Abstract
Neurons are metabolically active cells with high energy demands at locations distant from the cell body. As a result, these cells are particularly dependent on mitochondrial function, as reflected by the observation that diseases of mitochondrial dysfunction often have a neurodegenerative component. Recent discoveries have highlighted that neurons are reliant particularly on the dynamic properties of mitochondria. Mitochondria are dynamic organelles by several criteria. They engage in repeated cycles of fusion and fission, which serve to intermix the lipids and contents of a population of mitochondria. In addition, mitochondria are actively recruited to subcellular sites, such as the axonal and dendritic processes of neurons. Finally, the quality of a mitochondrial population is maintained through mitophagy, a form of autophagy in which defective mitochondria are selectively degraded. We review the general features of mitochondrial dynamics, incorporating recent findings on mitochondrial fusion, fission, transport and mitophagy. Defects in these key features are associated with neurodegenerative disease. Charcot-Marie-Tooth type 2A, a peripheral neuropathy, and dominant optic atrophy, an inherited optic neuropathy, result from a primary deficiency of mitochondrial fusion. Moreover, several major neurodegenerative diseases—including Parkinson's, Alzheimer's and Huntington's disease—involve disruption of mitochondrial dynamics. Remarkably, in several disease models, the manipulation of mitochondrial fusion or fission can partially rescue disease phenotypes. We review how mitochondrial dynamics is altered in these neurodegenerative diseases and discuss the reciprocal interactions between mitochondrial fusion, fission, transport and mitophagy.
INTRODUCTION
In the past decade, our view of mitochondrial dynamics has expanded from a curious phenomenon into an integral cell biological process influencing many cellular functions and ultimately survival (1,2). Once thought to be solitary and rigidly structured, mitochondria are now appreciated to constitute a population of organelles that migrate throughout the cell, fuse and divide, and undergo regulated turnover. These dynamic processes regulate mitochondrial function by enabling mitochondrial recruitment to critical subcellular compartments, content exchange between mitochondria, mitochondrial shape control, mitochondrial communication with the cytosol and mitochondrial quality control. As a result, mitochondria can readily adapt to changes in cellular requirements, whether due to physiological or environmental imperatives. When mitochondrial dynamics is disrupted, cellular dysfunction ensues. Here we discuss the human diseases associated with abnormalities in mitochondrial dynamics.
DISEASES OF MITOCHONDRIAL FUSION: CONVERGING PHENOTYPES
Three mammalian proteins are required for mitochondrial fusion (1): Mfn1 and Mfn2 for outer membrane fusion, and OPA1 for inner membrane fusion (3,4). Mouse knockouts of each of these three genes result in embryonic lethality and mitochondrial dysfunction (5–7). Mutations in Mfn2 cause Charcot-Marie-Tooth type 2A (CMT2A), a peripheral neuropathy affecting sensory and motor neurons of the distal extremities (8). Mutations in OPA1 are the predominant cause of autosomal dominant optic atrophy (DOA), a degeneration of retinal ganglia cells that leads to optic nerve atrophy (9,10). A frequently asked question is why mutations in two proteins both required for mitochondrial fusion should cause two diseases with such different tissue specificity. Potential answers include differential expression patterns between Mfn2 versus OPA1, functional differences between OPA1 (11) and Mfn2 (12), differences in an outer membrane fusion defect versus an inner membrane fusion defect (4) and redundancy between Mfn1 and Mfn2 (6,13).
Although all of these explanations may apply, more overlap in the clinical manifestations of CMT2A and DOA has emerged with the study of additional affected families. In addition to optic atrophy, some DOA patients present with additional symptoms, including peripheral motor-sensory neuropathy. Sensorineural deafness, cerebral atrophy, cerebellar ataxia, chronic progressive external ophthalmoplegia, mitochondrial myopathy and psychiatric involvement can also manifest (14,15). Similarly, some CMT patients with Mfn2 mutations suffer from optic atrophy as well as deafness, cognitive dysfunction, cerebral and cerebellar abnormalities, vocal cord paresis, scoliosis, parkinsonism and psychiatric involvement (16–21). In addition, the same mutation (e.g. R94W) can generate a large range of phenotypes even within the same family. Therefore, modifying factors clearly play a role in determining manifestation of the diseases. In fact, one patient with an OPA1 mutation notably does not have optic atrophy, but instead displays ptosis, hearing loss, proximal weakness, psychiatric involvement and muscle mitochondrial dysfunction (22).
In summary, mutations in either OPA1 or Mfn2 can impact a broad range of tissues beyond the optic and peripheral nerves. The overlapping symptoms in DOA and CMT2A families suggest that OPA1 and Mfn2 mutations can affect certain tissues in a similar way. Interestingly, the widespread CNS involvement and myopathies are reminiscent of mtDNA mutation syndromes. Indeed, OPA1 ‘plus’ patients actually harbor mitochondrial DNA (mtDNA) deletions in their muscle cells, suggesting a role for mitochondrial fusion in maintaining mtDNA integrity (14,15).
