Abstract
Context: Visceral adiposity is a strong determinant of GH secretion, and low endogenous GH secretion is associated with increased insulin resistance, a key component of the metabolic syndrome. Increased fat accumulation in skeletal muscle and liver may play an etiological role in the development of insulin resistance and other complications of the metabolic syndrome. Little is known about the role of decreased endogenous GH secretion in the pathogenesis of insulin resistance in obesity.
Objective: To investigate the relationship between intramyocellular lipids (IMCL), intrahepatic lipids, and peak-stimulated GH in premenopausal women with obesity.
Design and Setting: We conducted a cross-sectional study at a clinical translational research center.
Patients: Patients included 21 premenopausal women with obesity (mean body mass index, 34.0 ± 4.5 kg/m2) and 17 normal-weight controls (mean body mass index, 21.9 ± 2.0 kg/m2) of comparable mean age.
Main Outcomes Measures: IMCL and intrahepatic lipids were measured with proton magnetic resonance spectroscopy (1H-MRS). Body composition was measured with magnetic resonance imaging. Peak GH was measured after stimulation with GHRH-arginine.
Results: Obese subjects had higher IMCL, intrahepatic lipids, abdominal and thigh fat, and thigh muscle mass compared with normal-weight controls. There were strong inverse associations between peak GH and both IMCL and intrahepatic lipids independent of age and visceral adiposity. There were positive associations between IMCL and intrahepatic lipids with measures of insulin resistance and serum triglycerides.
Conclusion: In premenopausal women with obesity, peak GH is inversely associated with IMCL and intrahepatic lipids independent of age and visceral adiposity. This suggests that low GH may contribute to insulin resistance in obesity through effects on muscle and intrahepatic lipids.
Peak growth hormone after GHRH-arginine stimulation is inversely associated with intramyocellular and intrahepatic lipids in premenopausal women with obesity.
Obesity is a known risk factor for the development of insulin resistance and a key component of the metabolic syndrome. Several similarities exist between patients with the metabolic syndrome and patients with adult GH deficiency (1,2). Both conditions are characterized by increased visceral fat depots, nonalcoholic fatty liver disease, insulin resistance, high levels of triglycerides, and increased mortality from cardiovascular events (3,4). With increased visceral adiposity, physiological GH secretion is impaired, and GH response to stimuli is decreased (5,6). However, the effects of GH on glucose metabolism and insulin resistance are not well understood. GH increases adipocyte lipolysis and lipid turnover, resulting in increased plasma free fatty acids (FFA) (7,8). This increase in FFA has been implicated in GH-induced insulin resistance (9), which is observed acutely with GH administration. A lack of increased insulin resistance with chronic GH administration observed in several studies (10,11) has been attributed to the opposing effects of GH on body composition (10,11). Therefore, further studies are needed to investigate the mechanisms responsible for these effects.
Previous studies have shown that intramyocellular lipids (IMCL) and intrahepatic lipids are increased in obesity and non-insulin-dependent diabetes mellitus and that quantifications of IMCL and intrahepatic lipids obtained with proton magnetic resonance spectroscopy (1H-MRS) are reliable noninvasive surrogate markers of insulin sensitivity in individuals who are healthy or obese or have type 2 diabetes (3,4,12,13,14,15,16,17,18,19,20,21). It has been hypothesized that increased IMCL and intrahepatic lipids may play an etiological role in the development of insulin resistance (13). Little is known about the effects of low GH on these parameters in obesity. We have demonstrated an inverse association between IMCL and GH in adolescents with obesity (22). Franco et al. (11) have shown a decrease in intrahepatic lipids and visceral fat after GH treatment in postmenopausal women with obesity. However, the relationship between peak stimulated GH and either IMCL or intrahepatic lipids in healthy lean and obese women of reproductive age has not been investigated.
We hypothesize that in obese premenopausal women, low peak GH after stimulation with GHRH-arginine would be associated with higher IMCL and intrahepatic lipids, abdominal fat, and increased insulin resistance. We therefore evaluated calf muscle IMCL, intrahepatic lipid content, abdominal fat, thigh fat, and thigh muscle mass using 1H-MRS and magnetic resonance imaging (MRI) in obese premenopausal women and normal-weight controls to determine whether there is an association between these parameters and GH secretion and insulin resistance.
Subjects and Methods
The study was approved by the institutional review board of Partners Healthcare Inc. and was Health Insurance Portability and Accountability Act compliant. Written informed consent was obtained from all subjects before the study.
