Abstract
Context: As men age, the prevalence of frailty increases whereas levels of androgens decline. Little is known about the relation between these factors.
Objective: The aim of this study was to assess cross-sectional and longitudinal associations of estradiol, bioavailable estradiol, testosterone, bioavailable testosterone (bioT), and SHBG with frailty status.
Design and Setting: The Osteoporotic Fractures in Men (MrOS) study was conducted at six U.S. clinical centers.
Participants: A total of 1469 community-dwelling men at least 65 yr old with baseline data participated; 1245 men had frailty status reassessed 4.1 yr later.
Main Outcome Measure: Proportional odds models estimated the likelihood of greater frailty status. Frail men had at least three of the following: weakness, slowness, low activity, exhaustion, and shrinking/sarcopenia; intermediate men had one or two criteria; and robust men had none. At follow-up, death was included as an additional ordinal outcome. Sex hormones were assayed by spectrometry/chromatographic methods.
Results: In cross-sectional analyses, men in the lowest quartile of bioT had 1.39-fold (95% confidence interval, 1.02, 1.91) increased odds of greater frailty status compared to men in the highest quartile after adjustment for covariates including age, body size, health status, and medical conditions. In age-adjusted longitudinal analyses, men in the lowest quartile of bioT had 1.51-fold (95% confidence interval, 1.10, 2.07) increased odds of greater frailty status 4.1 yr later. This association was largely attenuated by adjustment for covariates. No other hormones were associated in a cross-sectional or longitudinal manner with frailty status after adjustment.
Conclusions: Low levels of bioT were independently associated with worse baseline frailty status. Frailty status should be considered as an outcome in trials of testosterone supplementation.
Low levels of bioavailable testosterone are associated with worse frailty status in older men. Neither total testosterone nor estradiol is related to frailty status.
Frailty is a state where an older individual is especially vulnerable to external stressors (1,2). A conceptual framework (1) and working definition for frailty has been described using data from the Cardiovascular Health Study (CHS) (3). This phenotype comprises five domains: weakness (grip strength), shrinking/sarcopenia (weight loss), slowness (walking speed), low activity level (self-reported physical activity questionnaire), and exhaustion (by self-report). Although this phenotype of frailty has been shown to be predictive of mortality, hospitalization, and other adverse health outcomes in a number of cohorts (4,5,6,7,8), factors that may be associated with frailty status have not been extensively investigated.
The age-related decline in physical function and increased prevalence of frailty parallel the androgen decline (9) seen in older men. Additionally, the symptoms associated with androgen deprivation therapy for prostate cancer (such as sarcopenia or lean mass loss, muscle weakness, fatigue, and decreased activity levels) are similar to the components of the frailty syndrome (10). These observations suggest that there may be an association between frailty and low sex hormone levels in older men. The only cross-sectional report that has investigated the association between testosterone, SHBG, and frailty status found no association between free or total testosterone and frailty status after adjustment for potential confounders (11). Higher SHBG was associated with an increased likelihood of frailty after multivariate adjustment. No longitudinal studies examining the prospective association between sex hormones and frailty status as defined by the CHS definition in older men have been published.
The aims of the present analyses were to describe the cross-sectional and longitudinal associations of estradiol, bioavailable estradiol, testosterone, bioavailable testosterone, and SHBG with frailty in community-dwelling older men using data from the Osteoporotic Fractures in Men (MrOS) study.
Subjects and Methods
Participants
The MrOS study initially recruited 5995 community-dwelling men aged 65 yr and older from six U.S. clinical centers for a baseline visit between March 2000 and April 2002. Participants were recruited from Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; the Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California. To be eligible to participate, men must have been able to walk without assistance and not have had bilateral hip replacements. A description of the MrOS cohort has been published elsewhere (12,13). All participants provided written informed consent, and the study has been approved by the institutional review boards of all clinical centers and the coordinating center. Participants returned for a second clinic visit (visit 2) between March 2005 and May 2006.
Clinical and questionnaire-based data
Height was measured using wall-mounted stadiometers, and weight was measured with balance beam or digital scales. Body mass index (BMI) was calculated as weight (kilograms)/height (meters)2. Appendicular skeletal mass and total and percentage body fat were determined from whole body dual-energy x-ray absorptiometry on Hologic QDR4500W scanners (Hologic Inc., Bedford, MA) using standardized protocols. Participants self-reported living situation (alone or with others), race/ethnicity, marital status, self-rated health (excellent/good vs. fair/poor/very poor), education (college education vs. less), walking for exercise, and the history of a variety of medical conditions (cancer, stroke, diabetes, low or high thyroid, Parkinson’s disease, heart attack, congestive heart failure, chronic obstructive pulmonary disease, and hypertension). The number of medical conditions was summed. Smoking status (current/never/past) and alcohol intake (no intake, <14 drinks/week, ≥14 drinks/week) were ascertained by self-report. Physical activity was quantified using the Physical Activity Scale for the Elderly (14); a higher score represents greater activity. The Medical Outcomes Study 12-item Short Form (SF-12) (15) was completed by all participants. Participants were considered to have a functional limitation if they reported inability to walk two or three blocks or climb 10 steps without resting and an instrumental activities in daily living limitation if they reported that they were unable to prepare meals, complete heavy housework, or shop.
Participants were asked to bring in all prescription and nonprescription medications taken daily or almost daily during the previous month. All medications recorded by the clinics were stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA). Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) (16).