IMPACT OF MITOCHONDRIAL FISSION ON FUNCTION
As in yeast (Dnm1), flies (Drp1) and plants (DRP3B), mitochondrial fission in mammals is mediated by a dynamin-like protein, Drp1 (1). Drp1 is a predominantly cytosolic protein that is recruited to mitochondria during fission. In yeast, this recruitment is clearly dependent on the mitochondrial outer membrane protein Fis1 (2). In mammalian cells, however, knockdown of Fis1 blocks mitochondrial fission without affecting Drp1 localization to mitochondria (23). Recent studies have identified another tail-anchored outer membrane protein, Mff, which is involved in mitochondrial fission. Knockdown of Mff causes mitochondrial elongation and resistance to CCCP-induced fragmentation (24). Blue native gel studies show that Fis1 and Mff reside in complexes of different sizes, suggesting distinct machineries for mitochondrial fission. The potential role of Mff in Drp1 recruitment to mitochondria remains to be tested.
Although no inherited diseases are known to result from mutation of these genes, one case of neonatal lethality has been attributed to a defect in Drp1 (25). This patient carried a dominant-negative allele that caused perinuclear tangles of elongated, large-diameter mitochondria. Symptoms included optic atrophy, a notably small head with abnormal brain development and hypoplasia. Lactic acid levels were elevated in the blood and even more so in the cerebral spinal fluid (CSF). Muscle and fibroblasts showed normal respiratory function, but it seems likely that neuronal cells had electron transport chain (ETC) abnormalities, given the extreme lactic acid concentrations in the CSF and the brain structural defects. In cell culture studies, down-regulation of Drp1 in HeLa cells causes slower cell growth, loss of mtDNA, uncoupling of the ETC, decreased cellular respiration and increased reactive oxygen species levels (26).
Therefore, like mitochondrial fusion, fission seems to be required to maintain mitochondrial function. The mechanisms, however, probably differ. Fusion is likely to protect function by providing a chance for mitochondria to mix their contents, thus enabling protein complementation, mtDNA repair and equal distribution of metabolites. Fission likely acts instead to facilitate equal segregation of mitochondria into daughter cells during cell division and to enhance distribution of mitochondria along cytoskeletal tracks. In addition, fission may help to isolate damaged segments of mitochondria and thus promote their autophagy as discussed below (27). When these protective mechanisms fail, mitochondrial fission can also promote apoptosis, an important topic that has been extensively reviewed (28).
EMERGING ASPECTS OF MITOCHONDRIAL DYNAMICS
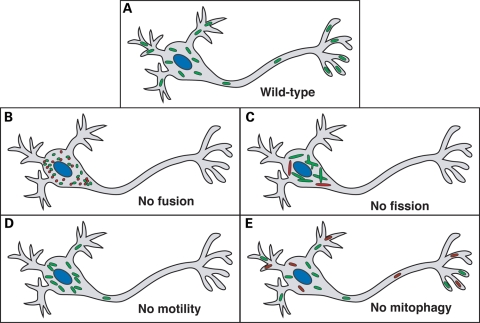
Perturbations in mitochondrial fusion, fission, motility and turnover can lead to distinctive defects in neurons (Fig. 1). However, there is also substantial overlap in these defects, because these four processes are interdependent. The reciprocal interactions between these processes are becoming appreciated, but our molecular understanding is still sparse.
Figure 1.
Defects in mitochondrial dynamics that lead to neuronal dysfunction. (A) In wild-type neurons, mitochondria travel long distances from the cell body out to dendritic and axonal termini, where they play important roles in ATP production and calcium homeostasis. (B) In the absence of fusion, the mitochondrial population fragments and a subset show ultrastructural defects and dysfunction (red). The mitochondria secondarily have transport defects that prevent proper distribution to the periphery. (C) In the absence of fission, the mitochondrial population is excessively long and interconnected, and a subset shows dysfunction (red). These large mitochondria cluster within the cell body and are not efficiently transported to the periphery. (D) Primary defects in mitochondrial motility prevent distribution of mitochondria to the periphery. (E) In the absence of mitophagy, abnormal mitochondria (red) accumulate.
Mitophagy
Autophagy is a process whereby cellular components are degraded by engulfment into autophagosomes. Autophagosomes fuse with lysosomes, which contain hydrolytic enzymes that break down cellular components. During nutrient deprivation, the products can be recycled into more urgently needed molecules. Although autophagy plays a particularly prominent role during starvation, it also appears to have a housekeeping role in maintaining quality control by turning over organelles and degrading protein aggregates. Mitophagy denotes the degradation of mitochondria through autophagy. Although the existence of mitophagy has been known for some time, it has been unclear whether mitochondria are randomly or selectively targeted for mitophagy.
Several recent findings indicate that mitophagy can selectively degrade defective mitochondria. In yeast cells, mitophagy is regulated independently from bulk autophagy (29). Mitochondria that are damaged by a laser irradiation in hepatocytes are selectively removed by mitophagy (30). Studies in pancreatic β-cells and COS7 cells show that mitochondrial fission can yield uneven products, with one depolarized daughter mitochondrion and one hyperpolarized mitochondrion (27). Such depolarized mitochondria are much less likely to fuse, have reduced levels of OPA1 protein, and are eventually autophagocytosed. This mitophagy is dependent on loss of fusion and the presence of fission, because OPA1-overexpression, Fis1 RNAi, and Drp1 dominant-negative expression all reduce levels of mitophagy. When mitophagy is thus compromised, oxidized proteins accumulate, and cellular respiration and insulin secretion decrease. It is important to note that although mitochondrial fragmentation is permissive for mitophagy, it is not a sufficient signal for mitophagy (27,31).