Subjects
The study group was comprised of 38 healthy premenopausal women who were recruited from the community through advertisements. There were 21 obese subjects [body mass index (BMI), 34.0 ± 4.5 kg/m2) and 17 normal-weight controls (BMI, 21.9 ± 2.0 kg/m2)]. Inclusion criteria for the obese group were ages 18–45 yr, female gender, and BMI higher than 25 kg/m2. Exclusion criteria included hypothalamic or pituitary disorders, diabetes mellitus or other chronic illnesses, estrogen or glucocorticoid use, and weight greater than 280 pounds (due to the limitations of the MRI scanner). Participants were admitted to the General Clinical Research Center at the Massachusetts General Hospital, where testing was performed. Each participant underwent 1H-MRS and MRI as detailed below, fasting blood tests, and an oral glucose tolerance test. A GHRH-arginine stimulation test was performed in 30 subjects, after which GHRH became unavailable in the United States.
Endocrine testing
Subjects underwent the following blood tests: fasting determination of lipid profile [total, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides], glucose, insulin, IGF-I, and a GHRH-arginine stimulation test. For the GHRH-arginine stimulation test (n = 30), GHRH 1 μg/kg plus arginine 0.5 g/kg (maximum 30 g) iv were administered and GH levels drawn at baseline and every 30 min for 2 h, the standard protocol used to diagnose GH deficiency in patients with hypopituitarism (23). In addition, a standard oral glucose tolerance test with 75 g glucose load was performed. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as [fasting glucose (mmol/l) × fasting insulin (μU/ml)]/22.5 (24). All hormones and metabolites were measured in the Clinical Laboratories at the Massachusetts General Hospital, as previously described (25).
1H-MRS of muscle and liver
1H-MRS was performed using a 3.0-T (Siemens Trio; Siemens Medical Systems, Erlangen, Germany) MRI system. After an 8-h overnight fast, each subject underwent 1H-MRS of calf muscle and 1H-MRS of the liver. For calf muscle 1H-MRS, a voxel measuring 15 × 15 × 15 mm (3.4 ml) was placed on the axial T1-weighted slice with largest muscle cross-sectional area of the tibialis anterior and subsequently the soleus, avoiding visible interstitial tissue, fat, or vessels. Single-voxel 1H-MRS data were acquired using point-resolved spatially localized spectroscopy pulse sequence as previously described (26). For liver 1H-MRS, a breath-hold true fast imaging with steady precession sequence was obtained. A voxel measuring 20 × 20 × 20 mm (8 ml) was placed within the right lobe of the liver, avoiding vessels or artifact. Breath-hold single-voxel 1H-MRS data were acquired using point-resolved spatially localized spectroscopy pulse sequence without water suppression with the following parameters: TE of 30 msec, TR of 1500 msec, eight acquisitions, 1024 data points, and receiver bandwidth of 2000 Hz.
1H MRS data analysis
Fitting of all 1H-MRS data were performed using LCModel (version 6.1-4A; Stephen Provencher, Oakville, Ontario, Canada) (27). Data were transferred from the MR scanner to a Linux workstation, and metabolite quantification was performed using eddy current correction and water scaling. The fitting algorithm was customized for muscle analysis providing estimates for lipid peaks (0.9, 1.1, 1.3, 1.5, 2.1, and 2.3 ppm), creatine (2.8 and ∼3.0 ppm), trimethylamines (3.2 ppm), and putative taurine signal (∼3.5 ppm). Data for IMCL (1.3 ppm) and extramyocellular lipids (EMCL) (1.5 ppm) methylene protons were used for statistical analyses. LCModel IMCL and EMCL estimates were automatically scaled to unsuppressed water peak (4.7 ppm) and expressed in lipid to water ratio (percent). For liver 1H-MRS, a customized fitting algorithm for liver analysis provided estimates for all lipid signals combined (0.9, 1.3, and 2.3 ppm). LCModel liver lipid estimates were automatically scaled to unsuppressed water peak (4.7 ppm), and lipid to water ratio (percent) is expressed.
Body composition measurement
Single-axial MRI slice through the abdomen at the level of L4 and a single slice through the left midthigh were obtained (Siemens Trio). For the single slice through the abdomen, visceral adipose tissue (VAT) and sc adipose tissue (SAT) areas and total adipose tissue (the sum of SAT and VAT) were determined based on offline analysis of tracings obtained using commercial software (VITRAK, Merge/eFilm, Milwaukee, WI). Abdominal fat determined by single-slice MRI has been shown to be closely correlated with total body abdominal fat volumes (28). From the single slice through the midthigh, SAT (thigh sc fat) and thigh muscle areas were determined using the same method.