Measurement of sex hormones
A random sample of 1602 was selected for assessment of sex hormone levels in 2007–2008 using previously stored serum from the baseline exam in 2000–2002. Participants provided a fasting morning serum sample that was stored at −70 C. Total estradiol and total testosterone were analyzed by gas chromatograph/mass spectrometry assay (Taylor Technology, Princeton NJ). Specifically, a combined gas chromatographic negative ionization tandem mass spectrometry and liquid chromatographic electrospray tandem mass spectrometry bioanalytical methods were used to measure steroids in serum. The lower limit of detection for total estradiol was 0.625 pg/ml and for total testosterone, 2.5 ng/dl. Duplicate aliquots from each participant were assayed, and the two results were averaged. Some participants had incomplete data for the sex hormone assays: 20 estradiol samples and 25 testosterone samples were missing due to a bad assay run or incomplete data entry.
SHBG was assayed by the Oregon Health and Science University’s Clinical and Translational Research Institute labs using an Immulite Analyzer with chemiluminescent substrate (Diagnostic Products Corp., Los Angeles, CA) on the same samples previously thawed for the sex hormone measurements. The standard curve ranged from 0.2–180 nm/liter. No values fell below the standard curve; two participants with values above the standard curve were excluded from analyses. A number of men (n = 21) had insufficient serum to have SHBG assays completed. Bioavailable estradiol and testosterone were calculated using mass action equations described by Sodergard et al. (17) with updated association constants for testosterone (18) and a fixed albumin level of 4.3 g/dl. The “bioavailable” amount of hormone refers to the amount that is either unbound (free) or loosely bound to albumin in the serum and excludes the amount of hormone that is tightly bound to SHBG. Testosterone deficiency was defined as total testosterone levels below 250 ng/dl as recommended by the Institute of Medicine (19).
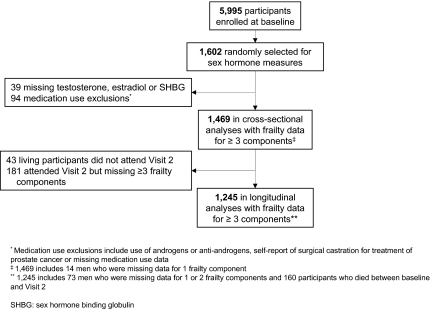
Of the 1602 men randomly selected to have sex hormones assays completed, 133 participants were missing sex hormone assay data (for either total testosterone, estradiol, or SHBG measures) or were excluded from analyses based on medication use (reported taking androgens or antiandrogens, missing medication use data), or reported surgical castration as a treatment for prostate cancer (Fig. 1). Thus, 1469 men were eligible for the cross-sectional analyses.
Figure 1.
Participants selected for cross-sectional and longitudinal analyses.
Frailty
Frailty was defined using modified criteria from the Cardiovascular Health Study (3) and previous analyses in MrOS (8) as described in Supplemental Table 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). The CHS definition uses fivecomponents to define the presence of frailty: shrinking/sarcopenia, weakness, slowness, low activity level, and exhaustion. Participants who have at least three components are defined as frail, those with one or two components are intermediate, and those with no components are robust. Cut-points to define frailty for this analysis were derived from the set of randomly selected MrOS participants who had valid sex hormone data and did not meet exclusion criteria for these analyses (n = 1469). Frailty at visit 2 was defined using cut-points from the baseline visit.
Participants included in cross-sectional analyses
Men who had sex hormone measures and frailty data for at least three of the frailty components were included in the cross-sectional analyses (n = 1469). (Fig. 1). At baseline, only 14 participants were missing data for only one frailty component; therefore all men with valid sex hormone measures were included in the cross-sectional analyses.
Participants included in the longitudinal analyses
Of the participants included in the baseline analyses, 160 participants died before visit 2. To jointly analyze the outcomes of frailty status at visit 2 and mortality data, four levels of frailty status were considered at the second clinic visit: robust, intermediate, frail, and dead. A total of 1245 men were included in the longitudinal analyses. This included 1085 men with data for at least three frailty components and 160 men who died between baseline and visit 2 (Fig. 1). Participants excluded from the longitudinal analyses included 43 men who were living but did not attend any part of visit 2 and 181 men who attended part of visit 2 but were missing data for at least three of the frailty components. Average time between visits was 4.1 yr.
Statistical analysis
Characteristics of participants were compared across quartiles of sex hormone measures: ANOVA was used for normally distributed continuous variables, Kruskall-Wallis tests for skewed continuous variables, and χ2 tests for categorical variables. Statistical analyses were completed using SAS v9.1.3 (SAS, Inc., Cary, NC) and Stata v10 (Stata Corporation, College Station, TX).
Each sex hormone was analyzed in a separate, age-adjusted model. To account for potentially confounding factors, additional covariates were included in multivariate models if they were associated with the majority of the sex hormone levels and frailty in this cohort. Covariates that were used to define the frailty syndrome (such as physical activity or percentage body fat) were not included in multivariate models. Multivariate baseline models were adjusted for age, BMI, self-rated health, education, smoking, marital status, and number of medical conditions. Longitudinal models were additionally adjusted for baseline frailty status.
To determine whether sex hormone levels were associated with an increased likelihood of greater frailty status at baseline, ordinal logistic regression was used (20). Proportional odds models were used to estimate the odds ratio (OR) for the sex hormone variables simultaneously across the ordinal levels of the frailty outcome. In the cross-sectional analyses, the levels of the frailty outcome were modeled as frail/intermediate (vs. robust) and frail (vs. robust/intermediate). In general, for proportional odds models to be valid, the assumption of proportionality (also known as the assumption of homogeneity of effect) must be met. For this assumption to hold, the relationship between the independent variable must be proportional across all levels of the outcome. In the cross-sectional analyses presented here, the assumption of proportionality was met for all the sex hormone variables and covariates at baseline. Thus, for all baseline models, a single OR that summarized the effect of the sex hormone over all levels of the ordinal frailty outcome was reported.