Mitochondrial motility
Another aspect of mitochondrial dynamics beyond fusion and fission is the motility of mitochondria. This aspect is critically important in highly polarized cells, such as neurons (32), which require mitochondria at sites distant from the cell body, but can also be crucial to cellular function in smaller cells (33). Defects in both fusion and fission have been shown to decrease mitochondrial movement. Presumably, the large tangle of highly interconnected mitochondria in fission-deficient cells prevents efficient movement, especially into small pathways such as neuronal processes (34,35). In fusion-deficient cells, the cause of decreased motility is less clear. Empirically, however, fusion-deficient mitochondria display loss of directed movement, instead hovering in a manner reminiscent of Brownian motion (6). In neurons lacking mitochondrial fusion, both increased mitochondrial diameter due to swelling and aggregations of mitochondria seem to block efficient entry into neurites, resulting in a dearth of mitochondria in axons and dendrites (36). These defects result in improperly developed neurons or gradual neurodegeneration.
Mitochondrial transport in mammalian cells is largely microtubule based (32). Anterograde motion is driven by the kinesin-1 motor (KHC, Kif5b), which interacts with mitochondria through the outer membrane proteins Miro1 and Miro2 (37). In Drosophila, Milton is an adapter protein that mediates binding of Miro to kinesin. High local concentrations of calcium act through the EF hands of Miro to halt mitochondrial movement, and thus retain mitochondria at sites where ATP production and Ca++ buffering are needed (38–40). Loss of this Miro-dependent transport pathway in neurons results in depletion of mitochondria from dendrites and axons, resulting in neurotransmission defects during prolonged stimulation (41).
How this mitochondrial transport apparatus interacts with the fusion/fission machinery is unclear, but most likely involves indirect interactions. Deletion of Miro in yeast greatly affects mitochondrial morphology without disrupting mitochondrial fusion or fission (42). Likewise in mammalian cells, manipulation of Miro can dramatically affect mitochondrial shape (39,43,44). Disruption of dynein function, which is necessary for retrograde transport of mitochondria, sequesters Drp1 in the cytoplasm and results in perinuclear, elongated mitochondria (45). Therefore, mitochondrial fusion and fission defects secondarily impair motility, and conversely, transport defects affect mitochondrial morphology. However, the mechanisms underlying this interplay remain to be determined.
NEURODEGENERATIVE DISEASES INVOLVING MITOCHONDRIAL DYNAMICS
Aside from diseases such as DOA and CMT2A caused by perturbation of mitochondrial fusion, mitochondrial dynamics seems to impact a wide variety of human diseases through interactions with other cellular processes. Many of these diseases are neurodegenerative and affect many distinct regions of the brain as well as the peripheral nervous system (Fig. 2), again emphasizing the importance of mitochondrial function in maintaining healthy neurons.
Figure 2.
Neurodegenerative disease associated with defects in mitochondrial dynamics. Neuronal systems affected in neurodegenerative disease are shown. For each disease, only the primary affected regions are indicated, but there is evidence for more widespread involvement.
Parkinson's disease
Progressive loss of dopaminergic neurons in the substantia nigra leads to Parkinson's disease (PD), the second most common neurodegenerative disease in humans. The most prominent PD symptoms include a resting tremor, rigidity, bradykinesia and a characteristic unsteady gait. Both chemical and genetic lines of evidence strongly suggest mitochondrial involvement. Drugs that inhibit complex I of the ETC produce PD-like symptoms in humans and animal models. The majority of PD cases are sporadic with unclear etiology. However, ∼10% of PD cases are inherited, and linkage analysis has identified a number of PD-associated genes. Two genes identified in hereditary PD are Pink1 and Parkin, both of which have been shown to be important for mitochondrial integrity (46). Pink1 is a serine/threonine kinase with an N-terminal mitochondrial targeting sequence and is localized to both mitochondria and the cytosol. Parkin is a cytosolic E3 ubiquitin ligase with two RING fingers, cysteine- and histidine-containing protein motifs that coordinate zinc ions.
Studies of mammalian Pink1 and Parkin have been complicated by the fact that mouse knockouts have little phenotype and fail to reproduce the common symptoms of PD (47–49). However, mitochondria from both knockouts demonstrate mild defects in respiration and sensitivity to oxidative stress (47,49–51). Additional studies show that loss of Pink1 can result in abnormalities in mitochondrial morphology, but the effects differ among cell types. Loss of Pink1 in human dopaminergic neurons or primary mouse neuronal cultures leads to reduced viability accompanied by abnormal, swollen mitochondria (52). Mitochondria from Pink1−/− mouse striatal neurons show no gross ultrastructural changes, although a small increase in the number of large mitochondria is observed (50). Knockdown of Pink1 in COS7 cells also increases mitochondrial size through tubulation. This effect is suppressed by hFis1 or Drp1 over-expression (53). In contrast, PD patient fibroblasts were reported to contain fragmented mitochondria (54). Similarly, knockdown of Pink1 in HeLa cells and human neuronal SH-SY5Y cells causes mitochondrial fragmentation, an effect reversed by over-expression of Parkin (54,55).
