α are associated with neuroinflammatory responses (Huang et al., 2000; Ambrosini and Aloisi, 2004; Cartier et al., 2005). Thus, there are numerous studies demonstrating that the neuroinflammatory response of the brain involves upregulation of MCP-1 in particular, with most of the chemokine synthesis coming from activated microglia and astrocytes (Mahad and Ransohoff, 2003). MCP-1 and other upregulated chemokines activate CCR2, CXCR3, and CCR1/CCR5 receptors, which we have shown, are normally expressed by neural progenitor cells. We and others have demonstrated that these chemokines, in addition to SDF-1, can act as chemoattractants for neural progenitors using cell culture assays (Tran et al., 2004; Widera et al., 2004; Pluchino et al., 2005). Furthermore, upregulation of MCP-1 synthesis has been shown to be important for the trafficking of leukocytes into the brain as part of the neuroinflammatory response (Babcock et al., 2003).
Thus, it is likely that chemokines such as MCP-1 that are upregulated in the compromised brain can act to guide neural progenitors to sites of injury for attempted repair purposes. Indeed, the expression of multiple types of chemokine receptors by neural progenitors will provide them with the flexibility to respond to numerous chemokines whose synthesis may be differentially regulated under different circumstances. It is interesting that the overall effect of the neuroinflammatory milieu on neurogenesis in the brain may be multifaceted. Chemokines may attract neural progenitor cells to these areas where they may develop into progeny that are appropriate for repair purposes. Thus, the migration and development of neural progenitors into neurons in models of stroke (Kokaia and Lindvall, 2003; Zhang et al., 2003, 2004a,b) and into oligodendrocytes in models of demyelination (Ben-Hur et al., 2003; Pluchino et al., 2003, 2005) have been demonstrated in several studies. On the other hand, many progenitor cells that migrate in the diseased brain fail to differentiate into the appropriate progeny owing to the production of cytokines such as IL-6 at neuroinflammatory sites that direct progenitors to become astrocytes, or other factors that lead to progenitor cell death (Ekdahl et al., 2003; Monje et al., 2003). Clearly, therefore, the interface between neurogenesis and neuroinflammation in the brain is a highly complex one.
In summary, the present studies demonstrate that chemokine receptors are expressed in the brain by cells with the characteristics of neural progenitor cells. This receptor expression may allow these cells to participate in chemokine-directed migration in the context of normal neurogenesis and also in the context of brain repair.
ACKNOWLEDGMENTS
We thank Drs. Ruth Yu and Ron Evans (Salk Inst.), Dr. Enrico Mugnaini (Northwestern Univ.), Dr. Mary E. Hatten (Rockefeller Univ.), and Dr. William Karpus (Northwestern Univ.) for the gift of transgenic mice. We also thank Dr. Stephane Melik Parsadaniantz (INSERM U732, France) for the gift of CCR2 antibody and Christopher Mauer for help with confocal microscopy. We thank Hosung Jung for help with the preparation of the probes for in situ hybridization studies and for preparation of the first figure.
Grant sponsor: National Institutes of Health (NIH); Grant numbers: R01NS043095, R01DA013141, and R37MH040165.
LITERATURE CITED
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–336. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Ambrosini E, Aloisi F. Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res. 2004;29:1017–1038. doi: 10.1023/b:nere.0000021246.96864.89. [DOI] [PubMed] [Google Scholar]
- Arakawa Y, Bito H, Furuyashiki T, Tsuji T, Takemoto-Kimura S, Kimura K, Nozaki K, Hashimoto N, Narumiya S. Control of axon elongation via an SDF-1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J Cell Biol. 2003;161:381–391. doi: 10.1083/jcb.200210149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Babcock AA, Kuziel WA, Rivest S, Owens Y. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001;22:147–184. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Schettini G. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. J Neurochem. 2002;82:1311–1329. doi: 10.1046/j.1471-4159.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Parsadaniantz M. Neuroanatomical distribution of CXCR4 in adult brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002ab;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Queraud-Lesaux F, Boutterin MC, Pelaprat D, Zalc B, Rostene W, Haour F, Parsadaniantz SM. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem. 2002b;81:257–269. doi: 10.1046/j.1471-4159.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Skrzydelski D, Kitabgi P, Rostene W, Parsadaniantz SM. Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur J Neurosci. 2003;18:1593–1606. doi: 10.1046/j.1460-9568.2003.02893.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Kitabgi P, Rostene W, Parsadaniantz SM. Neuroanatomical localization of the CCR2 chemokine receptor and its colocalization with neurotransmitters. J Comp Neurol. 2005;492:178–192. doi: 10.1002/cne.20729. [DOI] [PubMed] [Google Scholar]