Statistical analysis
JMP Statistical Database Software (version 5.0.1; SAS Institute, Cary, NC) was used for statistical analyses. Variables were tested for normality of distribution using the Wilk-Shapiro test. Variables that were normally distributed were compared using the Student’s t test. Variables that were not normally distributed were compared using the nonparametric Wilcoxon rank sum test. Nonparametric Spearman rank correlation coefficients are reported. Multivariate standard least squares regression modeling was performed to control for age, weight, and visceral adiposity. Forward stepwise regression modeling was also performed. P < 0.05 was used to denote significance.
Results
Clinical characteristics of study subjects
Subject characteristics are shown in Table 1. The age of study participants ranged from 22–45 yr, with a mean of 33.6 ± 7.0 yr. Study participants ranged in BMI from 18.1–42.0 kg/m2, with a mean of 28.6 ± 7.0 kg/m2 and in weight from 46–117 kg, with a mean of 78 ± 19 kg. Obese subjects had lower peak GH on the GHRH-arginine stimulation test, and there was no significant difference in mean IGF-I levels. Obese subjects had higher LDL cholesterol, lower mean HDL cholesterol, and higher mean triglyceride levels compared with normal-weight controls. The glucose area under the curve (AUC), insulin, and HOMA-IR were higher in obese subjects compared with normal-weight controls. Six subjects had impaired glucose tolerance, defined as a 2-h glucose value higher than 140 mg/dl, and no subject had a 2-h glucose higher than 200 mg/dl.
Table 1.
Clinical characteristics of obese subjects and normal-weight controls (means ± sd)
| Obese (n = 21) | Controls (n = 17) | P | |
|---|---|---|---|
| Age (yr) | 35.4 ± 7.0 | 31.5 ± 6.5 | NS |
| Weight (kg) | 91.6 ± 12.2 | 61.0 ± 8.6 | <0.0001 |
| BMI (kg/m2) | 34.0 ± 4.5 | 21.9 ± 2.0 | <0.0001 |
| GH stim peak (ng/ml) | 17.2 ± 9.7 | 28.8 ± 10.7 | 0.004 |
| IGF-I (ng/ml) | 130 ± 67 | 164 ± 28 | NS |
| Total cholesterol (mg/dl) | 172 ± 33 | 155 ± 31 | NS |
| HDL cholesterol (mg/dl) | 44 ± 11 | 53 ± 7 | 0.009 |
| LDL cholesterol (mg/dl) | 105 ± 28 | 87 ± 27 | 0.06 |
| Triglycerides (mg/dl) | 119 ± 59 | 77 ± 41 | 0.02 |
| Glucose AUC (mg/dl · 120 min) | 16,470 ± 3,531 | 13,089 ± 1,721 | 0.0005 |
| Insulin (μU/ml) | 14.6 ± 5.1 | 10.4 ± 3.7 | 0.02 |
| HOMA-IR | 3.5 ± 1.2 | 2.0 ± 0.7 | 0.0003 |
NS, Not significant.
IMCL and intrahepatic lipid content and body composition
Obese subjects had higher mean IMCL of the tibialis anterior and soleus muscles (IMCL-TA and IMCL-SOL, respectively) and higher mean intrahepatic lipids as determined by 1H-MRS compared with normal-weight controls (Figs. 1 and 2). Obese subjects had higher mean total, sc, and visceral abdominal fat, higher sc thigh fat, and higher mean thigh muscle mass as determined by MRI compared with normal-weight controls (Table 2).
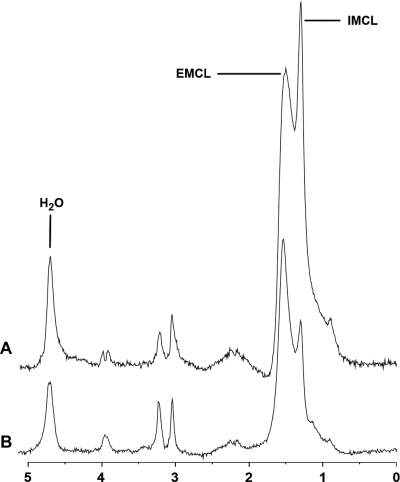
Figure 1.