In longitudinal analyses, the association between a given sex hormone and the ordinal frailty outcome (robust, intermediate, frail, dead) at visit 2 was assessed. In the longitudinal analyses presented here, the assumption of proportionality was violated for some of the independent variables; therefore, a partial proportional odds model was used. For the variables that met the assumption of proportionality (sex hormone variables, age, marital status, smoking status, education, and self-rated health), a single OR that relates the independent variable to the ordinal frailty outcome was reported. For the predictors that violated the homogeneity assumption (frailty status at baseline, BMI, and number of medical conditions), a separate OR was reported for each level of the ordinal frailty outcome. Finally, to illustrate the combined influence of baseline frailty status and sex hormone levels on frailty status at visit 2, predicted probabilities for frailty status at visit 2 were calculated from the partial proportional odds models and were displayed graphically.
For cross-sectional analyses, to graphically display the relationship between frailty and bioavailable testosterone, we fitted a series of restricted cubic spline models using knots specified at cut-points for declines, quintiles, and quartiles of bioavailable testosterone. All of the graphs were similar and showed nearly identical suggested associations. Thus, for simplicity, the graph with knots specified as the 5th, 25th, 75th, and 95th percentile cut-points (108.4, 165.4, 241.9, and 312.2 ng/dl) was presented. The cubic spline models were not adjusted for any covariates.
Results
Cross-sectional results
At baseline, 682 (46.4%) men were robust, 675 (46.0%) were classified as intermediate, and 112 (7.6%) were classified as frail. Characteristics of participants by quartile of bioavailable testosterone are presented in Table 1. Men in the lowest quartiles of bioavailable testosterone tended to be older and to have higher BMI and greater body fat than those with higher levels of bioavailable testosterone (P < 0.001 for all). They were also less likely to be married, have at least a college education, and report excellent or good health status. Additionally, men with bioavailable testosterone levels in the lowest quartiles were more likely to have lower physical activity, to walk more slowly, to report more medical conditions, and to have lower grip strength and leg power than men with higher bioavailable testosterone levels.
Table 1.
Characteristics of the MrOS cohort by quartile of bioavailable testosterone
| Characteristics | Quartile of bioavailable testosterone
|
Pvalue | |||
|---|---|---|---|---|---|
| Q1 (<165.4 ng/dl) | Q2 (165.4 to <201.5 ng/dl) | Q3 (201.5 to <241.9 ng/dl) | Q4 (≥241.9 ng/dl) | ||
| n
|
367 | 367 | 367 | 368 | |
| Age (yr) | 75.3 ± 6.3 | 73.5 ± 5.4 | 73.6 ± 5.9 | 72.4 ± 5.4 | <0.001 |
| BMI (kg/m2) | 28.8 ± 4.2 | 27.6 ± 3.6 | 27 ± 3.3 | 26.1 ± 3.1 | <0.001 |
| Height (cm) | 174.4 ± 6.7 | 174.7 ± 6.6 | 174.4 ± 7 | 174.1 ± 7.2 | 0.485 |
| Weight (kg) | 87.7 ± 14.3 | 84.5 ± 12.9 | 82.1 ± 12.2 | 79.2 ± 11.1 | <0.001 |
| Appendicular skeletal lean mass (kg) | 24.6 ± 3.5 | 24.5 ± 3.5 | 24.1 ± 3.4 | 24 ± 3.2 | 0.025 |
| Percentage fat (total body) | 28.6 ± 5.3 | 26.7 ± 4.9 | 25.8 ± 4.7 | 24.1 ± 4.7 | <0.001 |
| Married | 289 (78.7) | 321 (87.5) | 305 (83.1) | 301 (81.8) | 0.018 |
| Non-white race | 29 (7.9) | 42 (11.4) | 37 (10.1) | 41 (11.1) | 0.375 |
| Excellent/good self-rated health | 290 (79.2) | 319 (86.9) | 303 (82.6) | 334 (90.8) | <0.001 |
| College education | 172 (46.9) | 183 (49.9) | 197 (53.7) | 222 (60.3) | 0.002 |
| Feel full of energy | 34 (9.3) | 19 (5.2) | 22 (6) | 20 (5.4) | 0.069 |
| Walking speed (m/sec) | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | <0.001 |
| Grip strength (kg) | 40.4 ± 8.6 | 41.2 ± 7.6 | 41.5 ± 7.8 | 42.9 ± 8.4 | <0.001 |
| PASE score | 138 ± 68.9 | 147.6 ± 67.2 | 149.8 ± 71.6 | 151.9 ± 67.2 | 0.033 |
| Leg power (watts) | 200.9 ± 60.4 | 208.2 ± 61.5 | 210.9 ± 66.4 | 213.7 ± 59.3 | 0.048 |
| No. of medical conditions | 1.4 ± 1.2 | 1.3 ± 1.2 | 1.2 ± 1.1 | 1.0 ± 1.0 | <0.001 |
| Smoking status | |||||
| Never | 126 (34.3) | 126 (34.3) | 144 (39.2) | 150 (40.8) | 0.062 |
| Past | 229 (62.4) | 232 (63.2) | 207 (56.4) | 198 (53.8) | |
| Current | 12 (3.3) | 9 (2.5) | 16 (4.4) | 20 (5.4) | |
| Drinks per week | |||||
| None (<1/month) | 145 (39.5) | 125 (34.2) | 118 (32.2) | 111 (30.2) | 0.177 |
| 1/month to 14 drinks/week | 178 (48.5) | 200 (54.6) | 206 (56.1) | 207 (56.3) | |
| ≥14 drinks/week | 44 (12) | 41 (11.2) | 43 (11.7) | 50 (13.6) | |
| Frailty criteria | |||||
| Low activity level | 88 (25.6) | 66 (18.7) | 69 (19.9) | 57 (16.2) | 0.017 |
| Exhaustion | 43 (11.7) | 27 (7.4) | 29 (7.9) | 22 (6) | 0.031 |
| Weakness | 93 (25.3) | 63 (17.2) | 64 (17.4) | 53 (14.4) | <0.001 |
| Shrinking/sarcopenia | 86 (23.7) | 67 (18.4) | 77 (21.2) | 61 (16.7) | 0.092 |
| Slowness | 101 (27.5) | 79 (21.5) | 64 (17.4) | 51 (13.9) | <0.001 |
| No. of frailty components | 1.1 ± 1.1 | 0.8 ± 1 | 0.8 ± 1 | 0.7 ± 0.9 | <0.001 |
| Frailty classification | |||||
| Robust | 134 (36.5) | 171 (46.6) | 173 (47.1) | 204 (55.4) | <0.001 |
| Pre-frail | 193 (52.6) | 171 (46.6) | 167 (45.5) | 144 (39.1) | |
| Frail | 40 (10.9) | 25 (6.8) | 27 (7.4) | 20 (5.4) | |
Data are expressed as mean ± sd or number (%). PASE, Physical Activity Scale for the Elderly.