Whereas mouse knockouts of Pink1 or Parkin have subtle phenotypes, the corresponding mutants in flies show severe mitochondrial dysfunction in multiple tissues (56–59). Both mutants are remarkably similar, marked by flight muscle degeneration accompanied by swollen mitochondria with disrupted cristae. Double mutants have a similar phenotype. Parkin over-expression restores muscle integrity and flight to pink1 mutants, but Pink1 over-expression in parkin mutants has no effect. These findings have led to the model that Pink1 and Parkin likely act in the same pathway, with Parkin downstream of Pink1 (56,58,59). Consistent with this model, there is biochemical evidence for a direct interaction between Pink1 and Parkin (60).
How does the Pink1/Parkin pathway regulate mitochondrial function? Recent genetic studies in flies suggest that Pink1 and Parkin act to promote mitochondrial fission or inhibit fusion (53,61,62). Over-expression of Drp1 or down-regulation of Marf/Mfn2 (a fly homologue of mammalian Mfns) or Opa1 can dramatically ameliorate the phenotypes of pink1 or parkin mutant flies, including flight muscle degeneration, abnormal wing posture and defects in climbing and flying. These striking observations indicate a genetic interaction between the Pink1/Parkin pathway and mitochondrial dynamics. However, some observations suggest that the relationship may not be direct. For example, the epistatic relationship of Pink1/Parkin to mitochondrial fusion and fission is not straightforward. With regard to mitochondrial morphology in muscle, drp1, marf and opa1 mutations are epistatic to pink1 and parkin mutations. In the testicular mitochondria, however, a pink1 mutation is epistatic to an fzo mutation (61). Furthermore, although pink1 or parkin mutants have some enlargement of mitochondria, this morphological change is accompanied by increased apoptosis and is different from that of drp1 mutants. In addition, a synthetic lethal interaction is seen in pink1-null, drp1-heterozygous flies. These data indicate that Pink1/Parkin do not act in a simple linear pathway with either mitochondrial fission or fusion.
In the studies of PD, interpretation of the functions of Pink1 and Parkin have been complicated by the discrepancies in mitochondrial morphology defects found among various mammalian cell lines and between the fly and mammalian model systems. Such complexity is also apparent in studies of Alzheimer's disease (AD) and Huntington's disease (HD) (later). In part, these discrepancies may suggest that Pink1 and Parkin do not directly regulate mitochondrial fusion or fission in a straightforward manner. Mitochondrial morphology is highly dependent on mitochondrial physiology, and therefore it can be difficult to interpret changes in mitochondrial shape. In particular, the cause of mitochondrial fragmentation can be indirect, because it frequently accompanies mitochondrial dysfunction. In Caenorhabditis elegans, an RNAi screen revealed that disruption of over 80% of mitochondrial genes leads to mitochondrial fragmentation and/or aggregation, showing that this morphological phenotype is an extremely common one and not necessarily an indication of a specific effect on mitochondrial fusion or fission (63).
Recent studies implicate Parkin in the turnover of mitochondria through mitophagy. Parkin is specifically recruited to mitochondria with low membrane potential, and these targeted mitochondria are then destroyed through the autophagosome (31). The mitochondrial accumulation of Parkin is voltage-dependent, and does not depend on changes in pH or ATP levels (64). These experiments suggest that Parkin may act as a sensor for mitochondrial integrity and trigger mitophagy upon dysfunction. It is unclear whether Pink1 is involved in this pathway. Knockdown of Pink1 in SH-SyY5Y cells induces mitochondrial fragmentation, accumulation of mitochondrially-targeted autophagosomes and increased cell death (55). Inhibition of Drp1 prevents mitochondrial fragmentation and mitophagy, but exacerbates cell death. Co-expression of Parkin, though, increases mitophagy and decreases cell death while at the same time preventing mitochondrial fragmentation. In this case, mitochondrial morphology itself does not appear to be the critical component in determining cell fate. Rather, the functional state of the mitochondrial population appears to govern the cellular outcome. Taken together, these results suggest that Pink1 protects mitochondrial function, whereas Parkin promotes mitochondrial quality control by eliminating dysfunctional mitochondria. In future studies, it will be important to test whether Parkin-deficient cells from PD patients indeed have a defect in mitophagy.
Alzheimer's disease
The most common neurodegenerative disease, AD, is marked by cognitive dysfunction and memory loss caused by neuronal death in the cerebral cortex. Afflicted brains carry intracellular neurofibrillary tangles and extracellular amyloid plaques composed chiefly of beta-amyloid (Aβ) derived from amyloid precursor protein (APP). Although the pathological mechanism for AD is still unknown, the predominant hypothesis is that excess Aβ production results in cellular toxicity. Transgenic mouse models over-expressing APP lead to amyloid plaques associated with activation of inflammatory cells, localized loss of neurons, and some cognitive behavioral changes (65).