1H-MRS of soleus muscle obtained at 3.0 T from a subject with obesity (A) (BMI, 33 kg/m2) and age-matched normal-weight control (B) (BMI, 19 kg/m2). The obese subject shows relatively higher IMCL and EMCL resonances consistent with higher lipid content. IMCL, IMCL methylene protons at 1.3 ppm; EMCL, EMCL methylene protons at 1.5 ppm; H2O, residual water signal.
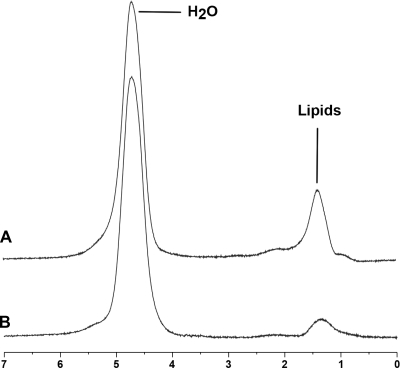
Figure 2.
1H-MRS of liver obtained at 3.0 T from a subject with obesity (A) (BMI, 32 kg/m2) and a normal-weight control (B) (BMI, 24 kg/m2) showing higher intrahepatic lipid content in the obese subject.
Table 2.
IMCL and intrahepatic lipids and body composition in obese subjects and normal-weight controls (means ± sd)
| Obese (n = 21) | Normal (n = 17) | P | |
|---|---|---|---|
| IMCL-TA/H2O (%) | 0.5 ± 0.2 | 0.3 ± 0.2 | 0.008 |
| IMCL-SOL/H2O (%) | 2.4 ± 0.9 | 1.4 ± 0.5 | 0.0006 |
| Liver lipids/H2O (%) | 8.6 ± 9.0 | 2.5 ± 3.3 | 0.01 |
| TAT (cm2) | 625.7 ± 204.4 | 231.9 ± 101.8 | <0.0001 |
| SAT (cm2) | 476.3 ± 156.4 | 189.3 ± 97.0 | <0.0001 |
| VAT (cm2) | 149.4 ± 75.2 | 42.6 ± 16.5 | <0.0001 |
| sc fat, thigh (cm2) | 179.3 ± 46.0 | 88.1 ± 26.5 | <0.0001 |
| Muscle, thigh (cm2) | 142.0 ± 18.0 | 107.8 ± 15.9 | <0.0001 |
TAT, Total adipose tissue.
Associations of IMCL and intrahepatic lipids with GH, body composition, serum lipids, and measures of insulin resistance
There was an inverse correlation between peak GH after stimulation with GHRH-arginine and IMCL-TA, IMCL-SOL, and intrahepatic lipids (Table 3 and Figs. 3 and 4). Because GH declines with increasing age and with increasing visceral adiposity, we corrected for age and visceral adiposity using multivariate analysis. After controlling for age and visceral adiposity, there remained a significant inverse association between GH and IMCL-TA (P = 0.04) and a trend between GH and intrahepatic lipids (P = 0.08). However, there was no association between GH and IMCL-SOL (P = 0.3) after correcting for age and visceral adiposity. There was an inverse correlation between IGF-I and intrahepatic lipids (r = −0.34; P = 0.04), which was not significant after controlling for age and visceral adiposity (P = 0.1). There were positive correlations between IMCL and intrahepatic lipids with abdominal fat and thigh muscle mass and measures of insulin resistance and triglycerides (Table 3). There were no significant correlations between IMCL and intrahepatic lipids with age or serum cholesterol.
Table 3.
Associations of IMCL and intrahepatic lipids with body composition, peak GH, measures of insulin resistance, and serum triglycerides
| IMCL-TA
|
IMCL-SOL
|
Intrahepatic fat
|
||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Peak GH | −0.50 | 0.005 | −0.44 | 0.02 | −0.52 | 0.004 |
| Weight | 0.42 | 0.009 | 0.62 | <0.0001 | 0.48 | 0.003 |
| BMI | 0.45 | 0.005 | 0.71 | <0.0001 | 0.55 | 0.0004 |
| TAT | 0.49 | 0.002 | 0.66 | <0.0001 | 0.54 | 0.0007 |
| SAT | 0.51 | 0.001 | 0.63 | <0.0001 | 0.54 | 0.0008 |
| VAT | 0.44 | 0.007 | 0.7 | <0.0001 | 0.54 | 0.0008 |
| sc fat, thigh | 0.37 | 0.03 | 0.62 | <0.0001 | 0.39 | 0.02 |
| Muscle, thigh | 0.40 | 0.02 | 0.55 | 0.0005 | 0.33 | 0.06 |
| Glucose AUC | 0.53 | 0.001 | 0.66 | <0.0001 | 0.51 | 0.002 |
| Insulin | 0.29 | NS | 0.50 | 0.01 | 0.16 | NS |
| HOMA-IR | 0.37 | 0.05 | 0.56 | 0.002 | 0.2 | NS |
| Triglycerides | 0.56 | 0.0003 | 0.37 | 0.03 | 0.64 | <0.0001 |
NS, Not significant; TAT, total adipose tissue.