Approximately 11% of men in the lowest quartile of bioavailable testosterone were frail at the baseline exam, compared with 5.4% in the highest quartile (Table 2). The prevalence of four of the five frailty components and the total number of frailty components was also highest in men in the lowest quartile of bioavailable testosterone. The average number of frailty components present decreased as the quartile of bioavailable testosterone increased. The prevalence of “shrinking/sarcopenia” was somewhat lower among men in the lowest quartile of bioavailable testosterone compared with higher quartiles, but this did not reach statistical significance.
Table 2.
Cross-sectional proportional ORs (95% CI) for greater frailty status by level of various sex hormones in elderly men
| Q1 | Q2 | Q3 | Q4 | Pfor trend | |
|---|---|---|---|---|---|
| E | <17.2 pg/ml | 17.2 to <21.7 pg/ml | 21.7 to <26.9 pg/ml | ≥26.9 pg/ml | |
| Age-adjusted | 1.05 (0.79, 1.40) | 0.85 (0.64, 1.13) | 0.88 (0.66, 1.17) | 1.00 (referent) | 0.801 |
| MV adjusted | 0.98 (0.73, 1.31) | 0.82 (0.61, 1.10) | 0.91 (0.68, 1.22) | 1.00 (referent) | 0.713 |
| Bioavailable E | <11.3 pg/ml | 11.3 to <14.0 pg/ml | 14.0 to <17.0 pg/ml | ≥17.0 pg/ml | |
| Age-adjusted | 1.17 (0.88, 1.56) | 1.00 (0.75, 1.33) | 0.86 (0.64, 1.14) | 1.00 (referent) | 0.178 |
| MV adjusted | 1.07 (0.79, 1.43) | 1.00 (0.74, 1.34) | 0.82 (0.61, 1.10) | 1.00 (referent) | 0.421 |
| T | <298 ng/dl | 298 to <388 ng/dl | 388 to <488 ng/dl | ≥488 ng/dl | |
| Age-adjusted | 1.48 (1.11, 1.98) | 1.10 (0.83, 1.47) | 1.15 (0.86, 1.53) | 1.00 (referent) | 0.013 |
| MV adjusted | 1.30 (0.95, 1.77) | 1.10 (0.81, 1.48) | 1.13 (0.84, 1.51) | 1.00 (referent) | 0.133 |
| Bioavailable T | <165.4 ng/dl | 165.4 to <201.5 ng/dl | 201.5 to <241.9 ng/dl | ≥241.9 ng/dl | |
| Age-adjusted | 1.68 (1.25, 2.25) | 1.27 (0.95, 1.70) | 1.24 (0.93, 1.66) | 1.00 (referent) | 0.001 |
| MV adjusted | 1.39 (1.02, 1.91) | 1.21 (0.90, 1.64) | 1.09 (0.81, 1.47) | 1.00 (referent) | 0.030 |
| SHBG | <35.7 nmol/liter | 35.7 to <46.5 nmol/liter | 46.5 to <59.3 nmol/liter | ≥59.3 nmol/liter | |
| Age-adjusted | 0.87 (0.65, 1.17) | 0.80 (0.60, 1.07) | 0.94 (0.70, 1.25) | 1.00 (referent) | 0.217 |
| MV adjusted | 0.78 (0.57, 1.06) | 0.78 (0.58, 1.05) | 0.97 (0.73, 1.31) | 1.00 (referent) | 0.052 |
Multivariate (MV) models are adjusted for age, BMI, self-rated health, marital status, education, smoking, and number of medical conditions. Number of participants is 1469 for age-adjusted models and 1468 for multivariate models. T, Testosterone; E, estradiol.
In age- and multivariate-adjusted models, there was no significant association between bioavailable estradiol, total estradiol, or SHBG and frailty status at baseline (Table 2). However, participants in the lowest quartile of total testosterone or bioavailable testosterone had a 48 and 68% increased likelihood of greater frailty status, respectively, compared with participants in the highest quartile of each sex hormone measure in age-adjusted models. The total testosterone association was no longer statistically significant after further adjustment for potential confounders including BMI, self-rated health, education, smoking, and number of medical conditions. However, bioavailable testosterone remained independently associated with frailty status after multivariate adjustment; participants in the lowest quartile of bioavailable testosterone had a 39% increased likelihood of greater frailty status compared with men in the highest quartile [Q1 vs. Q4, OR, 1.39; 95% confidence interval (CI), 1.02, 1.91; P for trend across quartiles, 0.030]. In multivariate models, men with clinically defined testosterone deficiency (total testosterone <250 ng/dl) had a somewhat increased likelihood of worse frailty status compared with men with higher testosterone levels (OR, 1.37; 95% CI, 1.00, 1.88; P = 0.054). In age-adjusted models, bioavailable testosterone was also related to the weakness, slowness, and exhaustion components of frailty individually at baseline (P < 0.05; data not shown); these associations were attenuated and no longer significant in multivariate models. Bioavailable testosterone was modestly associated with the shrinking component of frailty at baseline in age-adjusted models, and this association became significant after adjustment for covariates.