Abnormalities in mitochondrial structure are found in the brain of AD patients (66). Aβ can localize to mitochondria, and this interaction has been suggested to underlie in part their cytotoxic effects (67,68). Exposure of neuronal cells to conditioned medium from cells stably expressing mutant forms of APP leads to increased mitochondrial fission, loss of dendritic spines and eventually cell death (69). This increase in mitochondrial fission was traced to elevated levels of S-nitrosylated Drp1 (SNO-Drp1), which is suggested to have increase fission activity due to enhanced dimerization. Expression of Drp1(C644A), a mutant incapable of nitrosylation, prevents excessive mitochondrial fission and neuronal cell injury without interfering with normal, basal levels of mitochondrial fission. Moreover, increased levels of SNO-Drp1 were found in brain samples from AD patients and AD mouse models. These data suggest that the cytotoxic effects of Aβ result, in part, from generation of nitric oxide that leads to activation of Drp1 activity.
In contrast, another study found that fibroblasts from sporadic AD patients express lower levels of Drp1 and display elongated mitochondria which form collapsed perinuclear networks (70,71). The same group, however, found that APP over-expression in M17 neuroblastoma cells resulted in predominantly fragmented mitochondria, decreased levels of Drp1 and OPA1, and a defect in neuronal differentiation (72). Over-expression of Drp1 or OPA1 could partially rescue different aspects of these defects.
Huntington's disease
Progressive loss of striatal and cortical neurons leads to cognitive and motor impairment and eventually death in patients with HD. Whereas multiple genes have been associated with PD and AD, HD is an autosomal dominant disease caused by trinucleotide expansion [cytosine, adenine, and guanine (CAG)] within a single gene, huntingtin (Htt). Disease is associated with CAG expansion leading to a stretch of glutamines beyond 35 residues in Htt. In HD patients and mouse transgenic HD models, several lines of evidence indicate that expression of mutant Htt is associated with mitochondrial dysfunction (73). For example, Htt expression correlated with elevated lactate levels, decreased mitochondrial membrane potential, decreased respiratory function through complex II, defects in mitochondrial calcium uptake, reduced mitochondrial mobility and mitochondrial ultrastructural changes. In animal models, 3-nitropropionic acid, an irreversible inhibitor of complex II, can cause HD-like symptoms. Conversely, over-expression of complex II subunits can prevent cell death in striatal neurons carrying mutant Htt.
Recent studies uncover an interplay between HD and mitochondrial dynamics. Rat cortical neurons treated with 3-nitropropionic acid have fragmentation and condensation of mitochondria which can be prevented by antioxidant treatment (74). Likewise, mitochondria in HeLa cells over-expressing a mutant Htt with a 74 glutamine repeat (Htt74Q) show fragmentation of mitochondria, reduced mitochondrial fusion, reduced ATP and increased cell death (75). Remarkably, expression of either dominant-negative Drp1 or Mfn2 not only prevents this change in mitochondrial morphology, but also restores ATP levels and attenuates cell death. Inhibition of Drp1 can also rescue the mobility of worms expressing Htt74 in muscle cells.
Because increasing fusion or reducing fission can partially rescue mutant Htt over-expression, it is possible that mutant Htt disrupts fusion and fission. However, it is unclear whether such a direct relationship exists. Mutant Htt can associate with mitochondria (76), but the biochemical function of Htt remains poorly understood. It is possible that, in some cellular environments, manipulation of fusion and fission rates can improve the physiology of mitochondria even if the initial insult is not directly on fusion or fission. Mutant Htt expression has multiple effects on mitochondrial physiology, including respiratory dysfunction and reduced transport. Htt72Q transgenic mice display a generalized transport defect in neurons (77), including abnormal movement of mitochondria (76–78). The mitochondria move more slowly, stop more frequently and travel shorter distances. Defects in membrane potential, respiratory distress and inefficient transport can all secondarily contribute to the morphological defects associated with mutant Htt expression.
PERSPECTIVE
Taken together, the studies reviewed here clearly indicate that perturbations in mitochondrial dynamics are directly or indirectly involved in a host of human neurodegenerative diseases. However, our understanding of the mechanisms involved remains rudimentary at best. There exists much conflicting data among different disease models, especially regarding the mitochondrial morphological changes associated with disease states and the mechanisms underlying these changes. Several factors likely contribute to these discrepancies. First, the variations in mitochondrial phenotypes suggest that although PD, AD and HD clearly perturb mitochondrial dynamics, the effects are unlikely to result from simple, direct effects on the processes of fusion and fission. Second, it is becoming increasingly clear that a myriad of factors can affect mitochondrial shape. For each disease, it will be important to dissect the key underlying mechanisms.
As we learn more about how neurodegenerative diseases impact mitochondria, it is becoming clear that mitochondrial dynamics is a multi-factorial process that is integrated into cell physiology. Mitochondrial fusion and fission play prominent roles in controlling mitochondrial shape and function. However, these opposing processes have reciprocal interactions with mitochondrial transport and mitophagy. Therefore, several inter-related factors—fusion, fission, transport and turnover—form a complex interacting network that governs mitochondrial function and thereby cellular integrity. A challenge for future studies is to unravel the molecular nature of these interactions. Given that manipulation of genes controlling mitochondrial fusion and fission can ameliorate disease phenotypes, there is compelling reason to hope that efforts to artificially manipulate mitochondrial dynamics (79) will ultimately lead to new therapeutic approaches.
Conflict of Interest statement. None declared.