- Beech RD, Cleary MA, Treloar HB, Eisch AJ, Harrist AV, Zhong W, Greer CA, Duman RS, Picciotto MR. Nestin promoter/enhancer directs transgene expression to precursors of adult generated periglomerular neurons. J Comp Neurol. 2004;475:128–141. doi: 10.1002/cne.20179. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ. The chemokine stromal derived factor 1 regulates the migration of sensory neuron progenitors. J Neurosci. 2005;25:3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur T, Einstein O, Mizrachi-Kol R, Ben-Menachem O, Reinhartz E, Karussis D, Abramsky O. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephomyelitis. Glia. 2003;41:73–80. doi: 10.1002/glia.10159. [DOI] [PubMed] [Google Scholar]
- Berger B, Alvarez C. Neurochemical development of the hippocampal region in the fetal rhesus monkey, III. Calbindin-D28K, calretinin and parvalbumin with special mention of Cajal-Retzius cells and the retrosplenial cortex. J Comp Neurol. 1996;366:674–699. doi: 10.1002/(SICI)1096-9861(19960318)366:4<674::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Cacquevel M, Lebeurrier N, Cheenne S, Vivien D. Cytokines in neuroinflammation and Alzheimer’s disease. Curr Drug Targets. 2004;5:529–534. doi: 10.2174/1389450043345308. [DOI] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Sabelko KA, Sunshine MJ, Littman DR, Raper JA. A chemokine, SDF-1, reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding. J Neurosci. 2003;23:1360–1371. doi: 10.1523/JNEUROSCI.23-04-01360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell RM, Silverstein FS. Developmental changes in the expression of chemokine receptor CCR1 in the rat cerebellum. J Comp Neurol. 2003;457:7–23. doi: 10.1002/cne.10554. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Xu H, Galasso JM, Silverstein FS. Hypoxic-ischemic injury induces macrophage inflammatory protein-1 alpha expression in immature rat brain. Stroke. 2002;33:795–801. doi: 10.1161/hs0302.103740. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Dziembowska M, Tham TN, Lau P, Vitry S, Lazarini F, Dubois-Dalcq M. A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia. 2005;50:258–269. doi: 10.1002/glia.20170. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Fricker RA, Carpenter MK, Winkler C, Greco C, Gates MA, Bjorklund A. Site-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J Neurosci. 1999;19:5990–6005. doi: 10.1523/JNEUROSCI.19-14-05990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003;23:9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay L, Lowell S, Rubin LL, Anderson DJ. Deregulation of dorsoventral patterning by FGF confers trilineage differentiation capacity on CNS stem cells in vitro. Neuron. 2003;40:485–499. doi: 10.1016/s0896-6273(03)00637-8. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Glass R, Synowitz M, Kronenberg G, Walzlein JH, Markovic DS, Wang LP, Gast D, Kiwit J, Kempermann G, Kettenmann H. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J Neurosci. 2005;25:2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- Huang D, Han Y, Rani MR, Glabinski A, Trebst C, Sorensen T, Tani M, Wang J, Chien P, O’Bryan S, Bielecki B, Zhou ZL, Majumder S, Ransohoff RM. Chemokines and chemokine receptors in inflammation of the nervous system: manifold roles and exquisite regulation. Immunol Rev. 2000;177:52–67. doi: 10.1034/j.1600-065x.2000.17709.x. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JF, He BP, Dheen ST, Tay SS. Expression of chemokine receptors CXCR4, CCR2, CCR5 and CX3CR1 in neural progenitor cells isolated from the subventricular zone of the adult rat brain. Neurosci Lett. 2004;355:236–240. doi: 10.1016/j.neulet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA. Enhanced neurogenesis in Alzheimers disease transgenic mice. Proc Nat Acad Sci U S A. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata K, Ujikawa M, Egawa T, Kawamoto H, Tachibana K, Lizasa H, Katsura Y, Kishimoto T, Nagasawa T. A cell autonomous requirement for CXCR4 in long term lymphoid and myeloid reconstitution. Proc Natl Acad Sci U S A. 1999;96:5663–5667. doi: 10.1073/pnas.96.10.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003;13:127–132. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- Kolson DL. Neuropathogenesis of central nervous system HIV-1 infection. Clin Lab Med. 2002;22:703–717. doi: 10.1016/s0272-2712(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Toida K, Aika Y, Kosaka T. How simple is the organization of the olfactory glomerulus? The heterogeneity of so-called periglomerular cells. Neurosci Res. 1998;30:101–110. doi: 10.1016/s0168-0102(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells. 2004;22:109–118. doi: 10.1634/stemcells.22-1-109. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Casanova P, Tham TN, De Clercq E, Arenzana-Seisdedos F, Baleux F, Dubois-Dalcq M. Differential signalling of the chemokine receptor CXCR4 by stromal cell-derived factor 1 and the HIV glycoprotein in rat neurons and astrocytes. Eur J Neurosci. 2000;12:117–125. doi: 10.1046/j.1460-9568.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lieberam I, Agalliu D, Nagasawa T, Ericson J, Jessell TM. A CXCL12-CXCR4 signaling pathway defines the initial trajectory of mammalian motor axons. Neuron. 2005;47:667–679. doi: 10.1016/j.neuron.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Li G, Pleasure SJ. Morphogenesis of the dentate gyrus: what we are learning from mouse mutants. Dev Neurosci. 2005;27:93–99. doi: 10.1159/000085980. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders—how to make it work. Nat Med Suppl. 2004:S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- Liu Z, Martin LJ. Olfactory bulb core is a rich source of neural progenitor and stem cells in adult rodent and human. J Comp Neurol. 2003;459:368–391. doi: 10.1002/cne.10664. [DOI] [PubMed] [Google Scholar]
- Liu G, Rao Y. Neuronal migration from the forebrain to the olfactory bulb requires a new attractant persistent in the olfactory bulb. J Neurosci. 2003;23:6651–6659. doi: 10.1523/JNEUROSCI.23-16-06651.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A, Lemasson M. Inhibitory interneurons in the olfactory bulb: from development to function. Neuroscientist. 2004;10:292–303. doi: 10.1177/1073858404263460. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB. Neuroblasts of the postnatal mammalian forebrain: their phenotype and fate. J Neurobiol. 1998;36:221–233. [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Takashima N, Arai Y, Nomura T, Inokuchi K, Yuasa S, Osumi N. Pax6 is required for production and maintenance of progenitor cells in postnatal hippocampal neurogenesis. Genes Cells. 2005;10:1001–1014. doi: 10.1111/j.1365-2443.2005.00893.x. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213:442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;10:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Emsley JG, Magavi SS, Arlotta P, Macklis JD. Constitutive and induced neurogenesis in the adult mammalian brain: manipulation of endogenous precursors toward CNS repair. Dev Neurosci. 2004;26:101–117. doi: 10.1159/000082131. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Bjorklund A, Hokfelt T, editors. Handbook of chemical neuroanatomy. vol. 4. GABA and neuropeptides in the CNS. Amsterdam: Elsevier; 1985. [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B cell lymphopoiesis and bone marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Navarro-Quiroga I, Hernandez-Valdes M, Lin SL, Naegele JR. Postnatal cellular contributions of the hippocampus subventricular zone to the dentate gyrus, corpus callosum, fimbria, and cerebral cortex. J Comp Neurol. 2006;497:833–845. doi: 10.1002/cne.21037. [DOI] [PubMed] [Google Scholar]
- Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;30:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- Ni HT, Hu S, Sheng WS, Olson JM, Cheeran MC, Chan AS, Lokensgard JR, Peterson PK. High-level expression of functional chemokine receptor CXCR4 on human neural precursor cells. Brain Res Dev Brain Res. 2004;152:159–169. doi: 10.1016/j.devbrainres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Okada A, Lansford R, Weimann JM, Fraser SE, McConnell SK. Imaging cells in the developing nervous system with retrovirus expressing modified green fluorescent protein. Exp Neurol. 1999;156:394–406. doi: 10.1006/exnr.1999.7033. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Peng H, Huang Y, Rose J, Erichsen D, Herek S, Fujii N, Tamamura H, Zheng J. Stromal cell-derived factor 1-mediated CXCR4 signaling in rat and human cortical neural progenitor cells. J Neurosci Res. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- Peretto P, Merighi A, Fasolo A, Bonfanti L. Glial tubes in the rostral migratory stream of the adult rat. Brain Res Bull. 1997;42:9–21. doi: 10.1016/s0361-9230(96)00116-5. [DOI] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Baron-Van Evercooren A. Experimental autoimmune encephomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A. 2002;89:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalani A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Pujol F, Kitabgi P, Boudin H. The chemokine SDF-1 differentially regulates axonal regulation and branching in hippocampal neurons. J Cell Sci. 2005;118:1071–1080. doi: 10.1242/jcs.01694. [DOI] [PubMed] [Google Scholar]