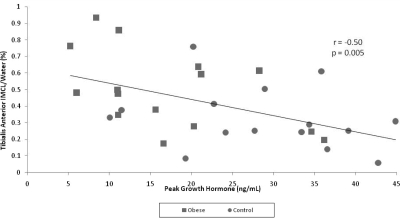
Figure 3.
Regression analysis between IMCL-TA and peak GH in obese subjects (squares) and subjects of normal weight (circles) (n = 30). There is an inverse correlation between IMCL-TA and peak GH.
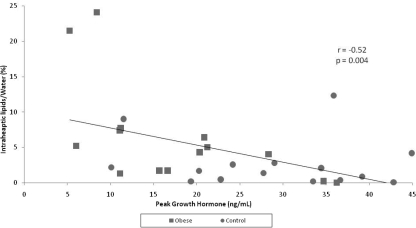
Figure 4.
Regression analysis between intrahepatic lipids and peak GH in obese subjects (squares) and subjects of normal weight (circles) (n = 30). There is an inverse correlation between intrahepatic lipids and peak GH.
When IMCL-TA was entered as a dependent variable and peak GH, HOMA-IR, visceral fat, and weight as independent variables in a forward stepwise regression model, GH explained 30% of the variability of IMCL-TA (r2 =0.30; P = 0.004). When IMCL-SOL was entered as a dependent variable and peak GH, HOMA-IR, visceral fat, and weight as independent variables in a forward stepwise regression model, weight explained 37% of the variability of IMCL-SOL (r2 = 0.37; P = 0.001).
When intrahepatic lipid content was entered into the model as a dependent variable and peak GH, HOMA-IR, visceral fat, and weight as independent variables, GH explained 25% of the variability of intrahepatic lipids (r2 = 0.25; P = 0.01).
When peak GH was entered into the model as a dependent variable and IMCL-TA, intrahepatic lipids, visceral fat, and HOMI-IR as independent variables in a forward stepwise regression model, visceral fat was a significant predictor of peak GH (P = 0.0001), and there was a trend of IMCL-TA as a significant predictor (P = 0.09).
There was an inverse correlation between peak GH after stimulation with GHRH-arginine and BMI (r = −0.45; P = 0.01), total abdominal adipose tissue (r = −0.47; P = 0.01), and visceral abdominal adipose tissue (r = −0.70; P < 0.0001). There were no correlations between IGF-I and measures of abdominal fat. Peak GH after stimulation with GHRH-arginine was inversely associated with total serum cholesterol (r = −0.42; P = 0.02), serum LDL cholesterol (r = −0.45; P = 0.01), serum triglycerides (r = −0.39; P = 0.03), and measures of insulin resistance (glucose-AUC r = −0.75, P < 0.0001; HOMA-IR r = −0.42, P = 0.03).
Discussion
Our study showed that GH is inversely associated with IMCL and intrahepatic lipid content in premenopausal women with obesity, independent of visceral fat, and age. Prior studies have established that visceral adiposity is a strong determinant of GH secretion (6,29) and that low endogenous GH secretion is associated with increased insulin resistance and cardiovascular risk markers in obesity (25). Increased insulin resistance is present in women and men with GH deficiency due to hypopituitarism and in low endogenous GH secretion due to obesity, despite the fact that acute GH administration increases insulin resistance, whereas chronic GH administration has been shown in some but not all studies to have beneficial effects on insulin resistance (30,31,32,33,34). The pathophysiological basis of these apparently contradictory findings is not well understood but is thought to be mediated in part by body composition abnormalities and changes with GH therapy. The role of decreased endogenous GH in the pathogeneses of insulin resistance in obesity has not been elucidated. Increased fat accumulation of skeletal muscle and liver may play an etiological role in the development of insulin resistance and other complications of the metabolic syndrome (3,4,14). We therefore studied the association between GH secretion and IMCL and intrahepatic fat content using 1H-MRS as a novel noninvasive technique in premenopausal women with obesity. We furthermore evaluated body composition using MRI and studied the association between decreased GH secretion and metabolic risk factors (serum lipids and measures of glucose homeostasis). Our data suggest that low GH may contribute to insulin resistance in obesity through effects on IMCL and intrahepatic lipid accumulation. However, interventional studies are needed to further investigate this hypothesis.