FUNDING
Work in the authors' laboratory was supported by funds from NIH (GM062967 and GM083121), the Ellison Medical Foundation, and HHMI. Funding to pay the Open Access publication charges for this article was provided by HHMI.
NOTE ADDED IN PROOF
Mihara (80) and colleagues have recently disrupted Drp1 in mice and have provided additional evidence for the role of mitochondrial fission in neuronal development. Embryonic neurons lacking Drp1 show improper mitochondrial distribution consistent with Figure 1 and increased sensitivity to apoptosis.
REFERENCES
- 1.Detmer S.A., Chan D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto K., Shaw J.M. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu. Rev. Genet. 2005;39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- 3.Meeusen S., DeVay R., Block J., Cassidy-Stone A., Wayson S., McCaffery J.M., Nunnari J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127:383–395. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Song Z., Ghochani M., McCaffery J.M., Frey T.G., Chan D.C. Mitofusins and OPA1 Mediate Sequential Steps in Mitochondrial Membrane Fusion. Mol. Biol. Cell. 2009 doi: 10.1091/mbc.E09-03-0252. May 28 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alavi M.V., Bette S., Schimpf S., Schuettauf F., Schraermeyer U., Wehrl H.F., Ruttiger L., Beck S.C., Tonagel F., Pichler B.J., et al. A splice site mutation in the murine Opa1 gene features pathology of autosomal dominant optic atrophy. Brain. 2007;130:1029–1042. doi: 10.1093/brain/awm005. [DOI] [PubMed] [Google Scholar]
- 6.Chen H., Detmer S.A., Ewald A.J., Griffin E.E., Fraser S.E., Chan D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies V.J., Hollins A.J., Piechota M.J., Yip W., Davies J.R., White K.E., Nicols P.P., Boulton M.E., Votruba M. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum. Mol. Genet. 2007;16:1307–1318. doi: 10.1093/hmg/ddm079. [DOI] [PubMed] [Google Scholar]
- 8.Zuchner S., Mersiyanova I.V., Muglia M., Bissar-Tadmouri N., Rochelle J., Dadali E.L., Zappia M., Nelis E., Patitucci A., Senderek J., et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 9.Alexander C., Votruba M., Pesch U.E., Thiselton D.L., Mayer S., Moore A., Rodriguez M., Kellner U., Leo-Kottler B., Auburger G., et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 10.Delettre C., Lenaers G., Griffoin J.M., Gigarel N., Lorenzo C., Belenguer P., Pelloquin L., Grosgeorge J., Turc-Carel C., Perret E., et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat. Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 11.Frezza C., Cipolat S., Martins de Brito O., Micaroni M., Beznoussenko G.V., Rudka T., Bartoli D., Polishuck R.S., Danial N.N., De Strooper B., et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 12.de Brito O.M., Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 13.Chen H., Chomyn A., Chan D.C. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 14.Amati-Bonneau P., Valentino M.L., Reynier P., Gallardo M.E., Bornstein B., Boissiere A., Campos Y., Rivera H., de la Aleja J.G., Carroccia R., et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy ‘plus’ phenotypes. Brain. 2008;131:338–351. doi: 10.1093/brain/awm298. [DOI] [PubMed] [Google Scholar]
- 15.Hudson G., Amati-Bonneau P., Blakely E.L., Stewart J.D., He L., Schaefer A.M., Griffiths P.G., Ahlqvist K., Suomalainen A., Reynier P., et al. Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain. 2008;131:329–337. doi: 10.1093/brain/awm272. [DOI] [PubMed] [Google Scholar]
- 16.Banchs I., Casasnovas C., Montero J., Martinez-Matos J.A., Volpini V. Two Spanish families with Charcot-Marie-Tooth type 2A: clinical, electrophysiological and molecular findings. Neuromuscul. Disord. 2008;18:974–978. doi: 10.1016/j.nmd.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Brockmann K., Dreha-Kulaczewski S., Dechent P., Bonnemann C., Helms G., Kyllerman M., Bruck W., Frahm J., Huehne K., Gartner J., et al. Cerebral involvement in axonal Charcot-Marie-Tooth neuropathy caused by mitofusin2 mutations. J. Neurol. 2008;255:1049–1058. doi: 10.1007/s00415-008-0847-1. [DOI] [PubMed] [Google Scholar]
- 18.Chung K.W., Kim S.B., Park K.D., Choi K.G., Lee J.H., Eun H.W., Suh J.S., Hwang J.H., Kim W.K., Seo B.C., et al. Early onset severe and late-onset mild Charcot-Marie-Tooth disease with mitofusin 2 (MFN2) mutations. Brain. 2006;129:2103–2118. doi: 10.1093/brain/awl174. [DOI] [PubMed] [Google Scholar]
- 19.Del Bo R., Moggio M., Rango M., Bonato S., D'Angelo M.G., Ghezzi S., Airoldi G., Bassi M.T., Guglieri M., Napoli L., et al. Mutated mitofusin 2 presents with intrafamilial variability and brain mitochondrial dysfunction. Neurology. 2008;71:1959–1966. doi: 10.1212/01.wnl.0000327095.32005.a4. [DOI] [PubMed] [Google Scholar]
- 20.Verhoeven K., Claeys K.G., Zuchner S., Schroder J.M., Weis J., Ceuterick C., Jordanova A., Nelis E., De Vriendt E., Van Hul M., et al. MFN2 mutation distribution and genotype/phenotype correlation in Charcot-Marie-Tooth type 2. Brain. 2006;129:2093–2102. doi: 10.1093/brain/awl126. [DOI] [PubMed] [Google Scholar]
- 21.Zuchner S., De Jonghe P., Jordanova A., Claeys K.G., Guergueltcheva V., Cherninkova S., Hamilton S.R., Van Stavern G., Krajewski K.M., Stajich J., et al. Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann. Neurol. 2006;59:276–281. doi: 10.1002/ana.20797. [DOI] [PubMed] [Google Scholar]