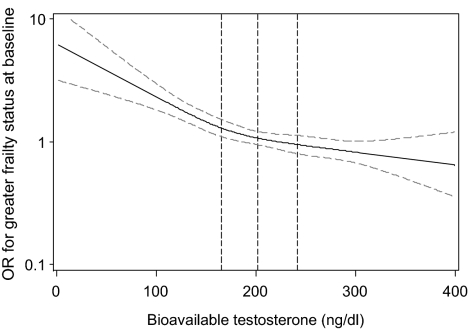
Consistent with the quartiles analysis, the graphical spline analysis demonstrated that the likelihood of greater frailty status at baseline increased as the level of bioavailable testosterone decreased (Fig. 2). No threshold level of bioavailable testosterone appears to exist.
Figure 2.
Restricted cubic spline plot of ORs for greater frailty status at baseline by level of bioavailable testosterone. Dotted vertical lines represent the quartile cut-points for bioavailable testosterone: Q1, ≤165.4 ng/dl; Q2, 165.4 to <201.5 ng/dl; Q3, 201.5 to <241.9 ng/dl; and Q4, ≥241.9 ng/dl. Gray dotted lines represent the 95% CI for the OR.
Longitudinal results
At the second clinic visit, 437 (35.1%) men were robust, 517 (41.5%) were intermediate, 131 (10.5%) were frail, and 160 (12.9%) had died. Consistent with the cross-sectional data, there was no association between bioavailable estradiol, total estradiol, SHBG, or total testosterone and frailty status at visit 2 in multivariate models adjusted for age, baseline frailty status, and confounding factors (Table 3). In contrast, lower levels of bioavailable testosterone were associated with an increased risk of frailty at visit 2 after adjustment for age and baseline frailty status. Men in the lowest quartile of bioavailable testosterone were 1.51-fold (95% CI, 1.10, 2.07) more likely to have worse frailty status at visit 2 than men in the highest quartile of bioavailable testosterone at baseline (P for trend across quartiles, 0.005). Adjustment for confounding factors (age, BMI, self-rated health, marital status, education, smoking, and number of medical conditions and baseline frailty status) attenuated the association; men in the lowest quartile of bioavailable testosterone appear to have a modest increase in the likelihood of worse frailty status that did not reach significance (Q1 vs. Q4, OR, 1.32; 95% CI, 0.94, 1.84), whereas the linear trend across quartiles remained of borderline significance (P for trend across quartiles = 0.055). Testosterone deficiency at baseline (total testosterone <250 ng/dl) was not associated with frailty status at visit 2. Finally, age at baseline was associated with increased odds of greater frailty status at follow-up; each 5-yr increase in baseline age was associated with a 1.76-fold (95% CI, 1.56, 2.00) increased likelihood of greater frailty status at follow-up in models that were also adjusted for baseline frailty status and bioavailable testosterone.
Table 3.
Longitudinal proportional ORs (95% CI) for greater frailty status at visit 2, by level of various sex hormones at baseline in elderly men
| Q1 | Q2 | Q3 | Q4 | Pfor trend | |
|---|---|---|---|---|---|
| E | <17.2 pg/ml | 17.2 to <21.7 pg/ml | 21.7 to <26.9 pg/ml | ≥26.9 pg/ml | |
| Age, baseline status adjusted | 1.17 (0.87, 1.60) | 0.99 (0.72, 1.34) | 1.18 (0.83, 1.69) | 1.00 (referent) | 0.282 |
| MV adjusted | 1.12 (0.82, 1.53) | 0.96 (0.70, 1.32) | 0.97 (0.71, 1.32) | 1.00 (referent) | 0.517 |
| Bioavailable E | <11.3 pg/ml | 11.3 to <14.0 pg/ml | 14.0 to <17.0 pg/ml | ≥17.0 pg/ml | |
| Age, baseline status adjusted | 0.96 (0.70, 1.30) | 0.99 (0.73, 1.34) | 0.72 (0.53, 0.99) | 1.00 (referent) | 0.751 |
| MV adjusted | 0.92 (0.67, 1.25) | 1.04 (0.76, 1.41) | 0.71 (0.52, 0.97) | 1.00 (referent) | 0.852 |
| T | <298 ng/dl | 298 to <388 ng/dl | 388 to <488 ng/dl | ≥488 ng/dl | |
| Age, baseline status adjusted | 1.25 (0.92, 1.69) | 1.57 (1.10, 2.24) | 1.05 (0.77, 1.42) | 1.00 (referent) | 0.093 |
| MV adjusted | 1.09 (0.79, 1.51) | 1.53 (1.06, 2.20) | 1.04 (0.76, 1.43) | 1.00 (referent) | 0.409 |
| Bioavailable T | <165.4 ng/dl | 165.4 to <201.5 ng/dl | 201.5 to <241.9 ng/dl | ≥241.9 ng/dl | |
| Age, baseline status adjusted | 1.51 (1.10, 2.07) | 1.29 (0.95, 1.76) | 1.06 (0.78, 1.45) | 1.00 (referent) | 0.005 |
| MV adjusted | 1.32 (0.94, 1.84) | 1.20 (0.87, 1.65) | 0.97 (0.71, 1.33) | 1.00 (referent) | 0.055 |
| SHBG | <35.7 nmol/liter | 35.7 to <46.5 nmol/liter | 46.5 to <59.3 nmol/liter | ≥59.3 nmol/liter | |
| Age, baseline status adjusted | 1.10 (0.80, 1.51) | 0.99 (0.73, 1.36) | 1.18 (0.86, 1.60) | 1.00 (referent) | 0.846 |
| MV adjusted | 0.94 (0.67, 1.32) | 0.98 (0.71, 1.36) | 1.22 (0.89, 1.68) | 1.00 (referent) | 0.465 |
Multivariate (MV) models are adjusted for age, BMI, self-rated health, marital status, education, smoking, number of medical conditions, and baseline frailty status. Number of participants is 1245 for age-adjusted models for estradiol, total testosterone, and SHBG, and 1244 for multivariate models. T, Testosterone; E, estradiol.