- Rallu M, Corbin JG, Fishell G. Parsing the prosencephalon. Nat Rev Neurosci. 2002;3:943–951. doi: 10.1038/nrn989. [DOI] [PubMed] [Google Scholar]
- Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Roy K, Kuznicki K, Wu Q, Sun Z, Bock D, Schutz G, Vranich N, Monaghan AP. The Tlx gene regulates the timing of neurogenesis in the cortex. J Neurosci. 2004;24:8333–8345. doi: 10.1523/JNEUROSCI.1148-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T. Hippocampal adult neurogenesis occurs in a microenvironment provided by PSA-NCAM-expressing immature neurons. J Neurosci Res. 2002a;69:772–783. doi: 10.1002/jnr.10366. [DOI] [PubMed] [Google Scholar]
- Seki T. Expression patterns of immature neuronal markers PSANCAM, CRMP-4 and NeuroD in the hippocampus of young adult and aged rodents. J Neurosci Res. 2002b;70:327–334. doi: 10.1002/jnr.10387. [DOI] [PubMed] [Google Scholar]
- Seki T. Microenvironmental elements supporting adult hippocampal neurogenesis. Anat Sci Int. 2003;78:69–78. doi: 10.1046/j.0022-7722.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7135–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Korn MJ, Shan Z, Ribak CE. GFAP-expressing radial glia-like cell bodies are involved in a one-to-one relationship with doublecortin-immunolabeled newborn neurons in the adult dentate gyrus. Brain Res. 2005;1040:81–91. doi: 10.1016/j.brainres.2005.01.098. [DOI] [PubMed] [Google Scholar]
- Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Lizasa H, Yoshida H, Kawabata K, Katoka Y, Kitamura Y, Matushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69:745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- Tissir F, Wang CE, Goffinet AM. Expression of the chemokine receptor Cxcr4 mRNA during mouse brain development. Brain Res Dev Brain Res. 2004;149:63–71. doi: 10.1016/j.devbrainres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors; signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are widely expressed by embryonic and adult neural progenitor cells. J Neurosci Res. 2004:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Miller RJ. The HIV-1 coat protein gp120 regulates CXCR4-mediated signaling in neural progenitor cells. J Neuroimmunol. 2005;160:68–76. doi: 10.1016/j.jneuroim.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Frost E, To V, Robinson S, Ffrench-Constant C, Geertman R, Ransohoff RM, Miller RH. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration. Cell. 2002;110:373–383. doi: 10.1016/s0092-8674(02)00838-3. [DOI] [PubMed] [Google Scholar]
- van Gassen KL, Netzeband JG, de Graan PN, Gruol DL. The chemokine CCL2 modulates Ca2+ dynamics and electrophysiological properties of cultured cerebellar Purkinje neurons. Eur J Neurosci. 2005;21:2949–2957. doi: 10.1111/j.1460-9568.2005.04113.x. [DOI] [PubMed] [Google Scholar]
- Widera D, Holtkamp W, Entschladen F, Niggemann B, Zanker K, Kaltschmidt B, Kaltschmidt C. MCP-1 induces migration of adult neural stem cells. Eur J Cell Biol. 2004;83:381–387. doi: 10.1078/0171-9335-00403. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Li Y, Zhang Z, Cui K, Wang S, Yuan XB, Wu CP, Poo MM, Duan S. Nerve growth cone guidance mediated by G protein coupled receptors. Nat Neurosci. 2002;5:843–848. doi: 10.1038/nn899. [DOI] [PubMed] [Google Scholar]
- Yaworsky PJ, Kappen C. Heterogeneity of neural progenitor cells revealed by enhancers in the nestin gene. Dev Biol. 1999;205:309–321. doi: 10.1006/dbio.1998.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang L, Zhang ZG, Morris D, Jiang Q, Wang L, Zhang LJ, Chopp M. Migration and differentiation of adult rat subventricular zone progenitor cells transplanted into the adult rat striatum. Neuroscience. 2003;116:373–382. doi: 10.1016/s0306-4522(02)00696-6. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004a;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Zhang C, Zhang L, Robin A, Wang Y, Lu M, Chopp M. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J Neurosci. 2004b;24:5810–5815. doi: 10.1523/JNEUROSCI.1109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicha D, Dunn GA, Jones GE. A new direct-viewing chemotaxis chamber. J Cell Sci. 1991;99:769–775. doi: 10.1242/jcs.99.4.769. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]