- 22.Milone M., Younge B.R., Wang J., Zhang S., Wong L.J. Mitochondrial disorder with OPA1 mutation lacking optic atrophy. Mitochondrion. 2009;9:279–281. doi: 10.1016/j.mito.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y.J., Jeong S.Y., Karbowski M., Smith C.L., Youle R.J. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1 and Opa1 in apoptosis. Mol. Biol. Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandre-Babbe S., van der Bliek A.M. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waterham H.R., Koster J., van Roermund C.W., Mooyer P.A., Wanders R.J., Leonard J.V. A lethal defect of mitochondrial and peroxisomal fission. N. Engl. J. Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 26.Parone P.A., Da Cruz S., Tondera D., Mattenberger Y., James D.I., Maechler P., Barja F., Martinou J.C. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE. 2008;3:e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Twig G., Elorza A., Molina A.J., Mohamed H., Wikstrom J.D., Walzer G., Stiles L., Haigh S.E., Katz S., Las G., et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suen D.F., Norris K.L., Youle R.J. Mitochondrial dynamics and apoptosis. Genes. Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanki T., Klionsky D.J. Mitophagy in yeast occurs through a selective mechanism. J. Biol. Chem. 2008;283:32386–32393. doi: 10.1074/jbc.M802403200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim I., Rodriguez-Enriquez S., Lemasters J.J. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narendra D., Tanaka A., Suen D.F., Youle R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollenbeck P.J., Saxton W.M. The axonal transport of mitochondria. J. Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campello S., Lacalle R.A., Bettella M., Manes S., Scorrano L., Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J. Exp. Med. 2006;203:2879–2886. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verstreken P., Ly C.V., Venken K.J., Koh T.W., Zhou Y., Bellen H.J. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Li Z., Okamoto K., Hayashi Y., Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Chen H., McCaffery J.M., Chan D.C. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Reis K., Fransson A., Aspenstrom P. The Miro GTPases: at the heart of the mitochondrial transport machinery. FEBS Lett. 2009;583:1391–1398. doi: 10.1016/j.febslet.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 38.MacAskill A.F., Brickley K., Stephenson F.A., Kittler J.T. GTPase dependent recruitment of Grif-1 by Miro1 regulates mitochondrial trafficking in hippocampal neurons. Mol. Cell. Neurosci. 2009;40:301–312. doi: 10.1016/j.mcn.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Saotome M., Safiulina D., Szabadkai G., Das S., Fransson A., Aspenstrom P., Rizzuto R., Hajnoczky G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc. Natl. Acad. Sci. U.S.A. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X., Schwarz T.L. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo X., Macleod G.T., Wellington A., Hu F., Panchumarthi S., Schoenfield M., Marin L., Charlton M.P., Atwood H.L., Zinsmaier K.E. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Frederick R.L., McCaffery J.M., Cunningham K.W., Okamoto K., Shaw J.M. Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway. J. Cell Biol. 2004;167:87–98. doi: 10.1083/jcb.200405100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fransson A., Ruusala A., Aspenstrom P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J. Biol. Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- 44.Fransson S., Ruusala A., Aspenstrom P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem. Biophys. Res. Commun. 2006;344:500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- 45.Varadi A., Johnson-Cadwell L.I., Cirulli V., Yoon Y., Allan V.J., Rutter G.A. Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J. Cell Sci. 2004;117:4389–4400. doi: 10.1242/jcs.01299. [DOI] [PubMed] [Google Scholar]
- 46.Dodson M.W., Guo M. Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson's disease. Curr. Opin. Neurobiol. 2007;17:331–337. doi: 10.1016/j.conb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Gispert S., Ricciardi F., Kurz A., Azizov M., Hoepken H.H., Becker D., Voos W., Leuner K., Muller W.E., Kudin A.P., et al. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS ONE. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitada T., Pisani A., Porter D.R., Yamaguchi H., Tscherter A., Martella G., Bonsi P., Zhang C., Pothos E.N., Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palacino J.J., Sagi D., Goldberg M.S., Krauss S., Motz C., Wacker M., Klose J., Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 50.Gautier C.A., Kitada T., Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mortiboys H., Thomas K.J., Koopman W.J., Klaffke S., Abou-Sleiman P., Olpin S., Wood N.W., Willems P.H., Smeitink J.A., Cookson M.R., et al. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann. Neurol. 2008;64:555–565. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood-Kaczmar A., Gandhi S., Yao Z., Abramov A.Y., Miljan E.A., Keen G., Stanyer L., Hargreaves I., Klupsch K., Deas E., et al. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE. 2008;3:e2455. doi: 10.1371/journal.pone.0002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y., Ouyang Y., Yang L., Beal M.F., McQuibban A., Vogel H., Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Exner N., Treske B., Paquet D., Holmstrom K., Schiesling C., Gispert S., Carballo-Carbajal I., Berg D., Hoepken H.H., Gasser T., et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J. Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dagda R.K., Cherra S.J., 3rd, Kulich S.M., Tandon A., Park D., Chu C.T. Loss of PINK1 Function Promotes Mitophagy through Effects on Oxidative Stress and Mitochondrial Fission. J. Biol. Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark I.E., Dodson M.W., Jiang C., Cao J.H., Huh J.R., Seol J.H., Yoo S.J., Hay B.A., Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 57.Greene J.C., Whitworth A.J., Kuo I., Andrews L.A., Feany M.B., Pallanck L.J. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park J., Lee S.B., Lee S., Kim Y., Song S., Kim S., Bae E., Kim J., Shong M., Kim J.M., et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y., Gehrke S., Imai Y., Huang Z., Ouyang Y., Wang J.W., Yang L., Beal M.F., Vogel H., Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc. Natl. Acad. Sci. U.S.A. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim Y., Park J., Kim S., Song S., Kwon S.K., Lee S.H., Kitada T., Kim J.M., Chung J. PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem. Biophys. Res. Commun. 2008;377:975–980. doi: 10.1016/j.bbrc.2008.10.104. [DOI] [PubMed] [Google Scholar]
- 61.Deng H., Dodson M.W., Huang H., Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poole A.C., Thomas R.E., Andrews L.A., McBride H.M., Whitworth A.J., Pallanck L.J. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ichishita R., Tanaka K., Sugiura Y., Sayano T., Mihara K., Oka T. An RNAi screen for mitochondrial proteins required to maintain the morphology of the organelle in Caenorhabditis elegans. J. Biochem. 2008;143:449–454. doi: 10.1093/jb/mvm245. [DOI] [PubMed] [Google Scholar]
- 64.Narendra D., Tanaka A., Suen D.F., Youle R.J. Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy. 2009;5 doi: 10.4161/auto.5.5.8505. [DOI] [PubMed] [Google Scholar]
- 65.Dodart J.C., May P. Overview on rodent models of Alzheimer's disease. Curr Protoc Neurosci. 2005;Chapter 9:Unit 9.22. doi: 10.1002/0471142301.ns0922s33. [DOI] [PubMed] [Google Scholar]
- 66.Baloyannis S.J. Mitochondrial alterations in Alzheimer's disease. J. Alzheimers Dis. 2006;9:119–126. doi: 10.3233/jad-2006-9204. [DOI] [PubMed] [Google Scholar]
- 67.Manczak M., Anekonda T.S., Henson E., Park B.S., Quinn J., Reddy P.H. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 68.Lustbader J.W., Cirilli M., Lin C., Xu H.W., Takuma K., Wang N., Caspersen C., Chen X., Pollak S., Chaney M., et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 69.Cho D.H., Nakamura T., Fang J., Cieplak P., Godzik A., Gu Z., Lipton S.A. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X., Su B., Fujioka H., Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer's disease patients. Am J Pathol. 2008;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X., Su B., Zheng L., Perry G., Smith M.A., Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J. Neurochem. 2009;109(Suppl 1):153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X., Su B., Siedlak S.L., Moreira P.I., Fujioka H., Wang Y., Casadesus G., Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl. Acad. Sci. U.S.A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bossy-Wetzel E., Petrilli A., Knott A.B. Mutant huntingtin and mitochondrial dysfunction. Trends. Neurosci. 2008;31:609–616. doi: 10.1016/j.tins.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liot G., Bossy B., Lubitz S., Kushnareva Y., Sejbuk N., Bossy-Wetzel E. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 2009;16:899–909. doi: 10.1038/cdd.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H., Lim P.J., Karbowski M., Monteiro M.J. Effects of overexpression of huntingtin proteins on mitochondrial integrity. Hum. Mol. Genet. 2009;18:737–752. doi: 10.1093/hmg/ddn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orr A.L., Li S., Wang C.E., Li H., Wang J., Rong J., Xu X., Mastroberardino P.G., Greenamyre J.T., Li X.J. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J. Neurosci. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trushina E., Dyer R.B., Badger J.D., 2nd, Ure D., Eide L., Tran D.D., Vrieze B.T., Legendre-Guillemin V., McPherson P.S., Mandavilli B.S., et al. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol. Cell. Biol. 2004;24:8195–8209. doi: 10.1128/MCB.24.18.8195-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang D.T., Rintoul G.L., Pandipati S., Reynolds I.J. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol. Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 79.Cassidy-Stone A., Chipuk J.E., Ingerman E., Song C., Yoo C., Kuwana T., Kurth M.J., Shaw J.T., Hinshaw J.E., Green D.R., et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev. Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishihara N., Nomura M., Jofuku A., Kato H., Suzuki S.O., Masuda K., Otera H., Nakanishi Y., Nonaka Y., Goto Y.I., et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009 doi: 10.1038/ncb1907. July 5 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]