We also investigated whether IMCL or intrahepatic lipid concentration were determinants of peak GH after controlling for VAT mass and age, two known strong determinants of peak GH in healthy and obese women. We found that IMCL-TA was a significant determinant of peak GH levels and that there was a trend toward intrahepatic lipid concentration as a determinant of peak GH levels. Therefore, it is possible that fat depots other than visceral fat may decrease GH secretion. Alternatively, our findings may reflect the effects of insulin secretion or resistance on endogenous GH production. Because ours is a cross-sectional study, we cannot determine causality or discern the details of a possible complex relationship of IMCL and intrahepatic lipid concentration with GH secretion and insulin resistance. Further studies are warranted to further characterize these relationships.
Despite a longstanding appreciation that obesity impairs insulin sensitivity, the relationship between body fat, glucose disposal, and GH remains unclear. Lipids are found in adipose stores (visceral, abdominal, and sc), in plasma (FFA and LDL and HDL cholesterol), and within tissues (both extra- and intracellular). In the muscle, EMCL occur in small groups of adipocytes found between muscle fibers. One published study (35) examined the relationship between intermuscular thigh fat and GH release in postmenopausal women with obesity and demonstrated an inverse relationship; however, the relationship between IMCL and GH has not been investigated in adults with obesity. IMCL differs from intermuscular thigh fat in that it occurs as triglyceride droplets within myocytes that are located close to muscle mitochondria and, therefore, serves as an important energy supply of FFA for oxidative metabolism. IMCL content has been found to be a better predictor of insulin resistance than either BMI or plasma FFA among normal-weight, obese, and diabetic men and women with sedentary or modestly active lifestyles (16,36). Furthermore, elevated IMCL has been hypothesized to play an etiological role in obesity-induced insulin resistance (13). In our study, there was a significant difference between IMCL-TA and IMCL-SOL in obese subjects and normal-weight controls as expected. IMCL was positively associated with measures of insulin resistance (glucose AUC, and HOMA-IR), abdominal fat, and serum triglycerides and inversely with GH.
We studied IMCL of tibialis anterior and soleus muscles. The tibialis anterior is predominately composed of type IIb (fast-twitch) fibers, and the orientation of tibialis anterior fibers is parallel to the magnetic field, which allows optimal peak separation. However, the fibers have a lower concentration of IMCL and are less insulin sensitive compared with soleus fibers (16,17,37). The soleus is predominantly composed of type I (slow-twitch) fibers, and soleus fibers have the highest concentration of IMCL and strongest correlation with insulin sensitivity (16,17,37). However, the pennate orientation of the muscle makes it more difficult to perform 1H-MRS (16,17). Despite this, we were able to obtain excellent quality spectra of both muscles. IMCL-TA showed a stronger inverse association with GH and a stronger positive correlation with intrahepatic lipids and serum triglycerides, whereas IMCL-SOL demonstrated stronger correlations with measures of insulin resistance and body fat. Therefore, the study of tibialis anterior and soleus muscles yields complementary and important insights into the pathophysiology of insulin resistance.
Visceral adiposity is associated with nonalcoholic fatty liver disease, an entity encompassing a broad spectrum ranging from simple steatosis to nonalcoholic steatohepatitis, which is strongly associated with insulin resistance, type 2 diabetes, and hypertriglyceridemia. Studies have shown that the degree of insulin sensitivity is strongly linked to hepatic lipid content (38,39). In our study of premenopausal obese and lean women, intrahepatic lipids determined by 1H-MRS showed a positive association with IMCL, body composition, triglycerides, and glucose AUC and an inverse correlation with GH and IGF-I. Franco et al. (35) did not find a correlation between GH release and intrahepatic lipid content as determined by computed tomography in postmenopausal women with obesity. A potential explanation for these findings could be the narrower range of BMI because only obese patients were studied, whereas we studied obese subjects and normal-weight controls. Of note, however, is that in our study, GH remained a significant predictor of intrahepatic lipid content after controlling for weight and age.
In conclusion, in premenopausal women with obesity, peak GH is inversely associated with IMCL and intrahepatic lipid content independent of visceral fat and age. This suggests that low GH may contribute to insulin resistance in obesity through effects on muscle and liver fat. However, further studies are necessary to investigate this hypothesis.