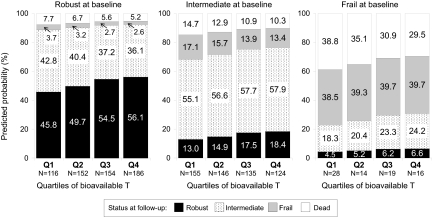
The probability of being classified in a given frailty category at visit 2 depended on both baseline bioavailable testosterone level and baseline frailty status (Fig. 3). To illustrate, a robust man of average age in the cohort (73.4 yr) with bioavailable testosterone in the highest quartile (Q4) had a 56.1% probability (95% CI, 50.0%, 62.1%) of being robust at visit 2, whereas a robust man of average age with bioavailable testosterone levels in the lowest quartile had only a 45.8% probability (95% CI, 39.2%, 52.4%) of being robust at visit 2. However, frailty status at baseline was a stronger determinant of frailty status at visit 2. For example, a frail man of average age in the cohort with bioavailable testosterone in the highest quartile had only a 6.6% (95% CI, 0.01%, 13.8%) chance of being robust at visit 2. A frail man at baseline with lower levels of bioavailable testosterone had an even lower probability of being robust at visit 2; a frail man of average age for the cohort with bioavailable testosterone values in the lowest quartile had only a 4.5% (95% CI, 0.01%, 9.5%) chance of being robust at visit 2.
Figure 3.
Predicted probability of frailty status at follow-up, by quartile of bioavailable testosterone and frailty status at baseline for a 73.4-yr-old man. Cut-points for quartiles of bioavailable testosterone: Q1, ≤165.4 ng/dl; Q2, 165.4 to <201.5 ng/dl; Q3, 201.5 to <241.9 ng/dl; and Q4, ≥241.9 ng/dl. Probabilities derived from a partial proportional odds model, with age and bioavailable testosterone meeting the proportionality assumption, and frailty status at baseline not meeting the proportional odds assumption. The age assumed for the model (73.4 yr) is the mean age of the population at baseline.
There was no interaction between baseline frailty status and bioavailable testosterone level. The interaction term between these factors (three level baseline frailty status × quartiles of bioavailable testosterone) was not significant (P > 0.10). Therefore, unstratified results summarizing the association of bioavailable testosterone on frailty status at visit 2 can be reported across all levels of baseline frailty status (as was done in Table 3).
Discussion
Low levels of bioavailable testosterone were independently associated with an increased likelihood of worse frailty status in older, community-dwelling men in cross-sectional analyses. Men with low baseline bioavailable testosterone levels had an increased likelihood of worse frailty status at follow-up, but adjustment for confounding factors reduced the strength of that association. The association between total testosterone and baseline frailty status was of borderline significance; no association between total testosterone and frailty status at visit 2 was observed. Estradiol, bioavailable estradiol, and SHBG concentrations were not associated with frailty status at baseline or at the follow-up visit.
A previous report from the Massachusetts Male Aging Study (MMAS) found no association between total or free testosterone and frailty status (11); bioavailable testosterone was not evaluated. High SHBG was associated with increased odds of being frail after adjustment for age, chronic disease, lifestyle factors, diet, and physical activity. Direct RIA methods were used to measure testosterone in the MMAS report, and such methods can be subject to measurement error, particularly at lower concentrations, compared with the gold standard of liquid chromatography-tandem mass spectrometry used in the current MrOS analyses (21,22,23). Additionally, unlike the MMAS report, the MrOS analyses also used statistical methods (i.e. proportional odds models) that accounted for the ordinal nature of the frailty outcome. Finally, the age ranges of the MMAS and MrOS cohorts differed: the mean age at the time of testosterone assays in MMAS was 67.9 yr (range, 50–89 yr), whereas the mean age in MrOS at baseline was 73.6 yr (range, 65–99 yr). These differences between the MMAS and MrOS analyses may explain the discrepancy in the findings between these two cohorts.
Many operational definitions for frailty exist, and each has its own strengths and limitations. The CHS definition used in these analyses has been widely studied in various modified forms (4,5,6,7,8), is based on a sound theoretical framework (1), and defines frailty as an entity distinct from disability or comorbidity. However, two main limitations of the CHS construct exist. First, not all cohort studies have collected information in the exact same manner as in CHS; thus, the small differences in how each component is defined may introduce measurement error and could limit the comparison of results between studies. Second, the CHS definition may be difficult to implement clinically because five separate domains must be assessed. A more parsimonious definition for frailty has been proposed using three measures: weight loss, poor energy, and repeated chair stands ability (24,25). How this simpler definition may be related to sex hormone levels has yet to be elucidated; however, the simplified index does predict adverse outcomes such as falls, fractures, disability, and mortality as well as the CHS index in older men and women (24,25). Additionally, not all alternative frailty definitions (26,27,28) consider frailty to be a separate entity from disability and comorbidity. Because the goals of these analyses were to identify associations between sex hormones and frailty per se, and not the associations between sex hormones and disability or comorbid illness, the modified CHS criteria were the best choice of several alternatives for this report.