Footnotes
This work was supported in part by National Institutes of Health Grants RO1 HL-077674, M01 RR-01066, and K23 RR-23090.
Disclosure summary: The authors have nothing to disclose.
First Published Online July 14, 2009
Abbreviations: AUC, Area under the curve; BMI, body mass index; EMCL, extramyocellular lipids; FFA, free fatty acids; HDL, high-density lipoprotein; 1H-MRS, proton magnetic resonance spectroscopy; HOMA-IR, homeostasis model assessment of insulin resistance; IMCL, intramyocellular lipids; IMCL-SOL, IMCL of soleus muscle; IMCL-TA, IMCL of tibialis anterior muscle; LDL, low-density lipoprotein; MRI, magnetic resonance imaging; SAT, sc adipose tissue; VAT, visceral adipose tissue.
References
- Christopher M, Hew FL, Oakley M, Rantzau C, Alford F 1998 Defects of insulin action and skeletal muscle glucose metabolism in growth hormone-deficient adults persist after 24 months of recombinant human growth hormone therapy. J Clin Endocrinol Metab 83:1668–1681 [DOI] [PubMed] [Google Scholar]
- Hoffman DM, Nguyen TV, O'Sullivan AJ, Baxter RC, Ho KK 1994 Diagnosis of growth hormone deficiency in adults. Lancet 344:482–483 [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL 2000 Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21:697–738 [DOI] [PubMed] [Google Scholar]
- Yu AS, Keeffe EB 2002 Nonalcoholic fatty liver disease. Rev Gastroenterol Disord 2:11–19 [PubMed] [Google Scholar]
- Hwu CM, Kwok CF, Lai TY, Shih KC, Lee TS, Hsiao LC, Lee SH, Fang VS, Ho LT 1997 Growth hormone (GH) replacement reduces total body fat and normalizes insulin sensitivity in GH-deficient adults: a report of one-year clinical experience. J Clin Endocrinol Metab 82:3285–3292 [DOI] [PubMed] [Google Scholar]
- Miller KK, Biller BM, Lipman JG, Bradwin G, Rifai N, Klibanski A 2005 Truncal adiposity, relative growth hormone deficiency, and cardiovascular risk. J Clin Endocrinol Metab 90:768–774 [DOI] [PubMed] [Google Scholar]
- Ho KK, O'Sullivan AJ, Hoffman DM 1996 Metabolic actions of growth hormone in man. Endocr J 43(Suppl):S57–S63 [DOI] [PubMed] [Google Scholar]
- Nørrelund H, Nielsen S, Christiansen JS, Jørgensen JO, Møller N 2004 Modulation of basal glucose metabolism and insulin sensitivity by growth hormone and free fatty acids during short-term fasting. Eur J Endocrinol 150:779–787 [DOI] [PubMed] [Google Scholar]
- Segerlantz M, Bramnert M, Manhem P, Laurila E, Groop LC 2003 Inhibition of lipolysis during acute GH exposure increases insulin sensitivity in previously untreated GH-deficient adults. Eur J Endocrinol 149:511–519 [DOI] [PubMed] [Google Scholar]
- Bengtsson BA, Johannsson G 1998 The use of growth hormone in adults: a review of the last 10 years, the present and a perspective for the future. Growth Horm IGF Res 8(Suppl B):27–35 [DOI] [PubMed] [Google Scholar]
- Franco C, Brandberg J, Lönn L, Andersson B, Bengtsson BA, Johannsson G 2005 Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab 90:1466–1474 [DOI] [PubMed] [Google Scholar]
- Boesch C, Slotboom J, Hoppeler H, Kreis R 1997 In vivo determination of intra-myocellular lipids in human muscle by means of localized 1H-MR-spectroscopy. Magn Reson Med 37:484–493 [DOI] [PubMed] [Google Scholar]
- Shulman GI 2000 Cellular mechanisms of insulin resistance. J Clin Invest 106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S 2002 Assessment of skeletal muscle triglyceride content by 1H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 51:1022–1027 [DOI] [PubMed] [Google Scholar]
- Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maerker E, Matthaei S, Schick F, Claussen CD, Häring HU 1999 Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 48:1113–1119 [DOI] [PubMed] [Google Scholar]
- Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI 1999 Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42:113–116 [DOI] [PubMed] [Google Scholar]
- Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L 1999 Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48:1600–1606 [DOI] [PubMed] [Google Scholar]
- Cowin GJ, Jonsson JR, Bauer JD, Ash S, Ali A, Osland EJ, Purdie DM, Clouston AD, Powell EE, Galloway GJ 2008 Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging 28:937–945 [DOI] [PubMed] [Google Scholar]
- Hwang JH, Stein DT, Barzilai N, Cui MH, Tonelli J, Kishore P, Hawkins M 2007 Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab 293:E1663–E1669 [DOI] [PubMed] [Google Scholar]
- Machann J, Stefan N, Schick F 2008 1H MR spectroscopy of skeletal muscle, liver and bone marrow. Eur J Radiol 67:275–284 [DOI] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI 2002 Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol 90:11G–18G [DOI] [PubMed] [Google Scholar]
- Misra M, Bredella MA, Tsai P, Mendes N, Miller KK, Klibanski A 2008 Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am J Physiol Endocrinol Metab 295:E385–E392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller BM, Samuels MH, Zagar A, Cook DM, Arafah BM, Bonert V, Stavrou S, Kleinberg DL, Chipman JJ, Hartman ML 2002 Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab 87:2067–2079 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Utz AL, Yamamoto A, Hemphill L, Miller KK 2008 Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab 93:2507–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torriani M, Thomas BJ, Bredella MA, Ouellette H 2007 Intramyocellular lipid quantification: comparison between 3.0- and 1.5-T 1H-MRS. Magn Reson Imaging 25:1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW 1993 Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679 [DOI] [PubMed] [Google Scholar]
- Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S 2004 Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr 80:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Utz AL, Torriani M, Thomas B, Schoenfeld DA, Miller KK 2009 Anthropometry, CT, and DXA as predictors of GH deficiency in premenopausal women: ROC curve analysis. J Appl Physiol 106:418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowelin J, Attvall S, Lager I, Bengtsson BA 1993 Effects of treatment with recombinant human growth hormone on insulin sensitivity and glucose metabolism in adults with growth hormone deficiency. Metabolism 42:1443–1447 [DOI] [PubMed] [Google Scholar]
- Johannsson G, Mårin P, Lönn L, Ottosson M, Stenlöf K, Björntorp P, Sjöström L, Bengtsson BA 1997 Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab 82:727–734 [DOI] [PubMed] [Google Scholar]
- Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P 2004 Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a metaanalysis of blinded, randomized, placebo-controlled trials. J Clin Endocrinol Metab 89:2192–2199 [DOI] [PubMed] [Google Scholar]
- Svensson J, Fowelin J, Landin K, Bengtsson BA, Johansson JO 2002 Effects of seven years of GH-replacement therapy on insulin sensitivity in GH-deficient adults. J Clin Endocrinol Metab 87:2121–2127 [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE 1990 Effects of human growth hormone in men over 60 years old. N Engl J Med 323:1–6 [DOI] [PubMed] [Google Scholar]
- Franco C, Veldhuis JD, Iranmanesh A, Brandberg J, Lönn L, Andersson B, Bengtsson BA, Svensson J, Johannsson G 2006 Thigh intermuscular fat is inversely associated with spontaneous GH release in post-menopausal women with abdominal obesity. Eur J Endocrinol 155:261–268 [DOI] [PubMed] [Google Scholar]
- Thamer C, Machann J, Bachmann O, Haap M, Dahl D, Wietek B, Tschritter O, Niess A, Brechtel K, Fritsche A, Claussen C, Jacob S, Schick F, Häring HU, Stumvoll M 2003 Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab 88:1785–1791 [DOI] [PubMed] [Google Scholar]
- Vermathen P, Kreis R, Boesch C 2004 Distribution of intramyocellular lipids in human calf muscles as determined by MR spectroscopic imaging. Magn Reson Med 51:253–262 [DOI] [PubMed] [Google Scholar]
- Goto T, Onuma T, Takebe K, Kral JG 1995 The influence of fatty liver on insulin clearance and insulin resistance in non-diabetic Japanese subjects. Int J Obes Relat Metab Disord 19:841–845 [PubMed] [Google Scholar]
- Johanson EH, Jansson PA, Lönn L, Matsuzawa Y, Funahashi T, Taskinen MR, Smith U, Axelsen M 2003 Fat distribution, lipid accumulation in the liver, and exercise capacity do not explain the insulin resistance in healthy males with a family history for type 2 diabetes. J Clin Endocrinol Metab 88:4232–4238 [DOI] [PubMed] [Google Scholar]