There are two hypotheses that would explain why the cross-sectional and longitudinal associations between bioavailable testosterone and frailty status are not completely consistent. The first hypothesis is that there is truly no relationship between bioavailable testosterone and frailty status. Accepting this hypothesis would lead us to conclude that our observed cross-sectional associations are due to residual confounding. Given our ability to accurately assess and account for potentially confounding factors in our well-characterized cohort, we consider this possibility to be unlikely. However, in an observational study, the possibility of residual confounding cannot be ruled out completely. The alternative hypothesis is that bioavailable testosterone is truly related to frailty status. Accepting this hypothesis would lead us to conclude that our longitudinal results are consistent with the cross-sectional results, but that the longitudinal associations do not reach statistical significance because of limited power to detect an association due to smaller sample size. In light of these two possibilities, we conclude that bioavailable testosterone levels appear to be related to frailty status. However, a causal association between these two factors can only be established in a randomized trial.
The implications of these results are many. These data provide further evidence that frailty is an outcome that should be considered in clinical trials of testosterone supplementation or other agents that alter sex hormone pathways. Testosterone supplementation has been proposed as a possible treatment for frailty (9,29), and a number of selective-androgen receptor modulators that are currently in development might also influence frailty status via testosterone-related pathways (30). These results suggest that whereas frailty should be considered as an outcome in clinical trials, interventions that do not alter the non-SHBG-bound fraction of testosterone (and presumably its bioavailability) may not have an impact on frailty status.
The strengths of this study include excellent characterization of a large number of older men and gold standard measures of sex hormones. However, a few limitations should be noted. A number of participants (17% of survivors) were missing frailty data at the second clinic visit. Previous reports from this cohort suggest that frailty at baseline is associated with having incomplete data at the second clinic visit (8). Therefore, the overall prevalence of frailty in this cohort is probably underestimated at the second clinic visit. Additionally, although frailty status was available at two time points, the concurrent change in sex hormone levels was not assessed. Thus, it was not possible to assess whether worsening frailty status was associated with changes in sex hormone levels.
Although the association between bioavailable testosterone at baseline and frailty status at follow-up were modest in age-adjusted models, few factors aside from baseline frailty status are strongly associated with frailty status at follow-up. For example, each 5-yr increase in baseline age was associated with only a 1.76-fold increased likelihood of greater frailty status at follow-up.
The biological mechanisms through which bioavailable testosterone may act on frailty status are not well elucidated. Testosterone may act to increase muscle size and strength [possibly through increased protein synthesis (31)], which could then influence each of the five components of frailty, most directly the shrinking and weakness components. Additionally, declining levels of testosterone may be associated with a proinflammatory state (32,33), and a proinflammatory state has been associated with poor physical performance (34) and frailty (35). Further research into the biological underpinnings of the bioavailable testosterone and frailty relationship is needed.
In summary, lower bioavailable testosterone levels were associated with an increased likelihood of worse frailty status in cross-sectional analyses, independent of potentially confounding factors. In longitudinal analyses, lower bioavailable testosterone was modestly associated with increased likelihood of worsening frailty status at the follow-up visit; however, this association was mostly explained by adjustment for potential confounders. In contrast to the modest associations seen between bioavailable testosterone and frailty status at follow-up, frailty status at baseline is very strongly associated with frailty status at follow-up.
Supplementary Material
Acknowledgments
Investigators in the Osteoporotic Fractures in Men (MrOS) Research Group: Coordinating Center (California Pacific Medical Center Research Institute and University of California, San Francisco): S. R. Cummings (Principal Investigator), D. C. Bauer (co-Investigator), D. M. Black (co-Investigator), P. M. Cawthon (co-Investigator), M. C. Nevitt (co-Investigator), K. L. Stone (co-Investigator), R. Fullman (Project Director), R. Benard, T. Blackwell, A. Chau, L. Christianson, L. Concepcion, J. Diehl, S. Ewing, M. Farrell, C. Fox, S. Hoffland, J. Ireland, M. Jaime-Chavez, E. Kwan, S. L. Harrison, W. Liu, L. Y. Lui, A. Mills, L. Nusgarten, L. Palermo, N. Parimi, L. Perreault, J. Schneider, R. Scott, D. Tanaka, C. Yeung. Administrative Center (Oregon Health & Sciences University): E. Orwoll (Principal Investigator), K. Phipps (co-Investigator), L. Marshall (co-Investigator), J. Babich Blank (Project Director), L. Lambert, C. Nielson, Y. Wang, C. Petersen, M. Powell. University of Alabama, Birmingham: C. E. Lewis (Principal Investigator), J. Shikany (co-Investigator), P. Johnson (Project Director), N. Webb, K. Hardy, S. Felder, J. Wilkoff, J. King, T. Johnsey, M. Young, C. Atkins, C. Collier, J. Smith, C. Sassaman. University of Minnesota: K. Ensrud (Principal Investigator), H. Fink (co-Investigator), N. Nelson (Clinic Coordinator), P. Van Coevering (Program Director), S. Fillhouer (Project Director), R. Andrews, C. Bowie, M. Forseth, R. Gran, F. Imker-Witte, S. Luthi, K. Moen, N. Muehlbauer, M. Paudel, M. Slindee, S. Ziesche. Stanford University: M. Stefanick (Principal Investigator), A. Hoffman (co-Investigator), K. Kent, N. Ellsworth, A. Krauss, R. Gupta, S. Hartley, M. Bowers. University of Pittsburgh: J. Cauley (Principal Investigator), J. Zmuda (co-Investigator), M. Danielson (Study Administrator), L. Harper (Project Director), L. Buck (Clinic Coordinator), M. Nasim, D. Cusick, M. Gorecki, N. Watson, C. Bashada, C. Newman. University of California, San Diego: E. Barrett-Connor (Principal Investigator), T. Dam (co-Investigator), M. L. Carrion-Petersen (Project Director), P. Miller, N. Kamantigue, K. Marksbury, M. Stephens, and Z. Torres.
The authors thank Ms. Liezl Concepcion for her administrative assistance with the manuscript.
Footnotes
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health (NIH) funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 15, 2009
Abbreviations: BMI, Body mass index; CHS, Cardiovascular Health Study; CI, confidence interval; MrOS, Osteoporotic Fractures in Men (study); OR, odds ratio; Q, quartile.
References
- Fried LP, Walston J 1998 Frailty and failure to thrive. In: Hazzard WR, Blass JP, Ettinger WHJ, Halter JB, Ouslander J, eds. Principles of geriatric medicine and gerontology. 4th ed. New York: McGraw Hill; 1387–1402 [Google Scholar]
- Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP 2006 Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 54:991–1001 [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA 2001 Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–156 [DOI] [PubMed] [Google Scholar]
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP 2006 Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci 61:262–266 [DOI] [PubMed] [Google Scholar]
- Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP 2005 The association between obesity and the frailty syndrome in older women: the Women’s Health and Aging Studies. J Am Geriatr Soc 53:927–934 [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, Tracy JK, Hochberg MC, Rodondi N, Cawthon PM 2007 Frailty and risk of falls, fracture and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 62:744–751 [DOI] [PubMed] [Google Scholar]
- Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB 2005 Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc 53:1321- 1330 [DOI] [PubMed] [Google Scholar]
- Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, Orwoll ES 2007 Frailty in older men: prevalence, progression and relation to mortality. J Am Geriatr Soc 55:1216–1223 [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A 2005 The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 26:833–876 [DOI] [PubMed] [Google Scholar]
- Bylow K, Mohile SG, Stadler WM, Dale W 2007 Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer? A conceptual review. Cancer 110:2604–2613 [DOI] [PubMed] [Google Scholar]
- Mohr BA, Bhasin S, Kupelian V, Araujo AB, O'Donnell AB, McKinlay JB 2007 Testosterone, sex hormone-binding globulin, and frailty in older men. J Am Geriatr Soc 55:548–555 [DOI] [PubMed] [Google Scholar]
- Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR 2005 Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26:557–568 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K 2005 Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26:569–585 [DOI] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA 1993 The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162 [DOI] [PubMed] [Google Scholar]
- Ware J, Kosinski M, Keller S 1998 SF-12: How to score the SF-12 Physical and Mental Health Summary Scores. 3rd ed. Lincoln, RI: QualityMetric Incorporated [Google Scholar]
- Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P 1994 Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 10:405–411 [DOI] [PubMed] [Google Scholar]
- Södergård R, Bäckström T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17 β to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM 1999 A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine 2004 Testosterone and aging: clinical research directions. In: Liverman CT, Blazer DG, eds. Washington, DC: National Academies Press [PubMed] [Google Scholar]
- Scott SC, Goldberg MS, Mayo NE 1997 Statistical assessment of ordinal outcomes in comparative studies. J Clin Epidemiol 50:45–55 [DOI] [PubMed] [Google Scholar]
- Matsumoto AM, Bremner WJ 2004 Serum testosterone assays—accuracy matters. J Clin Endocrinol Metab 89:520–524 [DOI] [PubMed] [Google Scholar]
- Taieb J, Mathian B, Millot F, Patricot MC, Mathieu E, Queyrel N, Lacroix I, Somma-Delpero C, Boudou P 2003 Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem 49:1381–1395 [DOI] [PubMed] [Google Scholar]
- Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS 2004 Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab 89:534–543 [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, Tracy JK, Cummings SR 2008 Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med 168:382–389 [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Ewing SK, Cawthon PM, Fink HA, Taylor BC, Cauley JA, Dam TT, Marshall LM, Orwoll ES, Cummings SR 2009 A comparison of frailty indexes for the prediction of falls, disability, fractures and mortality in older men. J Am Geriatr Soc 57:492–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnitski AB, Song X, Rockwood K 2004 The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci 59:M627–M632 [DOI] [PubMed] [Google Scholar]
- Rockwood K, Howlett SE, MacKnight C, Beattie BL, Bergman H, Hébert R, Hogan DB, Wolfson C, McDowell I 2004 Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci 59:1310–1317 [DOI] [PubMed] [Google Scholar]
- Studenski S, Hayes RP, Leibowitz RQ, Bode R, Lavery L, Walston J, Duncan P, Perera S 2004 Clinical global impression of change in physical frailty: development of a measure based on clinical judgment. J Am Geriatr Soc 52:1560–1566 [DOI] [PubMed] [Google Scholar]
- Muller M, Grobbee DE, Thijssen JH, van den Beld AW, van der Schouw YT 2003 Sex hormones and male health: effects on components of the frailty syndrome. Trends Endocrinol Metab 14:289–296 [DOI] [PubMed] [Google Scholar]
- Kilbourne EJ, Moore WJ, Freedman LP, Nagpal S 2007 Selective androgen receptor modulators for frailty and osteoporosis. Curr Opin Investig Drugs 8:821–829 [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ 2002 Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282:E601–E607 [DOI] [PubMed] [Google Scholar]
- Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, Valenti G, Ling SM, Ferrucci L 2006 Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab 91:345–347 [DOI] [PubMed] [Google Scholar]
- Maggio M, Basaria S, Ceda GP, Ble A, Ling SM, Bandinelli S, Valenti G, Ferrucci L 2005 The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 28:116–119 [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Guralnik JM, Ferrucci L 2004 Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 59:242–248 [DOI] [PubMed] [Google Scholar]
- Hubbard RE, O'Mahony MS, Savva GM, Calver BL, Woodhouse KW 6 March 2009 Inflammation and frailty measures in older people. J Cell Mol Med 10.1111/j.1582–4934.2009.00733.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.